Abstract
The species Kinosternon scorpioides (scorpion mud turtle) has potential for commercial farming in captivity. Restraint is a stressful factor that can cause fatal reactions. However, research on stress in Muçuãs is scarce. Therefore, electrocardiography (ECG) is relevant because it allows precise diagnoses of cardiac events. The results revealed an increase in heart rate and amplitude in both sexes, with higher heart rates in males and greater amplitude in females. The P-Q interval decreased in females and remained unchanged in males. The QRS interval did not show statistically significant differences, but there was a slight decrease in males. The Q-T interval decreased in females, while it did not show significant variations in males. These findings suggest that the variation in intervals and, consequently, heart rate occurs due to the regulation of the adrenergic pathway during periods of stress in turtles, mainly due to high concentrations of β-adrenergic receptors. Therefore, it was shown that both females and males experienced cardiovascular instability during restraint stress, with greater instability in males.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
The breeding of turtles worldwide is primarily targeted at the pet trade, however, in Asian countries, high demand for this product is also associated with the production of gastronomic dishes [1]. In South America, specifically in the Brazilian Amazon, many freshwater turtles are exploited for consumption, where chelonians are acquired at high prices, revealing enormous economic potential [2,3,4]. In addition, the lack of legalized breeding facilities allows for the search for illicit sources, and the indiscriminate consumption of these animals has exacerbated the population decline of many species [5,6,7].
Breeding turtles in captivity is an alternative approach for the conservation of this group, as it enables conservation management and provides strategies for alternative income sources [4, 7]. Of the eighteen species of chelonians found in the Amazon region, six have potential for management: five species from the Podocnemidae family and one from the Kinosternidae family, the Kinosternon scorpioides [3, 8].
The species K. scorpioides (scorpion mud turtle) arouses interest not only as a food item but also for supplementary income in small-scale family or community production. Furthermore, it is significant in commercial captive breeding [9]. It has a great zootechnical aptitude, reproducing well in captivity and responding to inputs for growth and weight gain [10, 11].
It is a Brazilian freshwater turtle, encompassing more than 10,000 species with an average adult carapace length of 11.5–15.6 cm and in the state of Maranhão, Brazil, these animals feed primarily on fish, tadpoles, insects, and algae [12,13,14,15]. They exhibit sexual dimorphism, where adult males are larger than females, with a concave plastron to facilitate mounting for copulation, and a longer, thicker tail with a horny appendage at its tip. In contrast, females have a flat plastron and a shorter tail [16, 17].
Although hunting is prohibited, in Maranhão and Pará, it is considered a delicacy in local cuisine and is served clandestinely in hotels and upscale restaurants. The meat of K. scorpioides is very popular, and this turtle is easily kept in captivity. Nevertheless, primarily due to hunting and deforestation, the number of individuals of this species has decreased drastically in the last decade [18, 19]. Moreover, the low growth rates and the long periods required to reach maturity, coupled with their generally long lifespan, are associated with a low replacement rate of individuals in the population. These characteristics can predispose species to the risk of extinction [20].
Confinement is the most stressful moment in the life of a wild animal, potentially leading to fatal reactions, and is a factor that is underexplored in current scientific research [21]. In a study conducted on salmonid fish, it was observed that confinement in their breeding environment inhibited behavioral thermoregulation and forced researchers to develop adaptation strategies to maintain productivity [22], which could possibly be considered a stress factor. What is widely found in scientific studies is stress in the overall handling process and the use of anesthetic substances to alleviate this context [23], however, there is a lack of research on confinement stress in scorpion mud turtle (K. scorpioides).
Electrocardiography (ECG) is highly relevant as it allows for conclusive diagnosis of any cardiac conditions such as arrhythmias, tachycardias, or bradycardias. High-quality ECG recordings are important to distinguish between normal and abnormal complexes in a living being [24]. This method of analysis has been used in cardiac research of various species, both aquatic, such as fish [25], and others terrestrial, such as mice [26]. In this context, given the environmental importance of the aquatic species scorpion mud turtle, it is interesting to analyze the electrocardiographic recording of this species when subjected to restraint stress.
Therefore, due to the scarcity of information about this chelonian combined with the risks of extinction, this article aimed to analyze the electrocardiographic (ECG) recordings of hatchling female and male K. scorpioides subjected to restraint stress. This analysis seeks to understand the effect of this condition on the animals and, consequently, on the environment.
2 Materials and methods
2.1 Animals
For this study, 8 animals were used 24 h after birth, consisting of 4 males (4.71 ± 0.83 g) and 4 females (3.65 ± 0.36 g), average carapace length for males (27.71 ± 1.49 mm) for females (26.51 ± 1.04 mm). The animals were obtained from the experimental farm of the Brazilian Agricultural Research Corporation (Embrapa Amazônia Oriental) (LO 7310/2014-SEMAS-PA), located on Marajó Island (Salvaterra, Pará, Brazil, 48° 33′ 23.32″ W and 0° 42′ 25.26″ S). This study was approved by the Ethics Committee for Animal Use (No. 001/2016) of Embrapa Amazônia Oriental. These animals were sent to the Laboratory of Pharmacology and Toxicology of Natural Products at UFPA-ICB, an environment with regulated temperature. (24–25 °C). The study complies with institutional, national or international guidelines.
2.2 Monitoring of incubation and morphometry of the animals
For the morphometry of the eggs (length and width), a manual caliper (precision of 0,2 mm) was used, and for weight measurement, a digital scale was employed. (8068-series Professional Jewelry scale). The newly hatched offspring (n = 8) were weighed and measured (length and width of the carapace and plastron). Incubation was performed in a controlled environment at a temperature of (+ 25 °C), with the temperature and humidity of the location recorded by a thermo-hygrometer (Inconterm model 7664.01.0.00).
In 8 eggs, two distinct temperatures were tested to verify temperature-dependent sex determination: 28.0 ± 0.5 °C (n = 4; males) and 30.0 ± 0.5 °C (females; n = 4) [27]. The newly hatched offspring (n = 10) were weighed and measured (length and width of the carapace and plastron). For sexing the newly hatched individuals, the gonad was dissected, fixed in 10% formaldehyde solution, preserved in 70% alcohol, and subsequently processed for routine histological examination [28].
2.3 Electrode fabrication
The electrodes for cardiac signal acquisition were made with metal composed of nickel/chromium in an 80:20 ratio, and then soldered to non-conjugated JST SM cables. Subsequently, they were insulated with self-curing dental acrylic resin. The non-insulated part of the electrode measured 0.3 mm for cardiac signal acquisition.
2.4 Electrode implantation and physical restraint method
The coordinates for electrocardiogram acquisition were made in lead D2, with the reference electrode positioned in the fissure between the Humeral and Pectoral plates 0.5 cm from the edge of the right plastron. The recording electrode was positioned in the abdominal and femoral fissure 0.5 cm from the edge of the left plastron, with a penetration of 2.5 mm for ECG recordings (Fig. 1).
For the physical restraint method, a support was used to suspend the animals, preventing their movement (Fig. 1). During restraint, the animals were in a prone position (normal position for these animals). At the beginning of the restraint, the animals attempted to move and became agitated, displaying stress behavior. During this period, electrocardiograms were performed, each lasting 15 min for both males and females. All recordings were made between 8:00 and 11:00 AM.
2.5 Electrocardiographic(ECG) signal acquisition
The entire procedure for obtaining the recording was performed inside a Faraday cage with a metal screen. The electrodes were connected to a high-impedance amplifier (Grass Technologies, P511) (using a 5000× amplification), and monitored by an oscilloscope (Protek, 6510). All recordings lasted 15 min for each animal subjected to restraint stress.
2.6 Processing of the obtained data
Offline analysis was performed using a tool created in the Python programming language (version 2.7). The'Numpy'and'Scipy'libraries were used for mathematical processing, and the'matplotlib'library was used for obtaining graphs and plots. A graphical interface was developed using the PyQt4 library. Spectrograms were calculated using the Hamming window with 256 points (256/1000 s). For power spectral density (PSD), each frame was generated with an overlap of 128 points per window. For each frame, the PSD was calculated using Welch's averaged periodogram method. Frequency histograms were obtained by calculating the PSD of the signal using the Hamming window with 256 points without overlap, producing a resolution of 1 Hz per bin. Each wave displayed in the PSD was an average of a set of experiments. The PSDs were calculated for each group, and the averages were shown in individual bins. Analyses were performed on the ECG regarding the power of the recordings that were analyzed in the periods of 0–300 s, 300–600 s, and 600–900 s between males and females. Morphographic parameters of the electrocardiogram were analyzed at the beginning of the recording in the period of 0–10 s and at the end of every 5 min of stress in the periods of 290–300 s, 590–600 s, and 890–900 s. This morphographic analysis corresponded to the following elements of the recordings: heart rate, recording amplitude, P-Q and Q-T intervals, and QRS complex duration during restraint stress.
2.7 Statistical analysis
Comparisons between the mean amplitude of the tracings and the control values were made using ANOVA followed by Tukey's test. Mean values were followed by their respective standard deviation values (mean ± SD). The significance level was established as, * p < 0.05 ** p < 0.01 *** p < 0.001.
The software GraphPad® Prism 8 was used for statistical tests and graph creation. For morphometric measurements and incubation periods between males and females, the T-test was used.
3 Results
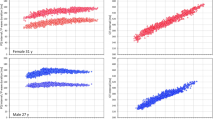
ECG recording with a duration of 15 min during the restraint of newly hatched female and male scorpion mud turtle (Kinosternon scorpioides). The tracings demonstrated a variation in amplitude during the course of restraint stress for females, starting at 2.3 mV and ending at 3.8 mV (Fig. 2A). The spectrogram showed energy variation mainly in the first 5 min for females (Fig. 2B). The recording of males showed more stability in the amplitude, ranging from 2.3 mV to 2.8 mV (Fig. 2C). The spectrogram showed a decrease in power between 300–600 s and an increase in power between 600–900 s (Fig. 2D). When comparing the average power observed in the recordings of females and males during the analyzed periods of 0–300 s (p = 0.382) and 300–600 s (p = 0.188), no difference was observed. In the period of 600–900 s, males showed higher power in cardiac activity recordings (136.4 ± 3.202 mV2/Hz × 10–3) compared to the averages of females (127.4 ± 2.211 mV2/Hz × 10–3) (Fig. 2E). To evaluate the effect of restraint stress on the cardiac activity of females during the analyzed periods, a decrease in power was observed in the period of 300–600 s (120.6 ± 6.199 mV2/Hz × 10–3) and 0–300 s (129.4 ± 3.148 mV2/Hz × 10–3). The average power between the periods 0–300 and 600–900 s (p = 0.177) and 300–600 s and 600–900 s (p = 0.113) (Fig. 2 F). For males, the average power in the period 0–300 s (134.9 ± 3.32 mV2/Hz × 10–3) decreased during the period 300–600 s (127.4 ± 4.167 mV2/Hz × 10–3) and increased again in the period of 600–900 s (136.4 ± 3.202 mV2/Hz × 10−3) (Fig. 2G).
Electrocardiographic tracing represented by 15 min of recording in females, showing the areas of the recordings that were analyzed for power (dotted in red): 0–300 s, 300–600 s, and 600–900 s (A); Energy distribution spectrogram showing areas with different intensities of cardiac energy during restraint stress in females (B); Demonstrative tracing of electrocardiographic recordings obtained from males for power analysis (dotted in red): 0–300 s, 300–600 s, and 600–900 s (C); and energy distribution spectrogram for males (D). Graph with average power comparing females and males according to the recording period (E); average power recorded in the ECG for females (F); average power recorded in the ECG for males (G) (After ANOVA followed by Tukey; *p < 0.05, ** p < 0.01, and ***p < 0.001 (n = 4 F and 4 M)
From each recording, 10 s fragments were taken for analysis at the end of every 5 min of recording to compare the effects of stress on cardiac activity, represented by the periods: 0–10 s, 290–300 s, 590–600 s, and 890–900 s. During these periods, we observed that the heart rhythm in both females and males was sinusoidal, with the presence of all cardiac deflections, represented by the P wave, which indicates atrial depolarization, the QRS complex, which indicates ventricular depolarization, and the T wave, which indicates ventricular repolarization (Fig. 3A). These same graph elements are observed in females and males in the periods 290–300 s (Fig. 3B), 590–600 s (Fig. 3C), and 890–900 s (Fig. 3D).
Tracing patterns found during the 15-min recording obtained at the end of every 5 min, corresponding to the periods: 0–10 s (A), 290–300 s (B), 590–600 s (C), 890–900 s (D), for females (left) and males (right). In each tracing, the graph elements of the electrocardiogram can be observed, such as the P wave (atrial contraction), the QRS complex (ventricular contraction), and the T wave (ventricular repolarization). Heart rate (BPM), Amplitude (mV), P-Q interval (ms), QRS duration (ms), and Q-T interval (ms) were analyzed (shown in red)
For each tracing change during restraint stress, the cardiac activity behavior pattern was analyzed (Fig. 4). For heart rate in the 0–10 s period, the average for females was 43.0 ± 3.46 bpm, which showed no difference compared to the average for males (448.0 ± 6.53 bpm) (p = 0.742). For the 290–300 s period, the average for females was 57.50 ± 5.260 bpm and for males was 61.0 ± 2.58 bpm (p = 0.944). For the 590–600 s period, females had an average of 56.0 ± 5.65 bpm, while males had an average of 59.5 ± 2.517 bpm (p = 0.948). For the 890–900 s period, females had an average of 53.5 ± 4.43 bpm, which showed no difference compared to the male group (58.0 ± 2.82 bpm) (p = 0.8271) (Fig. 4A Left). When comparing the average heart rates during the restraint stress periods, it can be observed that both females and males showed an increase in the 290–300 s, 590–600 s, and 890–900 s periods compared to the 0–10 s period (Fig. 4A center, 4 A right).
Evaluation of cardiac activity between female and male scorpion mud turtle (Kinosternon scorpioides) during the periods of 0–10 s, 290–300 s, 590–600 s, and 890–900 s of physical restraint: Heart rate bpm (A); QRS amplitude (mV) (B); P-Q interval (ms) (C); QRS duration (ms) (D); and Q-T interval (E). After ANOVA followed by Tukey's test. *p < 0.05, **p < 0.01, ***p < 0.001 (n = 4 F and 4M)
The average recording amplitude comparing females and males, in the period of 0–10 s, was 2.220 ± 0.315 mV for females and 2.20 ± 0.114 mV for males (p = 0.999). In the 290–300 s period, the average amplitude for females was 2.945 ± 0.079 mV and for males was 2.608 ± 0.397 mV (p = 0.227). In the 590–600 s period, females had an average of 3.320 ± 0.084 mV, which was higher than the average amplitude of the recordings in males (2.578 ± 0.211 mV).
During the final period of 15 min (890–900 s), females had an average of 3.49 ± 0.161 mV, which was higher than in males (2.608 ± 0.205 mV) (Fig. 4 B left). The recording amplitude in females increased over the course of the recording, as can be observed in Fig. 3B center. Males maintained a consistent average amplitude throughout all periods (Fig. 4B right).
The variation in the P-Q interval observed in the 0–10 s period for females (203.0 ± 30.1 ms) was similar to the average for males (229.5 ± 21.6 ms) (p = 0.895). For the 290–300 s period, the averages for females (161.0 ± 45.5 ms) and males (24.5 ± 7.141 ms) (p = 0.0818). In the 590–600 s period, the average for females (132.5 ± 17.71 ms) was lower than for males (213.5 ± 15.93 ms). However, in the 890–900 s period, the average for females was 180.8 ± 49.07 ms and was similar to the average for males (232.8 ± 18.45 ms) (p = 0.2341) (Fig. 4C, left).
For the P-Q interval, there was no difference between the measured periods for females and males (Fig. 4C, center; 4 C right).
The average duration of the QRS complex comparing females and males, in the period of 0–10 s, was 40.43 ± 3.146 ms for females and 39.53 ± 3.05 ms for males (p = 0.999). In the 290–300 s period, the average QRS duration for females was 37.25 ± 3.948 ms and for males was 38.50 ± 2.646 ms (p = 0.999). In the 590–600 s period, females had an average of 35.00 ± 5.033 ms, which was similar to the average for males (39.75 ± 4.349 ms) (p = 0.6384).
During the final period (890–900 s), females had an average of 42.50 ± 4.655 ms, while the average for males was 34.75 ± 2.50 ms (Fig. 4 D left). The duration of the QRS complex, which represents the speed of ventricular contraction, showed no difference between the measured periods for females and males individually (Fig. 4D, center; 4 D, right).
The average Q-T interval observed in the 0–10 s period for females (544.5 ± 45.86 ms) was similar to the average for males (449.0 ± 45.32 ms) (p = 0.0568). In the 290–300 s period, the average for females (348.30 ± 17.13 ms) was lower than for males (481.0 ± 13.29 ms). In the 590–600 s period, the average for females (375.3 ± 74.41 ms) was similar to the average for males (434.3 ± 44.06 ms). In the 890–900 s period, the average for females was 445.5 ± 28.49 ms and was similar to the average for males (436.0 ± 29.65 ms) (p = 0.999) (Fig. 4E, left).
Analyzing the average Q-T interval for females, the initial period (0–10 s) was longer than the other periods. Therefore, restraint stress caused a shortening of the Q-T interval in females (Fig. 4E, center). However, there was no significant difference in the Q-T interval for males (Fig. 4E, right).
In the histological analysis of the gonadal tissue, female gonads were observed in hatchlings subjected to feminizing temperatures (30.0 °C) during incubation. The ovaries displayed a developed cortical region filled with germ cells and primordial follicles (Fig. 5A). Similarly, the formation of male gonads was observed in hatchlings whose eggs were incubated at masculinizing temperatures (28.0 °C). In the testes, the medullary region of the organ was developed and presented seminiferous cords (Fig. 5B).
The males were observed hatching at about 120.6 ± 8.05 days of incubation (n = 4) while the females hatched at 108.2 ± 9.09 days (n = 4) (Table 1), with no significant difference in the morphometric variables between males and females analyzed and between the incubation periods.
4 Discussion
In this study, the difference in restraint stress between newly hatched male and female scorpion mud turtle was demonstrated for the first time through electrocardiographic recording. We found that during the 15 min of ECG restraint in newly hatched female and male K. scorpioides, the tracings showed amplitude variation over the course of the stress, and the spectrogram showed energy variation mainly in the first 5 min for females. In contrast, males showed more stability in the same amplitude of recording as females.
The turtle heart is regulated by cholinergic and adrenergic control [29, 30]. Hicks and Farrell demonstrated that the administration of atropine in turtles is related to an increase in systemic cardiac output and that the administration of adrenaline increases systemic blood pressure and systemic cardiac output, in addition to evidencing the presence of β-adrenergic receptors [29]. In the heart, β-adrenergic receptors are the most important due to their high concentration, and they function to modulate heart rate [31]. Additionally, the α-adrenergic pathway is associated with an increase in systemic vasomotor tone in turtles [32]. The function of the adrenergic pathway during periods of stress is well known [33], which supports our results.
Regarding the heart rate analyzed from the R-R interval electrocardiographic recordings, as used to assess this parameter in the cardiovascular health of other animals [34, 35], it is possible to identify a concordance between the pattern developed in the recordings of females compared to those of males, as both showed an increase in heart rate with the progression of the recording, with males maintaining a higher heart rate than females throughout all intervals. Changes in heart rate over the course of the electrocardiogram recording are a good indicator of stress [36, 37]. The present study presented results consistent with this fact.
The prolongation of the P-Q interval suggests changes in cardiac automaticity [35]. This interval represents the atrial contraction activity and the beginning of ventricular activity [38]. In the present study, there was a decrease in the duration of the P-Q interval in females and males during the restraint stress recording, with a greater decrease in females, indicating that the heart's ability to generate action potentials decreases in females and that cardiac functionality may be affected. This result corroborates the higher increase in heart rate in females observed in K. scorpioides, associated with a decrease in female frequency. It is important to note that several studies associate heart rate dependency with the duration of the P-Q interval [39,40,41].
The QRS complex, which indicates ventricular contraction, is intrinsically linked to ventricular excitability, as outlined by [42]. Its duration provides an indirect assessment of the speed of electrical impulse conduction through the ventricular tissue [43]. The analysis of the QRS complex in K. scorpioides did not reveal statistically significant differences between males and females during the recording intervals. Hicks et al. [29] revealed that the ventricular mass of a turtle species was relatively larger in males, but the density of β-adrenergic receptors was similar between sexes [29].
The QT interval represents depolarization and repolarization in the ECG, with variations mainly due to the end of the T wave rather than the beginning of the QRS complex [44].
Our findings demonstrated a decrease in the QT interval in female scorpion mud turtle and an increase in the interval in males. It is worth noting that the autonomic nervous system (ANS) modulates ventricular repolarization through its action on the myocardium and effects related to heart rate (HR) [45, 46]. The β-adrenergic pathway stimulates the adaptation of the QT interval to gradual changes in heart rate (HR) [47].
Therefore, electrocardiography in scorpion mud turtle during restraint stress revealed that both females and males experienced cardiovascular instability, with greater instability in males. These findings are possibly related to the high density of β-adrenergic receptors present in these animals, which are closely associated with the adrenergic pathway that is sensitized when exposed to restraint. Thus, this study showed that restraint stress can alter the hemodynamics of the species Kinosternon scorpioides, compromising its safety and increasing the risk of mortality. From this perspective, it highlights the need to seek less aggressive alternative methods for restraining these animals, as it is a species of significant environmental importance and is at risk of extinction.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung YH, Fong JJ. Assessing consumer trends and illegal activity by monitoring the online wildlife trade. Biol Conserv. 2018;2272:19–225.
Brito TP, Lima EBS, Santa Rosa JCG. Assessment of chelonian consumption in the municipality of Castanhal-Pará-Brazil. Rev Ouricuri. 2016;6(1):071–103.
Ferrara CR, Fagundes CK, Morcatty TQ, Vogt RC. Amazonian Chelonians: Identification and Distribution Guide. Wildlife Conservation Society, Manaus, Brazil. 2017. https://repositorio.inpa.gov.br/handle/1/36248
Dantas Filho JV, Pontuschka RB, Franck KM, Gasparotto PHG, Cavali J. Cultivation of chelonium promotes conservation and social and economic development of the Amazon. Rev Cienc Saúde Anim. 2020;2:09–31. https://doi.org/10.6084/m9.figshare.12058596.v1.
Cristo SS, Baia Junior PC, da Silva JS, Marques JRF, De Araujo Guimarães DA. The trade of Kinosternon scorpioides on Marajó island, Brazilian Amazon: from hunting to consumption. J Herpetol. 2017;27(4):361–7.
De Faria VA, Malvásio A. Aspects about hunting, commercialization and consumption of chelonians in the araguaia bananal ecological corridor region in the state of tocantins. Rev Ouricuri. 2018;8(2):026–48. https://doi.org/10.29327/ouricuri.v8.i2.a3.
Alves D, de Barros PBA. Analysis of the feasibility of cheloniculture in the municipality of tefé: species conservation and income generation. Rev Cient Acertte. 2022;2(1):1–15. https://doi.org/10.47820/acertte.v2i1.42.
Júnior GS, Balestra RAM, Luz VLF. Brief history of the conservation of Amazonian chelonians in Brazil. Conservation management and population monitoring of Amazonian chelonians. Brazilian Institute of Environment and Renewable Natural Resources. Brasília: Ibama, 2016:11–14.
Rodrigues AS, Ávila SGD. Physical-chemical characterization of chicken egg shell and use as a source for production of calcium compounds. Rev Virtual de Química. 2017;9(2):596–607. https://doi.org/10.21577/1984-6835.20170035.
Araújo JC, Palha M, Rosa PV. Nutrition in chelonian captive breeding: Review. Revista Eletrônica Nutritime. 2013;10(06):2833–71.
Costa JDS, Figueiró MR, Marques LC, Sales RL, Schierholt AS, Marques JRF. Productive behavior of Muçuãs (Kinosternon scorpioides spp. Linnaeus, 1766) on Marajó Island, Pará State. 2015. http://www.alice.cnptia.embrapa.br/alice/handle/doc/1056031.
Sousa AL, Campos-Junior PHA, Costa GMJ, França LR. Spermatogenic cycle length and sperm production in the freshwater turtle Kinosternon scorpioides. Biol Reprod. 2014;90(2):35. https://doi.org/10.1095/biolreprod.113.112391.
Chaves LPFA, Viana DC, Chaves EP, Miglino MA, Sousa AL. Reproductive morphophysiology of the male scorpion mud turtle (Kinosternon scorpioides Linnaeus, 1766) in captivity. Vet Med Sci. 2020;6(3):570–8. https://doi.org/10.1002/vms3.245.
Berry JF, Iverson JB. Kinosternon scorpioides. Catalogue of American Amphibians and Reptiles. 2001;725:1–11.
Ernst CH, Barbour RW. Turtlesof the World. Washington, D.C.: Smithsonian Institution Press; 1989.
Costa FB, Alves FR, Costa AP, Barros ACE, Guerra PC, Sousa AL, Oliveira AS. Ultrasonographic and radiographic determination of egg development of jurarás (Kinosternon scorpioides) in captivity1. Pesq Vet Bras. 2009;29(10):841–6. https://doi.org/10.1590/S0100-736X2009001000011.
Costa FB, Oliveira AS, Sousa AL, Costa AP, Araújo AVC, Rocha AL, Coelho GB. Biometric aspects correlated with sexual maturity in female Jurará (Kinosternon scorpioides Linnaeus, 1766) in captivity. (2006). UEMA, 18, São Luis. Anais... São Luis.
Pereira LA, Sousa AL, Lemos JJS. Extractivism of Jurará Kinosternon scorpioides Linnaeus, 1766 (Reptila, Chelonia, Kinosternidae) and socio-environmental assessment of fishermen in the Municipality of São Bento/MA. (2007). In: Silva, A.C., Fortes, J.L.O (eds.), Diversidade Biológica Uso e Conservação de Recursos Naturais no Maranhão. Projeto e Ações em Biologia e Química; 2:269–299.
Carvalho RC, de Oliveira SCR, Bombonato PP, Oliveira AS, de Sousa AL. Morphology of the male genital organs of the Scorpion Mud Turtle Kinosternon scorpioides (Chelonia: Kinosternidae). Pesquisa Veterinária Brasileira. 2010;30(4):289–94. https://doi.org/10.1590/S0100-736X2010000400001.
Salera Júnior G. Evaluation of reproductive biology, natural predation and social importance in turtles occurring in the Araguaia basin. Dissertation (Master's in Environmental Sciences) - Universidade Federal do Tocantins, Palmas. 2005.
Fischer CP, Romero LM. Chronic captivity stress in wild animals is highly species-specific. Conserv Physiol. 2019;7(1):coz093. https://doi.org/10.1093/conphys/coz093.
Pankhurst NW, King HR. Temperature and salmonid reproduction: implications for aquaculture. J Fish Biol. 2010;76(1):69–85. https://doi.org/10.1111/j.1095-8649.2009.02484.x.
DiGeronimo PM, Balko JA. Sedation of anesthesia of amphibians. Vet Clin North Am Exot Anim Pract. 2022;25(1):31–47. https://doi.org/10.1016/j.cvex.2021.08.008.
Mitchell KJ. Equine electrocardiography. Vet Clin N Am Equine Pract. 2019;35(1):65–83. https://doi.org/10.1016/j.cveq.2018.12.007.
Zhao Y, James NA, Beshay AR, Chang EE, Lin A, Bashar F, Wassily A, Nguyen B, Nguyen TP. Adult zebrafish ventricular electrical gradients as tissue mechanisms of ECG patterns under baseline vs oxidative stress. Cardiovasc Res. 2021;117(8):1891–907. https://doi.org/10.1093/cvr/cvaa238.
Calvet C, Seebeck P. What to consider for ECG in mice—with special emphasis on telemetry. Mamm Genome. 2023;34(2):166–79. https://doi.org/10.1007/s00335-023-09977-0.
Ewert MA, Nelson CE. Sex determination in turtles: diverse patterns and some possible adaptive values. Am Soc Ichthyol Herpetol. 1991;1991(1):50–69. https://doi.org/10.2307/1446248.
Prophet EB, Mills B, Arrington JB, et al. Afip laboratory methods in histotechnology. Washington: American Registry of Pathology; 1992. p. 278.
Hicks JMT, Farrel AP. The cardiovascular responses of the red-eared slider (Trachemis scripta) acclimated to either 22 or 5°C. II. Effects of anoxia on adrenergic and cholinergic control. J Exp Biol. 2000;203:3775–84. https://doi.org/10.1242/jeb.203.24.3775.
Garner M, Barber RG, Cussins J, Hall D, Reisinger J, Stecyk JAW. Does the ventricle limit cardiac contraction rate in the anoxic turtle (Trachemys scripta)? II. In vivo and in vitro assessment of the prevalence of cardiac arrhythmia and atrioventricular block. Curr Res Physiol. 2022;8(5):292–301. https://doi.org/10.1016/j.crphys.2022.07.002.
Szentmiklosi JA, Szentandrássy N, Hegyi B, Horvath B, Magyar J, Bányász T, Nanasi PP. Chemistry, physiology, and pharmacology of β-adrenergic mechanisms in the heart. Why are β-blocker antiarrhythmics superior? Curr Pharm Des. 2015;21(8):1030–41. https://doi.org/10.2174/1381612820666141029111240.
Stecyk JAW, Overgaard J, Farrell AP, Wang T. Alpha-adrenergic regulation of systemic peripheral resistance and blood flow distribution in the turtle Trachemys scripta during anoxic submergence at 5 degrees C and 21 degrees C. J Exp Biol. 2004;207(Pt 2):269–83. https://doi.org/10.1242/jeb.00744.
Krizanova O, Myslivecek J, Tillinger A, Jurkovicova D, Kubovcakova L. Adrenergic and calcium modulation of the heart in stress: from molecular biology to function. Stress. 2007;10(2):173–84. https://doi.org/10.1080/10253890701305754.
Xing N, Ji L, Song J, Ma J, Li S, Ren Z, Xu F, Zhu J. Cadmium stress assessment based on the electrocardiogram characteristics of zebra fish (Danio rerio): QRS complex could play an important role. Aquat Toxicol. 2017;191:236–44. https://doi.org/10.1016/j.aquatox.2017.08.015.
Cantanhêde SM, de Carvalho ISC, Hamoy M, Corrêa JAM, de Carvalho LM, Barbas LAL, Montag LFA, Amado LL. Evaluation of cardiotoxicity in Amazonian fish Bryconops caudomaculatus by acute exposure to aluminium in an acidic environment. Aquat Toxicol. 2022;242: 106044. https://doi.org/10.1016/j.aquatox.2021.106044.
Anusha AS, Sukumaran P, Sarveswaran V, Shyam A, Akl TJ, Preejith SP, Sivaprakasam M. Electrodermal activity based pre-surgery stress detection using a wrist wearable. IEEE J Biomed Health Inform. 2020;24(1):92–100. https://doi.org/10.1109/JBHI.2019.2893222.
Pourmohammadi S, Maleki A. Stress detection using ECG and EMG signals: a comprehensive study. Comput Methods Programs Biomed. 2020;193: 105482. https://doi.org/10.1016/j.cmpb.2020.105482.
Choi S, Adnane M, Lee GJ, Jang H, Jiang Z, Park HK. Development of ECG beat segmentation method by combining lowpass filter and irregular R-R interval checkup strategy. Expert Syst Appl. 2010;37(7):5208–18. https://doi.org/10.1016/j.eswa.2009.12.069.
Dilaveris PE, Färbom P, Batchvarov V, Ghuran A, Malik M. Circadian behavior of P-wave duration, P-wave area, and PR interval in healthy subjects. Ann Noninvasive Electrocardiol. 2001;6(2):92–7. https://doi.org/10.1111/j.1542-474x.2001.tb00092.x.
Soliman EZ, Rautaharju PM. Heart rate adjustment of PR interval in middle-aged and older adults. J Electrocardiol. 2012;45(1):66–9. https://doi.org/10.1016/j.jelectrocard.2011.06.003.
Malik M, Hnatkova K, Sisakova M, Schmidt G. Subject-specific heart rate dependency of electrocardiographic QT, PQ, and QRS intervals. J Electrocardiol. 2008;41:491–7. https://doi.org/10.1016/j.jelectrocard.2008.06.022.
Vilhena CS, da Silva RA, da Costa BMPA, Torres MF, de Mello VJ, Noronha RCR, da Silva JKR, Hamoy M, Barbas LAL, Nascimento LAS. Cardiac response in tambaqui Colossoma macropomum anaesthetised with Piper divaricatum essential oil. Fish Physiol Biochem. 2022;48(5):1413–25. https://doi.org/10.1007/s10695-022-01132-x.
Cotter PA, Rodnick KJ. Differential effects of anesthetics on electrical properties of the rainbow trout (Oncorhynchus mykiss) heart. Comp Biochem Physiol A Mol Integr Physiol. 2006;145(2):158–65. https://doi.org/10.1016/j.cbpa.2006.06.001.
Cowan JC, Yusoff K, Moore M, Amos PA, Gold AE, Bourke JP, Tansuphaswadikul S, Campbell RW. Importance of lead selection in QT interval measurement. Am J Cardiol. 1988;61(1):83–7. https://doi.org/10.1016/0002-9149(88)91309-4.
Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res. 2007;100(7):e72–80. https://doi.org/10.1161/01.RES.0000264101.06417.33.
Nayyar S, Roberts-Thomson KC, Hasan MA, Sullivan T, Harrington J, Sanders P, Baumert M. Autonomic modulation of repolarization instability in patients with heart failure prone to ventricular tachycardia. Am J Physiol Heart Circ Physiol. 2013;305(8):H1181–8. https://doi.org/10.1152/ajpheart.00448.2013.
Pérez C, Cebollada R, Mountris KA, Martínez JP, Laguna P, Pueyo E. The role of β-adrenergic stimulation in QT interval adaptation to heart rate during stress test. PLoS ONE. 2023;18(1): e0280901. https://doi.org/10.1371/journal.pone.0280901.
Acknowledgements
This work received financial support through research grants provided by the Coordination for the Improvement of Higher Education Personnel- CAPES and the Federal University of Pará- UFPA.
Author information
Authors and Affiliations
Contributions
Moisés Hamoy, Brenda Stefany dos Santos Braga, Deise de Lima Cardoso, Daniella Bastos de Araújo, Luana Souza de Vasconcelos, Gabriela Brito Barbosa, Laís Helena Baptista Amóras, Gabriela Brito Barbosa and Lucas Lima da Rocha contributed to the research design, conduction of experiments (animal experiments) and data collection. Moisés Hamoy, Maria Klara Otake Hamoy, Clarissa Araújo da Paz, Luciana Eiró-Quirino, Thaysa de Sousa Reis contributed to interpretation and manuscript revision. Moisés Hamoy, José Ribamar Felipe Marques and Diva Anelie de Araújo Guimarães contributed to research design, study supervision, funding acquisition, data interpretation, manuscript revision and funding support. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Animal guidelines
The study complies with institutional, national or international guidelines. The study complies with institutional, national or international guidelines. This study was approved by the Ethics Committee for Animal Use (No. 001/2016) of Embrapa Amazônia Oriental. These animals were sent to the Laboratory of Pharmacology and Toxicology of Natural Products at UFPA-ICB, an environment with regulated temperature. (24–25 °C).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
dos Santos Braga, B.S., de Lima Cardoso, D., de Araújo, D.B. et al. Electrocardiographic recording (ECG) of hatchling females and males of scorpion mud turtle (Kinosternon scorpioides) subjected to restraint stress. Discov Anim 2, 21 (2025). https://doi.org/10.1007/s44338-025-00066-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44338-025-00066-x