Abstract
Second-generation antipsychotics (SGAs) have been developed as an alternative to the first-generation antipsychotics due to their fewer side effects. However, clinical case reports suggested a connection between SGAs and ileus. The aim of this study is to explore the links between ileus and SGAs via a pharmacovigilance analysis. Adverse event reports from January 1st, 2014 to December 31st, 2023 were collected from the U.S. Food and Drug Administration Adverse Event Reporting System database. The demographic characteristics of cases were summarized to perform descriptive analysis. The reaction outcomes, actions taken with the drug, and onset time of ileus, were also extracted and analyzed. In disproportionality analysis, the algorithms of reporting odds ratio (ROR) and information component (IC) were applied for ileus risk signal detection. A total of 419 cases with SGAs-related ileus were obtained from the database. The median age for patients was 58 years (range 6 ~ 94) and the median onset time of ileus was 40 days (range 0 ~ 6070). After excluding reports with incomplete data, a total of 219 cases were identified in reaction outcomes and a total of 179 cases were included in the analysis of actions taken with the drug. A majority of patients reached recovered state (n = 119, 54.34%) in reaction outcomes, and most patients received drug withdrawn (n = 120, 67.04%) in actions taken with the drug. Clozapine showed the strongest risk signal of ileus (n = 131, ROR025 = 2.77, IC025 = 1.42), whereas this signal was not significant in risperidone (n = 29, ROR025 = 0.68, IC025 = −0.38). In summary, our data suggested that SGAs administration increased the risk of ileus. These results would provide valuable insight into the prognosis and safe use of SGAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
Antipsychotics are essential part of pharmacological treatment for schizophrenia and other psychotic disorders, such as bipolar disorders, dementia, anxiety, and depression (Solmi et al. 2017). Early synthesized antipsychotic drugs, including chlorpromazine, perphenazine, fluphenazine, trifluoperazine, and thioridazine, are known as first-generation antipsychotics. However, a large number of patients treated with first-generation antipsychotics developed severe side effects, including extrapyramidal adverse effects, tardive dyskinesia, and parkinsonism (Solmi et al. 2017; Lally and MacCabe 2015). Such adverse effects prompted the development of second-generation antipsychotics (SGAs), which have fewer side effects while maintaining the efficacy (Grajales et al. 2019).
Ileus is a serious gastrointestinal disease with potentially fatal consequences, such as shock, water-electrolyte disturbances, and intra-abdominal infections (Nielsen and Meyer 2012). Medication-induced ileus has been commonly seen in the use of anticholinergics, opioids, antihistamines, calcium antagonists, and antipsychotics (Isbister et al. 2001). Although SGAs have been widely used clinically, SGAs-associated ileus has only been reported occasionally from case reports and case-based reviews (McKinnon et al. 2009; Tyras et al. 2021). The safety assessment of antipsychotics in ileus has been reported in several studies previously (Nielsen and Meyer 2012; Hasegawa et al. 2024; Ingimarsson et al. 2018). However, these findings were derived from national medical information databases which might limit the generalizability of the results globally. Hence, a larger-scale real-world dataset is needed to reflect a more comprehensive situation of this rare adverse drug reaction (ADR). The United States Food and Drug Administration (FDA) defines “real-world data” as “data regarding the usage, or the potential benefits or risks, of a drug derived from sources other than traditional clinical trials” (Liu and Panagiotakos 2022). Since previous clinical trials were conducted in a limited number of participants with specific enrollment criteria, relevant results regarding SGAs-associated ileus might not provide an exhaustive presentation of real-world situations.
The FDA adverse event (AE) reporting system (FAERS) is a publicly available database of spontaneous AE reports, serving as an essential resource for real-world pharmacovigilance analysis (Khaleel et al. 2022). The method of pharmacovigilance analysis offers the advantage of large samples needed to assess rare ADR signals (Sakaeda et al. 2013). Thus, the aim of this study is to characterize and evaluate the risk signals of SGAs-related ileus through pharmacovigilance investigation using the FAERS database. We believe our study would provide referable evidence for the clinical monitoring and risk identification for SGAs-related ileus.
2 Materials and methods
2.1 Data acquisition
In this study, AE reports received from January 1 st, 2014 to December 31 st, 2023 were retrieved from the FAERS database (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers). The AE term of “ileus” was identified with code 1021328 from the Medical Dictionary for Regulatory Activities (Version 24.0). According to the list of World Health Organization (WHO) essential medicines, the following 14 medicines were considered as SGAs: amisulpride, aripiprazole, asenapine, brexiprazole, cariprazine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, sertindole, and zotepine (Todesco et al. 2023). Considering the very low number of reports for 9 of the SGAs (fewer than 15 reports each), we modified the SGAs list in our final analysis as follows: clozapine, risperidone, olanzapine, aripiprazole, and quetiapine (Fig. S1). In addition to monotherapy of the selected SGAs, we also analyzed combined therapy that contained more than one SGA. The detailed information of combined therapy was listed in Table S1.
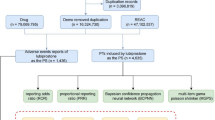
The data mining tool OpenVigil FDA was applied to calculate the onset time of ADR, which was defined as the interval from the first time of SGAs treatment until the onset of ileus (Böhm et al. 2016). Additionally, each AE was assigned with a unique case ID, and cases owning the same ID were excluded from the analysis (duplicate cases). The detailed process of data collection was depicted in Fig. 1. Since all data of our pharmacovigilance study was originated from public databases with non-identifiable datasets, no ethical approval is required.
2.2 Statistical analysis
In this study, descriptive analysis and disproportionality analysis were conducted to investigate the risk signals of ileus relevant to SGAs administration. In descriptive analysis, we analyzed the relationships between SGAs-related ileus and various variables, such as age (pediatric and adolescent (< 18 years), adult (18–44 years), middle-aged (45–64 years), and elderly (≥ 65 years)), gender (female and male), reporter type (consumer and healthcare professional), and reporting country/region.
Disproportionality analysis is a fundamental approach in pharmacovigilance research for detecting ADR signals of medications. In the current study, we employed two widely accepted algorithms to mine ADR signals, namely reporting odds ratio (ROR) and Bayesian confidence propagation neural network (BCPNN) (Bate et al. 1998; van Puijenbroek et al. 2002). ROR and information component (IC) were the two major measures in disproportionality analysis, and confidence interval (CI) is a range of upper and lower limits which describes the possible mean of a sample (Almenoff et al. 2007; Szumilas 2010; O'Brien and Yi 2016). ROR and IC were calculated according to the two-by-two contingency table (Table 1), and the detailed information of formulas and significant criteria were listed in Table 2. A statistically significant signal was considered if both the lower limit 95% CI of ROR (ROR025) > 1 and the lower limit 95% CI of IC (IC025) > 0 (Sakaeda et al. 2013). All analysis and visualization were performed using R studio (Version 4.1.0) and GraphPad Prism (Version 9.3.1).
3 Results
3.1 Descriptive analysis
After data processing, a total of 13,269,320 AE reports during 2014 ~ 2023 were obtained, with 492,802 reports related to SGAs medication. After removing the duplicate records, 419 cases of SGAs-related ileus were included in the final pharmacovigilance analysis. Figure 2 and Table 3 displayed the clinical characteristics of the 419 cases. The annual number of reported cases represented a fluctuating trend from 2014 to 2023. Specifically, the numbers of reported cases in 2014 and 2016 were the smallest (n = 27 each), while the number of reported cases in 2018 was the greatest (n = 68) (Fig. 2a). Clozapine constituted the vast majority of the suspected drugs (n = 131, 31.26%), followed by quetiapine (n = 99, 23.63%), combined therapy (n = 55, 13.13%), aripiprazole (n = 54, 12.89%), olanzapine (n = 51, 12.17%), and risperidone (n = 29, 6.92%) (Fig. 2b). Regarding reporting country/region, the United States reported the most AEs (n = 125, 29.83%), followed by Japan (n = 107, 25.54%), Germany (n = 39, 9.31%), Australia (n = 32, 7.64%), and the United Kingdom (n = 19, 4.53%) (Fig. 2c). The clinical characteristics of patients were displayed in Fig. 2d, the proportion of men was greater than that of women (46.78% vs 44.15%), and most patients were distributed in the age range of 45–64 years (n = 145, 34.61%). Furthermore, these cases were mainly reported by the healthcare professionals (n = 404, 96.42%), thus providing reliable data on SGAs-related ileus to some extent.
3.2 Reaction outcomes and actions taken with the drug
After removing the incomplete reports, a total of 219 cases were identified for the analysis of reaction outcomes. Figure 3a and Table S2 exhibited that most patients were observed in recovered state (n = 119, 54.34%), other outcomes included recovering (n = 56, 25.57%), fatal (n = 24, 10.96%), not recovered (n = 19, 8.68%), and recovered with sequelae (n = 1, 0.46%). Within each SGA treatment condition, quetiapine ranked first in the rate of fatalities (n = 10, 23.26%), followed by olanzapine (n = 3, 13.26%), clozapine (n = 6, 9.09%), combined therapy (n = 3, 8.33%), and aripiprazole (n = 2, 6.90%). As for recovered state, clozapine ranked first in proportion (n = 43, 65.15%), ahead of combined therapy (n = 22, 61.11%), quetiapine (n = 24, 55.81%), olanzapine (n = 11, 50.00%), risperidone (n = 9, 39.13%), and aripiprazole (n = 10, 34.48%). Risperidone showed the highest rate of recovering (n = 13, 56.52%), trailed by aripiprazole (n = 13, 44.83%), clozapine (n = 13, 19.70%), combined therapy (n = 7, 19.44%), olanzapine (n = 4, 18.18%), and quetiapine (n = 6, 13.95%). Moreover, olanzapine led in the rate of not recovered, (n = 4, 18.18%), followed by aripiprazole (n = 4, 13.79%), combined therapy (n = 4, 11.11%), quetiapine (n = 3, 6.98%), clozapine (n = 3, 4.55%), and risperidone (n = 1, 4.35%). There was only one case from clozapine treatment group containing outcome of recovered with sequelae.
After discarding reports with missing information, a total of 179 cases were included in the analysis of actions taken with the drug. As illustrated in Fig. 3b and Table S2, drug withdrawn (n = 120, 67.04%) was recorded in most cases, and the same trend was found within each SGA treatment group: aripiprazole (n = 16, 66.67%), clozapine (n = 30, 57.69%), olanzapine (n = 17, 68.00%), quetiapine (n = 26, 68.42%), risperidone (n = 12, 92.31%), and combined therapy (n = 19, 70.37%).
3.3 Time to onset of SGAs-related ileus
After excluding the reports with missing or inaccurate data, a total of 82 cases were analyzed for the time of onset. As demonstrated in Fig. 4 and Table S2, ileus was mostly observed within 30 days after the first-time SGAs administration (n = 35, 42.68%), and the same trend was found in treatment group of clozapine (n = 16, 42.11%), quetiapine (n = 3, 42.86%), risperidone (n = 2, 66.67%), and combined therapy (n = 11, 57.90%). Whereas in aripiprazole group, ileus was more frequently observed in 31 ~ 60 days after the first-time SGAs administration (n = 6, 75.00%). In olanzapine group, ileus was mostly found in 31 ~ 60 or 151 ~ 180 days after the first-time treatment of SGAs (n = 2 each, 28.57%). The median onset of SGAs-related ileus was 40 days (range 0 ~ 6070) for all cases. Quetiapine has the greatest median of onset (114 days, range 0 ~ 3838), while the median onset of risperidone was the smallest (0 day, range 0 ~ 43).
3.4 Disproportionality analysis
As shown in Fig. 5 and Table 4, SGAs including clozapine (ROR = 3.30, 95%Cl: 2.77 ~ 3.92), quetiapine (ROR = 1.93, 95%Cl:1.58 ~ 2.35), olanzapine (ROR = 1.66, 95%Cl: 1.26 ~ 2.18), aripiprazole (ROR = 1.36, 95%Cl: 1.04 ~ 1.78), and combined therapy (ROR = 1.84, 95%Cl: 1.41 ~ 2.40) showed significant signals of ileus. The ROR025 of these SGAs were greater than 1, indicating an increased risk of ileus after SGAs administration. However, we did not observe a significant ADR signal in risperidone (ROR = 0.68, 95%Cl: 0.47 ~ 0.98) (Fig. 5a). Likewise, IC exhibited similar results that clozapine (IC = 1.69, 95%Cl: 1.42 ~ 2.00), quetiapine (IC = 0.93, 95%Cl: 0.76 ~ 1.13), olanzapine (IC = 0.71, 95%Cl: 0.54 ~ 0.94), aripiprazole (IC = 0.44, 95%Cl: 0.33 ~ 0.57), and combined therapy (IC = 0.86, 95%Cl: 0.66 ~ 1.13) were significantly associated with an increased risk of ileus, whereas the ADR signal of risperidone was not significant (IC = −0.55, 95%Cl: −0.38 ~ −0.79) (Fig. 5b).
4 Discussion
To our knowledge, this is the first pharmacovigilance analysis investigating the links between the risk of ileus and SGAs medication. SGAs are effective agents in the treatment of psychotic disorders, and the commonly observed ADRs of SGAs contain metabolic abnormalities, prolactin elevation, dizziness and sedation, sexual dysfunction, cardiotoxicity, cataract, ileus, and constipation (Solmi et al. 2017). Although our results suggested a relatively low incidence of SGAs-related ileus in all cases of SGAs-associated AEs (419 out of 492,802), it warrants significant clinical attention due to the potential serious consequences of patients (Sarangi et al. 2021). In addition to the 419 AE cases we collected, several life-threatening cases of SGA-induced ileus have been documented (Every-Palmer et al. 2020; Osterman et al. 2017; Hibbard et al. 2009; Palmer et al. 2008; Shirazi et al. 2016), reflecting the necessity for clinical surveillance of this ADR. In our current study, the AE reports covered the populations that are typically excluded from clinical trials, such as children, pregnant women, and the elderly (Jacobson et al. 2024). Therefore, we investigated SGAs-related ileus in this large-scale dataset, aiming to broaden the understanding of this rare but serious ADR and minimize the risk of it.
Previous findings have revealed three major neurotransmitter mechanisms that might be involved in SGAs-related ileus: muscarinic cholinergic receptor antagonism, serotonin (5-hydroxytryptamine, 5-HT) receptor antagonism, and histamine H1 receptor antagonism (Xu et al. 2021). Acetylcholine is an intestinal excitatory transmitter that acts at muscarinic receptors on intestinal smooth muscle cells and adjacent Cajal cells (Harrington et al. 2010; Dome et al. 2007). The antagonism of muscarinic M1 and M3 cholinergic receptors can reduce intestinal contractility and delay intestinal transit, thereby causing ileus (Singh et al. 2022). Interestingly, we found that SGA with relatively lower muscarinic receptor affinity, such as aripiprazole, also displayed an increased risk of ileus, which might be linked to the antagonistic effects on 5-HT receptors (Every-Palmer et al. 2019; Keepers et al. 2020). 5-HT is capable of regulating gastrointestinal motility by acting on the vagal afferent pathways and slowing the colonic transport rate (Gershon 2004). Another mechanism contributing to ileus might be the antagonistic effect of SGAs on H1 receptor. Histamine acts on H1 receptors in gastrointestinal smooth muscles to promote intestinal peristalsis, and multiple SGAs have been shown to have H1 antagonism effect, including clozapine, olanzapine, and quetiapine (Keepers et al. 2020; Michl et al. 2014; Mandola et al. 2019). Of note, we should be aware that since SGAs can act on multiple receptors simultaneously, the mechanism of action of this ADR has pharmacologically complex properties (Taylor et al. 2005). Sedation is another common ADR of SGAs that cannot be ignored. The ADR of sedation can lead to gastrointestinal motility disturbances via the alterations of autonomic nervous motor patterns (Keepers et al. 2020; Arana 2000; Citrome 2017). The underlying pharmacological mechanisms may involve multiple gastrointestinal tract receptors, including GABA receptors, histamine receptors, and opioid receptors (Michl et al. 2014; Chang et al. 2020).
In the present study, we found the highest frequency and strongest risk signal of clozapine-associated ileus in FAERS database. We also exploratively collected data from other two widely applied ADR reporting systems, the EudraVigilance which primarily collects and analyzes AE reports within the European Economic Area (https://www.adrreports.eu/en/index.html) and the World Health Organization AE reporting system, namely VigiBase (https://www.who-umc.org). Interestingly, we found similar distribution patterns of SGA-related ileus among these three databases (Fig. S2). Indeed, ileus related to clozapine has been reported more frequently than other SGAs (Tyras et al. 2021; Tyras et al. 2021; Shirazi et al. 2016; Castillo-García et al. 2016; Khokhar et al. 2018; De Las et al. 2024). Although the underlying reason might be that clozapine has the highest anticholinergic affinity compared to other SGAs (Dean et al. 2023), we believe that potential reporting biases cannot be ignored. Due to influencing factors such as regulatory changes, media attention, Weber effect, and notoriety effect, medical staff and consumers would unconsciously pay more attention to ADR of clozapine-associated ileus (Neha et al. 2021; Matsuda et al. 2015). Our disproportionality analysis did not detect a strong link between the risk of ileus and risperidone (ROR025 = 0.68, IC025 = −0.38), which echoed the findings from a very recent pharmacovigilance study that risperidone has the least effect on gastrointestinal motility compared to other antipsychotics (He and Li 2025). Although risperidone has negligible affinity to muscarinic receptors, there were several case reports of severe intestinal motility inhibition caused by risperidone (Dome et al. 2007; Keepers et al. 2020; Rattehalli et al. 2016; Citrome et al. 2001). One possible reason could be the sympathetic hyperactivity triggered by risperidone-driven dopamine receptor blockade (Ramamourthy et al. 2013). Another reason could be that risperidone affects gastrointestinal motility through its relatively high affinity to 5-HT2 A receptors (Keepers et al. 2020; Guzel and Mirowska-Guzel 2022). Further, extensive evidence indicated that olanzapine has stronger muscarinic receptor affinity than quetiapine (Keepers et al. 2020; Dean et al. 2023; Lavrador et al. 2021). However, we found a much higher number of reports with quetiapine than olanzapine, which might support the view that there are growing cases of quetiapine misuse and abuse worldwide (Gjerden et al. 2017). Based on the above-mentioned findings derived from clinical data and pharmacokinetic results, we exploratively summarized the receptor binding properties and sedation effect of SGAs in Table S3. Moreover, we found that combined therapy did not show elevated risk of ileus versus aripiprazole (ROR025 = 0.93), clozapine (ROR025 = 0.41), olanzapine (ROR025 = 0.76), or quetiapine (ROR025 = 0.69), but displayed higher risk compared to risperidone (ROR025 = 1.72) (Fig. S3). Since the statistical power was limited by the small sample size of combined therapy group (n = 55), we performed descriptive analysis in reaction outcomes. We found that combined therapy showed a high rate in “recovered” state (61.11%), outperforming most monotherapies (except clozapine, 65.15%). As for fatality rate, combined therapy demonstrated a relatively low rate at 8.33%, only slightly higher than aripiprazole (6.90%) (Table S2). These findings collectively revealed the comparably safe profiles of combined therapy in terms of ileus risk and clinical outcomes. Taken together, we should reinforce the fact that proper monitoring is still needed while using SGAs other than clozapine.
Findings from previous retrospective analysis have suggested that the mortality rate of ileus was significantly higher in middle-aged and older adults compared to younger people (Koşar and Görgülü 2021, Elgar et al. 2022, Carroll et al. 2016), which is consistent with our data that the median age of patients with SGAs-associated ileus was 58 years. On the other hand, the connection between gender and SGAs-related ileus remains unclear, some studies observed that gastrointestinal hypomotility was independent of gender in patients receiving clozapine (Every-Palmer et al. 2016, 2019), whereas other study suggested that female patients receiving antipsychotics were more likely to develop constipation (Barbui et al. 2005). Although we did not find significant gender difference, it would be of interest to explore this point for follow-up studies. Of note, we noticed that although the median onset time of SGA-associated ileus was 40 days, a considerable proportion of AEs occurred more than 1 year after the first-time SGAs administration (n = 24, 29.27%), including clozapine, olanzapine, quetiapine, and combined therapy. Moreover, there were 3 cases involving clozapine medication exceeded 5 years in onset time of ileus, which was in line with previous evidence indicating that clozapine-associated ileus is highly likely to occur in the maintenance phase (more than 4 years) rather than acute phase (Nielsen and Meyer 2012). Considering the late onset property of SGAs-related ileus, we therefore recommend that healthcare professionals should increase their vigilance on patients with long-term use of SGAs. Several other SGAs-related factors of ileus could not be ignored either. Patients receiving SGAs medication tend to have sedentary lifestyle and low fiber diets which are considered as risk factors of ileus (Rognoni et al. 2021). Further, patients with schizophrenia are relatively insensitive to pain, which might reduce their somatic awareness of ileus (Coronado et al. 2021). As such, high-fiber diet, adequate fluid intake, and physical exercise would be beneficial in reducing the risk of SGAs-related ileus (Camilleri et al. 2017).
Regarding the dosage of SGAs, we found only a few cases marked with SGAs overdose (n = 19, 4.53%), thus indicating that our findings mostly reflected the profile of ADRs in the routine-dose SGAs scenario. Interestingly, we found 16 of these cases contained quetiapine extended-release dosage form. Since sustained/controlled-release form usually contains high dose of medications, patients are more likely to orally overdose themselves without a proper guidance from healthcare professionals (Ni et al. 2023). In addition, one literature review revealed that anticholinergic ADRs of SGAs appeared to be dose-dependent, including clozapine, olanzapine, and quetiapine (Yoshida and Takeuchi 2021). Unfortunately, due to our limited access to the specific dosage information or serum drug levels in databases, the dose-dependent profile between SGAs and ileus need to be further validated in the future.
We also identified several concomitant medications with potential risk of ileus in Table S4, including ondansetron, diphenhydramine, haloperidol, metoclopramide, benztropine, morphine, and fentanyl (de Alvarenga et al. 2017; Maheshwari and Sood 2023; Karunarathna 2024; de Boer et al. 2017). Besides, anticholinergics used to treat extrapyramidal side effects of schizophrenia patients might also increase the risk of ileus (Ali et al. 2021; Bolden et al. 1992). However, a firm influence of confounders (e.g., concomitant drugs and comorbidities) was difficult to determine as the detailed medical records were not reachable, such as administration timing, dosing sequence, blood concentration, allergy history, and drug interactions. Future investigations integrating comprehensive medical records, including patient baseline condition, medical history, and medication timeline, would enable deeper exploration of the confounding factors.
Despite the potential seriousness of ileus, knowledge about its clinical strategies or preventive measures remained poor, even among psychiatric clinical staff (De Hert et al. 2016). Based on our results of actions taken with the drug and the experience from other case reports (Tyras et al. 2021; Chiang and Lan 2018), we suggest that the focus of ileus management should be on discontinuing or switching SGAs to prevent potential fatalities. Early symptoms of ileus normally include abdominal pain, vomiting, abdominal distension, and nausea, which should be closely monitored during patient care (Vilz et al. 2017). Prophylactic laxatives, adjustment of electrolyte or acid–base disturbances, and early enteral feeding are considered effective for ileus (Vilz et al. 2017). Moreover, imaging of abdominal plain film, ultrasound, and computed tomography would be helpful for diagnosis, whereas the decision of surgical intervention should be made by experienced surgeons (Jang et al. 2010).
This study should be interpreted within its limitations. On the one hand, since that FAERS relies on voluntary reporting, underreporting, misreporting, and selection biases are thereby inevitable, which resulted a reduction in the number of cases collected. In addition, due to the lack of detailed clinical records and medication history of patients, the potential confounders of concomitant drugs and comorbidities were rarely analyzed. Therefore, education and training among healthcare staff and patients would be of great value to raise their awareness and improve the quality of AE reports. On the other hand, due to the intrinsic limitation of FAERS database, the data collected only allowed us to generate a statistical association of drug-AE correlation rather than a definite proof of causality. Further investigations integrating other data sources, such as electronic health records, medical insurance database, as well as causality assessment tools of WHO-Uppsala Monitoring Center scale and Naranjo scale, would provide more comprehensive safety assessment (Rezende de Menezes et al. 2021; Naranjo et al. 1981). Moreover, the newly developed machine learning model would be promising to optimally analyze FAERS data together with the tools and data sources described above (Ali and Aoun 2023).
5 Conclusion
In this study, we conducted a real-world pharmacovigilance analysis of SGAs-related ileus using the FAERS database. Our results suggested that SGAs administration increased the risk of ileus, and the characteristics of AEs would help clinicians understand the potential risk signals of SGAs-related ileus. Moreover, our results in the disproportionality analysis would assist clinicians in selecting SGAs for patients with relevant medication history, and provide valuable insight into the prognosis and safe use of SGAs.
Data availability
The data sets analyzed in this study are available upon request from the corresponding author.
References
Ali U, Aoun M (2023) Machine learning and FAERS data: revolutionizing health care analytics for adverse drug reaction prediction. J Appl Health Sci 8(3):1–18
Ali T, Sisay M, Tariku M, Mekuria AN, Desalew A (2021) Antipsychotic-induced extrapyramidal side effects: a systematic review and meta-analysis of observational studies. PLoS ONE 16(9):e0257129. https://doi.org/10.1371/journal.pone.0257129
Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N (2007) Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther 82(2):157–166. https://doi.org/10.1038/sj.clpt.6100258
Arana GW (2000) An overview of side effects caused by typical antipsychotics. J Clin Psychiatry 61(4):5–13
Barbui C, Nosè M, Bindman J, Schene A, Becker T, Mazzi MA et al (2005) Sex differences in the subjective tolerability of antipsychotic drugs. J Clin Psychopharmacol 25(6):521–526. https://doi.org/10.1097/01.jcp.0000185423.15891.02
Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A et al (1998) A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 54(4):315–321. https://doi.org/10.1007/s002280050466
Böhm R, von Hehn L, Herdegen T, Klein HJ, Bruhn O, Petri H et al (2016) OpenVigil FDA - inspection of U.S. American adverse drug events pharmacovigilance data and novel clinical applications. PloS One 11(6):e0157753. https://doi.org/10.1371/journal.pone.0157753
Bolden C, Cusack B, Richelson E (1992) Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther 260(2):576–580
Camilleri M, Ford AC, Mawe GM, Dinning PG, Rao SS, Chey WD et al (2017) Chronic constipation. Nat Rev Dis Primers 3:17095. https://doi.org/10.1038/nrdp.2017.95
Carroll AG, Kavanagh RG, Ni Leidhin C, Cullinan NM, Lavelle LP, Malone DE (2016) Comparative effectiveness of imaging modalities for the diagnosis of intestinal obstruction in neonates and infants: a critically appraised topic. Acad Radiol 23(5):559–568. https://doi.org/10.1016/j.acra.2015.12.014
Castillo-García IM, Maestro G, Puerta S, Ostos F, Nava P, Losada I (2016) Clozapine-induced paralytic ileus. Actas Esp Psiquiatr Acepsi 44(1):44–45
Chang H, Li S, Li Y, Hu H, Cheng B, Miao J et al (2020) Effect of sedation with dexmedetomidine or propofol on gastrointestinal motility in lipopolysaccharide-induced endotoxemic mice. BMC Anesthesiol 20(1):227. https://doi.org/10.1186/s12871-020-01146-z
Chiang ST, Lan CC (2018) Quetiapine related acute paralytic ileus in a Bipolar I disorder patient with successful low dose amisulpride substitution: a case report. Clin Psychopharmacol Neurosci 16(2):228–231. https://doi.org/10.9758/cpn.2018.16.2.228
Citrome L (2017) Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol 37(2):138–147. https://doi.org/10.1097/jcp.0000000000000665
Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer JP, McEvoy J et al (2001) Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv 52(11):1510–1514. https://doi.org/10.1176/appi.ps.52.11.1510
Coronado B, Dunn J, Veronin MA, Reinert JP (2021) Efficacy and safety considerations with second-generation antipsychotics as adjunctive analgesics: a review of literature. J Pharm Technol 37(4):202–208. https://doi.org/10.1177/87551225211004145
de Alvarenga K, Sacramento E, Rosa D, Souza B, de Rezende V, Romano-Silva M (2017) Effects of antipsychotics on intestinal motility in zebrafish larvae. Neurogastroenterol Motil 29(5):e13006. https://doi.org/10.1111/nmo.13006
de Boer HD, Detriche O, Forget P (2017) Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol 31(4):499–504. https://doi.org/10.1016/j.bpa.2017.07.002
De Hert M, De Beugher A, Sweers K, Wampers M, Correll CU, Cohen D (2016) Knowledge of psychiatric nurses about the potentially lethal side-effects of clozapine. Arch Psychiatr Nurs 30(1):79–83. https://doi.org/10.1016/j.apnu.2015.09.003
De Las CC, Sanz EJ, de Leon J (2024) Adverse drug reactions and their fatal outcomes in clozapine patients in VigiBase: comparing the top four reporting countries (US, UK, Canada and Australia). Schizophr Res 268:165–174. https://doi.org/10.1016/j.schres.2023.05.004
Dean B, Bakker G, Ueda HR, Tobin AB, Brown A, Kanaan RAA (2023) A growing understanding of the role of muscarinic receptors in the molecular pathology and treatment of schizophrenia. Front Cell Neurosci 17:1124333. https://doi.org/10.3389/fncel.2023.1124333
Dome P, Teleki Z, Kotanyi R (2007) Paralytic ileus associated with combined atypical antipsychotic therapy. Prog Neuropsychopharmacol Biol Psychiatry 31(2):557–560. https://doi.org/10.1016/j.pnpbp.2006.10.012
Elgar G, Smiley P, Smiley A, Feingold C, Latifi R (2022) Age increases the risk of mortality by four-fold in patients with emergent paralytic ileus: hospital length of stay, sex, frailty, and time to operation as other risk factors. Int J Environ Res Public Health 19(16):9905. https://doi.org/10.3390/ijerph19169905
Every-Palmer S, Nowitz M, Stanley J, Grant E, Huthwaite M, Dunn H et al (2016) Clozapine-treated patients have marked gastrointestinal hypomotility, the probable basis of life-threatening gastrointestinal complications: a cross sectional study. EBioMedicine 5:125–134. https://doi.org/10.1016/j.ebiom.2016.02.020
Every-Palmer S, Inns SJ, Grant E, Ellis PM (2019) Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs 33(1):81–91. https://doi.org/10.1007/s40263-018-0587-4
Every-Palmer S, Inns SJ, Ellis PM (2020) Constipation screening in people taking clozapine: a diagnostic accuracy study. Schizophr Res 220:179–186. https://doi.org/10.1016/j.schres.2020.03.032
Gershon MD (2004) Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 20(Suppl 7):3–14. https://doi.org/10.1111/j.1365-2036.2004.02180.x
Gjerden P, Bramness JG, Tvete IF, Slørdal L (2017) The antipsychotic agent quetiapine is increasingly not used as such: dispensed prescriptions in Norway 2004–2015. Eur J Clin Pharmacol 73(9):1173–1179. https://doi.org/10.1007/s00228-017-2281-8
Grajales D, Ferreira V, Valverde ÁM (2019) Second-generation antipsychotics and dysregulation of glucose metabolism: beyond weight gain. Cells 8(11). https://doi.org/10.3390/cells8111336
Guzel T, Mirowska-Guzel D (2022) The role of serotonin neurotransmission in gastrointestinal tract and pharmacotherapy. Molecules 27(5):1680. https://doi.org/10.3390/molecules27051680
Harrington AM, Hutson JM, Southwell BR (2010) Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem 44(4):173–202
Hasegawa T, Sawada S, Saito T, Kohama M, Kajiyama K, Ishiguro C et al (2024) Lower risks of gastrointestinal perforation and intestinal obstruction in patients with atypical antipsychotics in comparison with typical antipsychotics based on real-world data from the MID-NET® in Japan. Ther Innov Regul Sci 58(1):192–199. https://doi.org/10.1007/s43441-023-00586-2
He S, Li C (2025) Potential risk analysis of antipsychotics-related constipation from the FDA Adverse Event Reporting System. Expert Opin Drug Saf:1–6. https://doi.org/10.1080/14740338.2025.2468857
Hibbard KR, Propst A, Frank DE, Wyse J (2009) Fatalities associated with clozapine-related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics 50(4):416–419. https://doi.org/10.1176/appi.psy.50.4.416
Ingimarsson O, MacCabe JH, Sigurdsson E (2018) Constipation, ileus and medication use during clozapine treatment in patients with schizophrenia in Iceland. Nord J Psychiatry 72(7):497–500. https://doi.org/10.1080/08039488.2018.1517189
Isbister GK, Oakley P, Whyte I, Dawson A (2001) Treatment of anticholinergic-induced ileus with neostigmine. Ann Emerg Med 38(6):689–693. https://doi.org/10.1067/mem.2001.119458
Jacobson RM, Pignolo RJ, Lazaridis KN (2024) Clinical trials for special populations: children, older adults, and rare diseases. Mayo Clin Proc 99(2):318–335. https://doi.org/10.1016/j.mayocp.2023.03.003
Jang KM, Min K, Kim MJ, Koh SH, Jeon EY, Kim IG et al (2010) Diagnostic performance of CT in the detection of intestinal ischemia associated with small-bowel obstruction using maximal attenuation of region of interest. AJR Am J Roentgenol. 194(4):957–63. https://doi.org/10.2214/ajr.09.2702
Karunarathna I (2024) Fentanyl: clinical applications and pharmacological considerations. Uva Clinical Lab
Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R et al (2020) The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 177(9):868–872
Khaleel MA, Khan AH, Ghadzi SMS, Adnan AS, Abdallah QM (2022) A standardized dataset of a spontaneous adverse event reporting system. Healthcare 10(3):420. https://doi.org/10.3390/healthcare10030420
Khokhar JY, Henricks AM, Sullivan EDK, Green AI (2018) Unique effects of clozapine: a pharmacological perspective. Adv Pharmacol 82:137–162. https://doi.org/10.1016/bs.apha.2017.09.009
Koşar MN, Görgülü Ö (2021) Incidence and mortality results of intestinal obstruction in geriatric and adult patients: 10 years retrospective analysis. Turk J Surg 37(4):363–370. https://doi.org/10.47717/turkjsurg.2021.5177
Lally J, MacCabe JH (2015) Antipsychotic medication in schizophrenia: a review. Br Med Bull 114(1):169–179. https://doi.org/10.1093/bmb/ldv017
Lavrador M, Castel-Branco MM, Cabral AC, Veríssimo MT, Figueiredo IV, Fernandez-Llimos F (2021) Association between anticholinergic burden and anticholinergic adverse outcomes in the elderly: pharmacological basis of their predictive value for adverse outcomes. Pharmacol Res 163:105306. https://doi.org/10.1016/j.phrs.2020.105306
Liu F, Panagiotakos D (2022) Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol 22(1):287. https://doi.org/10.1186/s12874-022-01768-6
Maheshwari A, Sood MR (2023) Drugs acting on the gut: prokinetics, antispasmodics, laxatives. Pediatric neurogastroenterology: gastrointestinal motility disorders and disorders of gut brain interaction in children. Springer, pp 555–71. https://doi.org/10.1007/978-3-031-15229-0_43
Mandola A, Nozawa A, Eiwegger T (2019) Histamine, histamine receptors, and anti-histamines in the context of allergic responses. LymphoSign Journal 6(2):35–51. https://doi.org/10.14785/lymphosign-2018-0016
Matsuda S, Aoki K, Kawamata T, Kimotsuki T, Kobayashi T, Kuriki H et al (2015) Bias in spontaneous reporting of adverse drug reactions in Japan. PLoS ONE 10(5):e0126413. https://doi.org/10.1371/journal.pone.0126413
McKinnon ND, Azad A, Waters BM, Joshi KG (2009) Clozapine-induced bowel infarction: a case report. Psychiatry (Edgmont) 6(3):30–35
Michl J, Scharinger C, Zauner M, Kasper S, Freissmuth M, Sitte HH et al (2014) A multivariate approach linking reported side effects of clinical antidepressant and antipsychotic trials to in vitro binding affinities. Eur Neuropsychopharmacol 24(9):1463–1474. https://doi.org/10.1016/j.euroneuro.2014.06.013
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA et al (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30(2):239–245. https://doi.org/10.1038/clpt.1981.154
Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E (2021) Existence of notoriety bias in FDA adverse event reporting system database and its impact on signal strength. Hosp Pharm 56(3):152–158. https://doi.org/10.1177/0018578719882323
Ni J, Tang X, Chen L (2023) Medication overdose data analysis: a review of medication error reports in the FDA adverse event reporting system (FAERS). BMC Pharmacol Toxicol 24(1):41. https://doi.org/10.1186/s40360-023-00681-y
Nielsen J, Meyer JM (2012) Risk factors for ileus in patients with schizophrenia. Schizophr Bull 38(3):592–598. https://doi.org/10.1093/schbul/sbq137
O’Brien SF, Yi QL (2016) How do I interpret a confidence interval? Transfusion 56(7):1680–1683. https://doi.org/10.1111/trf.13635
Osterman MT, Foley C, Matthias I (2017) Clozapine-induced acute gastrointestinal necrosis: a case report. J Med Case Rep 11(1):270. https://doi.org/10.1186/s13256-017-1447-4
Palmer SE, McLean RM, Ellis PM, Harrison-Woolrych M (2008) Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry 69(5):759–768. https://doi.org/10.4088/jcp.v69n0509
Ramamourthy P, Kumaran A, Kattimani S (2013) Risperidone associated paralytic ileus in schizophrenia. Indian J Psychol Med 35(1):87–88. https://doi.org/10.4103/0253-7176.112214
Rattehalli RD, Zhao S, Li BG, Jayaram MB, Xia J, Sampson S (2016) Risperidone versus placebo for schizophrenia. Cochrane Database Syst Rev 12(12):Cd006918. https://doi.org/10.1002/14651858.CD006918.pub3
Rezende de Menezes R, Graciano Silva MdD, PinhoRibeiro AL, Martins Pinto Filho M, Martinho GH, Carvalho Ferreira LE et al (2021) Causality assessment of adverse drug reactions by applying a global introspection method in a high complexity hospital. Explor Res Clin Soc Pharm 3:100064. https://doi.org/10.1016/j.rcsop.2021.100064
Rognoni C, Bertolani A, Jommi C (2021) Second-generation antipsychotic drugs for patients with schizophrenia: systematic literature review and meta-analysis of metabolic and cardiovascular side effects. Clin Drug Investig 41(4):303–319. https://doi.org/10.1007/s40261-021-01000-1
Sakaeda T, Tamon A, Kadoyama K, Okuno Y (2013) Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci 10(7):796–803. https://doi.org/10.7150/ijms.6048
Sarangi A, Armin S, Vargas A, Chu VM, Fain K, Nelson J (2021) Management of constipation in patients with schizophrenia—a case study and review of literature. Middle East Current Psychiatry 28(1):17. https://doi.org/10.1186/s43045-021-00097-6
Shirazi A, Stubbs B, Gomez L, Moore S, Gaughran F, Flanagan RJ et al (2016) Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci 17(6):863
Singh R, Zogg H, Ghoshal UC, Ro S (2022) Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol 13:808195. https://doi.org/10.3389/fphar.2022.808195
Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M et al (2017) Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag 13:757–777. https://doi.org/10.2147/tcrm.S117321
Szumilas M (2010) Explaining odds ratios. J Am Acad Child Adolesc Psychiatry 19(3):227–229. https://doi.org/10.1111/trf.13635
Taylor C, Fricker AD, Devi LA, Gomes I (2005) Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal 17(5):549–557. https://doi.org/10.1016/j.cellsig.2004.12.007
Todesco B, Ostuzzi G, Gastaldon C, Papola D, Barbui C (2023) Essential medicines for mental disorders: comparison of 121 national lists with WHO recommendations. Arch Public Health 81(1):8. https://doi.org/10.1186/s13690-022-01014-x
Tyras S, Wierzchoń K, Jaroszewska A (2021) Cases of clozapine-induced gastrointestinal hypomotility in Europe: outcomes and fatality risk factors based on EudraVigilance data. Psychiatry Res 300:113911. https://doi.org/10.1016/j.psychres.2021.113911
van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Orre R, Egberts ACG (2002) A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 11(1):3–10. https://doi.org/10.1002/pds.668
Vilz TO, Stoffels B, Strassburg C, Schild HH, Kalff JC (2017) Ileus in adults: pathogenesis, investigation and treatment. Dtsch Arztebl Int 114(29–30):508. https://doi.org/10.3238/arztebl.2017.0508
Xu Y, Amdanee N, Zhang X (2021) Antipsychotic-induced constipation: a review of the pathogenesis, clinical diagnosis, and treatment. CNS Drugs 35(12):1265–1274. https://doi.org/10.1007/s40263-021-00859-0
Yoshida K, Takeuchi H (2021) Dose-dependent effects of antipsychotics on efficacy and adverse effects in schizophrenia. Behav Brain Res 402:113098. https://doi.org/10.1016/j.bbr.2020.113098
Acknowledgements
We would like to thank FAERS, EudraVigilance, and VigiBase, for the free access of data. We also thank Dr. Ruwen Böhm for the free use of OpenVigil FDA.
Funding
This work was supported by the National Natural Science Foundation of China (82104223 and 82204372), Guangdong Basic and Applied Basic Research Foundation (2020 A1515110008), Science and Technology Program of Guangzhou (202102021022, 2023 A04 J0601, 2024 A04 J10001, and 2025 A03 J3308), Guangzhou Municipal Science and Technology Project for Medicine and Healthcare (20211 A011044), and Guangzhou Municipal Key Discipline in Medicine (2025–2027).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YT, HX, and QS. The first draft of the manuscript was written by YT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Since all data of our study was originated from public databases with non-identifiable datasets, no ethical approval is required.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tan, Y., Xia, H. & Song, Q. Comparison of ileus risk in selected second-generation antipsychotics based on FAERS database. Saudi Pharm. J. 33, 13 (2025). https://doi.org/10.1007/s44446-025-00020-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44446-025-00020-8