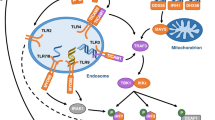
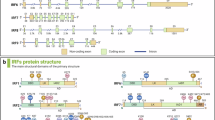
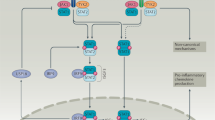
Abstract
Type III interferons (IFNs) or IFN-λs regulate a similar set of genes as type I IFNs, but whereas type I IFNs act globally, IFN-λs primarily target mucosal epithelial cells and protect them against the frequent viral attacks that are typical for barrier tissues. IFN-λs thereby help to maintain healthy mucosal surfaces through immune protection, without the significant immune-related pathogenic risk associated with type I IFN responses. Type III IFNs also target the human liver, with dual effects: they induce an antiviral state in hepatocytes, but specific IFN-λ4 action impairs the clearance of hepatitis C virus and could influence inflammatory responses. This constitutes a paradox that has yet to be resolved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kotenko, S.V. et al. IFN-λ s mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4, 69–77 (2003).
Sheppard, P. et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4, 63–68 (2003). These back-to-back papers reported the discovery of IFN-λs 1–3, describe how they use the IFNλR1 and IL-10R2 receptor chains for signaling and demonstrate their antiviral activity.
Fox, B.A., Sheppard, P.O. & O'Hara, P.J. The role of genomic data in the discovery, annotation and evolutionary interpretation of the interferon-λ family. PLoS ONE 4, e4933 (2009).
Prokunina-Olsson, L. et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 45, 164–171 (2013). This article described the discovery of the IFNL4 gene and demonstrates its association with HCV clearance.
Dumoutier, L. et al. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-λ 1: similarities with type I interferon signaling. J. Biol. Chem. 279, 32269–32274 (2004).
Zhou, Z. et al. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 81, 7749–7758 (2007).
Doyle, S.E. et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 44, 896–906 (2006).
Marcello, T. et al. Interferons α and λ inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131, 1887–1898 (2006).
Crotta, S. et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 9, e1003773 (2013).
Malakhova, O.A. et al. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 25, 2358–2367 (2006).
François-Newton, V. et al. USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon α response. PLoS ONE 6, e22200 (2011).
Makowska, Z., Duong, F.H., Trincucci, G., Tough, D.F. & Heim, M.H. Interferon-β and interferon-λ signaling is not affected by interferon-induced refractoriness to interferon-α in vivo. Hepatology 53, 1154–1163 (2011).
Bolen, C.R., Ding, S., Robek, M.D. & Kleinstein, S.H. Dynamic expression profiling of type I and type III interferon-stimulated hepatocytes reveals a stable hierarchy of gene expression. Hepatology 59, 1262–1272 (2014).
Jilg, N. et al. Kinetic differences in the induction of interferon stimulated genes by interferon-α and interleukin 28B are altered by infection with hepatitis C virus. Hepatology 59, 1250–1261 (2014).
Burkart, C. et al. Usp18 deficient mammary epithelial cells create an antitumour environment driven by hypersensitivity to IFN-λ and elevated secretion of Cxcl10. EMBO Mol. Med. 5, 967–982 (2013).
Gad, H.H. et al. Interferon-λ is functionally an interferon but structurally related to the IL-10 family. J. Biol. Chem. 284, 20869–20875 (2009). Presented the first crystal structure of an IFN-λ, here IFN-λ3, and mapped the IFNλR1 binding site by a mutagenesis approach.
Miknis, Z.J. et al. Crystal structure of human interferon-λ1 in complex with its high-affinity receptor interferon-λR1. J. Mol. Biol. 404, 650–664 (2010).
Mordstein, M. et al. λ interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 84, 5670–5677 (2010).
Sommereyns, C., Paul, S., Staeheli, P. & Michiels, T. IFN-λ (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4, e1000017 (2008). Showed that IFN-λs primarily act on epithelial cells and suggested that they evolved to protect epithelial tissues from viral infection.
Pulverer, J.E. et al. Temporal and spatial resolution of type I and III interferon responses in vivo. J. Virol. 84, 8626–8638 (2010).
Pott, J. et al. IFN-λ determines the intestinal epithelial antiviral host defense. Proc. Natl. Acad. Sci. USA 108, 7944–7949 (2011). Found that IFN-λ is required for control of rotavirus infection in mice and showed that type I IFN cannot substitute. This was the first demonstration of a unique role for IFN-λ.
Hermant, P. et al. Human but not mouse hepatocytes respond to interferon-λ in vivo. PLoS ONE 9, e87906 (2014).
Hermant, P. & Michiels, T. Interferon-λ in the context of viral infections: production, response and therapeutic implications. J. Innate Immun. 6, 563–574 (2014).
Anggakusuma et al. Control of hepatitis C virus replication in mouse liver-derived cells by MAVS-dependent production of type I and type III interferons. J. Virol. 89, 3833–3845 (2015).
Meager, A., Visvalingam, K., Dilger, P., Bryan, D. & Wadhwa, M. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine 31, 109–118 (2005).
Muir, A.J. et al. Phase 1b study of PEGylated interferon-λ1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology 52, 822–832 (2010).
Lauber, C. et al. Transcriptome analysis reveals a classical interferon signature induced by IFNλ4 in human primary cells. Genes Immun. doi:10.1038/gene.2015.23 (11 June 2015).
Witte, K. et al. Despite IFN-λ receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 10, 702–714 (2009).
Ding, S., Khoury-Hanold, W., Iwasaki, A. & Robek, M.D. Epigenetic reprogramming of the type III interferon response potentiates antiviral activity and suppresses tumor growth. PLoS Biol. 12, e1001758 (2014).
Durbin, R.K., Kotenko, S.V. & Durbin, J.E. Interferon induction and function at the mucosal surface. Immunol. Rev. 255, 25–39 (2013).
Ank, N. et al. λ interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 80, 4501–4509 (2006).
Donnelly, R.P. & Kotenko, S.V. Interferon-λ : a new addition to an old family. J. Interferon Cytokine Res. 30, 555–564 (2010).
Coccia, E.M. et al. Viral infection and Toll-like receptor agonists induce a differential expression of type I and λ interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34, 796–805 (2004).
Osterlund, P. et al. Gene expression and antiviral activity of α/β interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J. Virol. 79, 9608–9617 (2005).
Lauterbach, H. et al. Mouse CD8α+ DCs and human BDCA3+ DCs are major producers of IFN-λ in response to poly IC. J. Exp. Med. 207, 2703–2717 (2010).
Hillyer, P. et al. Expression profiles of human interferon-α and interferon-λ subtypes are ligand- and cell-dependent. Immunol. Cell Biol. 90, 774–783 (2012).
Melchjorsen, J., Siren, J., Julkunen, I., Paludan, S.R. & Matikainen, S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J. Gen. Virol. 87, 1099–1108 (2006).
Khaitov, M.R. et al. Respiratory virus induction of α-, β- and λ-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy 64, 375–386 (2009).
Wang, J. et al. Differentiated human alveolar type II cells secrete antiviral il-29 (IFN-1) in response to influenza A infection. J. Immunol. 182, 1296–1304 (2009).
Ioannidis, I., Ye, F., McNally, B., Willette, M. & Flano, E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J. Virol. 87, 3261–3270 (2013).
Jewell, N.A. et al. λ interferon is the predominant interferon induced by influenza A virus infection in vivo. J. Virol. 84, 11515–11522 (2010).
Osterlund, P.I., Pietila, T.E., Veckman, V., Kotenko, S.V. & Julkunen, I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-λ ) genes. J. Immunol. 179, 3434–3442 (2007).
Onoguchi, K. et al. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 282, 7576–7581 (2007).
Kotenko, S.V. IFN-λs. Curr. Opin. Immunol. 23, 583–590 (2011).
Thomson, S.J. et al. The role of transposable elements in the regulation of IFN-λ1 gene expression. Proc. Natl. Acad. Sci. USA 106, 11564–11569 (2009).
Siegel, R., Eskdale, J. & Gallagher, G. Regulation of IFN-λ1 promoter activity (IFN-λ1/IL-29) in human airway epithelial cells. J. Immunol. 187, 5636–5644 (2011).
Swider, A., Siegel, R., Eskdale, J. & Gallagher, G. Regulation of interferon λ-1 (IFNL1/IFN-λ1/IL-29) expression in human colon epithelial cells. Cytokine 65, 17–23 (2014).
Odendall, C. et al. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 15, 717–726 (2014).
Dixit, E. et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141, 668–681 (2010).
Lee, H.C., Narayanan, S., Park, S.J., Seong, S.Y. & Hahn, Y.S. Transcriptional regulation of IFN-λ genes in hepatitis C virus-infected hepatocytes via IRF-3.IRF-7.NF-κB complex. J. Biol. Chem. 289, 5310–5319 (2014).
Poss, Z.C., Ebmeier, C.C. & Taatjes, D.J. The Mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 48, 575–608 (2013).
Griffiths, S.J. et al. A systematic analysis of host factors reveals a Med23-interferon-λ regulatory axis against herpes simplex virus type 1 replication. PLoS Pathog. 9, e1003514 (2013).
Mahlakõiv, T., Hernandez, P., Gronke, K., Diefenbach, A. & Staeheli, P. Leukocyte-derived IFN-α/β and epithelial IFN-λ constitute a compartmentalized mucosal defense system that restricts enteric virus infections. PLoS Pathog. 11, e1004782 (2015).
Nice, T.J. et al. Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347, 269–273 (2015).
Baldridge, M.T. et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science 347, 266–269 (2015). Refs. 54 and 55 described that IFN-λ is necessary for a control of norovirus infection in mice. It was shown that recombinant IFN-λ can cure norovirus infection and thus has a significant therapeutic potential. The authors observed that sterilizing the gut by antibiotic treatment cures the persistent virus infection in a manner that requires the IFNλR1 receptor.
Ank, N. et al. An important role for type III interferon (IFN-λ/IL-28) in TLR-induced antiviral activity. J. Immunol. 180, 2474–2485 (2008).
Lazear, H.M. et al. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med. 7, 284ra259 (2015).
Mordstein, M. et al. Interferon-λ contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 4, e1000151 (2008).
Muir, A.J. et al. A randomized phase 2b study of peginterferon λ-1a for the treatment of chronic hcv infection. J. Hepatol. 61, 1238–1246 (2014).
Yin, Z. et al. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J. Immunol. 189, 2735–2745 (2012).
Liu, B.S., Janssen, H.L. & Boonstra, A. IL-29 and IFNα differ in their ability to modulate IL-12 production by TLR-activated human macrophages and exhibit differential regulation of the IFNγ receptor expression. Blood 117, 2385–2395 (2011).
Megjugorac, N.J., Gallagher, G.E. & Gallagher, G. Modulation of human plasmacytoid DC function by IFN-λ 1 (IL-29). J. Leukoc. Biol. 86, 1359–1363 (2009).
Koltsida, O. et al. IL-28A (IFN-λ2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol. Med. 3, 348–361 (2011).
Dring, M.M. et al. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 108, 5736–5741 (2011).
Krämer, B. et al. Do λ-IFNs IL28A and IL28B act on human natural killer cells? Proc. Natl. Acad. Sci. USA 108, E519–E520 (2011).
de Groen, R.A. et al. IFN-λ-mediated IL-12 production in macrophages induces IFN-γ production in human NK cells. Eur. J. Immunol. 45, 250–259 (2015).
Souza-Fonseca-Guimaraes, F. et al. NK cells require IL-28R for optimal in vivo activity. Proc. Natl. Acad. Sci. USA 112, E2376–E2384 (2015).
Blazek, K. et al. IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J. Exp. Med. 212, 845–853 (2015).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O'Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Dai, J., Megjugorac, N.J., Gallagher, G.E., Yu, R.Y. & Gallagher, G. IFN-λ1 (IL-29) inhibits GATA3 expression and suppresses Th2 responses in human naive and memory T cells. Blood 113, 5829–5838 (2009).
Egli, A. et al. IL-28B is a key regulator of B- and T-cell vaccine responses against influenza. PLoS Pathog. 10, e1004556 (2014).
Edwards, M.R. & Johnston, S.L. Interferon-λ as a new approach for treatment of allergic asthma? EMBO Mol. Med. 3, 306–308 (2011).
Davidson, S., Maini, M.K. & Wack, A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J. Interferon Cytokine Res. 35, 252–264 (2015).
Davidson, S., Crotta, S., McCabe, T.M. & Wack, A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat. Commun. 5, 3864 (2014).
Bosinger, S.E. et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Invest. 119, 3556–3572 (2009).
Jacquelin, B. et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119, 3544–3555 (2009).
Mandl, J.N. et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14, 1077–1087 (2008).
Micco, L. et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-α therapy of chronic hepatitis B. J. Hepatol. 58, 225–233 (2013).
Penna, A. et al. Peginterferon-α does not improve early peripheral blood HBV-specific T-cell responses in HBeAg-negative chronic hepatitis. J. Hepatol. 56, 1239–1246 (2012).
Teijaro, J.R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013).
Wilson, E.B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013).
Rayamajhi, M., Humann, J., Penheiter, K., Andreasen, K. & Lenz, L.L. Induction of IFN-γγ enables Listeria monocytogenes to suppress macrophage activation by IFN-γ. J. Exp. Med. 207, 327–337 (2010).
Teles, R.M. et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 339, 1448–1453 (2013).
Antonelli, L.R. et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120, 1674–1682 (2010).
Redford, P.S. et al. Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. J. Infect. Dis. 209, 270–274 (2014).
Shepard, C.W., Finelli, L. & Alter, M.J. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5, 558–567 (2005).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009). This was the first demonstration of a clear genetic association between the IFN-λ loci and the poor treatment outcome for chronic HCV infection.
Suppiah, V. et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nat. Genet. 41, 1100–1104 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to PEGylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41, 1105–1109 (2009).
Rauch, A. et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology 138, 1338–1345 (2010).
Thomas, D.L. et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801 (2009).
Urban, T.J. et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology 52, 1888–1896 (2010).
Terczyn´ska-Dyla, E. et al. Reduced IFN-λ4 activity is associated with improved HCV clearance and reduced expression of interferon-stimulated genes. Nat. Commun. 5, 5699 (2014). This article provided the first direct connection between the activity of the IFN-λ4 protein, the high expression of interferon stimulated genes in the liver and poor clearance rate of chronic HCV.
Smith, K.R. et al. Identification of improved IL28B SNPs and haplotypes for prediction of drug response in treatment of hepatitis C using massively parallel sequencing in a cross-sectional European cohort. Genome Med. 3, 57 (2011).
Ahlenstiel, G., Booth, D.R. & George, J. Will IL28B polymorphisms remain relevant to direct-acting antiviral treatment paradigms? Antivir. Ther. 17, 1163–1170 (2012).
Holmes, J.A., Desmond, P.V. & Thompson, A.J. Does IL28B genotyping still have a role in the era of direct-acting antiviral therapy for chronic hepatitis C infection? J. Viral Hepat. 19, 677–684 (2012).
Meissner, E.G. et al. IFNL4-ΔG genotype is associated with slower viral clearance in hepatitis C, genotype-1 patients treated with sofosbuvir and ribavirin. J. Infect. Dis. 209, 1700–1704 (2014).
Muir, A.J. IL28B in the era of direct-acting antivirals for hepatitis C. J. Clin. Gastroenterol. 47, 222–227 (2013).
Zeuzem, S. et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N. Engl. J. Med. 369, 630–639 (2013).
Bibert, S. et al. The IFNL3/4 ΔG variant increases susceptibility to cytomegalovirus retinitis among HIV-infected patients. AIDS 28, 1885–1889 (2014).
Manuel, O. et al. Influence of IFNL3/4 polymorphisms on the incidence of cytomegalovirus infection after solid-organ transplantation. J. Infect. Dis. 211, 906–914 (2015).
Hamming, O.J. et al. Interferon-λ4 signals via the IFN-λ receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J. 32, 3055–3065 (2013).
Bochud, P.Y. et al. IL28B alleles associated with poor hepatitis C virus (HCV) clearance protect against inflammation and fibrosis in patients infected with non-1 HCV genotypes. Hepatology 55, 384–394 (2012).
Eslam, M. et al. Interferon-λ rs12979860 genotype and liver fibrosis in viral and non-viral chronic liver disease. Nat. Commun. 6, 6422 (2015).
Petta, S. et al. IL28B and PNPLA3 polymorphisms affect histological liver damage in patients with non-alcoholic fatty liver disease. J. Hepatol. 56, 1356–1362 (2012).
Noureddin, M. et al. Association of IL28B genotype with fibrosis progression and clinical outcomes in patients with chronic hepatitis C: a longitudinal analysis. Hepatology 58, 1548–1557 (2013).
Jouvin-Marche, E. et al. Lymphocytes degranulation in liver in hepatitis C virus carriers is associated with IFNL4 polymorphisms and ALT levels. J. Infect. Dis. 209, 1907–1915 (2014).
Key, F.M. et al. Selection on a variant associated with improved viral clearance drives local, adaptive pseudogenization of interferon-λ4 (IFNL4). PLoS Genet. 10, e1004681 (2014).
Manry, J. et al. Evolutionary genetic dissection of human interferons. J. Exp. Med. 208, 2747–2759 (2011).
Kindler, E. et al. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. MBio 4, e00611–e00612 (2013).
Acknowledgements
We thank L. Heilesen, H.H. Gad and S. Davidson for critical reading of this manuscript and help with figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wack, A., Terczyńska-Dyla, E. & Hartmann, R. Guarding the frontiers: the biology of type III interferons. Nat Immunol 16, 802–809 (2015). https://doi.org/10.1038/ni.3212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3212