Abstract
The main objective of this multi-institutional study is to understand the effect of primary intravenous immunoglobulin (IVIG) replacement on clinical outcomes in recipients of BCMA-directed bispecific antibody (bsAb), where infection remains an important cause of morbidity and mortality. This is a retrospective study of patients treated with either standard of care teclistamab or BCMA-directed investigational bsAb between Nov 2017 and Dec 2023. Primary IVIG prophylaxis was defined as starting IVIG prior to first documented infection. All analyses were adjusted for immortal-time bias inherent in this grouping. A total of 225 patients were included in this analysis. Primary IVIG prophylaxis was defined as starting IVIG prior to first documented infection. The median follow-up of patients treated with and without primary IVIG prophylaxis was, 9 and 11 months, respectively. The cumulative incidence of all grade infections at 12 months with and without primary IVIG prophylaxis were 56% (95%CI 40%,78%) and 60% (95% CI 48%, 76%); p = 0.72, respectively. The 12-month cumulative incidence of ≥ grade 3 infections was 35% (95% CI 21%, 57%) with primary IVIG prophylaxis and 45% (95% CI 34%, 60%) without; p = 0.37. The median infection free survival (IFS) for all-grade infections was 7.7 (95% CI 3.3, 14) months with primary IVIG prophylaxis and 3 (95% CI 2.6, 4.5) months without (p = 0.021). The median ≥ grade 3 IFS was 14 (95% CI 8.8, NR) and 7.5 (95% CI 6.1, 14) months, with and without primary IVIG respectively; p = 0.022. Patients on primary IVIG prophylaxis had a superior progression-free-survival (PFS) [median PFS 15 vs 8 months; p = 0.026] and overall-survival (OS) [median OS 16 vs 44 months; p = 0.007]. On multivariate analysis, primary IVIG prophylaxis was independently associated with improved OS (HR = 0.37; p = 0.021), while the presence of extra-medullary (HR = 2.71; p = <0.001) and high-risk disease (HR = 1.88; p = 0.031) conferred poor outcomes. In recipients of BCMA-directed bsAb, IVIG supplementation was associated with an improved clinical outcome, including favorable IFS and OS.
Similar content being viewed by others
Introduction
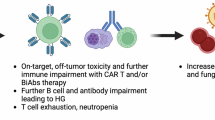
B-cell maturation antigen (BCMA) targeting bispecific antibodies (bsAb) for multiple myeloma (MM) have shown unprecedented clinical efficacy in patients with relapsed refractory disease [1,2,3,4]. However, these drugs are also associated with heightened risk of infections, including serious infections and infection related mortality [5,6,7,8]. Several factors such as profound humoral immunodeficiency secondary to B cell depletion, neutropenia, T and B-cell depletion, immune effector cell exhaustion in addition to several disease and host-related factors contribute to the heightened risk of infections [4, 9, 10]. Moreover, the risk of infection may vary depending on several factors, including the patient’s history of infections while on bsAb therapy, baseline bone marrow reserve (such as low blood counts), inflammatory markers (such as C-reactive protein and ferritin), the antigen targeted by bsAb, and any combination therapies involving bsAb [6, 11].
Data on clinical efficacy on intravenous immunoglobulin (IVIG) replacement in MM have been conflicting with most studies done in the post autologous stem cell transplant (ASCT) setting [12,13,14,15,16,17]. There is limited data in the context of BCMA directed chimeric antigen receptor (CAR) T-cell and bsAb therapies, which can lead to profound and prolonged humoral and innate immunodeficiency unlike what was seen with non-cellular therapies [9, 18]. In light of the evolving data, several consensus papers have been published regarding infection prophylaxis measures for recipients of BCMA targeting bsAb. These recommendations are heterogenous, with varying advice on use of IVIG replacement [19,20,21,22]. They vary from primary IVIG prophylaxis, which involves starting IVIG regardless of IgG levels and/or history of infection, to initiating IVIG in response to a low IgG level and /or infection trigger [20,21,22]. While the rationale for primary IVIG prophylaxis stems from near universal profound hypogammaglobinemia in virtually all responding patients, there is very limited data supporting its effectiveness [23].
The main objective of this multi-institutional study is to understand the effect of primary IVIG replacement on clinical outcomes in recipients of BCMA directed bsAb, where infection remains an important cause of morbidity and mortality.
Materials/Subjects and Methods
Patient and clinical end points
Patients in this study had relapsed and/or refractory MM and were treated with teclistamab as standard of care or alternative investigational BCMA targeting bsAb at one of 5 academic centers in the US-Medical College of Wisconsin, Milwaukee, WI, University of Arkansas for Medical Sciences, Little Rock, AR, Columbia University Irving Medical Center, New York, NY, Montefiore Medical Center and Albert Einstein College of Medicine, Bronx, NY, Rutgers Cancer Institute, New Brunswick, NJ. Of note, all teclistamab therapies were offered as standard of care. Patient who received at least one dose of teclistamab or investigational bsAb were included. Recipients of non-BCMA bsAb were excluded because these agents were associated with lower rates of hypogammaglobinemia and fewer infections compared to BCMA directed bsAb [6]. Baseline data including patient demographics, disease characteristics including fluorescence in-situ hybridization (FISH) studies at the most recent disease assessment prior to start of therapy were collected. Triple class refractory disease was defined as refractory to anti-CD38 monoclonal antibodies, proteasome inhibitor (PI) and immunomodulatory agents (IMiD) [24]. High risk disease was defined as the presence of t (4;14); t (14;16); t (14;20); 1q21 copy number abnormalities, or deletion (17 p) by FISH [25]. Neutropenia is defined as an absolute neutrophil count (ANC) of less than 1.0 × 10³/µL, while lymphopenia is defined as an absolute lymphocyte count (ALC) of less than 1.0 × 10³/µL.
For this study, any infection confirmed by clinical, imaging, microbiological and histopathological test was included from first day of first cycle until last follow up in patients on ongoing therapy, or 60 days after the last dose of bsAb in patients who discontinued treatment. Infections were graded by the Common Terminology Criteria for Adverse Events (CTCAE) version 5 NCI.
Cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) were graded according to the American Society for Transplantation and Cellular Therapy consensus and managed according to institutional guidelines [26]. Infection prophylaxis was implemented per institutional guidelines. The study was approved by the Institutional Review Board of the coordinating institution (Medical College of Wisconsin) and subsequently by all participating institutions. All methods were performed in accordance with the relevant guidelines/regulations and this retrospective study does not require informed consenting of patients.
For this analysis, the “primary IVIG prophylaxis” group was defined as IVIG after the start of bsAb but before any documented infection. Secondary prophylaxis was defined as administering IVIG after a documented infection. Patients who received IVIG before start of the bsAb were removed from the analyses (n = 9).
Statistical analysis
Demographic and disease characteristics of the study population were summarized using counts with percentages for categorical variables and median with range for continuous measures. Overall (OS), progression-free (PFS), and infection-free survival (IFS) were estimated using the Kaplan-Meier estimator with 95% confidence intervals based on the log-log transform. The cumulative incidence of infection was estimated using the Nelson-Aalen estimator with death without infection as competing risk. All analyses were adjusted for immortal-time bias inherent in this grouping as follows. The effect of primary IVIG prophylaxis on the cumulative incidence of infections was evaluated using a 60-days landmark analysis. Only patients alive without infection at 60 days post bsAb were included, and the IVIG prophylaxis was classified as having started IVIG before this day versus not. The estimated IFS, PFS and OS in hypothetical patients who never received IVIG prophylaxis versus received primary IVIG prophylaxis immediately was estimated using the Simon-Makuch method [27], with the Mantel-Byar test [28] for comparison. Covariate adjusted analysis was performed considering IVIG prophylaxis as a time-dependent variable. The risk factors for recurrent infections were modeled using a day-level Cox regression model with robust standard errors to account for within-subject repetitions. This model was selected as it allows the inclusion of recurrent infections and both baseline and time-varying covariates.
Results
We included 225 individual patients treated between Nov 2017 and Dec 2023 for this study. Of those, 133 (59%) did not receive primary IVIG prophylaxis, whereas the remainder did (n = 92, 41%). Baseline characteristics are as shown in Table 1. The group that did not receive primary IVIG prophylaxis had more patients that started treatment in earlier years (p < 0.001) and had also a higher percentage of investigational BCMA targeting bsAb (p < 0.001), whereas the majority of patients in the primary IVIG prophylaxis group received standard-of-care teclistamab (n = 78, 85%). The median follow-up of patients treated with and without primary IVIG prophylaxis was, 9 and 11 months, respectively. The groups were similar in terms of median age (69 years vs 71 years), racial distribution (23% vs 18% African Americans) and high-risk disease (63% vs 51%). There was enrichment for extramedullary disease in the group that was not started on primary IVIG prophylaxis (47% vs 28%; p = 0.006). Median lines of prior therapy were 5 in both groups and a higher percentage of triple class refractory patients in the group that did not receive primary IVIG prophylaxis (92% vs 79%; p = 0.004). Lymphopenia was seen in over 50% in both groups. The occurrence of CRS (54% vs 55%) and ICANS (9% vs 3.3%) was similar in both groups as was the use of tocilizumab (31% vs 24%) and systemic steroids (16% vs 18%). Median time to start of primary IVIG prophylaxis was 39 days (range 0–359) and most common dosing schedule was 400 mg/kg intravenously, every 4 weeks. The primary IVIG prophylaxis group had more patient with at least one prior ASCT (88% vs 98%; p = 0.024) with similar number of patients treated with a prior BCMA directed therapy (20% vs 27%; p = 0.2). BCMA directed CAR-T cell therapy (51%) and belantamab mafodotin (33%) were the most commonly used prior BCMA targeting agents in both groups. Interestingly, when looking at BCMA bsAb dosing, we found that the median number of given bsAb doses was statistically significantly higher in the group that received primary IVIG prophylaxis (19 vs 7; p < 0.001) and patients on primary IVIG prophylaxis remained longer on treatment (median duration of 6.8 vs 2 months; p < 0.001). It is noteworthy that 39 (29%) of patients in the group that did not receive primary IVIG prophylaxis went on to receive IVIG treatment after their first infection (secondary prophylaxis), for statistical purposes these patients remained in non-primary IVIG group.
A broad spectrum of infections occurs in BCMA bs Ab therapy
We next investigated the incidence of all infections in our patient cohort and observed 288 infections in 136 patients with a median time to infection of 97 (37–222) days from start of therapy. Bacterial and viral infection were common, noted in 53% (n = 147) and 43% (n = 119) patients, respectively. Respiratory tract infections were the most common infection observed in this cohort. COVID-19 infection accounted for 11% (n = 28) cases. About 2.7% (n = 8) had fungal infection including cases of Candida esophagitis, Candida tropicalis fungemia and Scedosporum spp lung infection. There were 5 cases of PJP pneumonia, all in patients who were not on PJP prophylaxis. The majority of patients (n = 175, n = 61%) required inpatient hospitalization for infectious complications. There were 11 grade 5 events, with 10 occurring in the group that did not receive primary IVIG prophylaxis. The 12-month cumulative incidence of all grades and ≥ grade 3 infection were 73% (95% CI 65%, 81%) and 53% (95% CI 45%, 63%), respectively (supplementary figs. 1 and 2). Further, the 12-month cumulative incidence of ≥ grade 3 bacterial and viral infection were 39% (95% CI 32%, 49%) and 22% (95% CI 15%, 32%), respectively. There were eleven (3.8%), grade 5 events noted, including nine bacterial and two viral infections.
Primary IVIG prophylaxis improved infection-free survival
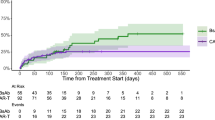
We then compared the two groups to understand the impact of primary IVIG prophylaxis on the occurrence and severity of infections. The median IFS for all-grade infections was 7.7 (95% CI 3.3, 14) months with primary IVIG prophylaxis and 3 (95% CI 2.6, 4.5) months without (Mantel-Byar test, p = 0.021). The median IFS for ≥ grade 3 infection was 14 (95% CI 8.8, NR) and 7.5 (95% CI 6.1, 14) months, with and without primary IVIG respectively; Mantel-Byar test p = 0.022, Fig. 1.
Next, we investigated other factors that could influence the risk of infection. Univariate analysis is as shown in supplementary table 1. On multivariate analysis including all covariates (Table 2), we found baseline lymphopenia (HR = 1.34, 95% CI 1.03,1.74; p = 0.029), administration of tocilizumab (HR = 1.53, 95% CI 1.11,2.10; p = 0.009), higher number of median prior lines of therapy (HR = 1.08; 95% CI 1.02,1.15; p = 0.014) and history of prior infection while on bsAb therapy (HR = 1.25; 95% CI 1.13,1.38; p = <0.001) were independently associated with an increased risk of all-grade infections. Interestingly, primary IVIG prophylaxis did not show a quite significant protective effect (HR = 0.71; 95% CI 0.48, 1.05; p = 0.085). However, the use of secondary IVIG prophylaxis after first infection was significantly associated with the reduction of further infections (HR = 0.41; 95% CI 0.22,0.74; p = 0.0.17).
Similarly, we found that baseline lymphopenia (HR = 1.53; 95% CI 1.04,2.26; p = 0.032), use of tocilizumab therapy (HR = 1.56; 95% CI 1.01,2.42; p = 0.047), prior history ≥ grade 3 infections on bs Ab therapy (HR = 1.53; 95% CI 1.32,1.77; p = < 0.001) increased the risk of ≥ grade 3 infection. Primary IVIG prophylaxis showed a trend toward being protective (HR = 0.58, 95% CI 0.33, 1.03; p = 0.064), while secondary IVIG prophylaxis (HR = 0.36; 95% CI 0.16, 0.81; p = 0.013) was associated with a lower risk of ≥ grade 3 infections.
IVIG predominantly protects against bacterial infections
We then investigated whether IVIG prophylaxis differ in its protective efficacy depending on the infectious origin being either bacterial and/or viral infections. Both primary (HR = 0.40 [95% CI 0.24, 0.66]; p = <0.001). and secondary IVIG prophylaxis (HR = 0.3 [95% CI 0.14, 0.56]; p = <0.002) were most effective in preventing bacterial infections. In contrast, neither primary (HR = 0.99 [95% CI 0.59, 1.65]; p = 0.96) or secondary IVIG (HR = 0.67 [95% CI 0.29, 1.56]; p = 0.36) prophylaxis were not significantly associated with a reduced risk of viral infections.
Additionally, a higher number of prior lines of therapy (HR = 1.12; 95% CI 1.02,1.22; p = 0.012), history of prior bacterial infections (HR = 1.23; 95% CI 1.07, 1.40; p = 0.003) and use of tocilizumab therapy (HR = 1.52; 95% CI 1, 2.29; p = 0.050) were independently associated with increased risk of bacterial infection. For viral infections, we identified only baseline neutropenia, HR = 2.96 [95%CI = 1.30, 6.7], p = 0.010) and tocilizumab (HR = 1.73, 95%CI 1.04, 2.88], p = 0.034) to be significantly associated with an increased risk of infections.
Primary IVIG supplementation decreases mortality in recipients of BCMA directed bsAb therapy
The PFS and OS survival of the entire cohort is shown in supplementary fig. 3. Patients who received primary IVIG prophylaxis had a superior PFS (15 [95% CI 13, NR] vs 8 [95% CI 3.9, NR]; Mantel-Byar test, p = 0.026) compared to patients in the no primary IVIG group (Fig. 2). However, as previously mentioned, the patient group without primary IVIG prophylaxis was enriched for EMD and triple class refractory disease, underscoring that these patients might have more resistant disease. Consequently, multi-variate analysis confirmed that primary IVIF prophylaxis was not significantly associated with an improved PFS (HR = 0.61; 95% CI 0.33, 1.12; p = 0.11) (Table 3), whereas the presence of EMD conferred an inferior PFS (HR = 2.30; 95% CI 1.43, 3.50; p = < 0.001).
More interestingly, primary IVIG prophylaxis was associated with an improved OS, showing a median OS was 44 (95% CI 27, NR) months for those on IVIG prophylaxis versus 16 (95% CI 13, NR) months for those not on prophylaxis; Mantel-Byar test, p = 0.007 (Fig. 3). Here, multi-variate analysis showed that the use of primary IVIG prophylaxis was associated with improved survival (HR = 0.37; 95% CI 0.16, 0.86; p = 0.021) emphasizing the beneficial effect of primary IVIG prophylaxis (Table 3).The presence of EMD (HR = 2.71; 95% CI 1.51, 4.87; p = <0.001) and high-risk disease (HR = 1.88; 95% CI 1.02, 3.46; p = <0.044) was associated with worse OS.
Discussion
In this study of patients treated with BCMA directed bsAb, we found primary IVIG prophylaxis, especially given pre-emptively prior to a documented infection and irrespective IgG levels was clinically beneficial. Our study highlights three major findings. Primary IVIG prophylaxis was found to be significantly associated with a reduction in both all-grade and ≥ grade 3 infection-free survival. The administration of tocilizumab for CRS, was linked to an increased risk of all-grade and ≥ grade 3 infections, including both bacterial and viral infections. Additionally, our findings demonstrate that primary IVIG prophylaxis was independently associated with improved OS in recipients of BCMA directed bsAb.
High-grade infections contribute to significant morbidity and mortality in patients treated with BCMA directed bsAb and our approach of early IVIG supplementation may help mitigate this risk. This aligns with increasing awareness of risk of infections with this class of drugs and adoption of best prevention practices [29]. Further, IVIG prophylaxis was associated with nearly a 59–64% reduction in all-grade infections and high grade. While the beneficial effect of primary IVIG prophylaxis was not quite significant on multi-variate analysis, this might have been due to the relatively small patient population receiving primary IVIG prophylaxis in our study. We believe that larger studies with longer follow up will likely support the independent beneficial effect of primary IVIG prophylaxis. Furthermore, primary IVIG administration had its most protective effect against bacterial infections, similar to what had been reported previously in patients with chronic lymphocytic leukemia with less effect on viral infections which could have blunted its effect on overall infections [30].
Use of tocilizumab in the current study, was associated with an increased risk of all-grade, ≥3 grade infections, bacterial and viral infections. Unsurprisingly, the incidence of high-grade CRS and the subsequent administration of tocilizumab may correlate with overall disease burden and thus indirectly to immunoparesis, and T cell dysfunction which may increase the risk of infectious complications [31]. However, there is increasingly lower clinical threshold for administration of tocilizumab for the first fever (or for low-grade CRS) in recipients of both bsAb and CAR-T cell therapy. As tocilizumab blunts early acute phase responses and blocks interleukin-6 (IL-6) these is a risk that tocilizumab may hamper the early recognition of infection and therefore heighten the risk of infections [32]. There is growing interest in using tocilizumab prophylactically for patients receiving bsAb for MM, primarily to facilitate safe outpatient step-up dosing [33, 34]. This approach should be carefully evaluated considering our data and further studies should evaluate the association of tocilizumab and infection.
Use of primary IVIG prophylaxis was also independently associated with improved OS. Of note, among the 11, grade 5 events, all but one death was observed in the group that did not receive primary IVIG prophylaxis. To our knowledge, this is the first time primary IVIG prophylaxis has been shown to improve mortality in the immunotherapy era. Although primary IVIG prophylaxis was initiated at the physician’s discretion, patients who received primary prophylaxis were predominantly treated with standard of care teclistamab therapy, reflecting the change in infectious prophylaxis practices in 2020 and beyond. Of note, while patients who received primary IVIG prophylaxis were more likely to have received a prior ASCT, there were fewer patients with triple class refractory disease and EMD compared to the group not on primary IVIG prophylaxis and this may have also influenced our results. Nevertheless, plausible reasons for improved survival with a primary IVIG approach include a reduction in the incidence of infections, especially high-grade infections, which may contribute to a longer therapy duration with fewer treatment interruptions, and discontinuations. Indeed, in our recent experience, the most common cause of treatment discontinuation was infectious complications of bsAb therapy in patients who are otherwise responding to treatment [35]. To collaborate with this, in the current study we observe that patients on primary IVIG prophylaxis remain on therapy for a longer duration, despite receiving fewer doses per month and longer interval between bsAb dose.
Based on these above findings, it is advisable to initiate IVIG prophylactically, regardless of an IgG levels or history of infection, in patients treated with BCMA directed bsAb therapy. On the flip side, it can be considered that broad application of infection prevention strategies also raises several concerns- such as significant burden on healthcare resources, particularly with the increasing use of these therapies and their potential approval for earlier lines of treatment [36, 37]. Additionally, IVIG therapy is administered over several hours, contributing to time toxicity and a substantial burden on healthcare resources [38]. Currently, IVIG will likely need to be administered throughout the duration of bsAb treatment, which may last about 2 years, with an extended duration for patients who achieve a complete response to therapy. This calls for clinical prediction models that can identify patients at the highest risk of infection allowing for targeted implementation of infection prevention strategies [11, 39, 40].
Our study has several limitations inherent to retrospective analyses. The use of IVIG and other infection prophylactic measures were at the discretion of the treating physicians. Additionally, we lacked data on the kinetics of immunoglobulin levels changes with IVIG therapy and had challenges in the exact attributes grade 5 events and mortality. We included patients treated on various early-phase 1/2 clinical trials of BCMA directed bsAb, however majority of those who received primary IVIG prophylaxis were treated with standard-of-care teclistamab, making our results generalizable to current clinical practice.
In conclusion, while we await clinical trials that demonstrates concrete benefits of IVIG prophylaxis in the era of the novel treatment strategies such as CAR T and bsAb therapies, our study demonstrates that effective supportive care measures such as these may further enhance therapeutic efficacies of these agents.
Data availability
Data will be made available upon reasonable request to the corresponding author.
References
Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl J Med. 2022;387:495–505.
Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29:2259–67.
Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodríguez-Otero P, et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl J Med. 2022;387:2232–44.
Lee H, Neri P, Bahlis NJ. Current use of bispecific antibodies to treat multiple myeloma. Hematology. 2023;2023:332–9.
Reynolds G, Cliff ERS, Mohyuddin GR, Popat R, Midha S, Liet Hing MN, et al. Infections following bispecific antibodies in myeloma: a systematic review and meta-analysis. Blood Adv. 2023;7:5898–903.
Hammons L, Szabo A, Janardan A, Bhatlapenumarthi V, Annyapu E, Dhakal B, et al. The changing spectrum of infection with BCMA and GPRC5D targeting bispecific antibody (bsAb) therapy in patients with relapsed refractory multiple myeloma. Haematologica. 2023;109:906–14.
Mohan M, Nagavally S, Dhakal B, Radhakrishnan SV, Chhabra S, D’Souza A, et al. Risk of infections with B-cell maturation antigen-directed immunotherapy in multiple myeloma. Blood Adv. 2022;6:2466–70.
Yee AJ. Improving outcomes with anti-BCMA bispecific antibodies with attention to infection. Blood Cancer J. 2024;14:110.
Hammons LR, Szabo A, Janardan A, Dhakal B, Chhabra S, D’Souza A, et al. Kinetics of Humoral Immunodeficiency With Bispecific Antibody Therapy in Relapsed Refractory Multiple Myeloma. JAMA Netw Open. 2022;5:e2238961-e.
Friedrich MJ, Neri P, Kehl N, Michel J, Steiger S, Kilian M, et al. The pre-existing T cell landscape determines the response to bispecific T cell engagers in multiple myeloma patients. Cancer Cell. 2023;41:711–25.e6.
Akhtar OS, Szabo A, Bhatlapenumarthi V, Forsberg M, Balev M, Patwari A, et al. Immune prognostic score predicts the risk of infection and survival outcomes in patients with relapsed multiple myeloma treated with bispecific antibodies. Br J Haematol. 2024;205:1830–34.
Chapel HM, Lee M, Hargreaves R, Pamphilon DH, Prentice AG. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. UK Group Immunoglobulin Replacement Ther Mult Myeloma Lancet. 1994;343:1059–63.
Blombery P, Prince HM, Worth LJ, Main J, Yang M, Wood EM, et al. Prophylactic intravenous immunoglobulin during autologous haemopoietic stem cell transplantation for multiple myeloma is not associated with reduced infectious complications. Ann Hematol. 2011;90:1167–72.
Howell JE, Gulbis AM, Champlin RE, Qazilbash MH. Retrospective analysis of weekly intravenous immunoglobulin prophylaxis versus intravenous immunoglobulin by IgG level monitoring in hematopoietic stem cell transplant recipients. Am J Hematol. 2012;87:172–4.
Park S, Jung CW, Jang JH, Kim SJ, Kim WS, Kim K. Incidence of infection according to intravenous immunoglobulin use in autologous hematopoietic stem cell transplant recipients with multiple myeloma. Transpl Infect Dis. 2015;17:679–87.
Na IK, Buckland M, Agostini C, Edgar JDM, Friman V, Michallet M, et al. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies. Eur J Haematol. 2019;102:447–56.
Wonnaparhown A, Hilal T, Squire J, Freeman C, Fonseca R. IgG replacement in multiple myeloma. Blood Cancer J. 2024;14:124.
Lancman G, Parsa K, Kotlarz K, Avery L, Lurie A, Lieberman-Cribbin A, et al. IVIg Use Associated with Ten-Fold Reduction of Serious Infections in Multiple Myeloma Patients Treated with Anti-BCMA Bispecific Antibodies. Blood Cancer Discov. 2023;4:440–51.
Ludwig H, Terpos E, van de Donk N, Mateos M-V, Moreau P, Dimopoulos M-A, et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: a consensus report of the European Myeloma Network. Lancet Oncol. 2023;24:e255–e69.
Raje N, Anderson K, Einsele H, Efebera Y, Gay F, Hammond SP, et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: consensus recommendations from an expert panel. Blood Cancer J. 2023;13:116.
Mohan M, Chakraborty R, Bal S, Nellore A, Baljevic M, D'Souza A, et al. Recommendations on prevention of infections during chimeric antigen receptor T-cell and bispecific antibody therapy in multiple myeloma. Br J Haematol. 2023;203:736–46.
Rodriguez-Otero P, Usmani S, Cohen AD, van de Donk N, Leleu X, Gállego Pérez-Larraya J, et al. International Myeloma Working Group immunotherapy committee consensus guidelines and recommendations for optimal use of T-cell-engaging bispecific antibodies in multiple myeloma. Lancet Oncol. 2024;25:e205–e16.
Mohan M, Monge J, Shah N, Luan D, Forsberg M, Bhatlapenumarthi V, et al. Teclistamab in relapsed refractory multiple myeloma: multi-institutional real-world study. Blood Cancer J. 2024;14:35.
Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–75.
Rees MJ, D’Agostino M, Leypoldt LB, Kumar S, Weisel KC, Gay F. Navigating High-Risk and Ultrahigh-Risk Multiple Myeloma: Challenges and Emerging Strategies. Am Soc Clin Oncol Educ Book. 2024;44:e433520.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25:625–38.
Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus non-responder bias. Stat Med. 1984;3:35–44.
Mantel N, Byar DP. Evaluation of Response-Time Data Involving Transient States: An Illustration Using Heart-Transplant Data. J Am Stat Assoc. 1974;69:81–6.
Nooka AK, Rodriguez C, Mateos MV, Manier S, Chastain K, Banerjee A, et al. Incidence, timing, and management of infections in patients receiving teclistamab for the treatment of relapsed/refractory multiple myeloma in the MajesTEC-1 study. Cancer. 2024;130:886–900.
Gale RP, Chapel HM, Bunch C, Rai KR, Foon K, Courter SG, et al. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. N. Engl J Med. 1988;319:902–7.
Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. Am Soc Clin Oncol Educ Book. 2019:39:433–44.
Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13:399–409.
Rifkin RM, Schade HH, Simmons G, Yasenchak CA, Fowler J, Lin TS, et al. OPTec: A phase 2 study to evaluate outpatient (OP) step-up administration of teclistamab (Tec), a BCMA-targeting bispecific antibody, in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2024;42:7528.
Scott SA, Marin EM, Maples KT, Joseph NS, Hofmeister CC, Gupta VA, et al. Prophylactic tocilizumab to prevent cytokine release syndrome (CRS) with teclistamab: A single-center experience. Blood Cancer J. 2023;13:191.
Chakraborty R, Cheruvalath H, Patwari A, Szabo A, Schinke C, Dhakal B, et al. Sustained remission following finite duration bispecific antibody therapy in patients with relapsed/refractory myeloma. Blood Cancer J. 2024;14:137.
Blackhouse G, Gaebel K, Xie F, Campbell K, Assasi N, Tarride JE, et al. Cost-utility of Intravenous Immunoglobulin (IVIG) compared with corticosteroids for the treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Canada. Cost Eff Resour Alloc. 2010;8:14.
Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C Jr. Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barré syndrome. BMC Health Serv Res. 2011;11:101.
Banerjee R, Cowan AJ, Ortega M, Missimer C, Carpenter PA, Oshima MU, et al. Financial Toxicity, Time Toxicity, and Quality of Life in Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2024;24:446–54.e3.
Frenking JH, Zhou X, Wagner V, Hielscher T, Kauer J, Mai EK, et al. EASIX-guided risk stratification for complications and outcome after CAR T-cell therapy with ide-cel in relapsed/refractory multiple myeloma. J Immunother Cancer. 2024;12:e009220.
Rejeski K, Hansen DK, Bansal R, Sesques P, Ailawadhi S, Logue JM, et al. The CAR-HEMATOTOX score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. J Hematol Oncol. 2023;16:88.
Acknowledgements
We thank our patients and families for the opportunity to be involved in their care and all the contribution to the advancement in the field. Advancing a Healthier Wisconsin Endowment-Clinical and Translational Science Institute KL2 award (MM).
Author information
Authors and Affiliations
Contributions
Conception and design: MM, CS, RC. Provision of study materials or patients: MM, NS, CS, MS, RC. Collection and assembly of data: HC, AC, VB, AP, MB, AS, AT: SV, AB. Data analysis and interpretation: AS, MM, CS, RC. Manuscript writing: MM, AS, HC, AC, VB, AP, MB, DB, AS, ST, BD, MZ, AT: SV, SA, SL, FR, AB, AD, NS, RC, MS, CS. Final approval of manuscript: MM, AS, HC, AC, VB, AP, MB, DB, AS, ST, BD, MZ, AT, SV, SA, SL, FR, AB, AD, NS, RC, MS, CS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohan, M., Szabo, A., Cheruvalath, H. et al. Effect of Intravenous Immunoglobulin (IVIG) Supplementation on infection-free survival in recipients of BCMA-directed bispecific antibody therapy for multiple myeloma. Blood Cancer J. 15, 74 (2025). https://doi.org/10.1038/s41408-025-01282-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-025-01282-0