Abstract
Delta opioid receptor (δOR) plays a pivotal role in modulating human sensation and emotion. It is an attractive target for drug discovery since, unlike Mu opioid receptor, it is associated with low risk of drug dependence. Despite its potential applications, the pharmacological properties of δOR, including the mechanisms of activation by small-molecule agonists and the complex signaling pathways it engages, as well as their relation to the potential side effects, remain poorly understood. In this study, we use cryo-electron microscopy (cryo-EM) to determine the structure of the δOR-Gi complex when bound to a small-molecule agonist (ADL5859). Moreover, we design a series of probes to examine the key receptor-ligand interaction site and identify a region involved in signaling bias. Using ADL06 as a chemical tool, we elucidate the relationship between the β-arrestin pathway of the δOR and its biological functions, such as analgesic tolerance and convulsion activities. Notably, we discover that the β-arrestin recruitment of δOR might be linked to reduced gastrointestinal motility. These insights enhance our understanding of δOR’s structure, signaling pathways, and biological functions, paving the way for the structure-based drug discovery.
Similar content being viewed by others
Introduction
Opioids, known for their potent analgesic properties, have been utilized for thousands of years in various cultural and medical settings. Their pharmacological action is primarily due to their affinity for opioid receptors, a crucial subgroup within the broad family of G protein-coupled receptors (GPCRs), which play a key role in modulating a wide array of physiological responses that underlie human sensation and emotion1. Opioids such as morphine, fentanyl, and codeine primarily target these receptors, each displaying unique affinity and selectivity profiles. For instance, morphine has a high affinity for the Mu opioid receptor (μOR), a particular subtype of opioid receptors, underscoring the complex dynamics between various opioids and receptor subtypes2. Among these subtypes, the δOR has garnered considerable scientific interest due to its unique modulation of pain perception, emotional states, and cognitive processes3,4. Unlike µOR, which is the primary target in traditional opioid analgesic development, δOR presents a promising alternative with potentially fewer adverse effects5. These include respiratory depression and dependency issues typically associated with µOR agonists. This differential risk profile enhances the therapeutic potential of δOR in neuroprotection and immunomodulation6,7. The distinct characteristics of δOR make it a strategic target for the development of innovative and more effective pain therapies, particularly given its demonstrated efficacy in treating chronic pain and its relatively lower potential for abuse8,9,10,11.
Despite the progression of several δOR-targeting compounds into clinical trials, our comprehension of the structure and functional signal transduction of the δOR remains limited. This presents significant challenges for the development of precisely targeted therapeutics12. The determination of several δOR crystal structures has shed light on the mechanisms of ligand recognition13,14,15,16. δOR is implicated in signaling through the Gi protein and β-arrestin pathways, reflecting its complex pharmacology. The advent of biased signaling or functional selectivity in GPCR represents a notable advancement in drug discovery, providing a potential pathway for more favorable therapeutic outcomes17,18,19. The recent disclosure of a fully activated δOR-Gi signaling complex bound to the exogenous opioid peptide [D-Ala2] deltorphin II, commonly known as deltorphin, marks significant progress in understanding δOR’s signal transduction20. However, the inherent relationships between the activation of δOR signaling pathways by small molecule ligands, particularly biased agonists, and their subsequent biological functions remain unclear.
In this study, we sought to clarify the relationship between the various pathways activated by δOR, their biological functions, and the potential for therapeutic applications. We present the first cryo-EM structure of δOR, stimulated by the subtype-selectivity agonist ADL5859, in complex with the Gi heterotrimer protein. This structure not only underscores a fundamental element essential for δOR activation but also pinpoints key residues contributing to subtype selectivity among opioid receptors. Our results shed light on the structural basis of ADL5859 functioning as a G-protein-biased agonist for δOR. Through mutagenesis studies and functional assays, we have identified potential elements influencing the biased signaling of ADL5859. This has led to the development of a series of ligands that significantly reduce β-arrestin recruitment. Among these ligands, ADL06 stands out. It not only demonstrates superior analgesic effects compared to ADL5859 in various pain models but also lacks convulsion activities and analgesic tolerance. Importantly, ADL06 does not exhibit the gastrointestinal (GI) inhibition commonly observed with ADL5859 and SNC80. Our findings suggest a potential relationship between the side effects of drugs and the β-arrestin pathway of δOR. Overall, our study enhances the understanding of the relationship between the structural characteristics of δOR and its biological properties. It lays a foundation framework for the rational ligand design, holding the promise for the development of a new class of analgesics without the side effects of traditional opioids.
Results
The structure of ADL5859-bound δOR-Gi complex
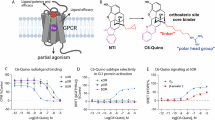
Previous studies have suggested that δOR agonists exhibit a significantly lower abuse potential, making them attractive candidates for the treatment of chronic pain, depression, and various neuropsychiatric conditions8,9,10,11,21 (Fig. 1a). Despite this potential, the clinical application of drugs targeting δOR remains a challenge. ADL5859, a δOR agonist, was in the phase II of clinical trials for pain treatment. It displayed an impressive affinity for δOR, which was 100-fold greater than that for μOR and kappa opioid receptor (κOR). This compound has shown remarkable performance in terms of tolerability and safety, making it a promising candidate in the field of δOR agonist research12. A thorough understanding of the pharmacological characteristics of ADL5859 is crucial for advancing the development of efficacious δOR-targeted therapies. We conducted assays to evaluate its effect on the Gi protein signaling pathway, using the Gαi1-Gγ9 dissociation assay, and on the β-arrestin pathway, employing the β-arrestin2 recruitment assay, GRK2 recruitment assay and receptor internalization assay. Compared with the reference agonist SNC80 and the endogenous ligand Leu-enkephalin, the results showed that ADL5859 is a Gi protein-biased agonist for δOR (Fig. 1b, Supplementary Fig. 1 and Supplementary Table 1).
a The side effects of opioid ligands targeting μOR and δOR are shown. The symbol of “○” reprents the common side effects for the receptor drugs, “×” indicates the infrequent side effects and “?” signifies that the side effects has not been reported for the receptor drugs. b Pharmacological investigation of ADL5859 with δOR. Web chart (bottom left panel) of bias illustrating distinctions in the pattern of ADL5859 at δOR. The web of plots log (pEC50) values for each ligand and for each signaling pathway tested at least three biologically independent experiments each performed in triplicate, n = 3. The signaling bias plot (bottom right panel) for ADL5859 on δOR relative to references SNC80 and Leu-enkephalin. The limon column chart refers to Leu-enkephalin as the reference, and the orange column chart refers to SNC80 as the reference. A BRET based Gαi1-Gγ9 dissociation assay was employed to assess Gi protein signaling, NanoBiT was utilized to measure β-arrestin2 recruitment, GRK2 recruitment and δOR internalization. **p < 0.01, ***p < 0.001 (one-way analysis of variance [ANOVA] followed by the Dunnett’s test). Data are presented as mean ± SEM from at least three biologically independent experiments each performed in triplicate, n = 3. The exact p value are provided in a Source Data file. c Cryo-EM map (left panel) and structural mode (right panel) of ADL5859-bound to δOR in complex with Gi heterotrimer and scFv16 and the density map of ADL5859 (middle panel). Medium aquamarine, δOR; khaki, Gαi; cornflower blue, Gβ; rosy brown, Gγ; sliver, scFv16. The EM density of δOR-Gi complex and ADL5859 was shown with the counter level of 0.15 and 0.15, respectively. Source data are provided as a Source Data file. Panels 1a and 1b were created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en.
To delve deeper insight into the molecular mechanism of ADL5859’s preference for the Gi protein, we present the cryo-EM structure of the ADL5859-bound δOR-Gi complex (Fig. 1c and Supplementary Fig. 2). We used a truncated δOR construct (residues from 36 to 352) to obtain a stable ADL5859-bound δOR-Gi complex. The cytochrome b562 RIL (BRIL) protein was fused at the N-terminus of this construct to enhance the receptor expression. The Gi heterotrimer and δOR were subsequently co-expressed in Spodoptera frugiperda (Sf9) insect cells. The δOR-Gi complex was formed in the presence of ADL5859 and the antibody scFv16, which further stabilized the complex. Finally, we resolved the ADL5859-bound δOR-Gi complex at a resolution of 3.11 Å. The density map allowed us to construct a model comprising the receptor, the agonist ADL5859, and the Gi heterotrimer (Fig. 1c and Supplementary Table 2). The binding model of ADL5859 at the orthosteric site was further validated by molecular dynamic (MD) simulations (Supplementary Fig. 3). Structural alignment with the inactive state of δOR14 (PDB: 4N6H) revealed a significant outward movement of TM6 (12.1 Å measured at the Cα atom of residue D2536.27) and a large inward movement of TM7 in the cytoplasmic region. These movements are common features during receptor activation (Supplementary Fig. 4a).
The overall architecture of the ADL5859-bound δOR-Gi complex is similar to the structure of the δOR-Gi complex bound to the opioid peptide (PDB: 8F7S)20. The root-mean-square deviation (RMSD) values are 1.1 for the receptor and 1.0 for the whole complex, respectively. Compared to the deltorphin peptide binding mode, ADL5859 occupies a relatively smaller binding pocket in δOR. This induces a contraction of TM7 and TM2 toward the core of the TM bundles (Supplementary Fig. 4b). These conformational changes further strengthen the direct interaction between Y1092.64 and H3017.36, resulting in a noticeable outward movement of TM1 in the ADL5859-bound δOR (Supplementary Fig. 4c).
Recognition of ADL5859 with δOR
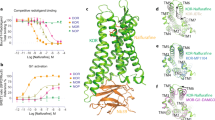
In the structure of the small-molecule ADL5859-bound δOR complex, ADL5859 adopts an inverted-T shape and deeply penetrates the narrow binding pocket formed by transmembrane domains TM3 and TM6-7 (Fig. 2a and Supplementary Fig. 5). Compared to deltorphin, ADL5859 undergoes a rotation, closing the cleft between TM6 and TM7. (Fig. 2a). As seen in Fig. 2a b, the component of ADL5859, the 4-phenylspiro[chromene-2,4’-piperidin]−5-ol moiety, is anchored at the base of the binding pocket. This mirrors the positioning of the N-terminal Y-[D-Ala2]-F segment of deltorphin in the δOR structure. The N, N-diethyl group, the upper segment of ADL5859, resides within the upper pocket region defined by the extracellular ends of TM6 and TM7 and the third extracellular loop (ECL3) (Fig. 2a-b). In detail, the 4-phenylspiro[chromene-2,4’-piperidin]−5-ol moiety of ADL5859, the bottom component, makes subtle contacts with the receptor through hydrophobic interactions and a salt bridge (Fig. 2b). Specifically, this moiety forms hydrophobic interactions with residues Y1293.33, I2776.51, H2786.52, and I3047.39, and a salt bridge with D1283.32. Notably, ADL5859 forms a unique face-to-end stacking interaction with residue Y3087.43, a residue that establishes a polar interaction with the amino nitrogen from Y1 in the deltorphin-bound δOR (Fig. 2b).
a Structural alignment of deltorphin and ADL5859-bound δOR. The binding pocket of ADL5859 composed by TM3, TM6 and TM7 (left panel) and the core moiety of ADL5859 makes a stacking, replacing by a hydrogen bond, with Y3087.43 (right panel). b The binding pocket of ADL5859 in δOR. The detailed interaction for the 4-phenylspiro[chromene-2,4’-piperidin]−5-ol moiety (left panel), N, N-diethyl group (right panel) of ADL5859. c Sequence alignment of residues in the binding pocket of δOR, μOR and κOR. The non-conserved residues of opioid receptors are shown in red. The positions of the residues were labeled using Ballesteros-Weinstein number at the top. d The activation effect of ADL5859 on δOR wild type (WT) and mutations (MT). Bars represent differences in calculated the value of ΔpEC50 (pEC50MT-pEC50WT). nd, not detected; ***p < 0.001 (one-way analysis of variance [ANOVA] followed by the Dunnett’s test). Data are presented as mean ± SEM from at least three biologically independent experiments each performed in triplicate, n = 3. The exact p value are provided in a Source Data file. e The cAMP inhibition assay for examining effects of ADL5859 on δOR, μOR and κOR. nd, not detected; ***p < 0.001(one-way analysis of variance [ANOVA] followed by the Dunnett’s test). Data are displayed as mean ± SEM from at least three biologically independent experiments each performed in triplicate, n = 3. The exact p value are provided in a Source Data file. Source data are provided as a Source Data file.
To delve deeper into the selectivity of ADL5859 for δOR, we examined its top component, the diethyl group. This group interacts with residue W2846.58 and forms a hydrophobic interaction with residue L3007.35 (Fig. 2b). This interaction results in an outward movement of the extracellular region of TM6 and extracellular loop 3 (ECL3) compared to the deltorphin-bound δOR structure (Supplementary Fig. 4d). Further sequence alignment of the opioid receptor family suggests that these distinct extracellular conformations of δOR may underpin the preferential affinity of ADL5859 for δOR, as opposed to μOR and κOR (Fig. 2c). We subsequently performed site-directed mutagenesis, and the results of cellular functional assays indicated that alanine mutagenesis or allelic substitution at these residues substantially reduces the activation potency of δOR in response to ADL5859 (Fig. 2d). In contrast, the K3056.58W mutation in μOR enhanced the potency approximately tenfold, and the replacement of Y3127.35 with leucine in κOR promoted activation (Fig. 2d). Our findings indicate that a sub-pocket, formed by residues W2846.58 and L3007.35, is integral to ligand specificity for δOR. This highlights the pivotal role of certain residues in δOR’s recognition of diverse agonists, including both peptides and small-molecule ligands, which are highly conserved within the opioid receptor family (Supplementary Fig. 4e). As illustrated in Fig. 2c and Supplementary Fig. 4e, these conserved residues are crucial for receptor activation. This is further supported by the marked decrease in δOR activity following alanine substitution of these key residues, which diminishes the receptor’s response to ADL5859 (Fig. 2e, Supplementary Fig. 6 and Supplementary Table 3). Additionally, the expression levels of the mutant used in our functional assays were comparable to those of the wild type (Supplementary Fig. 6b).
The pharmacological property of δOR
In the functional assays, ADL5859 demonstrated its role as a Gi protein-biased agonist, using both Leu-enkephalin and deltorphin as reference points (Fig. 1b, Supplementary Figs. 1 and 8a). When compared with the structure of the deltorphin-bound δOR complex, noticeable displacements were observed in the side chains of the residues M1323.36 and W2746.48 in the ADL5859-bound δOR structure. Specifically, the side chain of W2746.48 underwent a counterclock rotation of approximately 90°, while the side chain of M1323.36 moved away from residue W2746.48 (Fig. 3a). These observations suggest that the interaction between these two residues may play a pivotal role in the preference of ADL5859 on the Gi protein.
a The conformation changes of residues M1323.36 and W2746.48 between deltorphin and ADL5859-bound δOR structures. b–c Dose-dependence curves for δOR wild type (WT) and mutations (M1323.36 and N1313.35) induced by ADL5859. The Gi protein signal pathway was measured by using BRET based Gαi1-Gγ9 dissociation assay and β-arrestin pathway was detected by using NanoBiT based β-arrestin2 recruitment assay. Data are presented as the mean ± SEM from at least three biologically independent experiments each performed in triplicate, n = 3. d Statistical table for the bias factor of δOR mutations. e The conformation arrangement of the residues in sodium pocket between deltorphin and ADL5859-bound δOR. Source data are provided as a Source Data file.
To assess the role of the residue pair M1323.36-W2746.48 in Gi protein-biased signaling, we constructed serials of M1323.36 mutants with varying side chain sizes. We then analyzed their activation efficacy on both Gi protein signaling and β-arrestin pathways. All mutants exhibited reduced efficacy in both pathways (Fig. 3b and Supplementary Table 4). Interestingly, the bias factor correlated with the size of the side chain of the M1323.36 mutants (Fig. 3b, d and Supplementary Table 4). Mutants with larger side chain displacements of M1323.36 (M1323.36W/F) displayed a β-arrestin biased signaling property, while those with smaller side chains of M1323.36 mutants (M1323.36L/V/A) showed a Gi protein-biased signaling property. The larger side chain displacements of M1323.36 may strengthen the interaction with W2746.48, while the smaller side chains may weaken it. This is consistent with the W2746.48F mutant (Supplementary Fig. 8b). These findings suggest that the conformation of M1323.36 and W2746.48 is a critical determinant in regulating the signaling preference for δOR.
Furthermore, we employed MD simulations to explore the dynamic conformational changes of the transmembrane (TM) helices in the δOR receptor and focused on the differences between biased and unbiased activation. Our analysis revealed that the angular changes of the TM7 helical axes and its positional relationships with TM3 are closely associated with ligand bias (Supplementary Fig. 7a–b). These dynamic structural features are consistent with those reported in previous studies on μOR22. In the ADL5859-bound wild-type δOR and its mutants, we consistently observed that the M1323.36 residue, located on TM3, correlates with the conformational profiles of TM7 and TM3. These findings confirm that the preferential δOR signaling induced by agonist ligands is intricately associated with the interactions among TM helices, particularly between TM3 and TM7 (Supplementary Fig. 7c).
In the ADL5859-bound δOR complex, the rotation of W2746.48 triggers an arrangement of the residues N3107.45 and N3147.49 within the sodium binding pocket (Fig. 3e). Compared to the deltorphin-bound δOR complex, residue N3147.49 shifts to the center of the 7TM bundle and forms a hydrogen bond with D952.50, mirroring the naltrindole-bound δOR structure14 (Fig. 3e and Supplementary Fig. 8c). Additionally, we observed a density map suggesting the presence of sodium ions or water molecules in the ADL5859-bound δOR complex (Supplementary Fig. 8c). We further explored the role of the residues in the sodium pocket on Gi protein-biased signaling and found that residue N1313.35 plays a significant role in ADL5859’s Gi protein-biased signaling (Fig. 3c–d and Supplementary Table 4). Substituting residue N1313.35 with alanine and valine also enhances the activity of δOR on the β-arrestin pathway, aligning with previous research14,16 (Supplementary Fig. 8d). Our findings underscore the crucial role of the sodium binding pocket in modulating the signaling preference at δOR.
Structure-based design of ADL5859 derivatives with improved Gi protein bias
Drawing on insights from mutagenesis studies and MD simulations, we hypothesized that strategic substitution at the protonable N atom to orient the sodium binding pocket could be a viable strategy to modulate the bias property of the compound. To test this, we designed and synthesized sixteen compounds with different N substituents (ADL01-ADL016, Fig. 4a and Supplementary Fig. 9). Functional assays revealed that compounds with heterocyclic (ADL01-ADL02) or aromatic ring (ADL03-ADL04) substituents exhibited negligible β-arrestin recruitment, even at concentrations as high as 100 μM (Fig. 4b, Supplementary Fig. 10 and Supplementary Table 5). However, while their efficacy remained consistent, there was a significant decrease in the potency of Gi protein activation, as indicated by ΔpEC50 values (Fig. 4b, Supplementary Fig. 10 and Supplementary Table 5). This suggests potential steric interference with the receptor.
a Chemical structures of ADL5859 derivatives (ADL01-ADL16). The chemical groups of R1 are shown in in dashed boxes. b Efficacy and potency heat map for ADL5859 derivatives using BRET based Gαi1-Gγ9 dissociation assay and NanoBiT based β-arrestin2 recruitment assay. Raw curves and values for all compounds are presented in Supplementary Fig. 10 and Supplementary Table 5. Data are presented as mean ± SEM from at least three biologically independent experiments each performed in triplicate, n = 3. c Schematic diagram of bias factor of ADL5859 derivatives compared with ADL5859 at δOR. ns, no significant difference. *p < 0.05,**p < 0.01, ***p < 0.001 (one-way analysis of variance [ANOVA] followed by the Dunnett’s test). Data are presented as mean ± SEM from at least three biologically independent experiments, each performed in triplicate, n = 3. The exact p value are provided in a Source Data file. d Comparison of activating effect of ADL5859 and ADL06 on δOR in Gi protein signal pathway using BRET based Gαi1-Gγ9 dissociation assay and β-arrestin pathway using NanoBiT based β-arrestin2 recruitment assay. Data are presented as the mean ± SEM from at least three biologically independent experiments, each performed in triplicate, n = 3. e Change of BRET ratio induced by different dose of DOR agonists in Gi1 response or β-arrestin recuitment in cerebellum granule neurons (CGNs). Data are normalized to maximum of Leu-enkephalin response and mean ± SEM from at least three biologically independent experiments each performed in triplicate. For Gi1 response, Leu-enkephalin, SNC80, ADL06, ADL5859, n = 3; For β-arrestin recuitment, Leu-enkephalin, n = 5; SNC80, n = 5; ADL06, n = 3; ADL5859, n = 4. Source data are provided as a Source Data file.
To counteract this steric effect, we introduced smaller alkyl substituents in subsequent compounds. Functional tests revealed that these modifications (ADL05-ADL06) retained the potency and efficacy for Gi protein activation, although the activation potency was reduced by approximately 15–20 times compared to ADL5859. However, the capacity to recruit β-arrestin significantly diminished as the alkyl chain lengthened (ADL06 versus ADL05, Fig. 4b–c, Supplementary Fig. 10 and Supplementary Table 5). Building on these observations, we further increased the length of the alkyl substituent, and added groups capable of serving as hydrogen bond acceptors (donors) to enhance receptor interactions. The selected groups included some amino and guanidino groups, which have been proven effective in developing Gi protein-biased μOR agonists23 (ADL07-ADL14), and other similar moieties (ADL15-ADL16). Most of these compounds exhibited a favorable Gi protein bias property similar to ADL06 (Fig. 4b, Supplementary Fig. 10 and Supplementary Table 5). However, their potency and efficacy of Gi protein activation did not surpass that of ADL06 (Fig. 4b, Supplementary Fig. 10 and Supplementary Table 5). Molecular docking results showed that the alkyl side chain of the ADL06 parent core in the δOR-ADL06 complex extended into the sodium pocket (Supplementary Fig. 11). Collectively, our results validate the strategy of probing the sodium binding pocket to achieve signaling selectivity in δOR. Among the synthesized compounds, ADL06 stands out as the optimal Gi protein-biased agonist for δOR. It not only maintains the ideal agonism property of the Gi protein, which is necessary for pain management, but also reduces β-arrestin recruitment in terms of potency and efficacy (Fig. 4d). Furthermore, we assessed the signaling preference of ADL06 in cerebellar granule neurons (CGNs) and the detailed procedure referred to the methods. The results implied that ADL06 is a Gi protein-biased agonist for δOR when Leu-enkephalin, SNC80 and ADL5859 serve as references (Fig. 4e, Supplementary Fig. 13 and Supplementary Table 6).
As indicated by the cell-based functional assay (Supplementary Fig. 12 and Supplementary Table 7), ADL06 exhibited almost no activation ability for μOR and κOR. This maintains an ideal δOR subtype selectivity, similar to ADL5859. We further evaluated the δOR selectivity of ADL06 by testing its agonistic activities on other GPCRs that could also mediate the analgesic effects and side effects. These included α1A24, α2A25, CB126, CB227, 5-HT1A28, 5-HT1B29, 5-HT2B30, 5-HT2C31, 5-HT432, and 5-HT733. At a screening concentration of 10 μM, ADL5859 and ADL06 were inactive across this panel, with activation percentages of all less than 10% (Supplementary Fig. 14). This reaffirms the high δOR selectivity of ADL06.
In vivo pharmacology studies of ADL06
We conducted a comprehensive in vivo evaluation of compound ADL06, using SNC80 and ADL5859 as the reference compounds. The primary goal of these in vivo experiments was to determine whether an improved Gi signaling bias of δOR could mitigate side effects during pain treatment. SNC80, being among the well-characterized δOR agonists, is the primary choice for studying the δOR-related side effects. Therefore, we determined the test doses based on SNC80. We found that 30 mg/kg is a common and appropriate dose of SNC80 for studying its various side effects, such as convulsion34 and GI motility inhibition35. To ensure accurate comparison, we chose this dose for studying other ADL compounds as well, but we converted the unit (for SNC80: 30 mg/kg = 0.06 mmol/kg). This was necessary because using 30 mg/kg uniformly was not strictly applicable due to the varying molecular weights of the compounds (for ADL5859: 0.06 mmol/kg = 26 mg/kg, and for ADL06: 0.06 mmol/kg = 29 mg/kg).
First, we employed hindpaw complete Freund’s adjuvant (CFA)-injection induced inflammatory pain models and chronic constriction injury (CCI)-induced neuropathic pain models to assess the analgesic effects of ADL06. This methodology was chosen based on the well-documented efficacy of δOR agonism in managing chronic pain36. As depicted in Fig. 5, ADL06 significantly elevated the hindpaw mechanical withdrawal threshold of the hindpaw in CFA models (Fig. 5a) and CCI models (Fig. 5b). Moreover, ADL06 effectively alleviated visceral pain triggered by intraperitoneal injection of 1% glacial acetic acid (Fig. 5c), demonstrating an analgesic effect on par with SNC80 and ADL5859. Morphine, while exhibiting similar analgesic effects as selective δOR agonists, required a much lower dose (10 mg/kg, 0.027 mmol/kg), suggesting a more potent effect of μOR in pain modulation than δOR37. However, the opioid-related side effects of morphine were also more severe (Fig. 5d). In all three pain models, mice were pretreated with naltrindole, a highly selective δOR antagonist. The results showed that the analgesic effects of ADL06 were almost completely blocked in all assays (Fig. 5a–c), providing direct evidence for the specificity of ADL06 for δOR.
a The analgesic effect of morphine (s.c. 0.027 mmol/kg, 10 mg/kg, n = 6 mice), SNC80 (i.p., 0.06 mmol/kg, 30 mg/kg, n = 6 mice), ADL5859 (i.p., 0.06 mmol/kg, 26 mg/kg, n = 6 mice), and ADL06 (i.p., 0.06 mmol/kg, 29 mg/kg, n = 6 mice) in CFA models. b The analgesic effect of morphine (s.c. 0.027 mmol/kg, 10 mg/kg, n = 6 mice), SNC80 (i.p., 0.06 mmol/kg, 30 mg/kg, n = 6 mice), ADL5859 (i.p., 0.06 mmol/kg, 26 mg/kg, n = 6 mice), and ADL06 (i.p., 0.06 mmol/kg, 29 mg/kg, n = 6 mice) in CCI induced neuropathic pain models. c The analgesic effect of morphine (s.c. 0.027 mmol/kg, 10 mg/kg, n = 6 mice), SNC80 (i.p., 0.06 mmol/kg, 30 mg/kg, n = 6 mice), ADL5859 (i.p., 0.06 mmol/kg, 26 mg/kg, n = 6 mice), and ADL06 (i.p., 0.06 mmol/kg, 29 mg/kg, n = 6 mice) in 1% acetic acid-induced visceral pain models. d The influence of morphine, SNC80, ADL5859, and ADL06 on the GI function under analgesic dose reflected by GI transit% measurements, along with the mechanism exploration utilizing the selective δOR antagonist naltrindole (s.c., 10 mg/kg) and the selective μOR antagonist CTAP (s.c., 1 mg/kg) (n = 6 mice per group). e The latency to the convulsion of mice after injecting tested compound under analgecic dose (n = 6 mice per drug). f The maximum seizure class of mice after injecting tested compound under analgesic dose (n = 6 mice per drug). g The representative electroencephalography of mouse in each drug. h The investigation of analgesic tolerance of SNC80, ADL5859, and ADL06 utilizing CFA models (i.p., 0.06 mmol/kg for all compounds, n = 6 mice per drug). a–c *** p < 0.001 versus the vehicle group, ** p < 0.01 versus the vehicle group, * p < 0.05 versus the vehicle group, ### p < 0.001 versus the group pretreated by naltrindole, d *** p < 0.001 versus the vehicle group, ** p < 0.01 versus the vehicle group, ### p < 0.001 versus the group pretreated by naltrindole, ## p < 0.01 versus the group pretreated by naltrindole, f *** p < 0.001 versus the SNC80 group, ** p < 0.01 versus the SNC80 group, * p < 0.05 versus the SNC80 group, h ** p < 0.01 versus day 1, * p < 0.05 versus day 1, according to one-way (c, d) or two-way (a, b, f) ANOVA followed by Bonferroni’s post hoc test, or two-tailed paired t test (h). Each value is represented as the mean ± SEM. The exact p value are provided in a Source Data file. Source data are provided as a Source Data file.
We observed that despite the EC50 values of ADL5859 and ADL06 differing by approximately a factor of 15 in the Gi protein pathway, their analgesic effects were comparable (also in a dose-dependent manner, Supplementary Fig. 15). To gain insight into these results, we investigated their pharmacokinetics (PK) properties, respectively. According to the metabolism behaviors (Supplementary Fig. 16), ADL06 displayed better PK properties than ADL5859 with a faster absorption, a more systemic exposure, and a longer half-life (Supplementary Table 8), which may account for its maintained analgesic effects.
Given that adverse effects associated with opioids, such as constipation and tolerance, are primarily mediated by μOR38, we investigated whether δOR also contributes to these classical side effects. Furthermore, the tendency of δOR agonism to induce convulsions poses a significant challenge in the development of δOR analgesics39. Although this effect has been shown to be ligand-specific, δOR agonists biased toward Gi protein signaling have demonstrated potential for reduced convulsion behaviors11, mirroring the diminished adverse effects observed with μOR biased ligands. Using ADL06 as a representative compound, we conducted preliminary studies to investigate these crucial issues. Interestingly, while less severe than the μOR agonist (morphine), SNC80 and ADL5859 induced mild GI inhibition in mice (GI transit%: 33.9% ± 3.0% and 43.7% ± 2.8%, respectively). This effect was mitigated by the δOR-selective antagonist naltrindole (GI transit%: 78.4% ± 5.9% and 64.8% ± 4.1%, respectively), but not by the μOR-selective antagonist CTAP (GI transit%: 40.0% ± 3.9% and 30.7% ± 3.1%, respectively). On the contrary, ADL06, which is biased toward Gi protein signaling, did not induce significant GI inhibition (GI transit%: 66.1% ± 2.2%) (Fig. 5d). In convulsion studies, the reference compounds PTZ (latency: 76.2 ± 8.8 s, mean maximum seizure class: 4.7 ± 0.2) and SNC80 (latency: 156.2 ± 32.7 s, mean maximum seizure class: 3.0 ± 0.4) rapidly induced noticeable convulsion behaviors in mice. However, neither ADL5859 nor ADL06 induced any convulsion-like behaviors during a 30 min observation period post-treatment (Fig. 5e–f). To more accurately assess seizure severity, we also recorded the electroencephalography (EEG) of mice post-administration. Consistent with the observed phenotypes, there were significant EEG changes (paroxysmal bursts or inter-ictal spikes) in PTZ- and SNC80-treated mice, while the EEG of ADL5958- and ADL06-treated mice remained normal (Fig. 5g). These results suggest that ADL06 has lower convulsion activity during analgesia. During repeated treatment, ADL5859 and ADL06 did not exhibit analgesic tolerance, unlike the reduced efficacy observed with SNC80 (Fig. 5h). Despite the PK (Supplementary Fig. 16 and Supplementary Table 8) and off-target studies (Supplementary Fig. 14), we acknowledge that there may still be potential factors contributing to variations in their side effects, such as differences in receptor residence time. However, the current results suggest a potential relationship between the β-arrestin pathway and the side effects of δOR but needs further verification by more functional molecules in the future studies.
Discussion
Research into opioid receptors lies at the heart of analgesic drug discovery. Despite the prevalent use of drugs that target the μOR, their adverse side effects, such as dependency and respiratory depression, are the primary contributors to the opioid crisis. Therefore, a comprehensive study of the pharmacological properties of opioid receptors is not just a medical necessity, but also an urgent public health challenge. This urgency propels the innovation of more effective small-molecule drugs2,23,40,41. Simultaneously, the δOR has attracted significant research interest in research due to its unique role in modulating pain, emotions, and cognition. These capabilities suggest that δOR agonists could provide a novel analgesic strategy, potentially avoiding the adverse effects associated with traditional μOR agonists. However, the absence of detailed information on the structure of δOR-Gi signaling complexes with small molecules, particularly biased ligands, has hindered drug discovery and development targeting δOR.
In this context, our study offers pioneering insights, unveiling the unique interactions between biased small molecules and δOR. This mechanism diverges from the previously understood peptide-mediated δOR interactions. Leveraging the cryo-EM structure of ADL5859 bound to δOR, we characterized the pharmacological profile of δOR and developed a series of active compounds, including ADL06, a biased agonist. By using ADL06 as a chemical probe, we were able to elucidate the intrinsic relationships between the activation of different pathways by δOR and their biological functions. This compound demonstrated its efficacy in alleviating pain in vivo while minimizing side effects, such as reduced convulsion, tolerance, and GI inhibition. Significantly, our research not only deepens our understanding of GPCR pharmacological properties but also lays a scientific foundation for the development of new therapeutic strategies.
While we believe our study constitutes a significant advancement in δOR research, we recognize certain limitations. Specifically, our structural analysis was limited to a single small-molecule agonist, ADL5859, and its interactions with the δOR-Gi complex. Future studies are needed to investigate the interactions of δOR with a wider array of ligands, encompassing those with varying biases and pharmacological profiles. This broadening of scope will be crucial to fully harnessing the therapeutic potential of δOR modulation. Moreover, applying our findings to a more diverse range of physiological and pathological contexts will deepen our understanding of δOR’s role in pain management and potentially extend its applicability to other therapeutic areas.
Methods
Ethical statement
For the primary culture of cerebellar granule neurons (CGNs), all experiments were specifically designed to minimize the number of animals used and were approved by the Animal Experimentation Ethics Committee of the College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China. For all in vivo experiments, the handling, care, and treatment procedures of the mice were performed in compliance with the Agreement of the Experimental Animal Ethics Committee, West China Hospital, Sichuan University (20220706003).
GloSenor cAMP assay
To examine the intracellular cAMP level of cells overexpressing various constructs of δOR, the GloSensor cAMP assay (Promega) was performed42. Briefly, pcDNA3.1-HA-Flag-δOR or mutants were co-transfected with a cAMP biosensor plasmid GloSensor in HEK293 cells prepared in 6-well plates using Polyethylenimine Linear (PEI) MW4000 (YEASEN, 40816ES02). After 24 h, the cells were harvested and suspended in assay buffer (Hank’s Balanced Salt Solution (HBSS) containing 10 mM HEPES, pH 7.4), with additional 3% v/v dilution of the D-Luciferin-Potassium Salt (YEASEN, 40902ES01). 1 h later, corresponding agonists diluted in assay buffer containing 5 μM foskolin were added to the cells and incubated for 30 min at room temperature. The cAMP signals were read on Synergy H1 microplate reader (BioTek). To calculate the values of Emax and EC50, Data were processed using the nonlinear regression (curve fit) dose-response function in GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Each experiment was repeated at least 3 times.
BRET based Gα-Gγ dissociation assay
To determine δOR-mediated Gi protein activation, Gα-Gγ dissociation assay based on BRET2 was performed. In short, HEK293 cells were transiently transfected using a 1:1:1:1 DNA ratio of δOR: Gαi1-Rluc8: Gβ: Gγ9-GFP2 in 6-well plates. After 24 h transfection, cells were seeded onto 96-well white plates and cultured at 37 °C with 5% CO2. One day later, growth medium was replaced with assay buffer (25 mM HEPES, 1 mM CaCl2, 140 mM NaCl, 2.7 mM KCl, 0.9 mM MgCl2, 0.37 mM NaH2PO4, 5.5 mM d-glucose, 12 mM NaHCO3, pH 7.0) containing final concentration of 5 μM fluorescent substrate coelenterazine 400a (YEASEN, 40905ES). Pre-diluted agonists were then added and the BRET signals were read on Synergy H1 microplate reader (BioTek). The BRET signal was calculated by dividing the emission of the fluorescent acceptor at 515 nm by the emission of the fluorescent donor at 410 nm and agonist-induced change in BRET ratio was expressed as percentage of the basal signal. Data were analyzed using a simulation dose-response in GraphPad Prism 9.
NanoBiT β-arrestin2 recruitment assay
To measure δOR-mediated β-arrestin2 recruitment induced by different ligands, NanoBiT β-arrestin2 recruitment assay was performed. Firstly, the large (LgBiT) and small (SmBiT) fragment of the NanoBiT system was cloned at N-terminal of full-length of β-arrestin2 and C-terminal of full-length δOR vector respectively. The δOR-C-SmBiT and N-LgBiT-β-arrestin2 plasmids were co-transfected into HEK-293 cells prepared seeded onto 6-well plates. One day later, transfected cells were detached and re-seeded onto 96-well white plates and cultured in 37 °C, 5% CO2 incubator overnight. The next day, cells in each well which were washed once using Phosphate-Buffered Saline (PBS) in advance were incubated in assay buffer (25 mM HEPES, 1 mM CaCl2, 140 mM NaCl, 2.7 mM KCl, 0.9 mM MgCl2, 0.37 mM NaH2PO4, 5.5 mM d-glucose, 12 mM NaHCO3, pH 7.0) containing final concentration of 5 μM coelenterazine h (YEASEN, 40906ES) at room temperature for 30 min. Ligands diluted in the assay buffer were then added to the cells. The baseline and ligand-induced luminescence was read on Synergy H1 microplate reader (BioTek). Data were obtained from three replicates and analyzed using a simulation dose-response in GraphPad Prism 9.
NanoBiT GRK2 recruitment assay
To quantify ligand-dependent GRK recruitment for δOR, NanoBiT GRK2 recruitment assay was performed. In conclusion, the δOR-C-LgBiT and GRK2-C-SmBiT were co-transfected into HEK-293 cells prepared seeded onto 6-well plates. At 24 h post-transfection, the medium was aspirated and cells were re-seeded onto 96-well white plates and cultured in 37 °C overnight. The following day, cells were washed once using PBS and then incubated in assay buffer (25 mM HEPES, 1 mM CaCl2, 140 mM NaCl, 2.7 mM KCl, 0.9 mM MgCl2, 0.37 mM NaH2PO4, 5.5 mM d-glucose, 12 mM NaHCO3, pH 7.0) containing 5 μM coelenterazine h (YEASEN, 40906ES) at room temperature for 30 min. Ligands were then added to the cells and luminescence signals were read on Synergy H1 microplate reader (BioTek). Data were obtained from three replicates and analyzed using a simulation dose-response in GraphPad Prism 9.
NanoBiT based Internalization assay
To quantify β-arrestin-dependent internalization of δOR, NanoBiT Internalization assay was performed. The LgBiT and SmBiT of the NanoBiT system was respectively cloned at N-terminal of FYVE zinc finger domain and N-terminal of full-length β-arrestin2. HEK-293 cells prepared seeded onto 6-well plates were transfected with δOR-WT, N-LgBiT-FYVE and N-SmBiT-β-arrestin2 at a ratio of 1:1:1. The following day, transfected cells were re-seeded onto 96-well white plate and cultured in 37 °C overnight. The next day, cells were washed once using PBS ahead of time and incubated in assay buffer (25 mM HEPES, 1 mM CaCl2, 140 mM NaCl, 2.7 mM KCl, 0.9 mM MgCl2, 0.37 mM NaH2PO4, 5.5 mM d-glucose, 12 mM NaHCO3, pH 7.0) containing 5 μM coelenterazine h (YEASEN, 40906ES) for 30 min. Ligands were added to the cells and luminescence was read on Synergy H1 microplate reader (BioTek). Data were obtained from three replicates and analyzed using a simulation dose-response in GraphPad Prism 9.
Bias calculation
The bias factor (β value) was calculated to score signaling bias (Eq. (1)).
where P1 is δOR-Gi protein signaling data and P2 is β-arrestin signaling data; β value > 0 indicates Gi signaling biased while β value < 0 implies β-arrestin signaling biased.
Statistical analysis was performed using a two-sided, one-way ANOVA with Dunnett’s multiple comparison test to make pairwise comparisons between two pathways activated by a given ligand, where P < 0.05 was considered to be statistically significant.
Constructs and expression
To achieve stable expression of the δOR-G protein complex in sf9 cells, the truncated δOR (36–352) was cloned into a pFastBac1 vector containing a haemagglutinin (HA), Flag tag, and BRIL at the N-terminus. The dominant-negative Gαi2 (S47N, G204A, E246A and A327S) and Gβ1, Gγ2 were inserted into pFastBac1 and pFastBac dual vectors, respectively. Antibody scFv16, which follows a GP67 signal peptide at the N-terminus and 10×His tag at the C-terminus, was cloned into pFastBac1 vector. The Bac-to-Bac Baculovirus Expression System (Invitrogen) was employed to generate high-titer recombinant baculovirus, facilitating the co-expression of the δOR-G protein complex and scFv16.
Purification of scFv16
With the GP67 signal peptide, scFv16 is secreted outside the cell. Thus, after 60 hours of sf9 cells being transfected with viral infiltration, we harvested the supernatant by centrifugation at 4000 × g for 30 min. The filtered supernatant was incubated with HisPur Ni-NTA Resin (Thermo Fisher Scientific) for more than 2 hours and then passing through the empty column to gather the resin. The resin was washed with the buffer containing 50 mM HEPES pH 7.5, 300 mM NaCl and 50 mM imidazole and the protein was eluted by increasing the concentration of imidazole to 300 mM. The final protein was concentrated to 10 mg/mL, and changed to a solution buffer containing 20 mM HEPES pH 7.5, and 100 mM NaCl, flash-frozen in liquid nitrogen, and stored at −80 °C.
Formation of δOR-Gi heterotrimer
Sf9 cells were infected at a density of around 3.0 × 106 cells/mL with the mixed high-titer recombinant baculovirus of δOR, Gi1 and Gβ1γ2 at the ratio of 1:1:1, and then cultured for 48 h at 27 °C with shaking at 110 rpm. The cells were harvested by centrifugation when the vitality reached approximately 85%. For purification of δOR-Gi complex and scFv16, the cell pellet was lysed in a lysis buffer containing 20 mM HEPES pH 7.5, 50 mM NaCl, 5 mM CaCl2, 5 mM MgCl2, 20 μM ADL5859 (TargetMol, USA, T6175), 25 mU/mL Apyrase (Sigma-Aldrich, A6535) and Protease Inhibitor Cocktail (MCE, HY-K0010). After 2 h of stirring and 10 min of centrifugal precipitation at 100,000 × g, the cell membrane precipitate was homogenized and incubated for 2 h at 4 °C with solubilization buffer. This buffer is based on the lysis buffer but with the addition of 50 mM NaCl, 0.5% (w/v) lauryl maltose neopentyl glycol (LMNG, Anatrace; NG310), 0.1% (w/v) cholesteryl hemisuccinate (CHS), 10% (v/v) glycerol, and 4 mg scFv16. The insoluble part was eliminated by ultracentrifugation at 100,000 × g for 30 minutes. The supernatant was loaded on a M1 anti-Flag antibody-coupled Sepharose Resin, pre-equilibrated with wash buffer (20 mM HEPES pH 7.5, 100 mM NaCl, 0.1% (w/v) LMNG, 0.02% (w/v) CHS, 5% (w/v) glycerol). The incubation was rotated for 2 h to allow the complex to bind to the resin (Millipore, A4596). The resin was collected into an empty column and washed with 30 column volumes of wash buffer freshly added 5 mM CaCl2 and 1 μM ADL5859, as well as Protease Inhibitor Cocktail. The protein was eluted using 5 column volumes of buffer, including 20 mM HEPES pH 7.5, 100 mM NaCl, 0.1% (w/v) LMNG, 0.02% (w/v) CHS, 5% (w/v) glycerol, 10 mM EDTA, 0.2 mg/mL Flag peptide, 10 μM ADL5859 and Protease Inhibitor Cocktail. The complex sample was then concentrated to 0.5 mL using an AMICON ULTRA-15 Centrifugal Filter (Millipore, UFC910024) and loaded into the Superose6 increase 10/300 size exclusion column (GE Healthcare, 29091596). The column was pre-equilibrated with SEC buffer (20 mM HEPES pH 7.5, 100 mM NaCl 0.00075% (w/v) LMNG, 0.00025% digtonin (Biosynth, D-3203), 0.0002% (w/v) CHS and 10 µM ADL5859). The purified complex was collected and concentrated to 10 mg/mL for electron microscopy experiments.
Cryo-EM data collection and 3D reconstruction
In the Vitrobot Mark IV (Thermo Fisher), 3 μL of ADL5859-bound δOR-G protein complex, at a concentration of approximately 10 mg/mL, was loaded onto the glow-discharged (60 s at 10 mA) 300-mesh Au holey carbon grids (R1.2/1.3, Quantifoil) at 4 °C and under 100% humidity. After a 2.5 s blotting step, the grids were immediately plunge-frozen in liquid ethane and then stored in liquid nitrogen until data collection. Cryo-EM imaging was performed on a Titan Krios microscope (Thermo Fisher) with a K2 direct electron detector (Gatan) operating at 300 kV accelerating voltage and a magnification of 165,000, which corresponds to a pixel size of 0.85 Å.
A total of 4466 movies were imported into RELION 3.043 and subjected to motion correction using MotionCor244, followed by contrast transfer function (CTF) estimation with Gctf45. Subsequently, 4,765,652 particles were automatically picked and extracted from the micrographs. Following two rounds of 2D classification, classes exhibiting clear transmembrane helix features, totaling 1,368,151 particles, were selected. These selected particles underwent further classification into four classes during 3D classification, identifying three high-quality classes containing 676,428 particles for 3D auto refinement. Then, the refined particles were iteratively sieved by cryoSeive software46, and particles reported with the best resolution from the 5th iteration were selected and imported into cryoSPARC 4.4.047. Following a round of non-uniform refinement and local refinement, the final sharpened map was generated with a global resolution of 3.11 Å, as indicated by a Fourier shell correlation of 0.143. The local resolution was estimated using cryoSPARC 4.4.0.
Model building and structure refinement
The active-state structure of Deltorphin-bound δOR-Gi complex (PDB: 8F7S) was used as an initial ADL5859-bound δOR-Gi complex model. In Chimera, the model was docking into the cryo-EM map, and then in Coot, ADL5859 was matched into the map, initiating manual refinement. After several times of manual refinement, the model was loaded into Phenix for real-space refinement. The final model statistics were validated using MolProbity, and comprehensive refining statistics were provided in Supplementary Table 2. UCSF ChimeraX 1.3, UCSF Chimera 1.17.1 and PyMOL 2.5.2 (https://pymol.org/2/) software were used to prepare the structural figures.
Enzyme-linked immunosorbent assay
To estimate cell surface expression of wild-type δOR and its mutants, Enzyme-linked immunosorbent assay was performed. HEK-293 cells were transiently transfected the plasmid of δOR wild type or mutants using PEI according to the manufacturer’s instructions in 6-well plates. 24 h later, transfected cells were re-seeded onto poly-d-lysine-coated 96-well plates and cultured at 37 °C in 5% CO2 for another 24 h. The cells were washed using PBS following fixed by 4% (w/v) paraformaldehyde. Then, after being washed with PBS, the cells were blocked with 5% (w/v) BSA at room temperature for 1 h. Afterwards, the cells were incubated with anti-Flag HRP conjugated monoclonal antibody (1:2,000, Sigma-Aldrich) overnight at 4 °C. After washing, HRP substrate 3,30,5,50-tetramethylbenzidine (TMB) was added for color reaction, which was 15 min later stopped by addition of same volume of 0.5 M HCl. The absorbance at 450 nm was read on the Synergy H1 microplate reader (BioTek).
Chemical general methods
All chemicals and solvents were commercially available and used directly without further purification. The reactions were monitored by the thin-layer chromatography (TLC) and visualized under the ultraviolet light at 254 nm. Column chromatography for separation and purification was carried out on silica gel (200 − 300 mesh) eluting with dichloromethane (DCM) and MeOH. The 1H and 13C NMR spectra of compounds were collected at 400 MHz and 101 MHz on a Bruker Avance spectrometer (Bruker Company, Germany), respectively. Chemical shifts (δ values) and coupling constants (J values) were given in ppm and hertz with tetramethylsilane as the internal standard. For all final products, their purities were determined to be all over 95% by reversed-phase high-performance liquid chromatography (HPLC) analysis (SHIMADZU) and high-resolution mass spectrometry (HRMS) were recorded on a high-resolution quadrupole-orbitrap tandem mass spectrometer (Q-Exactive; Thermo Fisher Scientific, Waltham, Massachusetts, USA) with an electrospray ionization (ESI) probe operated in the positive-ion mode. The detailed chemical syntheses and compound validation are available in the Supplementry Methods.
Molecular docking and molecular dynamics simulation
The structures obtained by cryo-electron microscopy were used as templates to study the molecular dynamics of several protein-ligand complexes to explore the mechanism of δOR dynamic conformational changes. By docking the agonist ligands SNC-80, TAN67, and KNT127 into the binding pocket of the δOR-ADL5859 complex using the CDOCKER docking program in Discovery Studio 2019. CHARMM-GUI48 was used to assemble the above complex systems, wild-type and mutant systems of the δOR-ADL5859 complex, the models of the δOR and the ligands were constructed and optimized using the CHARMM3649 and CGenFF50 force fields, respectively. These constructed complex systems were embedded in a 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) bilayer. The system was solvated using the TIP3P water model and neutralized with potassium and chloride ions. Prior to the production simulation, a minimization step was conducted after system preparation to eliminate unnatural collisions, utilizing the steepest descent algorithm. Subsequently, the system underwent equilibration using the six-step process provided by CHARMM-GUI. Each atom in the system was randomly assigned initial velocities, and the temperature was gradually increased from 0 K to 303.15 K over 2500 ps during the first two steps of the NVT ensemble simulation, with a time step of 1 fs. Following the NVT ensemble, the next four equilibration steps were conducted at 303.15 K and 1 bar pressure, involving a 10,000 ps NPT ensemble simulation with a time step of 2 fs. The particle mesh ewald (PME) method was used for calculating electrostatic interactions and constraining bonds involving hydrogen atoms with the LINCS algorithm. A 500 ns molecular dynamics simulation was then performed on all system based on parallel simulations. All simulations were executed using the Gromacs software package, version 2018.851. UCSF ChimeraX 1.3 software was used to prepare the figures. Simulation data related to this study have been uploaded to GPCRmd52 (https://www.gpcrmd.org) for peer analysis and use.
Primary cerebellar granule neuron culture and transfection
Kunming mice were obtained from the Center for Disease Control and Prevention of Hubei Province. The mice were raised in a specific pathogen free (SPF) environment with an ambient temperature of 18–22 °C, a humidity of 50%–60%, and a 12 h light-dark cycle.
Primary cerebellar granule neuronal cultures were prepared from one-week-old newborn Kunming mice. The dissected tissue was gently triturated after Versene (Gibco) treatment for 5 min at 37 °C, and the homogenate was centrifuged at 170 g for 5 min. In the meantime, mixtures of the DNA and Lipofectamine 2000 (Thermo Fisher Scientific) in a 1:3 ratio in an Opti-minimal essential medium (Opti-MEM) (Thermo Fisher Scientific) were prepared following the manufacturer’s protocols. The pellet was re-suspended in culture medium DMEM-F12 (Gibco) supplemented with 2 mM glutamine, 30 mM KCl, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS) and seeded into 96-well plates (100 μL/well) previously coated with poly-L-ornithine (Sigma-Aldrich). Then, the mixtures of DNA and Lipofectamine 2000 (50 μL/well) were added to the wells. BRET experiments were performed three or four days after transfection. BRET experiments were performed at DIV3. The cDNA amounts used per well in 96-well plate were as following: for Gi1 response: δOR (80 ng), Gαi1Nluc (50 ng) and VenusGγ2 (50 ng); for β-arrestin1 recruitment: δOR (80 ng), Nlucβ-arrestin1 (30 ng) and VenusCAAX (50 ng).
BRET measurement in CGNs
CGNs were starved in HEPES-buffered saline (HBS) containing 10 mM HEPES pH 7.4, 140 mM NaCl, 4 mM KCl, 2 mM MgSO4, and 1 mM KH2PO4. BRET measurements were performed using PHERAstar FS (BMG Labtech) with the program PHERAstar control (Version 4.00 R4)53. The signals emitted by the donor (460–500 nm band-pass filter, Em 480 nm) and the acceptor (510–550 nm band-pass filter, Em 530 nm) were recorded after the addition of 10 μM furimazine. All measurements were performed at 37 °C. The BRET signal was determined by calculating the ratio between the emission of acceptor and donor (Em 530 nm / Em 480 nm). The basal BRET ratio (BRETbasal) of cells was recorded before the stimulation with drugs or buffer. The change in BRET ratio was obtained by subtracting the BRET ratio after agonist treatment from the basal BRET (BRETbasal-BRETagonist). The net BRET was further calculated by subtracting the change in BRET ratio in buffer only group.
Animals and drug administration
All in vivo experiments in this study were carried out on mice provided by Chengdu Dossy Experimental Animals Co., Ltd. The mice had free access to sterile food and water and were fed under a light-dark cycle of 12 h (light off at 7:00 p.m.), temperature at 25 ± 1 °C, and humidity of 40%–70%. All behavior-related experiments were performed in a soundproof room. All ADL compounds and SNC80 were dissolved in a vehicle composed of DMSO and dH2O (V/V = 1/9), and other compounds were dissolved in saline. All compounds were administrated intraperitoneally (i.p.) or subcutaneously (s.c.).
CFA induced chronic pain models
One day before the experiment, the basal hindpaw mechanical withdrawal threshold (MWT) of each mouse was measured by the von-Frey filament, and then 20 μL CFA was given via intraplantar injection into the left hindpaw to induce pain. On the day of the experiment, the basal hindpaw MWT of each mouse was tested again, and a significant decrease in MWT indicated the successful modeling. Then, the mice were grouped randomly and received an i.p. injection of the vehicle or the tested compound, after which the hindpaw MWT was measured at 20, 40, 60, 90, 120, 180 min after treatment. Each MWT was the average of the three repeated tests at a 20 s interval. A significant recovery of the MWT compared with the vehicle group indicated the analgesic effects of the tested compound. The analgesic effect was expressed as MPE% calculated by the formula: MPE% = test MWT (g) − model MWT (g) / basal MWT (g) − model MWT (g) × 100%. The analgesic ED50 were fitted by GraphPad Prism 9.
During the experiments for mechanism verification, the selective δOR antagonist naltrindole (10 mg/kg) was s.c. injected 10 min before tested compound (the same below).
CCI induced neuropathic pain models
Briefly, the mice were narcotized by isoflurane via inhalation. The left hind leg was shaved with an electric shaver, and the surface of the skin was disinfected with iodophor. The sciatic nerve was isolated through the glass dissecting tool after blunt separation of the skin and muscle. Two ligations were made on the nerve with chrome catgut. The tightness was judged by the observation of the slight twitch of the left hind limb during ligation. One week after the surgery, the similar MWT tests were conducted as illustrated above.
1% acetic acid induced visceral pain models
The mice were grouped randomly and received an i.p. injection of the vehicle or the tested compound. After 15 min, 1% acetic acid was i.p. injected from another side to each mouse to cause visceral pain, which was characterized and quantified by the writhing. The writhing number of each mouse was recorded 5–20 min after 1% acetic acid injection. A significant decrease of the writhing number compared with the vehicle group indicated the analgesic effects of the tested compound.
Assessment of the GI transit rate
The mice were fasted (free access to water) for 16 h before the experiment. Then, the mice were grouped randomly and received an i.p. injection of the vehicle or the tested compound. After 30 min, 300 μL of the GI marker (10% charcoal suspension in 5% aqueous gum arabic) was given through oral to each mouse and the mice were sacrificed after another 30 min. The abdomen was opened and the intestine was separated from the pylorus to the ileocecal junction. The distance from the pylorus to the most forward of the marker (D1) and the total length of the intestine (D2) were measured by a ruler. The GI transit rate was obtained by the equation: GI transit% = D1/D2 × 100%. A GI transit% of less than 55% indicated the occurrence of constipation54.
For the mechanism exploration, CTAP (1 mg/kg) or naltrindole (10 mg/kg) was s.c. injected 10 min before the tested drug.
Evaluation of δOR related convulsions
The mice were grouped randomly and received an i.p. injection of tested compound. According to the well-established characterization methods for convulsion, the behaviors of mice were observed for 30 min with the latency to the first convulsion being recorded. The convulsion severity was also determined according to the Racine scale55,56,57 (1: immobility followed by facial clonus; 2: masticatory movements and head nodding; 3: continuous body tremor; 4: unilateral or bilateral forelimb clonus; 5: rearing and falling, and death caused by tonic-clonic seizure).
Mice were implanted with extradural electroencephalogram electrodes for electroencephalogram recordings. Briefly, under anesthesia using 1.5% isoflurane, two stainless steel screws were placed in the primary motor cortex (AP: + 1.0 mm, ML: ± 1.5 mm, and DV: – 1.5 mm) and cerebellum. The mice were allowed to recover for at least 5 days before experiments. For recording, the baseline was recorded for 1 min. Then, the tested compound was i.p. injected to mice, and the electrophysiological signals were recorded by a Pinnacle EEG recording system (Part #8200-SL; Pinnacle Technology, United States) for 10 min. A preamplifier unit was rigidly attached to the miniature plug. The sampling frequency of the signals was set to 400 Hz.
Investigation of analgesic tolerance
On day 1, the compounds were tested for their analgesic activities on CFA models. On next two days, all mice were i.p. injected the same compound twice daily. On day 4, the analgesic activities of the tested compounds on CFA models were evaluated again. A decreased analgesic performance indicates the analgesic tolerance.
Pharmacokinetic study
The tested compound was i.p. injected to male ICR mice (n = 3). After injection, blood samples of all mice were collected from the cheek at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 h, which were standing for 15 min on ice and then centrifuged at 3500 × g for 3 min to obtain plasma. The samples were analyzed by using LC-MS/MS (Triple Quad 6500 + ) to establish the metabolism behavior. The pharmacokinetic parameters were calculated by the WinNolin software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data produced or analyzed in our study is included in the main text or the supplementary materials. The cryo-EM density map and atomic coordinate of ADL5859-δOR-Gi complex data in this study have been deposited in the Electron Microscopy Data Bank (EMDB) and Protein Data Bank (PDB) under accession numbers EMD-38909 and 8Y45, respectively. Molecular dynamics simulations are deposited in the GPCRmd repository (https://www.gpcrmd.org/). Source data are provided in this paper.
References
Snyder, S. H. & Pasternak, G. W. Historical review: opioid receptors. Trends Pharm. Sci. 24, 198–205 (2003).
Zhuang, Y. et al. Molecular recognition of morphine and fentanyl by the human mu-opioid receptor. Cell 185, 4361–4375 e4319 (2022).
Valentino, R. J. & Volkow, N. D. Untangling the complexity of opioid receptor function. Neuropsychopharmacology 43, 2514–2520 (2018).
Darcq, E. & Kieffer, B. L. Opioid receptors: drivers to addiction? Nat. Rev. Neurosci. 19, 499–514 (2018).
Blaine, A. T. & van Rijn, R. M. Receptor expression and signaling properties in the brain, and structural ligand motifs that contribute to delta opioid receptor agonist-induced seizures. Neuropharmacology 232, 109526 (2023).
Horiuchi, T. et al. The effects of the delta-opioid agonist SNC80 on hind-limb motor function and neuronal injury after spinal cord ischemia in rats. Anesth. Analg. 99, 235–240 (2004).
Eisenstein, T. K. The role of opioid receptors in immune system function. Front Immunol. 10, 2904 (2019).
Longoni, R., Cadoni, C., Mulas, A., Di Chiara, G. & Spina, L. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 2. Place-preference and brain microdialysis studies in rats. Behav. Pharm. 9, 9–14 (1998).
Saitoh, A. & Nagase, H. Delta opioid receptor (DOR) ligands and pharmacology: development of indolo- and quinolinomorphinan derivatives based on the message-address concept. Handb. Exp. Pharm. 247, 3–19 (2018).
Stevenson, G. W., Folk, J. E., Rice, K. C. & Negus, S. S. Interactions between delta and mu opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: studies with SNC80 [(+)−4-[(alphaR)-alpha-((2S,5R)−4-allyl-2,5-dimethyl-1-piperazinyl)−3-methoxybenzyl]-N,N-diethylbenzamide] and heroin. J. Pharmacol. Exp. Ther. 314, 221–231 (2005).
Conibear, A. E. et al. A novel G protein-biased agonist at the delta opioid receptor with analgesic efficacy in models of chronic pain. J. Pharm. Exp. Ther. 372, 224–236 (2020).
Spahn, V. & Stein, C. Targeting delta opioid receptors for pain treatment: drugs in phase I and II clinical development. Expert Opin. Investig. Drugs 26, 155–160 (2017).
Granier, S. et al. Structure of the delta-opioid receptor bound to naltrindole. Nature 485, 400–404 (2012).
Fenalti, G. et al. Molecular control of delta-opioid receptor signalling. Nature 506, 191–196 (2014).
Fenalti, G. et al. Structural basis for bifunctional peptide recognition at human delta-opioid receptor. Nat. Struct. Mol. Biol. 22, 265–268 (2015).
Claff, T. et al. Elucidating the active delta-opioid receptor crystal structure with peptide and small-molecule agonists. Sci. Adv. 5, eaax9115 (2019).
Keov, P., Sexton, P. M. & Christopoulos, A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60, 24–35 (2011).
Slosky, L. M., Caron, M. G. & Barak, L. S. Biased allosteric modulators: new frontiers in GPCR drug discovery. Trends Pharm. Sci. 42, 283–299 (2021).
Slosky, L. M. et al. beta-arrestin-biased allosteric modulator of NTSR1 selectively attenuates addictive behaviors. Cell 181, 1364–1379 e1314 (2020).
Wang, Y. et al. Structures of the entire human opioid receptor family. Cell 186, 413–427 e417 (2023).
Dripps, I. J. & Jutkiewicz, E. M. Delta opioid receptors and modulation of mood and emotion. Handb. Exp. Pharm. 247, 179–197 (2018).
Cong, X. et al. Molecular insights into the biased signaling mechanism of the mu-opioid receptor. Mol. Cell 81, 4165–4175 e4166 (2021).
Faouzi, A. et al. Structure-based design of bitopic ligands for the micro-opioid receptor. Nature 613, 767–774 (2023).
Huang, Y., Chen, H., Chen, S. R. & Pan, H. L. Duloxetine and amitriptyline reduce neuropathic pain by inhibiting primary sensory input to spinal dorsal horn neurons via alpha1- and alpha2-adrenergic receptors. ACS Chem. Neurosci. 14, 1261–1277 (2023).
Fink, E. A. et al. Structure-based discovery of nonopioid analgesics acting through the alpha(2A)-adrenergic receptor. Science 377, eabn7065 (2022).
Landi, M. et al. Modulation of gastric emptying and gastrointestinal transit in rats through intestinal cannabinoid CB(1) receptors. Eur. J. Pharm. 450, 77–83 (2002).
Guindon, J. & Hohmann, A. G. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharm. 153, 319–334, (2008).
Linciano, P. et al. Identification of a potent and selective 5-HT(1A) receptor agonist with in vitro and in vivo antinociceptive activity. ACS Chem. Neurosci. 11, 4111–4127 (2020).
Kayser, V., Aubel, B., Hamon, M. & Bourgoin, S. The antimigraine 5-HT 1B/1D receptor agonists, sumatriptan, zolmitriptan and dihydroergotamine, attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. Br. J. Pharm. 137, 1287–1297, (2002).
Urtikova, N. et al. Antinociceptive effect of peripheral serotonin 5-HT2B receptor activation on neuropathic pain. Pain 153, 1320–1331 (2012).
Silenieks, L. B. et al. Evaluation of selective 5-HT(2C) agonists in acute seizure models. ACS Chem. Neurosci. 10, 3284–3295 (2019).
Shin, A. et al. Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharm. Ther. 39, 239–253 (2014).
Santello, M. et al. The brain-penetrant 5-HT(7) receptor agonist LP-211 reduces the sensory and affective components of neuropathic pain. Neurobiol. Dis. 106, 214–221 (2017).
Saitoh, A. et al. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav. Brain Res 223, 271–279 (2011).
Broccardo, M., Improta, G. & Tabacco, A. Central effect of SNC 80, a selective and systemically active delta-opioid receptor agonist, on gastrointestinal propulsion in the mouse. Eur. J. Pharm. 342, 247–251 (1998).
Gendron, L., Cahill, C. M., von Zastrow, M., Schiller, P. W. & Pineyro, G. Molecular Pharmacology of delta-Opioid Receptors. Pharm. Rev. 68, 631–700 (2016).
Manglik, A. et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190 (2016).
Chan, H. C. S., McCarthy, D., Li, J., Palczewski, K. & Yuan, S. Designing Safer Analgesics via mu-Opioid Receptor Pathways. Trends Pharm. Sci. 38, 1016–1037 (2017).
Comer, S. D. et al. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J. Pharm. Exp. Ther. 267, 888–895 (1993).
Wang, H. et al. Structure-based evolution of G protein-biased mu-opioid receptor agonists. Angew. Chem. Int Ed. Engl. 61, e202200269 (2022).
Qu, Q. et al. Insights into distinct signaling profiles of the microOR activated by diverse agonists. Nat. Chem. Biol. 19, 423–430 (2023).
Xu, Z. et al. Ligand recognition and G-protein coupling of trace amine receptor TAAR1. Nature 624, 672–681 (2023).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zhu, J. et al. A minority of final stacks yields superior amplitude in single-particle cryo-EM. Nat. Commun. 14, 7822 (2023).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput Chem. 29, 1859–1865 (2008).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J. Chem. Theory Comput 8, 3257–3273 (2012).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput Chem. 26, 1701–1718 (2005).
Rodriguez-Espigares, I. et al. GPCRmd uncovers the dynamics of the 3D-GPCRome. Nat. Methods 17, 777–787 (2020).
Xu, C. et al. Specific pharmacological and G(i/o) protein responses of some native GPCRs in neurons. Nat. Commun. 15, 1990 (2024).
Miao, Z. et al. A novel bifunctional muOR agonist and sigma(1)R antagonist with potent analgesic responses and reduced adverse effects. J. Med Chem. 66, 16257–16275 (2023).
Racine, R. J. Modification of seizure activity by electrical stimulation. II. motor seizure. Electroencephalogr. Clin. Neurophysiol. 32, 281–294 (1972).
Raedt, R. et al. Seizures in the intrahippocampal kainic acid epilepsy model: characterization using long-term video-EEG monitoring in the rat. Acta Neurol. Scand. 119, 293–303 (2009).
Levesque, M. & Avoli, M. The kainic acid model of temporal lobe epilepsy. Neurosci. Biobehav Rev. 37, 2887–2899 (2013).
Acknowledgements
The cryo-EM data was collected at Cryo-EM Center of Sichuan University West China Cryo-EM Center, and processed at Duyu High Performance Computing Center of Sichuan University. This work was supported by the Ministry of Science and Technology of the People’s Republic of China (STI2030-Major Projects2021ZD0201900 to X.T.), National Natural Science Foundation of China (T2221004 and 31972916 to Z.S., 82425054 and 82273784 to B.K., 32371288 and 32100988 to W.Y., 82271190 32100965 to L.C., 32330049 and 82320108021 to J.L., 323B2038 to C.Z.), Ministry of Technology department of China grant (2019YFA0508800 to Z.S.), Sichuan Science and Technology Program (2023ZYD0168 to B.K., 2024NSFJQ0052 to Z.S.), Frontiers Medical Center, Tianfu Jincheng Laboratory Foundation (TFJC2023010010 to Z.S.), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYYC21002 and ZYGD23025 to B.K., ZYYC20023 to Z.S.).
Author information
Authors and Affiliations
Contributions
L.C. and H.F. designed the expression constructs, purified the ADL5859-bound δOR-Gi complex and prepared the final sample for data collection toward the structure; S.L. designed and performed signaling assays with the assistance of H.F. and H.W.; C.X. performed the signaling assays in CGNs with assistance of Y. L.; C.Z. prepared the cryo-EM grids, collected cryo-EM images and performed map calculations; L.C. and C.Z. built and refined the structure model under the supervision of Z.S. and W.Y.; Z.M. designed compounds; S.H. prepared compounds synthesis with assistance of T.Z. and Z.M.; Z.L. performed the MD simulations with assistance of W.L. and R.L.; Z.M. prepared the pharmacology studies in vivo under the supervision of B.K.; L.C., H.F, S.L., Z.M., Z.L., X.T. helped with manuscript editing; J. L., Z.S. and B.K. supervised the overall project, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Communications thanks Hideaki Fujii and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, L., Miao, Z., Liu, S. et al. Cryo-EM structure of small-molecule agonist bound delta opioid receptor-Gi complex enables discovery of biased compound. Nat Commun 15, 8284 (2024). https://doi.org/10.1038/s41467-024-52601-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52601-1
This article is cited by
-
Structure-guided design of partial agonists at an opioid receptor
Nature Communications (2025)