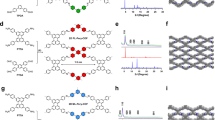
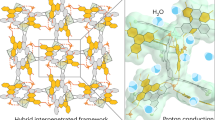
Abstract
Progress over the past decades in proton-conducting materials has generated a variety of polyelectrolytes1,2,3,4,5 and microporous polymers6,7,8,9,10. However, most studies are still based on a preconception that large pores eventually cause simply flow of proton carriers rather than efficient conduction of proton ions, which precludes the exploration of large-pore polymers for proton transport. Here, we demonstrate proton conduction across mesoporous channels in a crystalline covalent organic framework. The frameworks are designed to constitute hexagonally aligned, dense, mesoporous channels that allow for loading of N-heterocyclic proton carriers. The frameworks achieve proton conductivities that are 2–4 orders of magnitude higher than those of microporous and non-porous polymers. Temperature-dependent and isotopic experiments revealed that the proton transport in these channels is controlled by a low-energy-barrier hopping mechanism. Our results reveal a platform based on porous covalent organic frameworks for proton conduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
12 April 2016
In the version of the Letter originally published, a word was erroneously repeated in the sentence beginning 'Our results reveal...' in the first paragraph. This has been corrected in all versions of the Letter.
References
Higashihara, T., Matsumoto, K. & Ueda, M. Sulfonated aromatic hydrocarbon polymers as proton exchange membranes for fuel cells. Polymer 50, 5341–5357 (2009).
Fang, J. et al. Novel sulfonated polyimides as polyelectrolytes for fuel cell application. 1. Synthesis, proton conductivity, and water stability of polyimides from 4,4’-diaminodiphenyl ether-2,2’-disulfonic acid. Macromolecules 35, 9022–9028 (2002).
Xing, P., Robertson, G. P., Guiver, M. D., Mikhailenko, S. D. & Kaliaguine, S. Sulfonated poly(aryl ether ketone)s containing the hexafluoroisopropylidene diphenyl moiety prepared by direct copolymerization, as proton exchange membranes for fuel cell application. Macromolecules 37, 7960–7967 (2004).
Mader, J. A. & Benicewicz, B. C. Sulfonated polybenzimidazoles for high temperature PEM fuel cells. Macromolecules 43, 6706–6715 (2010).
Matsumoto, K., Higashihara, T. & Ueda, M. Locally and densely sulfonated poly(ether sulfone)s as proton exchange membrane. Macromolecules 42, 1161–1166 (2009).
Shimizu, G. K., Taylor, J. M. & Kim, S. Proton conduction with metal-organic frameworks. Science 341, 354–355 (2013).
Horike, S., Umeyama, D. & Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal-organic frameworks. Acc. Chem. Res. 46, 2376–2384 (2013).
Yoon, M., Suh, K., Natarajan, S. & Kim, K. Proton conduction in metal-organic frameworks and related modularly built porous solids. Angew. Chem. Int. Ed. 52, 2688–2700 (2013).
Xu, G., Otsubo, K., Yamada, T., Sakaida, S. & Kitagawa, H. Superprotonic conductivity in a highly oriented crystalline metal-organic framework nanofilm. J. Am. Chem. Soc. 135, 7438–7441 (2013).
Jeong, N. C., Samanta, B., Lee, C. Y., Farha, O. K. & Hupp, J. T. Coordination-chemistry control of proton conductivity in the iconic metal-organic framework material HKUST-1. J. Am. Chem. Soc. 134, 51–54 (2012).
Maffeo, C., Bhattacharya, S., Yoo, J., Wells, D. & Aksimentiev, A. Modeling and simulation of ion channels. Chem. Rev. 112, 6250–6284 (2012).
Mauritz, K. A. & Moore, R. B. State of understanding of nafion. Chem. Rev. 104, 4535–4585 (2004).
Schmidt-Rohr, K. & Chen, Q. Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nature Mater. 7, 75–83 (2008).
Xu, H. & Jiang, D. Covalent organic frameworks: crossing the channel. Nature Chem. 6, 564–566 (2014).
Bureekaew, S. et al. One-dimensional imidazole aggregate in aluminium porous coordination polymers with high proton conductivity. Nature Mater. 8, 831–836 (2009).
Hurd, J. A. et al. Anhydrous proton conduction at 150 °C in a crystalline metal-organic framework. Nature Chem. 1, 705–710 (2009).
Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012).
Cote, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Colson, J. W. et al. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 332, 228–231 (2011).
Chandra, S. et al. Phosphoric acid loaded azo (–N = N–) based covalent organic framework for proton conduction. J. Am. Chem. Soc. 136, 6570–6573 (2014).
Kuhn, P., Antonietti, M. & Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47, 3450–3453 (2008).
Zhu, X. et al. A superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation. J. Am. Chem. Soc. 134, 10478–10484 (2012).
Xu, H., Gao, J. & Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nature Chem. 7, 905–912 (2015).
DeBlase, C. R. et al. Rapid and efficient redox processes within 2D covalent organic framework thin films. ACS Nano 9, 3178–3183 (2015).
Medina, D. D. et al. Room temperature synthesis of covalent-organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 137, 1016–1019 (2015).
Kawada, W., McGhie, A. R. & Labes, M. M. Protonic conductivity in imidazole single crystal. J. Chem. Phys. 52, 3121–3125 (1970).
Li, S., Zhou, Z., Zhang, Y. & Liu, M. 1H-1,2,4-triazole: an effective solvent for proton-conducting electrolytes. Chem. Mater. 17, 5884–5886 (2005).
Aradi, B., Hourahine, B. & Frauenheim, T. DFTB +, a sparse matrix-based implementation of the DFTB method. J. Phys. Chem. A 111, 5678–5684 (2007).
Lukose, B., Kuc, A. & Heine, T. Stability and electronic properties of 3D covalent organic frameworks. J. Mol. Model. 19, 2143–2148 (2013).
Kuhnert, N., Rossignolo, G. M. & Lopez-Periago, A. The synthesis of trianglimines: on the scope and limitations of the [3 + 3] cyclocondensation reaction between (1R,2R)-diaminocyclohexane and aromatic dicarboxaldehydes. Org. Biomol. Chem. 1, 1157–1170 (2003).
Acknowledgements
D.J. acknowledges the support of a Grant-in-Aid for Scientific Research (A) (24245030) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Author information
Authors and Affiliations
Contributions
D.J. supervised and supported the project. H.X. conducted the experiments and computational calculations. S.T. performed porosity, conductivity and durability experiments. D.J. and H.X. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4621 kb)
Rights and permissions
About this article
Cite this article
Xu, H., Tao, S. & Jiang, D. Proton conduction in crystalline and porous covalent organic frameworks. Nature Mater 15, 722–726 (2016). https://doi.org/10.1038/nmat4611
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4611