Key Points
-
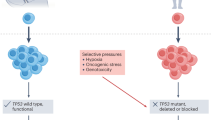
The problem of curing cancer is equivalent to the extinction of a genetically diverse, single-celled organismal species
-
Most extinctions are thought to occur through a 'press-pulse' dynamic in which multiple stressors reduce population size and habitat, and then an abrupt perturbation finally causes population collapse
-
Cancer therapy can be improved by mimicking causes of species extinction, including reducing neoplastic evolvability, destroying habitat, targeting escape phenotypes, and maintaining multiple, diverse selective pressures for many cell generations
-
The characteristics that make a species resistant to extinction should also be useful prognostic markers for a neoplasm's resistance to therapy
-
Targeting a tumour's habitat and evolvability remain promising, but relatively unexplored, avenues for future research
Abstract
Although we can treat cancers with cytotoxic chemotherapies, target them with molecules that inhibit oncogenic drivers, and induce substantial cell death with radiation, local and metastatic tumours recur, resulting in extensive morbidity and mortality. Indeed, driving a tumour to extinction is difficult. Geographically dispersed species of organisms are perhaps equally resistant to extinction, but >99.9% of species that have ever existed on this planet have become extinct. By contrast, we are nowhere near that level of success in cancer therapy. The phenomena are broadly analogous—in both cases, a genetically diverse population mutates and evolves through natural selection. The goal of cancer therapy is to cause cancer cell population extinction, or at least to limit any further increase in population size, to prevent the tumour burden from overwhelming the patient. However, despite available treatments, complete responses are rare, and partial responses are limited in duration. Many patients eventually relapse with tumours that evolve from cells that survive therapy. Similarly, species are remarkably resilient to environmental change. Paleontology can show us the conditions that lead to extinction and the characteristics of species that make them resistant to extinction. These lessons could be translated to improve cancer therapy and prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Merlo, L. M., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935 (2006).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Crespi, B. & Summers, K. Evolutionary biology of cancer. Trends Ecol. Evol. 20, 545–552 (2005).
Anderson, K. et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 469, 356–361 (2011).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Gerlinger, M. et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 46, 225–233 (2014).
Dewhurst, S. M. et al. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discov. 4, 175–185 (2014).
Diaz, L. A. Jr et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Sepkoski, J. J. Jr in Patterns and processes in the history of life (eds Raup, D. M. & Jablonski, D.) 277–295 (Springer, 1986).
Raup, D. M. Extinction: bad genes or bad luck? (W. W. Norton & Company, 1991).
Wiens, D. & Slaton, M. R. The mechanism of background extinction. Biol. J. Linnean Soc. 105, 255–268 (2012).
Raup, D. M. Biological extinction in earth history. Science 231, 1528–1533 (1986).
Weinbauer, M. G. & Rassoulzadegan, F. Extinction of microbes: evidence and potential consequences. Endanger. Species Res. 3, 205–215 (2007).
Quental, T. B. & Marshall, C. R. How the Red Queen drives terrestrial mammals to extinction. Science 341, 290–292 (2013).
Bambach, R. K. Phanerozoic biodiversity mass extinctions. Annu. Rev. Earth Planet. Sci. 34, 127–155 (2006).
Sodhi, N. S., Brook, B. W. & Bradshaw, C. in Princeton Guide to Ecology (ed. Levin, S. A.) 514–520 (Princeton University Press, 2009).
Sheehan, P. M. The late Ordovician mass extinction. Annu. Rev. Earth Planet. Sci. 29, 331–364 (2001).
Arens, N. C. & West, I. D. Press-pulse: a general theory of mass extinction? Paleobiology 34, 456–471 (2008).
Barnosky, A. D. et al. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57 (2011).
Wake, D. B. & Vredenburg, V. T. Colloquium paper: are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA 105 (Suppl. 1), 11466–11473 (2008).
Barnosky, A. D., Koch, P. L., Feranec, R. S., Wing, S. L. & Shabel, A. B. Assessing the causes of late Pleistocene extinctions on the continents. Science 306, 70–75 (2004).
Thomas, C. D. et al. Extinction risk from climate change. Nature 427, 145–148 (2004).
Wasik, B. R. & Turner, P. E. On the biological success of viruses. Annu. Rev. Microbiol. 67, 519–541 (2013).
Aktipis, C. A., Boddy, A. M., Gatenby, R. A., Brown, J. S. & Maley, C. C. Life history trade-offs in cancer evolution. Nat. Rev. Cancer 13, 883–892 (2013).
Flessa, K. W. et al. in Patterns and processes in the history of life (eds Raup, D. M. & Jablonski, D.) 235–257 (Springer, 1986).
McKinney, M. L. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516 (1997).
Jablonski, D. Mass extinctions and macroevolution. Paleobiology 31, 192–210 (2005).
Soltis, D. E. & Soltis, P. S. Polyploidy: recurrent formation and genome evolution. Trends Ecol. Evol. 14, 348–352 (1999).
Otto, S. P. The evolutionary consequences of polyploidy. Cell 131, 452–462 (2007).
Flessa, K. W. & Jablonski, D. Declining Phanerozoic background extinction rates: effect of taxonomic structure? Nature 313, 216–218 (1985).
Payne, J. L. & Finnegan, S. The effect of geographic range on extinction risk during background and mass extinction. Proc. Natl Acad. Sci. USA 104, 10506–10511 (2007).
Wang, T. L. et al. Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. Proc. Natl Acad. Sci. USA 101, 3089–3094 (2004).
Silva, A. S. & Gatenby, R. A. A theoretical quantitative model for evolution of cancer chemotherapy resistance. Biol. Direct 5, 25 (2010).
Cunningham, J. J., Gatenby, R. A. & Brown, J. S. Evolutionary dynamics in cancer therapy. Mol. Pharm. 8, 2094–2100 (2011).
Orlando, P. A., Gatenby, R. A. & Brown, J. S. Cancer treatment as a game: integrating evolutionary game theory into the optimal control of chemotherapy. Phys. Biol. 9, 065007 (2012).
Simon, R. & Norton, L. The Norton–Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat. Clin. Pract. Oncol. 3, 406–407 (2006).
Liow, L. H., Fortelius, M., Lintulaakso, K., Mannila, H. & Stenseth, N. C. Lower extinction risk in sleep-or-hide mammals. Am. Nat. 173, 264–272 (2009).
Perez, C. A., Grigsby, P. W., Castro-Vita, H. & Lockett, M. A. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 32, 1275–1288 (1995).
Overgaard, J. et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 362, 933–940 (2003).
Tomlinson, J. S. et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 25, 4575–4580 (2007).
Kopetz, S. et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 27, 3677–3683 (2009).
Pagani, O. et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J. Natl Cancer Inst. 102, 456–463 (2010).
Sinha, P., Clements, V. K., Miller, S. & Ostrand-Rosenberg, S. Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol. Immunother. 54, 1137–1142 (2005).
Swanton, C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 72, 4875–4882 (2012).
Gatenby, R. A., Brown, J. & Vincent, T. Lessons from applied ecology: cancer control using an evolutionary double bind. Cancer Res. 69, 7499–7502 (2009).
Lavergne, S. & Molofsky, J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc. Natl Acad. Sci. USA 104, 3883–3888 (2007).
Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W. & Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl Acad. Sci. USA 98, 15155–15160 (2001).
Patyar, S. et al. Bacteria in cancer therapy: a novel experimental strategy. J. Biomed. Sci. 17, 21 (2010).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Soria, J. C. et al. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1) [abstract]. Eur. J. Cancer 49 (Suppl. 2), a3408 (2013).
Alemany, R. Viruses in cancer treatment. Clin. Transl. Oncol. 15, 182–188 (2013).
Kim, M. K. et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci. Transl. Med. 5, 185ra63 (2013).
Eierhoff, T., Hrincius, E. R., Rescher, U., Ludwig, S. & Ehrhardt, C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 6, e1001099 (2010).
Hiley, C. T. et al. Vascular endothelial growth factor A promotes vaccinia virus entry into host cells via activation of the AKT pathway. J. Virol. 87, 2781–2790 (2013).
Andtbacka, R. H. I. et al. OPTiM: a randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma [abstract]. J. Clin. Oncol. 31 (Suppl.), LBA9008 (2013).
Bourke, M. G. et al. The emerging role of viruses in the treatment of solid tumours. Cancer Treat. Rev. 37, 618–632 (2011).
Heise, C. et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 3, 639–645 (1997).
Harrington, K. J. et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 16, 4005–4015 (2010).
Ferguson, M. S., Lemoine, N. R. & Wang, Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv. Virol. 2012, 805629 (2012).
Tysome, J. R. et al. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin. Cancer Res. 18, 6679–6689 (2012).
Komarova, S., Kawakami, Y., Stoff-Khalili, M. A., Curiel, D. T. & Pereboeva, L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 5, 755–766 (2006).
Mok, W., Boucher, Y. & Jain, R. K. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 67, 10664–10668 (2007).
Dunn, R. R., Harris, N. C., Colwell, R. K., Koh, L. P. & Sodhi, N. S. The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. Biol. Sci. 276, 3037–3045 (2009).
Camacho, D. F. & Pienta, K. J. Disrupting the networks of cancer. Clin. Cancer Res. 18, 2801–2808 (2012).
Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Cell 7, 513–520 (2005).
Pienta, K. J., McGregor, N., Axelrod, R. & Axelrod, D. E. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl. Oncol. 1, 158–164 (2008).
Kareva, I. What can ecology teach us about cancer? Transl. Oncol. 4, 266–270 (2011).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004).
Gatenby, R. A. & Gillies, R. J. Glycolysis in cancer: a potential target for therapy. Int. J. Biochem. Cell Biol. 39, 1358–1366 (2007).
Jin, S., DiPaola, R. S., Mathew, R. & White, E. Metabolic catastrophe as a means to cancer cell death. J. Cell Sci. 120, 379–383 (2007).
Cheong, H., Lu, C., Lindsten, T. & Thompson, C. B. Therapeutic targets in cancer cell metabolism and autophagy. Nat. Biotechnol. 30, 671–678 (2012).
Semenza, G. L. Tumor metabolism: cancer cells give and take lactate. J. Clin. Invest. 118, 3835–3837 (2008).
Gerlinger, M. et al. Genome-wide RNA interference analysis of renal carcinoma survival regulators identifies MCT4 as a Warburg effect metabolic target. J. Pathol. 227, 146–156 (2012).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Knoll, A. H., Bambach, R. K., Payne, J. L., Pruss, S. & Fischer, W. W. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313 (2007).
Kleber, M. et al. Challenging the current approaches to multiple myeloma- and other cancer-related bone diseases: from bisphosphonates to targeted therapy. Leuk. Lymphoma 53, 1057–1061 (2012).
Coleman, R. et al. Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: a meta-analysis of individual patient data from randomised trials [abstract]. Cancer Res. 73, S4–S7 (2013).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Coussens, L. M., Fingleton, B. & Matrisian, L. M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295, 2387–2392 (2002).
Egeblad, M. & Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174 (2002).
Kaye, S. B. et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin. Cancer Res. 18, 6509–6518 (2012).
Madden, J. I. Infinity Pharmaceuticals: Infinity reports update from phase 2 study of saridegib plus gemcitabine in patients with metastatic pancreatic cancer [online], (2012).
Amakye, D., Jagani, Z. & Dorsch, M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 19, 1410–1422 (2013).
Berlin, J. et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin. Cancer Res. 19, 258–267 (2013).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Al-Husein, B., Abdalla, M., Trepte, M., Deremer, D. L. & Somanath, P. R. Antiangiogenic therapy for cancer: an update. Pharmacotherapy 32, 1095–1111 (2012).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
De Bock, K., Mazzone, M. & Carmeliet, P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat. Rev. Clin. Oncol. 8, 393–404 (2011).
Ebos, J. M. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Boccaccio, C. & Comoglio, P. M. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 6, 637–645 (2006).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Smith, D. C. et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J. Clin. Oncol. 31, 412–419 (2013).
Chen, J., Sprouffske, K., Huang, Q. & Maley, C. C. Solving the puzzle of metastasis: the evolution of cell migration in neoplasms. PLoS ONE 6, e17933 (2011).
Aktipis, C. A., Maley, C. C. & Pepper, J. W. Dispersal evolution in neoplasms: the role of disregulated metabolism in the evolution of cell motility. Cancer Prev. Res. (Phila.) 5, 266–275 (2012).
Hildebrandt, B. et al. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 43, 33–56 (2002).
Wust, P. et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 3, 487–497 (2002).
Coffey, D. S., Getzenberg, R. H. & DeWeese, T. L. Hyperthermic biology and cancer therapies: a hypothesis for the “Lance Armstrong effect”. JAMA 296, 445–448 (2006).
Mosser, D. D., Caron, A. W., Bourget, L., Denis-Larose, C. & Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17, 5317–5327 (1997).
Scaltriti, M., Dawood, S. & Cortes, J. Molecular pathways: targeting hsp90—who benefits and who does not. Clin. Cancer Res. 18, 4508–4513 (2012).
Ostberg, J. R. & Repasky, E. A. Use of mild, whole body hyperthermia in cancer therapy. Immunol. Invest. 29, 139–142 (2000).
van der Zee, J. Heating the patient: a promising approach? Ann. Oncol. 13, 1173–1184 (2002).
Sardari, D. & Verga, N. in Current cancer treatment—novel beyond conventional approaches (ed. Ozdemir, O.) 455–474 (InTech, 2011).
Jones, E. L. et al. Randomized trial of hyperthermia and radiation for superficial tumors. J. Clin. Oncol. 23, 3079–3085 (2005).
Valdagni, R. & Amichetti, M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int. J. Radiat. Oncol. Biol. Phys. 28, 163–169 (1994).
van der Zee, J. et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 355, 1119–1125 (2000).
Issels, R. D. et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 11, 561–570 (2010).
Ibrahim-Hashim, A. et al. Systemic buffers inhibit carcinogenesis in TRAMP mice. J. Urol. 188, 624–631 (2012).
Scharovsky, O. G., Mainetti, L. E. & Rozados, V. R. Metronomic chemotherapy: changing the paradigm that more is better. Curr. Oncol. 16, 7–15 (2009).
Kerbel, R. S. & Kamen, B. A. The anti-angiogenic basis of metronomic chemotherapy. Nat. Rev. Cancer 4, 423–436 (2004).
Pasquier, E., Kavallaris, M. & André, N. Metronomic chemotherapy: new rationale for new directions. Nat. Rev. Clin. Oncol. 7, 455–465 (2010).
Lien, K., Georgsdottir, S., Sivanathan, L., Chan, K. & Emmenegger, U. Low-dose metronomic chemotherapy: a systematic literature analysis. Eur. J. Cancer 49, 3387–3395 (2013).
Kotler, B. P., Blaustein, L. & Brown, J. S. Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Ann. Zool. Fennici 29, 199–206 (1992).
Ashworth, A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 26, 3785–3790 (2008).
Kaelin, W. G. Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 5, 689–698 (2005).
Felsenstein, J. The evolutionary advantage of recombination. Genetics 78, 737–756 (1974).
Birkbak, N. J. et al. Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res. 71, 3447–3452 (2011).
Roylance, R. et al. Relationship of extreme chromosomal instability with long-term survival in a retrospective analysis of primary breast cancer. Cancer Epidemiol. Biomarkers Prev. 20, 2183–2194 (2011).
Harris, R. S. & Liddament, M. T. Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 4, 868–877 (2004).
Roberts, S. A. et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet. 45, 970–976 (2013).
Buckler, A. J. et al. The use of volumetric CT as an imaging biomarker in lung cancer. Acad. Radiol. 17, 100–106 (2010).
Eaves, C. J. Cancer stem cells: here, there, everywhere? Nature 456, 581–582 (2008).
Quintana, E. et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008).
Maley, C. C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat. Genet. 38, 468–473 (2006).
Merlo, L. M. et al. A comprehensive survey of clonal diversity measures in Barrett's esophagus as biomarkers of progression to esophageal adenocarcinoma. Cancer Prev. Res. (Phila.) 3, 1388–1397 (2010).
Mroz, E. A. et al. High intratumor genetic heterogeneity is related to worse outcome in patients with head and neck squamous cell carcinoma. Cancer 119, 3034–3042 (2013).
Rabinovitch, P. S., Longton, G., Blount, P. L., Levine, D. S. & Reid, B. J. Predictors of progression in Barrett's esophagus III: baseline flow cytometric variables. Am. J. Gastroenterol. 96, 3071–3083 (2001).
A'Hern, R. P. et al. Taxane benefit in breast cancer—a role for grade and chromosomal stability. Nat. Rev. Clin. Oncol. 10, 357–364 (2013).
Schwarzenberg, J. et al. 3′-deoxy-3′-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J. Nucl. Med. 53, 29–36 (2012).
Etzioni, R. et al. The case for early detection. Nat. Rev. Cancer 3, 243–252 (2003).
Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 20, 548–554 (2014).
Turley, E. A., Veiseh, M., Radisky, D. C. & Bissell, M. J. Mechanisms of disease: epithelial–mesenchymal transition—does cellular plasticity fuel neoplastic progression. Nat. Clin. Pract. Oncol. 5, 280–290 (2008).
Sahai, E. Illuminating the metastatic process. Nat. Rev. Cancer 7, 737–749 (2007).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Sottoriva, A., Spiteri, I., Shibata, D., Curtis, C. & Tavare, S. Single-molecule genomic data delineate patient-specific tumor profiles and cancer stem cell organization. Cancer Res. 73, 41–49 (2013).
Zhou, M. et al. Radiologically defined ecological dynamics and clinical outcomes in glioblastoma multiforme: preliminary results. Transl. Oncol. 7, 5–13 (2014).
Gatenby, R. A., Silva, A. S., Gillies, R. J. & Frieden, B. R. Adaptive therapy. Cancer Res. 69, 4894–4903 (2009).
Caplan, M. L. & Bustin, R. M. Devonian–Carboniferous Hangenberg mass extinction event, widespread organic-rich mudrock and anoxia: causes and consequences. Palaeogeogr. Palaeocl. 148, 187–207 (1999).
Streel, M., Caputo, M. V., Loboziak, S. & Melo, J. H. Late Frasnian–Famennian climates based on palynomorph analyses and the question of the Late Devonian glaciations. Earth Sci. Rev. 52, 121–173 (2000).
Stigall, A. L. Speciation collapse and invasive species dynamics during the Late Devonian “Mass Extinction”. GSA Today 22, 4–9 (2012).
Bambach, R. K., Knoll, A. H. & Sepkoski, J. J. Anatomical and ecological constraints on Phanerozoic animal diversity in the marine realm. Proc. Natl Acad. Sci. USA 99, 6854–6859 (2002).
Erwin, D. H., Bowring, S. A. & Yugan, J. in Catastrophic events and mass extinctions: impacts and beyond (eds Koeberl, C. & MacLeod, K. G.) 363–383 (Geological Society of America, 2002).
Shen, S. Z. et al. Calibrating the end-Permian mass extinction. Science 334, 1367–1372 (2011).
Burgess, S. D., Bowring, S. & Shen, S. Z. High-precision timeline for Earth's most severe extinction. Proc. Natl Acad. Sci. USA 111, 3316–3321 (2014).
Benton, M. J. More than one event in the late Triassic mass extinction. Nature 321, 857–861 (1986).
Deng, S., Lu, Y. & Xu, D. Progress and review of the studies on the end-Triassic mass extinction event. Sci. China Ser. D 48, 2049–2060 (2005).
Hautmann, M. Extinction: end-Triassic mass extinction. eLS http://dx.doi.org/10.1002/9780470015902.a0001655.pub3 (2012).
Blackburn, T. J. et al. Zircon U-Pb geochronology links the end-Triassic extinction with the Central Atlantic Magmatic Province. Science 340, 941–945 (2013).
Alvarez, L. W., Alvarez, W., Asaro, F. & Michel, H. V. Extraterrestrial cause for the cretaceous-tertiary extinction. Science 208, 1095–1108 (1980).
Melosh, H. J., Schneider, N. M., Zahnle, K. J. & Latham, D. Ignition of global wildfires at the Cretaceous/Tertiary boundary. Nature 343, 251–254 (1990).
Robertson, D. S., McKenna, M. C., Toon, O. B., Hope, S. & Lillegraven, J. A. Survival in the first hours of the Cenozoic. Geol. Soc. Am. Bull. 116, 760–768 (2004).
Schulte, P. et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 327, 1214–1218 (2010).
Burrell, R. A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345 (2013).
Kostadinov, R. L. et al. NSAIDs modulate clonal evolution in Barrett's esophagus. PLoS Genet. 9, e1003553 (2013).
Acknowledgements
The work of C.T.H. is supported by a National Institute for Health Research Academic Clinical Fellowship. C.S. receives funding from Cancer Research UK, the Rosetrees Trust, the Breast Cancer Research Foundation, the Prostate Cancer Foundation, European Union Framework program 7 grants PREDICT and RESPONSIFY, and the European Research Council. The work of P.E.T. is supported in part by grants from the National Science Foundation (DEB-1021243) and NIH (R01-AI091646). The work of C.C.M. is supported in part by Research Scholar Grant #117209-RSG-09-163-01-CNE from the American Cancer Society, NIH grants P01 CA91955, R01 CA149566, R01 CA170595 and R01 CA140657, and CDMRP Breast Cancer Research Program Award BC132057.
Author information
Authors and Affiliations
Contributions
V.W. researched the data for the article. All authors contributed substantially to discussion of content. V.W., C.T.H., P.E.T. and C.C.M. wrote the article. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Walther, V., Hiley, C., Shibata, D. et al. Can oncology recapitulate paleontology? Lessons from species extinctions. Nat Rev Clin Oncol 12, 273–285 (2015). https://doi.org/10.1038/nrclinonc.2015.12
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.12