Abstract
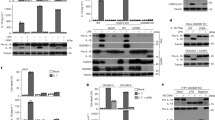
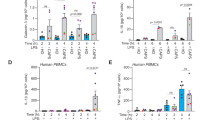
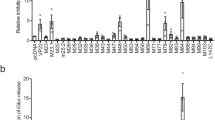
A crucial part of the innate immune response is the assembly of the inflammasome, a cytosolic complex of proteins that activates caspase-1 to process the proinflammatory cytokines interleukin (IL)-1β and IL-18. The adaptor protein ASC is essential for inflammasome function1,2, binding directly to caspase-1 (refs 3, 4), but the triggers of this interaction are less clear. ASC also interacts with the adaptor cryopyrin (also known as NALP3 or CIAS1)5,6. Activating mutations in cryopyrin are associated with familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal onset multisystem inflammatory disease, diseases that are characterized by excessive production of IL-1β5,7. Here we show that cryopyrin-deficient macrophages cannot activate caspase-1 in response to Toll-like receptor agonists plus ATP, the latter activating the P2X7 receptor to decrease intracellular K+ levels8,9. The release of IL-1β in response to nigericin, a potassium ionophore, and maitotoxin, a potent marine toxin, was also found to be dependent on cryopyrin. In contrast to Asc-/- macrophages, cells deficient in the gene encoding cryopyrin (Cias1-/-) activated caspase-1 and secreted normal levels of IL-1β and IL-18 when infected with Gram-negative Salmonella typhimurium or Francisella tularensis. Macrophages exposed to Gram-positive Staphylococcus aureus or Listeria monocytogenes, however, required both ASC and cryopyrin to activate caspase-1 and secrete IL-1β. Therefore, cryopyrin is essential for inflammasome activation in response to signalling pathways triggered specifically by ATP, nigericin, maitotoxin, S. aureus or L. monocytogenes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004)
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002)
Srinivasula, S. M. et al. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 277, 21119–21122 (2002)
Wang, L. et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 (2002)
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004)
Dowds, T. A., Masumoto, J., Zhu, L., Inohara, N. & Nunez, G. Cryopyrin-induced interleukin 1β secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J. Biol. Chem. 279, 21924–21928 (2004)
Ting, J. P. & Davis, B. K. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu. Rev. Immunol. 23, 387–414 (2005)
Perregaux, D. & Gabel, C. A. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 (1994)
Solle, M. et al. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 276, 125–132 (2001)
Hogquist, K. A., Nett, M. A., Unanue, E. R. & Chaplin, D. D. Interleukin 1 is processed and released during apoptosis. Proc. Natl Acad. Sci. USA 88, 8485–8489 (1991)
Yamamoto, M. et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9, 1055–1067 (2004)
Poltorak, A. et al. Defective LPS signalling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998)
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998)
Schilling, W. P., Wasylyna, T., Dubyak, G. R., Humphreys, B. D. & Sinkins, W. G. Maitotoxin and P2Z/P2X7 purinergic receptor stimulation activate a common cytolytic pore. Am. J. Physiol. 277, C766–C776 (1999)
Verhoef, P. A., Kertesy, S. B., Estacion, M., Schilling, W. P. & Dubyak, G. R. Maitotoxin induces biphasic interleukin-1β secretion and membrane blebbing in murine macrophages. Mol. Pharmacol. 66, 909–920 (2004)
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995)
Martinon, F., Agostini, L., Meylan, E. & Tschopp, J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14, 1929–1934 (2004)
Girardin, S. E. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003)
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005)
Maeda, S. et al. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1beta processing. Science 307, 734–738 (2005)
Damiano, J. S., Newman, R. M. & Reed, J. C. Multiple roles of CLAN (caspase-associated recruitment domain, leucine-rich repeat, and NAIP CIIA HET-E, and TP1-containing protein) in the mammalian innate immune response. J. Immunol. 173, 6338–6345 (2004)
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nature Genet. 29, 301–305 (2001)
McDermott, M. F. Genetic clues to understanding periodic fevers, and possible therapies. Trends Mol. Med. 8, 550–554 (2002)
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002)
Aganna, E. et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452 (2002)
Acknowledgements
We thank members of the Dixit laboratory for discussions, and M. Bauer, J. Starks, C. Olsson, M. Osborn, J. Hongo, M. Bever, J. Cupp and H. Maecker for technical assistance. S. aureus strains were provided by T. Foster. L. monocytogenes LLO mutant was provided by D. Portnoy. This work was supported by NIH grants awarded to D.M.M. and a fellowship from the Giannini Family Foundation awarded to D.S.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Methods and Supplementary Figures 1–6. (PDF 1354 kb)
Rights and permissions
About this article
Cite this article
Mariathasan, S., Weiss, D., Newton, K. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006). https://doi.org/10.1038/nature04515
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04515
This article is cited by
-
Diverse proinflammatory response in pharyngeal epithelial cells upon interaction with Neisseria meningitidis carriage and invasive isolates
BMC Infectious Diseases (2024)
-
P2X7 receptor knockout does not alter renal function or prevent angiotensin II-induced kidney injury in F344 rats
Scientific Reports (2024)
-
A palmitoylation–depalmitoylation relay spatiotemporally controls GSDMD activation in pyroptosis
Nature Cell Biology (2024)
-
Nuclear receptor coactivator 6 is a critical regulator of NLRP3 inflammasome activation and gouty arthritis
Cellular & Molecular Immunology (2024)
-
Using Pictorial Representations as Story-Telling
Foundations of Science (2024)