Abstract
Objective
Metacognitive training for psychosis (MCT) targets cognitive biases implicated in the pathogenesis of psychosis, e.g., jumping to conclusions, overconfidence in errors, and inflexibility. This systematic meta-review investigated the current meta-analytic evidence for the effectiveness of MCT with respect to core symptom features in schizophrenia (i.e., positive symptoms, delusions and hallucinations, negative symptoms, and overall psychotic symptoms).
Data sources
This meta-review was registered with PROSPERO (CRD42023447442) on July 28, 2023. Articles were searched across five electronic databases from January 1, 2007 to September 1, 2023.
Study selection
Meta-analyses addressing metacognitive interventions targeting psychotic symptoms were eligible for meta-review.
Data extraction and synthesis
PRISMA guidelines were followed when applicable. Data extraction was done independently by two authors (AM, AS). A random-effects model was used to pool data within meta-analyses.
Main outcomes and measures
Main outcomes were levels/severity of positive symptoms, delusions and hallucinations, negative symptoms, and overall psychotic symptoms after intervention.
Results
Eight meta-analyses and two re-analyses were included for meta-review. A total of eight analyses provided sufficient data for analysis. Significant evidence was found in favor of MCT for positive symptoms (85.71%; N = 35, g = 0.473 [0.295, 0.651], I2 = 74.64), delusions (60%; N = 24, g = 0.639 [0.389, 0.889], I2 = 80.01), hallucinations (100%; N = 9, g = 0.265 [0.098, 0.432], I2 = 6.1), negative symptoms (100%; N = 17, g = 0.233 [0.1, 0.366], I2 = 34.78), and overall symptoms (50%; N = 37, g = 0.392 [0.245, 0.538], I2 = 65.73). None of the meta-analyses included a large enough sample size to meet the criteria for ‘suggestive’, ‘convincing’, or ‘highly convincing’ evidence according to metaumbrella.org guidelines (required sample size > 1000 cases). None of the meta-analyses scored ‘moderate’ or ‘high’ on methodological quality. Meta-analyses with significant results were more recent and/or considered more primary studies.
Conclusions and relevance
There is consistent evidence that MCT ameliorates positive symptoms and delusions in schizophrenia.
Similar content being viewed by others
Introduction
Schizophrenia and related psychotic disorders have been ranked among the most debilitating mental disorders worldwide, requiring long-term disability adjustments for 1.5% of men and women aged between 25 and 49 years [1]. Moreover, schizophrenia has been linked to lifetime suicide rates as high as 10% [2], with rates of suicide attempts for people with schizophrenia ranging from 18–55% [3]. Guidelines from the American Psychiatric Association (APA), as well as the British NICE guidelines, recommend the use of antipsychotic drugs as first-line treatment for schizophrenia and psychosis [2]. In the past, psychotherapy for psychosis was often not offered due to the belief that the condition is neither psychologically explicable nor treatable [4]. Recent reviews with predominantly positive results [5, 6] have contradicted this viewpoint and discussed the inclusion of Cognitive Behavioral Therapy (CBT) in the treatment guidelines for psychosis. Yet, some dissenting findings have been reported [7], and effects of psychotherapy for psychosis are lower than for other disorders.
CBT targets symptoms by highlighting maladaptive beliefs or maladaptive appraisal and dysfunctional coping with events or situations [8,9,10]. A new trend in CBT is especially concerned with cognitive biases, such as jumping to conclusions (JTC), overconfidence in errors, and a bias against disconfirmatory evidence (BADE), which have been linked to the formation and maintenance of positive and negative symptoms [11,12,13,14] and are largely unresponsive to antipsychotic medication [15]. Metacognitive training for psychosis (MCT), a CBT variant, aims to raise (meta)cognitive awareness of these cognitive biases. The core principles and objectives of MCT relate to the concept of metacognition or the act of “thinking about thinking” [16]. MCT encompasses several aspects of self-awareness and problem-solving targeted at correcting cognitive distortions and overconfidence, as patients with schizophrenia spectrum disorders may not be aware of their cognitive biases or may tend to be overconfident in their assumptions [17,18,19] (see eTable 1 of the supplementary material for a complete overview of the MCT modules and objectives).
While an abundance of meta-analyses and reviews have investigated the effectiveness of CBT [5, 20, 21], less evidence is available for metacognitive approaches such as MCT. Several meta-analyses investigating the effectiveness, adherence, and feasibility of MCT have been published during the past decade, with the majority showing favorable results for MCT [22,23,24,25,26]; however, a few meta-analyses reported either unfavorable [27, 28] or inconclusive [29, 30] findings. To date, a critical meta-review addressing the methodological quality and robustness of these quantitative reviews is lacking. Meta-reviews provide a summary and grading of meta-analytic results and methodological quality in order to synthesize and identify the most credible evidence base for controversial or differentiated findings within the literature base [31]. Subsequently, meta-reviews may identify research domains in need of further evaluation as well as provide a direction for future research. Thus, the current study aimed to weigh the meta-analytic evidence on MCT’s effectiveness in reducing delusions as well as other positive, negative, and overall symptoms in patients with schizophrenia spectrum or related (non-affective) psychotic disorders.
Methods
Search strategy and selection criteria
This study was registered with PROSPERO (ID: CRD42023447442) on July 28, 2023. The following databases were searched between August 25 and September 1, 2023: PubMed, Web of Science, EMBASE, PsycINFO, and MEDLINE. The following search was conducted on PubMed: ((“Schizophrenia Spectrum and Other Psychotic Disorders”[Mesh]) OR (schizo* or delusion* or psychosis or psychoses or psychotic* or first episode* or first-episode* or FEP)) AND (((“metacognitive” train*) OR (“meta-cognitive” train*) OR (MCT)) AND (“2007”[Date - Publication] : “3000”[Date - Publication])) AND (meta-analys* OR metanalys* OR review). Similar searches were conducted in all databases (see eTable 2 in the supplementary material for a detailed description of the search strategy). We adhered to PRISMA guidelines, when applicable for this meta-review. Two authors (AM, AS) screened titles, abstracts, and full-text records independently. Any disagreements were resolved in discussion with two additional authors (RF, SM). Two search objectives were established. The first objective was to conduct a meta-review of MCT meta-analyses. The outcome of interest was defined as the severity of delusions after intervention compared to before intervention or to a different intervention/no treatment/treatment as usual (TAU)/active control. Secondary outcomes of interest included positive, negative, and overall psychotic symptoms. Outcomes at follow-up were also of interest. Due to the lack of studies investigating possible moderating effects of other baseline symptoms on the effectiveness of MCT for psychotic symptoms, particularly delusions, a second objective addressing moderating effects of baseline symptoms on the change in delusions only, as well as positive (i.e., delusions and hallucinations), negative, and overall symptoms was initially intended. The current study presents results for the first of the two objectives; the second objective was not further pursued in the framework of this article due to the aforementioned lack of studies.
Meta-analyses on metacognitive interventions were included for meta-review if they fulfilled the following inclusion and exclusion criteria: (i) samples comprised participants (mean age 18+ years) with a DSM/ICD diagnosis of a schizophrenia spectrum or related (non-affective) psychotic disorder, (ii) included interventions were group or individualized MCT+, which were either assessed separately or in combination against a control condition (no treatment, treatment as usual, active control), (iii) participants were compared before and after intervention or between conditions (no treatment, TAU, or active control, including treatments such as cognitive remediation, supportive therapy, and psychoeducation), (iv) included studies were meta-analyses that examined only MCT or psychological interventions targeting cognitive biases (i.e., metacognitive interventions) underlying delusions more broadly but included MCT as one of the interventions that measured clinical symptoms (including overall symptomatology, positive symptoms [i.e., delusions and hallucinations], negative symptoms) in people with psychosis. It has to be noted that results for effects on delusions, hallucinations, and positive symptoms (i.e., delusions and hallucinations) were separately evaluated for the purpose of this study.
Studies were excluded from the meta-review if they were conducted before 2007 (as the first MCT trial was published in 2007). Network meta-analyses were also excluded to minimize confounds pertaining to indirect evidence and inferences. Additionally, studies were excluded if they comprised participants with a diagnosis of affective psychosis or comprised samples made up of 60%+ of patients without a diagnosis of non-affective psychosis (as may be the case in studies recruiting participants with first-episode psychosis). Other literature to be excluded were single randomized controlled trials (RCTs), letters to the editor, study protocols, qualitative studies, case studies, editorial articles, book chapters, and systematic reviews if they were not coupled with a meta-analysis. Additionally, we excluded articles not written in English or German. Reference lists were searched for relevant studies, and experts in the field were contacted if necessary. See eTable 3, which provides a complete list and overview of all included articles, and eTable 4 which provides a list of the records and articles excluded based on the full text, both of which can be found in the supplementary material.
Data analysis
The following data were extracted from the meta-analyses: meta-analysis authors, primary study authors, year of publication, effect size values, 95% confidence intervals for effect sizes, and sample sizes (total, cases, controls). Additionally, in cases with missing or incomplete data, the authors of the meta-analyses were contacted for further information. We needed to contact four authors [25, 26, 32, 33] for additional data and information, three of whom [25, 26, 32] provided datasets that were not available in the original meta-analyses. One meta-analysis [29] included only three primary studies, the data of which was too limited to be used for further statistical analysis. Another study [27] was excluded from further analysis due to multiple misclassifications and other methodological problems [10].
Study overlap, i.e., the extent to which the meta-analyses included the same primary studies, was assessed using a calculation of the corrected covered area (CCA) [34]. An assessment of study overlap is recommended when conducting meta-reviews [31] to critically evaluate the results of meta-analyses that have included and assessed most of the same primary studies. Study overlap can be categorized as slight (0–5%), moderate (6–10%), high (11–15%), and very high (>15%).
Methodological quality was independently assessed by two authors (AM, AS) using the revised A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) checklist [35] intended for meta-analyses that include randomized and non-randomized trials. Additionally, we used the adapted AMSTAR-Plus Content score [36]. The AMSTAR-2 is scored on 16 items and classifies study quality into four categories: high (no or one non-critical weakness), moderate (more than one non-critical weakness), low (one critical flaw with or without non-critical weaknesses), and critically low (more than one critical flaw with or without non-critical weaknesses). The AMSTAR-2 regards the following seven key components as critical items for assessment: pre-registered protocol, literature search adequacy, study exclusion justification, risk of bias for individual studies, appropriateness of meta-analytical methods, consideration of risk of bias in the discussion/interpretation of results, and assessment of likelihood and impact of publication bias. Correll et al.’s [36] AMSTAR-Plus Content scores range from 0 to 8 on the following items: double-blindness, total number of participants, significance of large study, observed cases, heterogeneity, and publication bias. See eTable 5 and eTable 6 in the supplementary material for full lists of the AMSTAR-2 and AMSTAR-Plus Content items.
Analyzable data were entered into metaumbrella.org, a statistical browser-based tool and its associated R package specifically designed for conducting meta-reviews [37]. To combine effect sizes from studies with different methods and sample characteristics, a random effects model was used. This model allows for the entered data to be converted into a common effect size [38] (Hedges’ g), and to provide a 95% confidence interval, as well as an assessment of heterogeneity using the I2 statistic [39]. Additionally, risk of bias, i.e., small study bias (smaller studies revealing more significant effects than larger studies), excess significance bias (excess of significant findings in the literature and publication bias), prediction intervals, and large study effects were assessed. Effect sizes were interpreted as ranging from small (g = 0.2) to medium (g = 0.5) to large (g = 0.8). A classification of evidence is provided, which can be divided into the following five categories: (i) convincing evidence (Class I: sample size >1000, p-value < 10e-6, I2 < 50%, p-value Egger’s test >0.05 and p-value Ioannidis test >0.05), (ii) highly suggestive evidence (Class II: sample size >1000, p-value < 10e-6, largest study with a statistically significant effect and Class I criteria not met), (iii) suggestive evidence (Class III: sample size >1000, p-value < 10e-3, Classes I–II criteria not met), (iv) weak evidence (Class IV: p-value < 0.05, Classes I–III criteria not met), and (v) non-significant (ns: p-value > 0.05). With the use of a classification and hierarchical grading of the available meta-analytic evidence, we aimed to provide a comparable and comprehensive overview of the more or less inconsistent findings regarding the effectiveness of MCT for psychotic symptom reduction.
Role of the funding source
This project was supported by the Federal Ministry of Education and Research (BMBF), under the framework of ERA PerMed (ERAPERMED2022-292). The funding agency was not involved in the planning or execution of this study or in the evaluation of the results.
Results
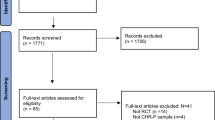
The initial data search yielded a total of 349 results, of which 199 duplicates were removed. After screening the articles based on title and abstract, 138 publications were excluded. After 12 records were screened based on full text, eight meta-analyses and two re-analyses were eventually included for meta-review. One study [29] provided unstandardized mean differences for three primary studies that were not convertible or analyzable for meta-review.
After further assessment, one meta-analysis [27] was excluded from the analysis on the basis of extensive misclassification of interventions and imprecise administering of inclusion and exclusion criteria of primary studies [10]. Burlingame et al. [32] performed a re-analysis of their study [27], which was included in the meta-review and for which the original authors provided additional evidence upon request. Van Oosterhout et al. [28] were contacted but did not provide additional data for further analysis of their re-analysis [33]. Please refer to Fig. 1 for a flowchart of the screening and selection process. Additionally, Table 1 provides an overview of study characteristics for all studies included in the meta-review.
Study overlap
The overlap of primary studies included in the meta-analyses was calculated using the corrected covered area (CCA) [34]. Study overlap was assessed for all ten meta-analyses and across all outcome parameters, as well as for each outcome parameter (i.e., overall symptoms, positive symptoms, delusions, hallucinations, negative symptoms) separately (see Table 2). The total study overlap, calculated for all included meta-analyses and re-analyses as well as for all included primary studies assessing the effectiveness of MCT, was very high (22%). Study overlap for overall symptoms was slight (2%). While study overlap for positive symptoms (17.8%) and delusions (17.8%) was very high, the CCA for hallucinations (0%) and negative symptoms (0%) is none, which can be attributed to the fact that only one study (Penney et al. [26]) assessed hallucinations and negative symptoms as part of its outcome parameters.
Methodological quality (AMSTAR-2)
Table 3 and Table 4 depict the AMSTAR-2 assessments regarding critical and non-critical domains for each analyzable meta-analysis, respectively. Table 5 depicts the AMSTAR-Plus Content scores for each meta-analysis. Two re-analyses [32, 33] could not be assessed using the AMSTAR-2 and AMSTAR-Plus Content guidelines, as the provided information for methodological quality in these two articles was not sufficient to be conclusively scored on the relevant items of these checklists.
All meta-analyses described the characteristics of PICO (population, intervention, comparator, outcome), three meta-analyses (42.85%) pre-registered a comprehensive protocol, two meta-analyses (28.57%) employed a comprehensive search strategy, four meta-analyses (57.14%) provided a list of and justifications for excluded studies, one meta-analysis (14.28%) comprehensively assessed risk of bias for the included primary studies, one meta-analysis (14.28%) employed appropriate statistical methods for combining results, five meta-analyses (71.42%) discussed risk of bias for the included primary studies when interpreting their results, and eight meta-analyses (85.71%) assessed publication bias. One meta-analysis provided a comprehensive explanation for inclusion of study design (14.28%), five meta-analyses (71.42%) performed study selection in duplicate, six meta-analyses (85.71%) performed data extraction in duplicate, none of the meta-analyses provided a detailed description of the included studies’ populations, none of the meta-analyses reported funding for the included primary studies, six meta-analyses (85.71%) assessed impact of included primary studies’ risk of bias on the results, six meta-analyses (85.71%) comprehensively discussed and explained heterogeneity in their results, and six meta-analyses (85.71%) appropriately reported conflicts of interest or funding for their study.
None of the meta-analyses had most primary studies of double-blind design, four meta-analyses (57.14%) had a total sample size n = 500–999, three meta-analyses (42.85%) had a total sample size of n > 1 000, none of the meta-analyses had results confirmed in an at least one- or two-arm large study with n > 200, one meta-analysis (14.28%) included observed cases in their study, two meta-analyses (14.28%) showed homogeneous results, and one meta-analysis (14.28%) did not show significant publication bias.
Evidence classification
Table 6 provides an overview of the classification of the meta-analytic evidence. eFigs 1–10 of the supplementary material present all outcomes via forest plots (eFigs 1–5) and histograms (eFigs 6–10) by outcome parameter. We note that all calculations were performed using unweighted effect sizes. Thus, results may differ from the meta-analyses’ original results by a one-hundredth decimal. Additionally, two meta-analyses [22, 30] used weighted effect sizes in their original reports, whereas none of the effect sizes in the current meta-review have been weighted.
Significant evidence was found in favor of MCT for overall symptoms (50%; N = 37, g = 0.392 [0.245, 0.538], I2 = 65.73), positive symptoms (71.43%; N = 36, g = 0.473 [0.295, 0.651], I2 = 74.64), delusions (60%; N = 24, g = 0.639 [0.389, 0.889], I2 = 80.01), hallucinations (100%; N = 9, g = 0.265 [0.098, 0.432], I2 = 6.11), and negative symptoms (100%; N = 17, g = 0.233 [0.1, 0.366], I2 = 34.78). These effect sizes correspond to the study with the highest classification of evidence and largest sample size [26]; reporting of effect sizes has been adapted from Berendsen et al. [5]. Resulting effect sizes may slightly differ from the original published results, due rounding to the one-hundredth decimal of primary studies’ results and the application of a random-effects model used by metaumbrella.org. Meta-analyses with significant results were more recent and/or considered more primary studies.
Overall symptoms
One of the meta-analyses [26] that assessed overall symptoms reported significant, small to medium-sized effects for overall symptoms, specifically for proximal (g = 0.392, 95% CI [0.245, 0.538], p < 0.001, I2 = 65.73) and distal symptoms (g = 0.313, 95% CI [0.197, 0.43]), p < 0.001, I2 = 46.73) at end of treatment (EoT). The second meta-analysis [32] reported non-significant effect sizes for the effectiveness of MCT for overall symptoms of schizophrenia. Heterogeneity was considerable (I2 > 45%) for both significant effect sizes. None of the meta-analyses showed excess significance bias or small study effects, however the largest primary studies included were not significant.
Positive symptoms
Six of the seven (85.71%) meta-analyses [22, 24,25,26, 30, 32] that assessed positive symptoms reported significant, small to medium-sized effects (g = 0.473, 95% CI [0.295, 0.651], p < 0.001, I2 = 74.64; g = 0.302, 95% CI [0.117, 0.486], p < 0.005, I2 = 49.25; g = 0.273, 95% CI [0.099, 0.446], p < 0.005, I2 = 47.32; g = 0.361, 95% CI [0.157, 0.565], p < 0.005, I2 = 7.13; g = 0.19, 95% CI [0.01, 0.37], p < 0.05, I2 = 0; g = 0.406, 95% CI [0.054, 0.758], p < 0.05, I2 = 34.3) for the effectiveness of MCT for positive symptoms at EoT. Small study effects were detected for one meta-analysis [30] (16%). Nonetheless, all of these were ranked as ‘weak’ (Class IV) according to the grading criteria [37], which only allows a sample size of n experimental condition >1000 to be classified as Class III or higher. One meta-analysis [28] (14.28%) reported non-significant effect sizes. No excess significance bias was detected for any of the meta-analyses. None of the largest primary studies were significant.
Delusions
Three out of five (60%) meta-analyses [22, 23, 26] assessing delusions reported significant small to large-sized (g = 0.639, 95% CI [0.389, 0.889], p < 0.001, I2 = 80.01; g = 0.38, 95% CI [0.125, 0.635], p < 0.005, I2 = 64.84; g = 0.407, 95% CI [0.066, 0.748], p < 0.05, I2 = 75.93) effects for the effectiveness of MCT for delusions at EoT. Heterogeneity was considerable (I2 > 45%) for all three significant effect sizes. All evidence was ranked as ‘weak’ (Class IV) according to the grading criteria [37]. Two meta-analyses [28, 30] (40%) reported non-significant results. Neither small study effects, excess significance bias, nor the largest primary studies were significant for any of the meta-analyses.
Hallucinations
One meta-analysis [26] investigated the effectiveness of MCT for hallucinations, showing a significant, small to medium-sized effect in favor of MCT at EoT (g = 0.265, 95% CI [0.098, 0.432], p < 0.005, I2 = 6.1). The evidence was classified as ‘weak’ (Class IV). Neither small study effects nor excess significance bias was detected. The meta-analysis’ largest primary study [40] did not find significant effects for hallucinations.
Negative symptoms
One meta-analysis [26] investigated the effectiveness of MCT for negative symptoms. The results showed a significant but small effect in favor of MCT at EoT (g = 0.233, 95% CI [0.1, 0.366], p < 0.001, I2 = 34.77). The evidence was classed as ‘weak’ (Class IV). Neither small study effects nor excess significance bias was detected. The meta-analysis’ largest primary study [41] did not find significant effects for negative symptoms.
Discussion
This meta-review aimed to provide a comprehensive overview of the meta-analytic evidence on the effectiveness of MCT in reducing the symptoms of schizophrenia, specifically delusions and positive symptoms. A total of eight meta-analyses and two re-analyses were considered for this meta-review.
Seven (87.5%; six meta-analyses and one re-analysis) out of eight statistically analyzable studies (seven meta-analyses (complemented by one re-analysis) and one re-analysis) provided evidence in favor of MCT at end of treatment (EoT), specifically for overall symptoms [26], positive symptoms [22, 24,25,26, 30, 32], delusions [22, 23, 26], hallucinations [26], and negative symptoms [26]. However, heterogeneity was substantial throughout all outcome measures. One study [32] reported no heterogeneity in their findings. Penney et al. [26] reported the highest amount of heterogeneity in their outcomes, while also encompassing the largest total sample size (N = 1816). Differences in heterogeneity could be ascribed several factors, including large sample sizes and increasing number of included studies, the variety of delivery formats of the intervention (e.g., in-person vs. online, number of sessions, in conjunction with other treatments vs. alone), as well as the setting (e.g., group vs. individual, inpatient vs outpatient setting) and staffing (e.g., nurse, psychologist) utilized during the treatment. Similar to other meta-reviews on interventions for schizophrenia symptoms (e.g., Berendsen et al. [5]), none of the meta-analytic evidence was classified as being ‘convincing’ (Class I) or ‘highly suggestive’ (Class II). All meta-analytic evidence was either classified as ‘weak (Class IV)’ or non-significant owing to an insufficient number of participants in the experimental conditions (n < 1 000) that is needed to be ranked Class III (‘suggestive’ evidence) or higher. None of the meta-analytic evidence regarding the effectiveness of CBT was ranked as ‘convincing’ (Class I) or ‘highly suggestive’ (Class II). Similar criteria have been used for the research and assessment of genetics (see Ioannidis et al. [42]) and dementia (see Bellou et al. [43]). Nonetheless, sample sizes for less prevalent disorders, such as psychosis and schizophrenia, are generally small (see Sauvé et al. [44]) and therefore less likely to fulfill the necessary classification criteria. Additionally, only 5–10% of treatments available in the medical literature show high levels of evidence, most of which (80%) are pharmacological interventions [45]. In combination with patients diagnosed with a psychotic disorder, who tend to show limited adherence to psychological interventions or treatments, these factors can considerably influence the observed effectiveness of metacognitive and other interventions in schizophrenia and psychosis. Thus, future studies should investigate patients’ satisfaction levels with MCT, which in turn may also influence adherence levels and treatment outcomes.
The most recent and broadest meta-analysis on the effectiveness of MCT to date was conducted by Penney et al. [26] and included 43 studies. The total sample size of that meta-analysis comprised 1816 participants and included 932 cases for proximal outcomes and 894 cases for positive symptoms. Penney et al. [26] found significant small (hallucinations, negative symptoms), medium-sized (proximal outcomes, distal outcomes, positive symptoms), and medium-to-large (delusions) effect sizes for all outcome variables of interest at EoT. All three meta-analyses reporting non-significant findings included less than ten primary studies [28, 30, 32].
Overlap of primary studies in our meta-review was very high for positive symptoms and delusions, suggesting that any discrepant results for meta-analyses addressing these outcomes might be due to methodological differences (i.e., effect size values, data analysis, and synthesis) rather than differences in samples and population. Total overlap for all meta-analyses and primary studies was accordingly classified as very high. Nonetheless, effects varied in size and even in status of significance, which is likely attributable to an increase in primary literature over time (e.g., differences in sample size and sample characteristics affecting results), methodology and data stratification. Hallucinations and negative symptoms were assessed by one meta-analysis [26]. Hence, overlap was none for these two outcomes.
Methodological quality of the meta-analyses was assessed using the AMSTAR-2 [35] and AMSTAR-Plus Content [36] checklists. Using these criteria, none of the meta-analyses were ranked as having high or moderate methodological quality. Two meta-analyses [24, 26] were ranked low, and all other meta-analyses received a critically low rank. The mean AMSTAR-Plus Content score was 2.44. None of the meta-analyses scored 5 or higher. Only two meta-analyses [24, 25] achieved a score of 4 (the maximum score can be achieved is 9). Additionally, double-blind primary studies were lacking, but this is inherently difficult to achieve in psychological intervention studies. While low scores indicate limited reliability of the data due to weaknesses in methodology, the AMSTAR-2 checklist [35] is subject to quite strict guidelines. Nonetheless, the authors state that “[…] our listing is a suggestion and appraisers may add or substitute other critical domains.” However, we decided to follow the suggested guidelines and a supplement to the AMSTAR-Plus Content [36] scores to ensure a comprehensive assessment.
Strengths and Limitations
The main strengths of this meta-review are its systematic approach and the use of two assessment tools for the appraisal of methodological quality as well as a classification and overview of the analyzable evidence, with which we aimed to provide a critical assessment of the effectiveness of MCT for psychotic symptom reduction.
Nonetheless, we are also aware of some limitations to this meta-review. A comprehensive statistical analysis could not be conducted for two meta-analyses [29, 33] due to insufficient data. Moreover, none of the meta-analyses had a large enough sample size to be classified as Class III or higher (n cases > 1000). Any computations generated with metaumbrella.org [37] are subject to the use of a random-effects model. Also, we did not consider long-term studies in our meta-review because of a lack of relevant studies.
Conclusion
The current study employed a systematic meta-review approach to investigate and evaluate the available meta-analytic evidence regarding the effectiveness of metacognitive training for psychosis (MCT) for reducing psychotic symptoms in schizophrenia spectrum or related, non-affective psychotic disorders. Seven out of eight analyzable studies (seven meta-analyses (one complemented by a re-analysis) and one re-analysis) provided evidence in terms of low-to-moderate effect sizes in favor of MCT at EoT and across all outcome parameters of interest. While MCT shows promising results for schizophrenia symptoms, showing the most favorable results for effects on delusions and positive symptoms, and represents a potentially cost-effective and easy-to-administer interventional tool, more studies with long-term outcomes and large sample sizes are needed for more powerful results, as well as more meta-analyses fulfilling AMSTAR-2 criteria.
Data sharing
Supplementary data and results will be made available on our corresponding website at https://clinical-neuropsychology.de/metacognitive_training-psychosis/.
References
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–22. https://doi.org/10.1016/s0140-6736(20)30925-9
Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177:868–72. https://doi.org/10.1176/appi.ajp.2020.177901
Sher L, Kahn RS. Suicide in schizophrenia: an educational overview. Medicina. 2019;55:361. https://doi.org/10.3390/medicina55070361
Moritz S, Vitzthum F, Randjbar S, Veckenstedt R, Woodward TS. Detecting and defusing cognitive traps: metacognitive intervention in schizophrenia. Curr Opin Psychiatry. 2010;23:561–9. https://doi.org/10.1097/yco.0b013e32833d16a8
Berendsen S, Berendse S, van der Torren J, Vermeulen J, de Haan L. Cognitive behavioural therapy for the treatment of schizophrenia spectrum disorders: an umbrella review of meta-analyses of randomised controlled trials. EClinicalMedicine. 2024;67:102392. https://doi.org/10.1016/j.eclinm.2023.102392
Turner DT, Burger S, Smit F, Valmaggia LR, van der Gaag M. What constitutes sufficient evidence for case formulation–driven CBT for psychosis? cumulative meta-analysis of the effect on hallucinations and delusions. Schizophr Bull. 2020;46:1072–85. https://doi.org/10.1093/schbul/sbaa045
Jones C, Hacker D, Meaden A, Cormac I, Irving CB, Xia J, et al. Cognitive behavioural therapy plus standard care versus standard care plus other psychosocial treatments for people with schizophrenia. Cochrane Database Syst Rev. 2018;11:CD008712. https://doi.org/10.1002/14651858.cd008712.pub3
Lemmers-Jansen ILJ, Moritz S. A practice-oriented review on effectiveness of metacognitive training (MCT) for psychosis. BPA Applied Psychology Bulletin. 2021;69:24–34. https://doi.org/10.26387/bpa.290.3
Moritz S, Klein JP, Lysaker PH, Mehl S. Metacognitive and cognitive-behavioral interventions for psychosis: new developments. Dialogues Clin Neurosci. 2019;21:309–17. https://doi.org/10.31887/dcns.2019.21.3/smoritz
Moritz S, Turner D, Bechdolf A, Mueller DR, Woodward TS, Penney D, et al. Group therapy for schizophrenia: Why Burlingame et al. (2020) should redo their meta-analysis. Psychother. 2022;59:133–5. https://doi.org/10.1037/pst0000401
Köther U, Vettorazzi E, Veckenstedt R, Hottenrott B, Bohn F, Scheu F, et al. Bayesian analyses of the effect of metacognitive training on social cognition deficits and overconfidence in errors. J Exp Psychopathol. 2017;8:158–74. https://doi.org/10.5127/jep.054516
McLean BF, Mattiske JK, Balzan RP. Association of the jumping to conclusions and evidence integration biases with delusions in psychosis: a detailed meta-analysis. Schizophr Bull. 2016;43:344–54. https://doi.org/10.1093/schbul/sbw056
Moritz S, Veckenstedt R, Andreou C, Bohn F, Hottenrott B, Leighton L, et al. Sustained and “sleeper” effects of group metacognitive training for schizophrenia. JAMA Psychiatry. 2014;71:1103. https://doi.org/10.1001/jamapsychiatry.2014.1038
Thibaudeau É, Achim AM, Parent C, Turcotte M, Cellard C. A meta-analysis of the associations between theory of mind and neurocognition in schizophrenia. Schizophr Res. 2020;216:118–28. https://doi.org/10.1016/j.schres.2019.12.017
Faden J, Citrome L. Resistance is not futile: treatment-refractory schizophrenia – overview, evaluation and treatment. Expert Opin Pharmacother. 2018;20:11–24. https://doi.org/10.1080/14656566.2018.1543409
Flavell JH. Metacognition and cognitive monitoring: a new area of cognitive-development inquiry. American Psychologist. 1979;34:906–11.
Moritz S, Menon M, Balzan R, Woodward TS. Metacognitive training for psychosis (MCT): past, present, and future. Eur Arch Psychiatry Clin Neurosci. 2023;273:811–7.
Moritz S, Balzan RP, Bohn F, Veckenstedt R, Kolbeck K, Bierbrodt J, et al. Subjective versus objective cognition: evidence for poor metacognitive monitoring in schizophrenia. Schizophr Res. 2016;178:74–79.
Freeman D, Garety P, Kuipers E, Colbert S, Jolley S, Fowler D, et al. Delusions and decision-making style: use of the need for closure scale. Behav Res Ther. 2006;44:1147–58.
Bighelli I, Salanti G, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry. 2018;17:316–29. https://doi.org/10.1002/wps.20577
Valiente C, Espinosa R, Trucharte A, Nieto J, Martínez-Prado L. The challenge of well-being and quality of life: a meta-analysis of psychological interventions in schizophrenia. Schizophr Res. 2019;208:16–24. https://doi.org/10.1016/j.schres.2019.01.040
Eichner C, Berna F. Acceptance and efficacy of metacognitive training (MCT) on positive symptoms and delusions in patients with schizophrenia: a meta-analysis taking into account important moderators. Schizophr Bull. 2016;42:952–62. https://doi.org/10.1093/schbul/sbv225
Liu Y, Tang C, Hung T, Tsai P, Lin M. The efficacy of metacognitive training for delusions in patients with schizophrenia: a meta‐analysis of randomized controlled trials informs evidence‐based practice. Worldviews Evid Based Nurs. 2018;15:130–9. https://doi.org/10.1111/wvn.12282
Philipp R, Kriston L, Lanio J, Kühne F, Härter M, Moritz S, et al. Effectiveness of metacognitive interventions for mental disorders in adults—a systematic review and meta‐analysis (METACOG). Clin Psychol Psychother. 2018;26:227–40. https://doi.org/10.1002/cpp.2345
Sauvé G, Lavigne KM, Pochiet G, Brodeur MB, Lepage M. Efficacy of psychological interventions targeting cognitive biases in schizophrenia: a systematic review and meta-analysis. Clin Psychol Rev. 2020;78:101854. https://doi.org/10.1016/j.cpr.2020.101854
Penney D, Sauvé G, Mendelson D, Thibaudeau É, Moritz S, Lepage M. Immediate and sustained outcomes and moderators associated with metacognitive training for psychosis. JAMA Psychiatry. 2022;79:417. https://doi.org/10.1001/jamapsychiatry.2022.0277
Burlingame GM, Svien H, Hoppe L, Hunt I, Rosendahl J. Group therapy for schizophrenia: a meta-analysis. Psychother. 2020;57:219–36. https://doi.org/10.1037/pst0000293
van Oosterhout B, Smit F, Krabbendam L, Castelein S, Staring AB, van der Gaag M. Metacognitive training for schizophrenia spectrum patients: a meta-analysis on outcome studies. Psychol Med. 2016a;46:47–57. https://doi.org/10.1017/s0033291715001105
Barnicot K, Michael C, Trione E, Lang S, Saunders T, Sharp M, et al. Psychological interventions for acute psychiatric inpatients with schizophrenia-spectrum disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2020;82:101929. https://doi.org/10.1016/j.cpr.2020.101929
Jiang J, Zhang L, Zhu Z, Li W, Li C. Metacognitive training for schizophrenia: a systematic review. Shanghai Arch Psychiatry. 2015;27:149–57. https://doi.org/10.11919/j.issn.1002-0829.215065
Hennessy EA, Johnson BT, Keenan C. Best practice guidelines and essential methodological steps to conduct rigorous and systematic meta‐reviews. Appl Psychol Health Well Being. 2019;11:353–81. https://doi.org/10.1111/aphw.12169
Burlingame GM, Rosendahl J. A scientific response to Moritz et al. (2022). Psychother. 2022;59:136–9. https://doi.org/10.1037/pst0000433
van Oosterhout B, Smit F, Krabbendam L, Castelein S, Staring AB, van der Gaag M. Letter to the editor: should we focus on quality or quantity in meta-analyses? Psychol Med. 2016b;46:2003–5. https://doi.org/10.1017/s003329171600009x
Pieper D, Antoine S-L, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67:368–75. https://doi.org/10.1016/j.jclinepi.2013.11.007
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. https://doi.org/10.1136/bmj.j4008.
Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia. JAMA Psychiatry. 2017;74:675. https://doi.org/10.1001/jamapsychiatry.2017.0624
Gosling CJ, Solanes A, Fusar-Poli P, Radua J. Metaumbrella: the first comprehensive suite to perform data analysis in umbrella reviews with stratification of the evidence. BMJ Ment Health. 2023;26:e300534. https://doi.org/10.1136/bmjment-2022-300534
Hedges LV. Distribution theory for glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6:107. https://doi.org/10.2307/1164588
Higgins JPT, Altman DG, Deeks JJ, Thompson SG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557
Van Oosterhout B, Krabbendam L, De Boer K, Ferwerda J, Van Der Helm M, Stant AD, et al. Metacognitive group training for schizophrenia spectrum patients with delusions: a randomized controlled trial. Psychol Med. 2014;44:3025–35. https://doi.org/10.1017/S0033291714000555
Chen Q, Sang Y, Ren L, Wu J, Chen Y, Zheng M, et al. Metacognitive training: a useful complement to community-based rehabilitation for schizophrenia patients in China. BMC Psychiatry. 2021;21:38. https://doi.org/10.1186/s12888-021-03039-y
Ioannidis JP, Boffetta P, Little J, O’Brien TR, Uitterlinden AG, Vineis P, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2007;37:120–32. https://doi.org/10.1093/ije/dym159
Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta‐analyses. Alzheimers Dement. 2016;13:406–18. https://doi.org/10.1016/j.jalz.2016.07.152
Sauvé G, Kline RB, Shah JL, Joober R, Malla A, Brodeur MB, et al. Cognitive capacity similarly predicts insight into symptoms in first- and multiple-episode psychosis. Schizophr Res. 2019;206:236–43. https://doi.org/10.1016/j.schres.2018.11.013
Howick J, Koletsi D, Ioannidis JP, Madigan C, Pandis N, Loef M, et al. Most healthcare interventions tested in cochrane reviews are not effective according to high quality evidence: a systematic review and meta-analysis. J Clin Epidemiol. 2022;148:160–9. https://doi.org/10.1016/j.jclinepi.2022.04.017
Acknowledgements
The PERMEPSY project was supported under the frame of ERA PerMed by: Instituto de Salud Carlos III (ISCIII), Spain; German Federal Ministry of Education and Research (BMBF), Germany; Agence Nationale de la Recherche (ANR), France; National Centre for Research and Development (NCBR), Poland; Agencia Nacional de Investigación y Desarrollo (ANID), Chile. The authors would like to acknowledge the contributions of all PERMEPSY members: Susana Ochoa, Maria Lamarca, Belen Ramos Josemaria, Judith Usall, Regina Vila Badia, Raquel Lopez Carrilero, Trini Pelaez Martinez, Irene Birulés; Partner 2 members Fabrice Berna, Adrien Goncalves; Partner 3 members: Łukasz Gawęda, Marytna Krezolek, Hanna Gelner, Adrianna Aleksandrowicz, Justyna Piwinska; Partner 4 members Caroline König, Pedro Copado, Àngela Nebot, Alfredo Bellido, Cecilio Angulo; and Partner 5 members Vanessa Acuña and Alvaro Cavieres. Additionally, we would like to acknowledge Eirini Karyotaki and Clara Miguel Sanz. We acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf.
Funding
This project was supported by the Federal Ministry of Education and Research (BMBF), under the framework of ERA PerMed (ERAPERMED2022-292). The PERMEPSY project was supported under the frame of ERA PerMed by: Instituto de Salud Carlos III (ISCIII), Spain; German Federal Ministry of Education and Research (BMBF), Germany; Agence Nationale de la Recherche (ANR), France; National Centre for Research and Development (NCBR), Poland; Agencia Nacional de Investigación y Desarrollo (ANID), Chile. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AM and AS independently performed the data search and study selection. AM and AS had access to the dataset and conducted all statistical analyses, independently. Any disagreements were resolved by RF and SM. AM, AS, RF, and SM contributed to interpretation of results. AM, RF, and SM wrote the manuscript. AM conceptualized figures and tables. GS, DP, SM, RF, FB, LG, ML, SO, CK, and VA provided comments and feedback on the manuscript at different stages and AM had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
SM is the main developer of metacognitive training for psychosis (MCT) and has conducted a number of the studies that were included into the underlying meta-analyses. FB, DP, and GS have conducted meta-analyses on MCT previously. RF regularly conducts paid workshops on facilitating MCT.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meinhart, A., Sauvé, G., Schmueser, A. et al. Metacognitive training for psychosis (MCT): a systematic meta-review of its effectiveness. Transl Psychiatry 15, 156 (2025). https://doi.org/10.1038/s41398-025-03344-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03344-0