Abstract
Cryptorchidism is associated with an increased risk of male infertility and testicular cancer. Persistent exposure to high temperature in cryptorchidism can lead to the apoptosis of spermatogenic cells. Transient receptor potential vanilloid 1 (TRPV1), a thermosensitive cation channel, has been found to have differential effects on various apoptosis processes. However, whether TRPV1 is involved in spermatogenic cell apoptosis induced by cryptorchidism remains unclear. Herein, we first observed the expression pattern of TRPV1 in the testes of mice with experimental cryptorchidism, and then investigated the role and mechanism of TRPV1 in spermatogenic cell apoptosis by using Trpv1−/− mice. The results showed that TRPV1 was highly expressed on the membrane of spermatocytes in mouse testis, and the expression increased significantly in the testis of mice with experimental cryptorchidism. After the operation, Trpv1−/− mice exhibited less reproductive damage and fewer spermatogenic cell apoptosis compared to the wild-type (WT) mice. Transcriptome sequencing revealed that the expression of apoptosis-related genes (Capn1, Capn2, Bax, Aifm1, Caspase 3, Map3k5, Itpr1 and Fas) was down-regulated in spermatocytes of cryptorchid Trpv1−/− mice. Our results suggest that TRPV1 promotes the apoptosis of spermatocytes in cryptorchid mice by regulating the expression of apoptosis-related genes.
Similar content being viewed by others
Introduction
Spermatogenesis is a heat-sensitive process, and the testicular temperature in most mammals must be maintained at a range from 2 to 8 °C below the core body temperature [1, 2]. Anything that raises the temperature of the testes and scrotum, including exposure to elevated ambient temperatures in occupational settings [3], clothing coverage [4], body posture [5], and pathologies such as varicocele [6] and cryptorchidism [7], could lead to the interruption of spermatogenesis in mammals. Studies have found that higher temperature can lead to a variety of adverse consequences in the testes, such as increasing oxidative stress levels [8], promoting DNA breaks and cell apoptosis [9]. Spermatogenic cells are highly susceptible to heat stress damage due to their frequent division [10]. It has been reported that meiosis in spermatocytes requires a low temperature to occur, and that spermatocytes in pachytene and diplotene stages are more susceptible to temperature variations [11]. At elevated testicular temperatures, double-strand breaks (DSBs) increase significantly, leading to an increase in aberrant chromosome binding and the elimination of heat-damaged spermatocytes via activation of meiotic checkpoints in the form of apoptosis [12].
Cryptorchidism is a common male genitourinary abnormality in which the testes do not descend completely but remain in the abdominal cavity or inguinal canal [13], leading to persistent damage to the spermatogenic epithelium and clinical infertility in men [14,15,16]. After surgery, 80% of adult men with a history of bilateral cryptorchidism and 30% of men with a history of unilateral cryptorchidism exhibit abnormal sperm counts [17]. In addition, abnormal testicular development and dysregulation of growth factor expression in cryptorchid males may lead to infertility and testicular cancer [18]. It has been established that the primary cause of impaired spermatogenesis in cryptorchidism is the exposure of the testis to the abdominal cavity, resulting in elevated temperatures. The surgery-induced cryptorchidism animal model induces an increase in scrotal temperature, which leads to the formation of multinucleated giant cells and the death of spermatogenic cells [19], ultimately causing a decrease in sperm production [20]. Apoptosis is generally considered the predominant mechanism of germ cell death in cryptorchidism, rather than atrophy or necrosis [21]. Furthermore, spermatogenic cell apoptosis is influenced by oxidative stress [22, 23], varying reactions to gonadotropins [24], and activation of nuclear factor κB in testes of experimentally induced cryptorchidism [25]. The primary apoptosis pathways of cryptorchid spermatogenic cells include the intrinsic mitochondrial pathway [26, 27], the Fas receptor system [28], and the p53 signaling pathway [26].
The heat-activated protein TRPV1, belonging to the TRP family, functions as a tetrametric transmembrane ion channel that remains closed and polarized at lower temperatures. The activation of TRPV1 is known to contribute significantly to the transduction of inflammatory pain [29,30,31]. Furthermore, it plays a crucial role in various processes, including apoptosis and cell proliferation [32, 33]. Additionally, it is involved in physiological functions such as thermoregulation [34]. In general, TRPV1 can be activated at temperatures greater than 42 °C [35]. Inflammatory conditions lower the temperature threshold for TRPV1 activation, resulting in its activation at body temperature [36]. In addition, it was found that TRPV1 channels can be rapidly activated at 33–39 °C in the presence of 2.5 μM phosphatidylinositol 4,5-bisphosphate [37]. Trpv1−/− mice exhibit decreased sensitivity to heat and inflammation stimuli [38]. Due to its thermal sensitivity, TRPV1 has attracted increasing interest in the field of reproduction. Previous studies have demonstrated the predominant localization of TRPV1 in the cytoplasm of human testicular germ cells, the mid-posterior region of the sperm acrosome, and the flagella [39,40,41]. Furthermore, TRPV1 has been detected in the seminiferous epithelium and post-acrosomal region of the sperm in adult mice [42, 43]. Previous research showed that aged Trpv1−/− mice exhibit a reproductive phenotype similar to that of youthful counterparts, characterized by reduced cell death in testicular spermatogenic cells [44]. Besides, the apoptosis of spermatogenic cells in Trpv1−/− mice increased under heat stimulation at 42 °C, suggesting that TRPV1 plays a crucial role in the defense of testis against heat stress [45]. However, the TRPV1 agonist capsaicin can prompt the apoptosis of rat spermatogonium stem cells in vitro [46]. Together, these studies indicated that TRPV1 has different effects on spermatogenic cells under different stimulus conditions.
In this study, we determined that TRPV1 is predominantly expressed in the spermatocytes of the mouse testis and plays an important role in spermatogenic injury caused by cryptorchidism. We found that TRPV1 can promote spermatocyte apoptosis in cryptorchid mice by regulating the transcription of apoptosis-related genes. The study provides a new research perspective on the role of TRPV1 in male reproduction.
Results
TRPV1 is predominantly expressed in spermatocytes of mouse testis
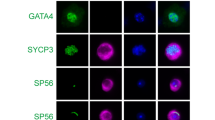
Previous studies have indicated that TRPV1 is expressed in the testicular tissues of humans [47] and mice [43], but the specific expression and localization of TRPV1 in germ cells of mice remain unclear. Therefore, we first investigated the expression and localization of TRPV1 in the mouse testis. RT-qPCR and Western blot analysis were performed using testicular tissues from 7-, 14-, 21-, 28-, 35-, and 56-day-old mice. The results showed that both TRPV1 mRNA and protein were expressed during testicular development in mice. Specifically, TRPV1 could be detected in the testis as early as postnatal day 7, gradually increased after day 14, reached a peak at day 28, and slightly decreased with the development of mice to adulthood (Fig. 1A, B). IHC analysis of TRPV1 in adult mice testis showed that the immunostaining signal of TRPV1 was observed in the membrane of spermatogenic cells at stages I to XII of the spermatogenic cycle, in which spermatocyte immunostaining was stronger at stages IV to VII and relatively weak at other stages (Fig. 1C). Co-staining of TRPV1 with SOX9 (Sertoli cell marker), PLZF (spermatogonium marker) and SYCP3 (spermatocyte marker) in testis of adult mice showed that TRPV1 was localized on the membranes of spermatogonia and spermatocytes, but not on Sertoli cells (Fig. 1D). In order to further understand the expression of TRPV1 in different spermatogenic cells, we isolated different types of spermatogenic cells, including spermatogonium (SPG), spermatocyte (SPC), round spermatid (rST) and elongated spermatid (eST) from adult mice testes. By RT-qPCR and Western blot analysis in these cells, we observed that TRPV1 was strongly expressed in spermatocytes and weakly expressed in other spermatogenic cells (Fig. 1E, F).
A, B TRPV1 mRNA and protein expression along testicular development. A mRNA expression was assessed using RT-qPCR (n = 3). Amplification of β-actin mRNA was used as an internal control. B Protein expression was determined using Western blot (n = 3). Protein expression levels were normalized to β-actin and densitometric analyses were performed with ImageJ software. C Localization of TRPV1 protein in testicular sections from adult mice (8-week-old) was assessed using IHC assay. IgG is shown in the right lower panel. SPG spermatogonium, SPC spermatocyte, rST round spermatid, eST elongated spermatid. D Co-stained of TRPV1 (red) with Sertoli cell marker SOX9 (green), spermatogonium marker PLZF (green), and spermatocyte marker SYCP3 (green) in testicular sections of WT mice (8-week-old) was detected by IF. Nuclei were stained with DAPI (blue). Dashed boxes showed localization of the enlarged images. E, F The expression profile of TRPV1 in spermatogenic cells. Different types of spermatogenic cells in WT testes (8-week-old) were isolated using the STA-PUT method. E, mRNA expression was assessed using RT-qPCR (n = 3). Amplification of β-actin mRNA was used as an internal control. F, Protein expression was assessed using Immunoblot analysis (n = 3). Protein expression levels were normalized to β-actin and densitometric analyses were performed with ImageJ software. Data were represented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by Student’s t test.
Germ cell apoptosis is induced by experimental cryptorchidism
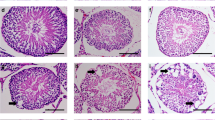
Given the high sensitivity of testis to temperature, we hypothesized that TRPV1 may play a role in the apoptosis of testicular spermatogenic cells under heat stress. In the study, we established an endogenous heat injury apoptosis model using mouse experimental cryptorchidism. Bilateral cryptorchidism was surgically induced in 8-week-old mice (Fig. 2A), and the testicular volume, weight, the ratio of testis weight/body weight, and sperm count were measured on 3 days, 6 days, 9 days, 12 days, and 15 days after surgery. In cryptorchid mice, we observed a marked decline in testes volume from day 9 (Fig. 2B), testis weight began to decrease from day 6 and significantly reduced from day 9 until day 15 (Fig. 2C). Furthermore, the ratio of testis weight/body weight (Fig. 2D) and sperm count (Fig. 2E) declined from day 9 and continued to decline over time compared to the sham group. We then examined the morphology of the seminiferous tubules and cauda epididymis in mice after cryptorchidism. The H&E staining results showed that there were no significant differences in the morphology of seminiferous tubules and sperm count between the cryptorchidism group and the sham group on day 3. After 6 days of cryptorchidism surgery, spermatogenic cells began to degenerate, and immature and degenerative spermatogenic cell debris appeared in the lumen of epididymides. After 9 days of cryptorchidism, the seminiferous tubule further atrophied, and the number of spermatogenic cells in the seminiferous tubules decreased, while the number of multinucleated giant cells increased significantly. Additionally, immature and degenerative spermatogenic cell debris appeared in the lumen of epididymides. On day 12 and 15, there were only a few Sertoli cells and spermatogonia in the spermatogenic epithelium of cryptorchid mouse, and the amount of spermatogenic cell debris in the epididymal lumen increased further (Fig. 2F). In order to observe the apoptosis of spermatogenic cells more directly, we used TUNEL to analyze the seminiferous tubules of cryptorchid testis. The results showed that spermatogenic cells began apoptosis on the 6 days after cryptorchidism, and the number of apoptosis cells increased with the duration of cryptorchidism. Both the number of TUNEL positive cells per tubule and the number of TUNEL positive tubules were significantly increased in the day 9 cryptorchid testis compared with the sham group (Fig. 2G–I). Altogether, these findings indicate that serious damage to spermatogenic cells begins at day 9 after cryptorchid surgery.
A Schematic diagram of surgery-induced cryptorchidism in mice. Bilateral cryptorchidism was performed in 8-week-old mice. Comparison of the testicular size (B), testicular weight (C), testicular weight/body weight ratio (D) and the number of spermatozoa in cauda epididymis (E) on days 3, 6, 9, 12 and 15 after cryptorchidism (n = 5). F H&E staining of mouse testes and cauda epididymis on days 3, 6, 9, 12 and 15 after cryptorchidism. The arrows represent multinucleated giant cells. G Sections of testes on days 3, 6, 9, 12 and 15 after cryptorchidism were stained with the TUNEL probe (green), and nuclei were stained with DAPI (blue). Histogram showing the quantification of TUNEL positive tubules (H) and TUNEL positive cells per tubule (I) on days 3, 6, 9, 12 and 15 in cryptorchid testes (n = 3). Data were represented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test.
TRPV1 is upregulated in cryptorchid testis
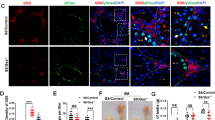
To investigate whether TRPV1 is related to the apoptosis of spermatogenic cells induced by cryptorchidism, the expression profile of TRPV1 in testes was detected on days 9 after cryptorchidism. RT-qPCR and Western blot analysis indicated that TRPV1 mRNA and protein expression levels were moderate in the testes of sham group, but significantly up-regulated in the cryptorchid testes (Fig. 3A, B). IF staining also revealed increased TRPV1 fluorescence intensity at 9 days after cryptorchid surgery compared to the sham group (Fig. 3C).
A, B TRPV1 mRNA and protein expression in the testis on day 9 after cryptorchidism (n = 3). A mRNA expression was assessed using RT-qPCR. Amplification of β-actin mRNA was used as an internal control. B Protein expression was determined using Western blot. Protein expression levels were normalized to β-actin and densitometric analyses were performed with ImageJ software. C IF staining was applied to detect TRPV1 (red) in the 9-day cryptorchid testis. Dashed boxes showed localization of the enlarged images. White arrows indicated cells with strong positive signals. Data were represented as mean ± SD from three independent experiments. **p < 0.01 by Student’s t test.
TRPV1 is dispensable for male fertility
To further study the relationship between TRPV1 and spermatogenic cell apoptosis, we obtained the Trpv1+/− mice from the Jackson laboratory. Trpv1+/− male mice were bred with Trpv1+/− female mice to generate Trpv1−/− mice. We first used Western blot to confirm the knockout efficiency of TRPV1 in the Trpv1−/− testis. The result showed that TRPV1 was expressed in wild-type (WT) mice testis, but almost disappeared in Trpv1−/− mice testis (Fig. 4A). Then, we explored the reproductive phenotypes of Trpv1−/− mice. We found that there were no differences in the litter size between Trpv1−/− mice and WT mice (Fig. 4B). Besides, the testicular volume, testicular weight, testicular weight/body weight ratio and sperm counts of Trpv1−/− mice were comparable to the WT mice (Fig. 4C–F). Furthermore, the histological analysis of Trpv1−/− mice also did not reveal any remarkable abnormalities in the testis, cauda epididymis and sperm of Trpv1−/− mice (Fig. 4G, H). Moreover, we found that apoptosis in spermatogenic cells of Trpv1−/− mice and WT mice had no significant difference (Fig. 4I–K). Hence, we conclude that TRPV1 is dispensable for male fertility in normal conditions.
A Protein expression of TRPV1 in the testes of WT and Trpv1−/− mice was determined using Western blot. β-actin served as a loading control. Comparison of average pups (B), testicular size (C), testicular weight (D), testicular weight/body weight ratio (E), and the sperm count in cauda epididymis (F) of 8-week-old Trpv1−/− and WT mice (n = 5). G H&E staining of testis and cauda epididymis of 8-week-old Trpv1−/− and WT mice. H H&E staining of spermatozoa from 8-week-old Trpv1−/− and WT mice. I Sections of testes from 8-week-old WT mice and Trpv1−/− mice were stained with a TUNEL probe (green), and nuclei were stained with DAPI (blue). Histogram showing the quantification of TUNEL positive tubules (J) and TUNEL positive cells per tubule (K) of 8-week-old WT mice and Trpv1−/− mice (n = 3). Data were represented as mean ± SD from three independent experiments. The data were analyzed with Student’s t test. ns not significant.
The deficiency of TRPV1 alleviates reproductive damage induced by experimental cryptorchidism
To reveal the function of TRPV1 in spermatogenic cell apoptosis after cryptorchidism, we further compared the reproductive phenotypes of Trpv1−/− mice and WT mice at 9 days after cryptorchidism. We found that the testicular volume of Trpv1−/− cryptorchid mice decreased less than that of WT cryptorchid mice (Fig. 5A). In addition, the testis weight, the ratio of testis weight/body weight and sperm count were increased in Trpv1−/− cryptorchid mice compared with WT cryptorchid mice, suggesting that the injury of cryptorchid testis was reduced after TRPV1 deletion (Fig. 5B–D). H&E staining showed that the seminiferous tubules had fewer luminal vacuoles and multinucleated giant cells in Trpv1−/− mice compared with WT mice after cryptorchidism. Besides, compared with WT cryptorchid mice, Trpv1−/− cryptorchid mice had more spermatogenic cells in the testis and more sperm in cauda epididymis (Fig. 5E). Next, we performed TUNEL staining of testes from Trpv1−/− mice and WT mice after cryptorchid surgery. Our results showed that positive TUNEL signals were reduced in Trpv1−/− cryptorchid mice (Fig. 5F). Statistical analysis of TUNEL staining showed that the number of TUNEL positive cells (Fig. 5G) and positive tubules (Fig. 5H) were significantly reduced in Trpv1−/− mice compared to WT mice after cryptorchid surgery. We further observed by periodic acid-schiff (PAS)-hematoxylin and PNA staining that compared with WT cryptorchid mice, the stages of seminiferous epithelium in Trpv1−/− mice after cryptorchid were recovered, and the acrosome of sperm was well developed (Supplementary Fig. S1A, B). These findings suggest that TRPV1 plays a role in promoting the apoptosis of spermatogenic cells after cryptorchid surgery.
Comparison of the testicular size (A), testicular weight (B), testicular weight/body weight ratio (C), and the number of spermatozoa in cauda epididymis (D) on day 9 after cryptorchidism in 8-week-old WT mice and Trpv1−/− mice (n = 5). E H&E staining of testes and cauda epididymis in cryptorchid surgery groups and sham groups of 8-week-old WT mice and Trpv1−/− mice. F Sections of testes from 8-week-old WT mice and Trpv1−/− mice on day 9 after cryptorchidism were stained with a TUNEL probe (green), and nuclei were stained with DAPI (blue). Histogram showing the quantification of TUNEL positive tubules (G) and TUNEL positive cells per tubule (H) on day 9 of cryptorchid testis in 8-week-old WT mice and Trpv1−/− mice (n = 3). Data were represented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test.
Apoptosis-related genes are downregulated in the spermatocytes of Trpv1 −/− mice with cryptorchidism
To gain insight into the molecular mechanism underlying TRPV1 regulation of spermatocyte apoptosis in cryptorchid mice, we used purified spermatocytes obtained from the testes of Trpv1−/− and WT mice at 9 days after cryptorchidism and extracted total RNA for RNA-seq (Fig. 6A). Transcriptome analysis showed that 430 genes were up-regulated and 3176 genes were down-regulated in spermatocytes of Trpv1−/− cryptorchid mice compared with WT mice (Fig. 6B, C). KEGG pathway analysis revealed that the apoptosis pathway was enriched in the down-regulated DEGs, and the major down-regulated genes in apoptosis pathways included Capn1, Capn2, Bax, Aifm1, Caspase 3, Map3k5, Itpr1 and Fas (Fig. 6C, D). To validate the RNA-seq findings, we conducted RT-qPCR and Western blot analyses on these genes. The results confirmed that the mRNA and protein levels of these genes were significantly decreased in spermatocytes of Trpv1−/− cryptorchid mice (Fig. 6E, F), which was consistent with the RNA-seq results. Hence, we conclude that TRPV1 can induce the increased expression of apoptosis-related molecules in the mouse model of cryptorchidism to participate in spermatocyte apoptosis.
A Schematic diagram depicting transcriptome analysis of spermatocytes. Briefly, total RNA from WT and Trpv1−/− spermatocytes of cryptorchid testes on day 9 were collected for the RNA-seq. B Heatmaps depicting significantly upregulated and downregulated genes in WT and Trpv1−/− spermatocytes. The genes with |log2 Fold change|≥1 and q < 0.05 were determined to generate the heatmap. C Volcano plot showing DEGs in WT and Trpv1−/− spermatocytes. The significant changed downregulated genes associated with apoptosis were labeled with gene name. D KEGG pathway analysis of 3176 downregulated genes in Trpv1−/− spermatocytes. The apoptosis pathway was labeled with asterisks. E, F Expression of CAPN1, CAPN2, BAX, AIFM1, Caspase 3, MAP3K5, ITPR1 and FAS in spermatocytes of WT and Trpv1−/− cryptorchid mice were detected by RT-qPCR and Western blot analysis (n = 3). Data were represented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test. G Cryptorchidism induces the increase of TRPV1 expression in mouse spermatocytes, leading to the increase of [Ca2+]i, which participates in the endogenous mitochondrial apoptosis pathway through up-regulation of CAPN1, CAPN2, BAX, AIFM1, MAP3K5 and ITPR1 on the one hand, and in exogenous apoptosis pathway through up-regulation of FAS on the other hand, ultimately leading to spermatocyte apoptosis. This picture was drawn by figdraw.
Discussion
Cryptorchidism is a common congenital disease of the male reproductive system, in which the testes are exposed to a relatively high-temperature environment, leading to apoptosis of spermatogenic cells and subsequent infertility. However, the underlying mechanism of spermatogenic cell apoptosis caused by increased testicular temperature remains unclear. In this study, we observed the phenotype of Trpv1−/− cryptorchid mice and found that TRPV1 knockout can alleviate the reduction in sperm count, damage to the seminiferous epithelium, and spermatogenic apoptosis caused by cryptorchidism. Furthermore, we preliminarily elucidated the molecular mechanism by which TRPV1 exerts pro-apoptotic effects on spermatogenic cells in response to thermal stimulation by regulating apoptotic molecules in both the death receptor pathway and mitochondrial pathway of apoptosis.
In this study, we observed that TRPV1 expression was detected in the testes of mice at day 7, significantly increased from day 21 to day 28, and then gradually decreased as the mice became adults. Throughout the developmental stages of mice, all types of spermatogonia (undifferentiated, A1, A2, A3, A4, intermediate, and B spermatogonia) were seen in the testis as early as postnatal day 5–6, followed by the differentiation of spermatogonia and meiosis in spermatocytes, and then by day 27, the first elongated spermatids were observed [48]. The expression profile of TRPV1 during postnatal testicular development suggests its potential significance in spermatogenesis. Our further examination showed that TRPV1 was expressed on the cell membrane of all spermatogenic cells, and the expression was relatively high in primary spermatocytes, suggesting that TRPV1 may play an important role in spermatocytes. Nevertheless, we found that the male Trpv1−/− mice exhibited normal spermatogenesis and fertility. The number of litters, testis weight, testis weight/body weight, and sperm count of Trpv1−/− mice were not different from those of WT mice. Moreover, no significant morphological abnormality was observed in the testis, epididymis, and sperm in Trpv1−/− mice. These findings suggest that knocking out TRPV1 does not affect mouse fertility, which is consistent with a previous report [38].
Thermal stimulation is considered a significant factor leading to reproductive damage following cryptorchidism. Moreover, spermatogenic cells are more susceptible to apoptosis induced by thermal stimulation than other cells, attributable to their high rate of division [49]. TRPV1, as a thermoreceptor, plays an important role in sensing temperature [50]. Therefore, we examined the function of TRPV1 in cryptorchid mice. We found that cryptorchid mice at 9 days had significantly decreased testicular volume, testis weight/body weight, and sperm count in cauda epididymis, with a large amount of spermatogenic epithelium detached, multinucleated giant cells in the lumen of the seminiferous tubules, and a significant increase in the number of apoptotic spermatogenic cells. The presence of multinucleated giant cells and apoptosis in spermatogenic cells are indicative morphological features of seminiferous epithelium damage in cryptorchid testes [19]. Our results show that severe damage to spermatogenic cells begins at 9 days of cryptorchidism. Moreover, our investigation of TRPV1 expression at 9 days of cryptorchidism revealed a significant increase in its levels within cryptorchid testes, which suggests that TRPV1 may be involved in spermatogenic cell damage caused by cryptorchidism. In order to clarify the role of TRPV1 in spermatogenic damage caused by cryptorchidism, we further constructed the cryptorchid model of Trpv1−/− mice, and found that there were obvious phenotypic differences between Trpv1−/− mice and WT mice after cryptorchidism. In Trpv1−/− cryptorchid mice, the damage of the seminiferous epithelium, the reduction of spermatozoa in cauda epididymis and the apoptosis of spermatogenic cells were all less severe than those in WT cryptorchid mice. It is evident that TRPV1 promotes spermatogenic cell apoptosis in cryptorchid mice, which is consistent with the results that TRPV1 promotes testicular cell apoptosis in aged mice found by Adrian S. Siregar et al. [44]. However, it is worth noting that the role of TRPV1 on spermatogenic apoptosis under different conditions appears to be quite complex. When the scrotum of Trpv1−/− mice was exposed to a 42 °C hot water bath, spermatogenic apoptosis increased, indicating that TRPV1 inhibits spermatogenic apoptosis under 42 °C thermal stimulation [45], which was contrary to our findings that TRPV1 could promote spermatogenic apoptosis under cryptorchidism. This difference suggests that the effect of TRPV1 on spermatogenic apoptosis depends on the specific activation conditions.
Cryptorchidism induces germ cell apoptosis, which mainly affects spermatocytes and round spermatids, eventually leading to testicular atrophy [51, 52]. Given our observation of high TRPV1 expression in spermatocytes, we further investigated the molecular mechanisms underlying TRPV1-induced spermatocyte apoptosis in cryptorchidism. TRPV1 is a ligand-gated cation channel, whose activation results in Ca2+ influx into the cell [53]. Ca2+ signals are involved in important checkpoints in cell death pathways and promote apoptosis [54]. There are two main pathways of apoptosis, intrinsic mitochondria-mediated pathway and extrinsic death receptor-mediated pathway [55]. Our RNA-Seq data revealed that genes related to apoptosis, such as Capn1, Capn2, Bax, Aifm1, Caspase 3, Map3k5, Itpr1 and Fas were reduced in the spermatocytes of Trpv1−/− cryptorchid mice. CAPN1 and CAPN2 are Ca2+ dependent cysteine proteases that cleave and activate Bax, allowing the translocation of Bax to the mitochondria [56, 57]. Bax is a pro-apoptotic protein, primarily functioning to enhance the permeability of the mitochondrial membrane [58]. The damage to the mitochondria leads to the exposure of their inner membrane, which causes the release of mitochondrial intermembrane space proteins that can either trigger caspase 3 activation, or caspase-independent pathways, such as AIFM1 [59]. Previous study has shown that Ca²⁺ influx induced by TRPV1 causes mitochondrial hyperpolarization and depolarization, leading to upregulation of Bax and release of AIFM1 [53]. This is consistent with our finding that the expression of Bax and AIFM1 is reduced in spermatocytes of Trpv1−/− cryptorchid mice. MAP3K5, known as apoptosis signal-regulating kinase 1, can also be activated in response to calcium overload [60], leading to mitochondrial dysregulation and eventually apoptosis [61]. ITPR1 is an intracellular Ca2+ release channel that regulates mitochondrial calcium-dependent apoptosis by facilitating calcium transport from the endoplasmic reticulum lumen to the mitochondria intermembrane space [62]. One study has found that the elevated apoptosis of spermatogenic cells in non-obstructive azoospermia patients was associated with increased expression of ITPR1 and Bax [63]. Fas functions as a death receptor in the extrinsic pathway of apoptosis. Upon binding to its ligand FASL, Fas initiates the caspase cascade to drive apoptosis [64]. In this study, reduced expression of Fas was detected in the spermatocytes of Trpv1−/− cryptorchid mice. This corresponds to a previous study that [Ca2+]i induced by TRPV1 activation could promote the aggregation of Fas death receptors and induce apoptosis in urothelial cancer cells [65]. Threrfore, our results suggest that TRPV1 is involved in spermatocyte apoptosis in mice with cryptorchidism through both intrinsic mitochondrial-mediated pathway and extrinsic death receptor-mediated pathway (Fig. 6G). Further molecular mechanisms need to be explored in future studies.
In summary, our study clarifies that the thermosensitive protein TRPV1 is mainly expressed on the membrane of mouse spermatocytes. And under cryptorchid conditions, TRPV1 can enhance the transcription of apoptotic molecules through both mitochondrial and death receptor apoptosis pathways, leading to spermatogenic cell apoptosis. This study demonstrates for the first time that TRPV1 plays an important role in spermatogenic cell apoptosis after cryptorchidism, providing a new target for the treatment of cryptorchidism.
Materials and methods
Animals
WT (C57BL/6J) mice were obtained from the Laboratory Animal Center of Air Force Military Medical University, and Trpv1+/− mice (Strain#: 003770) were obtained from Jackson Laboratory. All mice were housed in a specific pathogen-free (SPF) environments with a 12 h:12 h light/dark cycle and an ambient temperature of 23 ± 2 °C. Mice were randomly assigned to different experimental groups.
Reverse transcriptase - quantitative PCR (RT-qPCR)
Total RNA was extracted from the testes and spermatogenic cells using Trizol (Takara, Kyoto, Japan). RNA was reverse transcribed into cDNA using SmArt RT Master Premix (5×) (Deeyee, Shanghai, China) according to the manufacturer’s instructions. The synthesized cDNA was amplified with specific primers (Supplementary Table S1) and SYBR Green (Deeyee, Shanghai, China) using a QuantStudio 3 real-time fluorescence quantitative PCR system (Thermo Fisher Scientific, MA, USA). Triplicate samples were examined for each condition. The relative mRNA expression level was calculated using the 2−ΔΔCt method.
Western blot
Total proteins were extracted from testes or spermatogenic cells using cold-RIPA buffer (Beytime, Shanghai, China) with 1 mM PMSF (Beytime, Shanghai, China) and 1 mM protease inhibitor cocktail (Solarbio, Beijing, China). The lysates were centrifuged at 12,000 × g for 15 min. The protein concentration was determined using a BCA Protein Detection Kit (Beyotime, Shanghai, China). Proteins were separated by SDS-PAGE electrophoresis and transferred to a nitrocellulose membrane. The membrane was blocked with 5% (w/v) nonfat milk at room temperature for 1 h and then incubated with the appropriate primary antibodies at 4 °C overnight. Then, the membrane was incubated with HRP-conjugated secondary antibody at room temperature for 1 h. Protein bands were visualized using an enhanced chemiluminescence kit (InCellGenE, TX, USA) and the membrane was photographed by Clinx chemiluminescence instrument (Clinx Science Instruments Co., Ltd, Shanghai, China). The antibodies used in this study are listed in Table S2. Uncropped immunoblot gels are shown in supplementary file.
Tissue fixation and histological analysis
Testes and caudal epididymides were fixed in 4% (m/v) paraformaldehyde (PFA) for 24 h, dehydrated with gradient alcohol, embedded in paraffin, and processed into 5 μm sections. After deparaffinization, the tissue sections were stained with hematoxylin and eosin (H&E). For periodic acid-Schiff (PAS) staining, deparaffinized slides were stained with PAS and hematoxylin according to the protocol of PAS kit (G1280, Solarbio, China).
Immunofluorescence (IF), immunohistochemistry (IHC) and TUNEL assay
For IF assay, after routine deparaffin and rehydration, testicular sections were microwave-repaired in 10 mM sodium citrate solution (pH 6.0) for 15 min. Sections were block with 5% (m/v) BSA (Biofroxx, Einhausen, DE) for 30 min and incubated overnight at 4 °C with the appropriate primary antibodies. Subsequently, the sections were incubated with the secondary antibody at room temperature for 2 h. PNA dye (Sigma, MO, USA) was used to label the acrosomes of sperm cells at room temperature for 2 h. DAPI (abcam, Cambridge, UK) staining was used to visualize the nuclei of cells. The sections were mounted in 50% (v/v) glycerol and examined with a VS200 microscope (Olympus, Tokyo, Japan) and a FV1000 confocal microscope (Olympus, Tokyo, Japan).
For IHC assay, testicular sections were routinely deparaffinized, rehydrated, and microwave-repaired. Sections were incubated in 0.3% (m/v) H2O2 for 30 min at room temperature, and blocked with 5% (m/v) BSA at room temperature for 30 min. Sections were incubated with primary antibody at 4 °C overnight. Control sections were incubated with serum from the same source as the primary antibody instead of the primary antibody, followed by sequential incubation with biotinylated secondary antibody and streptavidin peroxidase complex provided with the VECTASTAIN Elite ABC HRP Kit (PK-4001, VECTASTAIN, USA). Sections were incubated with 0.02% (m/v) DAB (Sigma, MO, USA) solution, followed by staining of nuclei with hematoxylin for 1 min. And images were acquired using VS200 microscope (Olympus, Tokyo, Japan). The antibodies used for IF and IHC assay in this study are listed in Table S2.
For TUNEL assay, after the routinely deparaffinized, rehydrated, and microwave-repaired, enzyme and labeling solutions were mixed followed the TUNEL kit protocol (Roche, Basel, CH). Then, testicular sections were incubated in the mixture for 1.5 h at 37 °C under dark conditions. DAPI was used to stain the nuclei. Images were acquired using a VS200 microscope (Olympus, Tokyo, Japan).
Separation of spermatogenic cells
Spermatogenic cells were isolated by the STA-PUT method as previously described [66]. In brief, mouse testes were suspended in DMEM medium (Gibco, NYC, USA) containing 1.5 mg/ml hyaluronidase (InCellGenE, TX, USA) and 1.5 mg/ml collagenase IV (Gibco, NYC, USA). The sample was then digested in a 37 °C-water bath for 15 min. The digested cells were centrifuged and filtered through a 200-mesh filter (Solarbio, Beijing, China). A 2–4% (m/v) BSA gradient was applied to the separation tube, and the cells were left in the separation tube for 3 h. Different types of cells were collected from the bottom of the separator. The purity and type of cells in each tube were assessed by light microscopy according to the size and morphological characteristics of the cells. The collected cells were used for subsequent experiments.
Surgery-induced cryptorchidism
Eight-week-old WT or Trpv1−/− mice were randomly assigned to sham and cryptorchid groups. For the cryptorchid group, mice were anesthetized with 3% pentobarbital sodium. A cut was made along the skin in the right and left upper abdominal region and the adipose tissue of the caput epididymis was sutured to the inner peritoneal wall. For the sham group, mice were also anesthetized and bilateral abdominal incisions were made, but no further treatment was applied before suturing. The testes and epididymides of the mice were collected on days 3, 6, 9, 12, and 15 after cryptorchidism.
Fertility assay
Male WT mice or Trpv1−/− mice were mated with WT female mice at a ratio of 1:2. Vaginal plugs were checked the next day. After mating, each male mouse rested separately for 1–2 days before starting the next round of mating. The number of litters of female mice with positive vaginal plugs were recorded.
Sperm collection, sperm count and morphology test
The cauda epididymis of WT mice or Trpv1−/− mice was incised, and the spermatozoa were extruded in PBS. The spermatozoa were incubated for 10 min at 37 °C in a CO2 incubator, and then filtered through a 100 μm aperture cell sieve. Sperm counts were performed using a hemocytometer under a microscope. Additionally, sperm samples from the cauda epididymis of WT mice or Trpv1−/− mice were smeared onto slides, fixed for 30 min with 4% (m/v) paraformaldehyde in PBS, then rinsed and subjected to H&E staining.
Isolation of primary spermatocytes
Isolation of primary spermatocytes was performed as described as in a previous article [67]. In brief, mouse testes were isolated, digested with 1× Krebs buffer containing 1 mg/ml collagenase IV (InCellGenE, Texas, USA) at 37 °C for 3 min. Subsequently, 1× Krebs buffer was added to the sample and left for 1 min at room temperature. The sample was then centrifuged at 600 g for 5 min, and the supernatant was discarded. Afterward, the sample was diluted with 1× Krebs buffer containing 0.6 mg/ml trypsin (Solarbio, Beijing, China) and digested on a tube rotator inside the 34 °C incubator for 15–20 min. The sample was then filtered through a 40 μm cell strainer (Biosharp, Anhui, China). The sample was centrifuged again at 600 × g for 5 min, the supernatant was discarded, and this step was repeated 2–3 times. A gradient of 1–5% concentration of BSA was prepared, the cells were resuspended with 0.5% BSA, and loaded onto the gradient. After 1.5 h, the cells were collected and observed under a microscope.
RNA-seq library construction and data analysis
Total RNA from spermatocytes of Trpv1−/− and WT mice testes at 9 days after cryptorchidism was extracted using Trizol. Libraries were constructed using the TruseqTM RNA Library Preparation Kit (Illumina, CA, USA). Sequencing was performed on the Illumina Novaseq 6000. Data quality control was constructed by comparing the fast and pure data to the reference genome Mus_musculus. Gene expression was analyzed using RSEM. DEGseq was used to analyze differential gene expression between biological replicates. Genes with |log2 fold change|≥1 and p (value) <0.05 were identified as differentially expressed genes. The sequencing and analysis were performed by Megi Biomedicine Technology Co., Ltd (Shanghai, China).
Statistical analysis
All quantitative analyses were performed under blinded conditions. Detailed n values for each panel in the figures were stated in the corresponding legends. Sample sizes were based in standard protocols in the field and no sample or animal was excluded. Quantitative data with normal distribution were expressed as mean ± SD and Student’s t test was used for comparison between groups. All statistical analyses were performed by GraphPad Prism 9.0 Software. P < 0.05 was considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The RNA-seq datasets generated for this study can be found in the SRA metadata under accession PRJNA1216033.
References
Aldahhan RA, Stanton PG. Heat stress response of somatic cells in the testis. Mol Cell Endocrinol. 2021;527:111216.
Gopalakrishnan NP, Chung IM. Alteration in the expression of antioxidant and detoxification genes in chironomus riparius exposed to zinc oxide nanoparticles. Comp Biochem Physiol B Biochem Mol Biol. 2015;190:1–7.
Hoang-Thi AP, Dang-Thi AT, Phan-Van S, Nguyen-Ba T, Truong-Thi PL, Le-Minh T, et al. The impact of high ambient temperature on human sperm parameters: a meta-analysis. Iran J Public Health. 2022;51:710–23.
Mieusset R, Bengoudifa B, Bujan L. Effect of posture and clothing on scrotal temperature in fertile men. J Androl. 2007;28:170–5.
Palnitkar G, Phillips CL, Hoyos CM, Marren AJ, Bowman MC, Yee BJ. Linking sleep disturbance to idiopathic male infertility. Sleep Med Rev. 2018;42:149–59.
Paick S, Choi WS. Varicocele and testicular pain: a review. World J Mens Health. 2019;37:4–11.
Trsinar B, Muravec UR. Fertility potential after unilateral and bilateral orchidopexy for cryptorchidism. World J Urol. 2009;27:513–9.
Ziaeipour S, Piryaei A, Aliaghaei A, Nazarian H, Naserzadeh P, Ebrahimi V, et al. Chronic scrotal hyperthermia induces azoospermia and severe damage to testicular tissue in mice. Acta Histochem. 2021;123:151712.
Paul C, Teng S, Saunders PTK. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death1. Biol Reprod. 2009;80:913–9.
Leisegang K, Dutta S. Do lifestyle practices impede male fertility? Andrologia. 2021;53:e13595.
Pino JA, Osses N, Oyarzun D, Farias JG, Moreno RD, Reyes JG. Differential effects of temperature on reactive oxygen/nitrogen species production in rat pachytene spermatocytes and round spermatids. Reproduction. 2013;145:203–12.
Hirano K, Nonami Y, Nakamura Y, Sato T, Sato T, Ishiguro K, et al. Temperature sensitivity of dna double-strand break repair underpins heat-induced meiotic failure in mouse spermatogenesis. Commun Biol. 2022;5:504.
Serrano T, Chevrier C, Multigner L, Cordier S, Jegou B. International geographic correlation study of the prevalence of disorders of male reproductive health. Hum Reprod. 2013;28:1974–86.
Agoulnik AI, Huang Z, Ferguson L. Spermatogenesis in cryptorchidism. Methods Mol Biol. 2012;825:127–47.
Loebenstein M, Thorup J, Cortes D, Clasen-Linde E, Hutson JM, Li R. Cryptorchidism, gonocyte development, and the risks of germ cell malignancy and infertility: a systematic review. J Pediatr Surg. 2020;55:1201–10.
Kunej T, Zorn B, Peterlin B. Y chromosome microdeletions in infertile men with cryptorchidism. Fertil Steril. 2003;79:1559–65.
Cortes D, Thorup J, Lindenberg S, Visfeldt J. Infertility despite surgery for cryptorchidism in childhood can be classified by patients with normal or elevated follicle-stimulating hormone and identified at orchidopexy. BJU Int. 2003;91:670–4.
Ferguson L, Agoulnik AI. Testicular cancer and cryptorchidism. Front Endocrinol. 2013;4:32.
Chaki SP, Misro MM, Ghosh D, Gautam DK, Srinivas M. Apoptosis and cell removal in the cryptorchid rat testis. Apoptosis. 2005;10:395–405.
Ogi S, Tanji N, Yokoyama M, Takeuchi M, Terada N. Involvement of fas in the apoptosis of mouse germ cells induced by experimental cryptorchidism. Urol Res. 1998;26:17–21.
Kocak I, Dundar M, Hekimgil M, Okyay P. Assessment of germ cell apoptosis in cryptorchid rats. Asian J Androl. 2002;4:183–6.
Peltola V, Huhtaniemi I, Ahotupa M. Abdominal position of the rat testis is associated with high level of lipid peroxidation. Biol Reprod. 1995;53:1146–50.
Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–67.
Iizuka A, Park MK, Mori T. Effects of unilateral cryptorchidism on the expression of gonadotropin receptor mrna. Biochem Biophys Res Commun. 1996;221:290–4.
Mizuno K, Hayashi Y, Kojima Y, Nakane A, Tozawa K, Kohri K. Activation of nf-kappab associated with germ cell apoptosis in testes of experimentally induced cryptorchid rat model. Urology. 2009;73:389–93.
Absalan F, Movahedin M, Mowla SJ. Germ cell apoptosis induced by experimental cryptorchidism is mediated by molecular pathways in mouse testis. Andrologia. 2010;42:5–12.
Jung KY, Yon J, Lin C, Jung AY, Lee JG, Baek I, et al. Phospholipid hydroperoxide glutathione peroxidase is involved in the maintenance of male fertility under cryptorchidism in mice. Reprod Toxicol. 2015;57:73–80.
Yin Y, Stahl BC, Dewolf WC, Morgentaler A. P53-mediated germ cell quality control in spermatogenesis. Dev Biol. 1998;204:165–71.
Lee SG, Kim J, Lee YI, Kim J, Choi YS, Ham S, et al. Cutaneous neurogenic inflammation mediated by TRPV1-NGF-TRKA pathway activation in rosacea is exacerbated by the presence of demodex mites. J Eur Acad Dermatol Venereol. 2023;37:2589–600.
Zafar S, Luo Y, Zhang L, Li CH, Khan A, Khan MI, et al. Daidzein attenuated paclitaxel-induced neuropathic pain via the down-regulation of TRPV1/P2Y and up-regulation of Nrf2/HO-1 signaling. Inflammopharmacology. 2023;31:1977–92.
Wei H, Liu B, Yin C, Zeng D, Nie H, Li Y, et al. Electroacupuncture improves gout arthritis pain via attenuating ros-mediated NLRP3 inflammasome overactivation. Chin Med. 2023;18:86–104.
Qian K, Lei X, Liu G, Fang Y, Xie C, Wu X, et al. Transient receptor potential vanilloid-1 (TRPV1) alleviates hepatic fibrosis via TGF-β signaling. Dis Markers. 2022;2022:3100943.
Ma L, Li B, Ma J, Wu C, Li N, Zhou K, et al. Novel discovery of schisandrin a regulating the interplay of autophagy and apoptosis in oligoasthenospermia by targeting SCF/c-kit and TRPV1 via biosensors. Acta Pharm Sin B. 2023;13:2765–77.
Guihur A, Rebeaud ME, Goloubinoff P. How do plants feel the heat and survive? Trends Biochem Sci. 2022;47:824–38.
Numazaki M, Tominaga M. Nociception and trp channels. Curr Drug Targets CNS Neurol Disord. 2004;3:479–85.
Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3.
Sun X, Zakharian E. Regulation of the temperature-dependent activation of transient receptor potential vanilloid 1 (TRPV1) by phospholipids in planar lipid bilayers. J Biol Chem. 2015;290:4741–7.
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13.
Lewis SE, Rapino C, Di Tommaso M, Pucci M, Battista N, Paro R, et al. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS One. 2012;7:e47704.
Francavilla F, Battista N, Barbonetti A, Vassallo MR, Rapino C, Antonangelo C, et al. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology. 2009;150:4692–700.
De Toni L, Garolla A, Menegazzo M, Magagna S, Di Nisio A, šabović I, et al. Heat sensing receptor TRPV1 is a mediator of thermotaxis in human spermatozoa. PLoS One. 2016;11:e167622.
Catanzaro G, Battista N, Rossi G, Di Tommaso M, Pucci M, Pirazzi V, et al. Effect of capacitation on the endocannabinoid system of mouse sperm. Mol Cell Endocrinol. 2011;343:88–92.
Grimaldi P, Orlando P, Di Siena S, Lolicato F, Petrosino S, Bisogno T, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci USA. 2009;106:11131–6.
Siregar AS, Nyiramana MM, Kim E, Shin E, Kim C, Lee DK, et al. TRPV1 is associated with testicular apoptosis in mice. J Anim Reprod Biotechnol. 2019;34:311–7.
Mizrak SC, van Dissel-Emiliani FMF. Transient receptor potential vanilloid receptor-1 confers heat resistance to male germ cells. Fertil Steril. 2008;90:1290–3.
Mizrak SC, Gadella BM, Erdost H, Ozer A, van Pelt AM, van Dissel-Emiliani FM. Spermatogonial stem cell sensitivity to capsaicin: an in vitro study. Reprod Biol Endocrinol. 2008;6:52–61.
Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol. 2004;172:1175–8.
Drumond AL, Meistrich ML, Chiarini-Garcia H. Spermatogonial morphology and kinetics during testis development in mice: a high-resolution light microscopy approach. Reproduction. 2011;142:145–55.
Yadav SK, Pandey A, Kumar L, Devi A, Kushwaha B, Vishvkarma R, et al. The thermo-sensitive gene expression signatures of spermatogenesis. Reprod Biol Endocrinol. 2018;16:56–78.
Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–76.
Hou W, Hu J, Li Y, Zhao J, Li Z, Liu X, et al. Altered expression of NDRG2 in the testes of experimental rat model of cryptorchidism. Urology. 2010;75:985–91.
Shikone T, Billig H, Hsueh AJ. Experimentally induced cryptorchidism increases apoptosis in rat testis. Biol Reprod. 1994;51:865–72.
Zhai K, Liskova A, Kubatka P, Büsselberg D. Calcium entry through TRPV1: A potential target for the regulation of proliferation and apoptosis in cancerous and healthy cells. Int J Mol Sci. 2020;21:4177.
Shi M, Zhang T, Sun L, Luo Y, Liu DH, Xie ST, et al. Calpain, Atg5 and Bak play important roles in the crosstalk between apoptosis and autophagy induced by influx of extracellular calcium. Apoptosis. 2013;18:435–51.
Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–30.
Arnandis T, Ferrer-Vicens I, Garcia-Trevijano ER, Miralles VJ, Garcia C, Torres L, et al. Calpains mediate epithelial-cell death during mammary gland involution: mitochondria and lysosomal destabilization. Cell Death Differ. 2012;19:1536–48.
Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem. 2002;277:27217–26.
Spitz AZ, Gavathiotis E. Physiological and pharmacological modulation of BAX. Trends Pharm Sci. 2022;43:206–20.
Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923–9.
Shiizaki S, Naguro I, Ichijo H. Activation mechanisms of ASK1 in response to various stresses and its significance in intracellular signaling. Adv Biol Regul. 2013;53:135–44.
Wang T, Pang L, He M, Wang Z. Small-molecule inhibitors targeting apoptosis signal-regulated kinase 1. Eur J Med Chem. 2023;262:115889.
Ismail M, Zhang X, Taha R, Elhafiz M, Zhang Q, Yousef BA, et al. Expression profiles of lncRNAs and their possible regulatory role in monocrotaline-induced HSOS in rats. Front Genet. 2023;14:1041266.
Maleki B, Modarres P, Salehi P, Vallian S. Identification of ITPR1 gene as a novel target for hsa-mir-34b-5p in non-obstructive azoospermia: a Ca2+/apoptosis pathway cross-talk. Sci Rep. 2023;13:21873.
Ranjan K, Pathak C. Cellular dynamics of FAS-associated death domain in the regulation of cancer and inflammation. Int J Mol Sci. 2024;25:3228–51.
Amantini C, Ballarini P, Caprodossi S, Nabissi M, Morelli MB, Lucciarini R, et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009;30:1320–9.
Hohmann LK, Shows TB. Complementation of genetic disease: a velocity sedimentation procedure for the enrichment of heterokaryons. Somat Cell Genet. 1979;5:1013–29.
Da RM, Lehtiniemi T, Olotu O, Meikar O, Kotaja N. Enrichment of pachytene spermatocytes and spermatids from mouse testes using standard laboratory equipment. J Vis Exp. 2019. https://doi.org/10.3791/60271.
Funding
The study was supported by the Natural Science Foundation of Shaanxi province (Grant number 2024JC-YBMS-617), Scientific research project of Health Committee of Shaanxi province (Grant number 2022A012) and Natural Science Basic Research Program of Shaanxi (Grant number 2023-JC-QN-0940).
Author information
Authors and Affiliations
Contributions
Zhen Li and Yanqiu Zhao designed the study. Yanqiu Zhao, Jinhua Wei and Pang Cheng conducted the study and collected the data. Junxian Ma, Bo Liu, Mingxiang Xiong and Tianchen Sun analyzed the data. Ting Gao and Jingqi Yao performed the experiments of isolating spermatocytes. Yanqiu Zhao, Jinhua Wei and Zhen Li wrote the paper. All authors contributed to the article and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods in this study were performed in accordance with the relevant guidelines and regulations. All animal care and experimental procedures were conducted according to the guidelines approved by the Institutional Animal Care and Use Committee of Air Force Military Medical University (IACUC-20220159).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Wei, J., Cheng, P. et al. The involvement of TRPV1 in the apoptosis of spermatogenic cells in the testis of mice with cryptorchidism. Cell Death Discov. 11, 135 (2025). https://doi.org/10.1038/s41420-025-02447-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-025-02447-3