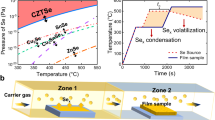
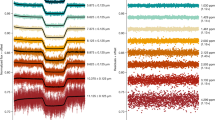
Abstract
THE fact that selenium dioxide, a white solid, gives a brown vapour has been discussed by Meyer and Langner (Ber., 60 B, 285; 1927), who conclude that the colour must be due to the dioxide and cannot be attributed to selenium liberated by dissociation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
EVANS, S. The Absorption Spectrum of Selenium Dioxide. Nature 125, 528 (1930). https://doi.org/10.1038/125528b0
Issue Date:
DOI: https://doi.org/10.1038/125528b0
This article is cited by
-
Absorption and Afterglow Spectra of Selenium Dioxide
Nature (1962)