Abstract
The molecular basis of increased hemoglobin in Andean Aymara highlanders is unknown. We conducted an integrative analysis of whole-genome-sequencing and granulocytes transcriptomics from Aymara and Europeans in Bolivia to explore genetic basis of the Aymara high hemoglobin. Differentially expressed and spliced genes in Aymaras were associated with inflammatory and hypoxia-related pathways. We identified transcripts with 4th or 5th exon skipping of NFKB1 (AS-NFKB1), key part of NF-kB complex, and their splicing quantitative trait loci; these were increased in Aymaras. AS-NFKB1 transcripts correlated with both transcripts and protein levels of inflammatory and HIF-regulated genes, including hemoglobin. While overexpression of the AS-NFKB1 variant led to increased expression of inflammatory and HIF-targeted genes; under inflammatory stress, NF-kB protein translocation to the nucleus was attenuated, resulting in reduced expression of these genes. Our study reveals AS-NFKB1 splicing events correlating with increased hemoglobin in Aymara and their possible protective mechanisms against excessive inflammation.
Similar content being viewed by others
Introduction
There are three widely studied populations living at high altitudes: Tibetans, Ethiopians, and Andeans. The molecular mechanisms related to oxygen sensing are the foundation of evolutionary adaptations in these populations to the hypoxic high-altitude environment1. Regulation of erythropoiesis plays significant roles in adaptations for oxygen-sensing and tissue oxygen delivery2. High hemoglobin (Hb) levels in a hypoxic environment offer the potential benefit of increased oxygen retention in the human body. However, a potentially harmful effect of increased blood viscosity from high hematocrit (Hct) also exists and may contribute to chronic mountain sickness3.
It was assumed that some high-altitude populations, such as Tibetans and Ethiopians, have undergone natural selection that blunted the normal physiological response maintaining of elevated Hb levels at high altitude1. In Tibetans, two genetic variants in the hypoxia sensing pathway, regulated by hypoxia-inducible factors (HIFs), that regulate erythropoiesis, have undergone positive selection to prevent high Hb and Hct. These are gain-of-function variants in the principal HIFs negative regulator EGLN1 (encoding prolyl hydroxylase 2; PHD2), and a Denisovan-originated haplotype of the EPAS1 gene4, that encodes the principal regulator of erythropoietin (EPO), HIF-2α. However, as previously reported, Tibetan Sherpas maintain normal Hb concentration at high altitudes, but exhibit increases in both plasma and red cell volumes, i.e., true erythrocytosis that is masked by increased plasma volume. This combination is advantageous for delivering oxygen to tissues due to extended capillary circulation5, while avoiding the potential harmful effects of high Hct and blood viscosity. In contrast to Tibetans and other high-altitude adapted populations, Andeans have distinct adaptations for Hb phenotypes. The Andeans have Hb levels that are not only higher than either Tibetans or Ethiopians1 but even higher than European sojourners to high altitude6. In contrast to Tibetans, Andeans' higher red cell volume is not accompanied by a beneficial expansion of plasma volume, resulting in their elevated Hb levels7. This leads to high blood oxygen saturation6,7. Andeans’ erythroid progenitors were reported to be hypersensitive or even to grow without EPO in vitro, suggesting that they also have some features of primary erythrocytosis8. However, the molecular mechanisms responsible for the high Hb observed in Andeans have remained elusive. We hypothesized that the elucidation of these unique Andean molecular mechanisms would enhance our understanding of human hypoxia adaptations.
The Aymara and Quechuas are two distantly related but distinct and well-studied Andean indigenous highland populations9. We previously reported genomic signatures of natural selection in Aymara10 that differ in some respects from those of Quechuas9. In this study, we focus solely on Aymara high altitude adaptation and their Hb levels.
Erythron production is controlled by HIFs to assure optimal tissue oxygen delivery, with HIF-2 being the principal regulator of EPO transcription1,11. Whole-genome-sequencing (WGS) analysis of high altitude populations including Tibetans12,13,14, Ethiopians1, and Quechuas15 have identified signals of natural selection: strong single nucleotide polymorphisms (SNPs) selection signals in EPAS1 and ELGN1 in Tibetans and SENP1 and ANP32D genes in Quechuas. These genes except ANP23D are all involved in the HIF pathway and are associated with regulation of Hb at high altitudes. However, these genes were not found to be evolutionary selected in Andean Aymara16. Our previous WGS study failed to discover the mechanisms of high Hb levels in Aymara; however, we found strong selection signals of BRINP3, NOS2, and TBX5, which are related to cardiovascular function and development10. While BRINP3 also regulates inflammation by modulating NF-kB activity in humans17, BRINP3 haplotype did not correlate with Aymara high Hb16.
Transcriptomic changes, i.e., alternatively regulated genes: decreased or increased transcripts, or multiple transcript isoforms, may also play a role in response to the hypoxic environment. The CASK gene has differential expression and alternative splicing forms (i.e., exon skipping or inclusion) under hypoxia and was reported to be enriched in hypoxia-regulated angiogenesis18,19,20,21. We hypothesized that the integration of studies of evolutionary selected genetic variants (i.e., SNPs) analysis with concomitant analysis of transcriptional regulation at the splicing level could further expand our understanding of evolutionary adaptation to hypoxia. We applied quantitative trait locus analysis of genes and spliced exons to develop a more precise estimation of these two genetic markers, SNPs, and gene expression in an integrative framework. We postulated this approach might also identify previously undescribed gene expression and splicing variants and provide new insights into the mechanism underlying the distinct Aymaras’ high Hb levels at high altitude.
As transcriptomes are cell lineage- and differentiation-specific, we attempted to isolate pure cell lineages from small amount of available peripheral blood but were only able to obtain high-quality granulocytes’ RNA that we used for whole-transcriptome analysis. Granulocytes release various pro-inflammatory cytokines and chemokines to initiate the inflammatory response; these inflammatory changes may also influence erythropoiesis. We performed an integrative analysis of granulocytes transcriptomes to prioritize candidate genes linked to high Hb in Aymara.
Results
Differentially expressed genes in Aymara
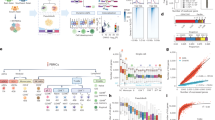
The overall design of the computational analysis in this study is described in Supplementary Fig. S1. We first compared transcript expression levels from the unbiased granulocyte transcriptomes of 10 Aymara and 4 European controls living in La Paz (3650 m) and later confirmed these pilot data in a larger number of subjects (67 samples of Bolivian Aymara and 18 Europeans; see “Materials and Methods”) to identify differentially expressed genes (DEGs). We found 1935 genes upregulated and 666 downregulated in Aymara compared to the European controls (adjusted p value [Adj. p] < 0.05, |fold change| > 1.5; Fig. 1a and Supplementary Data 1). Gene set enrichment analysis identified these dysregulated genes as enriched in hematopoiesis, immune-related, and inflammatory-related Gene Ontology terms along with canonical pathways including T helper (Th17, Th1, and Th2) cell differentiation, T-cell receptor signaling pathway, human T-cell leukemia virus 1 infection, leukocyte activation, interleukin pathways, regulation of cytokine, and NF-κB related signaling pathway (Fig. 1d and Supplementary Data 2).
a Identified differentially expressed genes (DEGs) and their expression quantitative trait loci (eQTLs). b Identified differentially alternatively spliced genes (DSG) and their splicing quantitative trait loci (sQTLs). c Heatmaps of DEG expression (left) and DSG exon usage (precent splicing in; PSI) (right). d Gene Ontology terms and canonical pathways enriched in DEGs (left) and DSGs (right). P values were calculated using a one-sided Fisher’s exact test. e Venn diagram of DEGs and DSGs (top), and of eQTL DEGs and sQTL DSGs (bottom). f RegulomeDB scores of eQTL regions (top) and sQTL regions (bottom).
Differential alternative splicing of genes in Aymara
We further analyzed the transcriptomic data to identify differential alternatively spliced genes (DSGs). This yielded a total of 1922 genes with alternative splicing events, comprising 1099 exon skipping events, 409 intron retention events, 161 alternative 3’ splice sites, 136 alternative 5’ splice sites, and 663 mutually exclusive exons (Fig. 1b, Supplementary Data 3). These alternatively spliced genes were also significantly over-represented in hematopoietic progenitor cell differentiation, HIF-1 signaling, immune-related, and inflammatory-related pathways including CD4 T cell receptor signaling, leukocyte activation, interleukin pathways, regulation of cytokine, TNF receptor, and NF-κB related signaling (Fig. 1d and Supplementary Data 4).
A heatmap with hierarchy clustering showed stratification of the Aymara and European samples based on DEG expression and percent spliced in (PSI) exon usage (Fig. 1c). Only a small portion of DEGs (10.2%) and DSGs (13.8%) overlapped at the gene level (upper Venn diagram in Fig. 1e), while at the pathway analysis multiple immune-related and NF-κB signaling pathways were shared by the two gene sets (Fig. 1d). Thus, although many individual genes may be exclusively regulated via expression or splicing mechanisms, the Aymara’s differentially regulated genes may be possibly involved in those pathways that contribute to Aymara high-altitude adaptation.
Cis-genetic regulators for DEGs and DSGs in Aymara
Since genetic variants (i.e., haplotypes and single nucleotide polymorphisms; SNPs) could act as regulators of DEGs and DSGs, we explored the SNPs associated with each individual DEG and DSG through integrative analysis of paired RNA-seq and WGS data. We considered SNPs within promoter regions as potential expression quantitative trait loci (eQTLs) for DEGs and those within alternatively spliced (AS) exons and their flanking introns as potential splicing quantitative trait loci (sQTLs) for DSGs (see Materials and Methods). Of the set of 2601 DEGs, a total of 160 SNPs were found to act as eQTLs associated with expression of 129 genes (Supplementary Data 5). Of the 1922 DSGs, a total of 858 SNPs were identified as sQTLs associated with altered exon usage of 340 genes (Supplementary Data 6). Only 7 genes were regulated by both eQTLs and sQTLs (bottom Venn diagram in Fig. 1e). Additionally, 57% of eQTL and 34% of sQTLs had a score less than or equal to 4 in RegulomeDB22, which suggests they are in regulatory regions (Fig. 1f). Furthermore, among eQTLs and sQTLs, 37 eQTLs and 309 sQTLs were detected as Aymara adapted SNPs that showed differential allele frequencies compared to the general European populations (see “Methods”) (Supplementary Data 7).
NFKB1 as a candidate hub gene in Aymara adaptation
We constructed a protein-protein interaction (PPI) network using the human phenotype resource in StringDB23 for the 129 DEGs and 340 DSGs, respectively regulated by eQTLs and sQTLs to further gain insight into the molecular signature of these genes. The constructed network suggested NFKB1 to be a hub gene; this gene has established roles in erythrocyte production, granulocyte count, and immune responses (Supplementary Fig. S2).
We detected previously unreported three alternative splicing of NFKB1 (AS-NFKB1) in our RNA-seq analysis skipping either exon 4 (NFKB1-AS1), exon 5 (NFKB1-AS2), or both (NFKB1-AS3) (Supplementary Fig. S3a). Exons 4 and 5 together encode the Rel homology domain (RHD), DNA binding domain found in Pfam database24, and therefore, all three exon skipping events may lead to the loss-of-function NFKB1 RHD domain. In addition, NFKB1-AS1 and NFKB1-AS3 encode peptides out-of-frame NFKB1 mRNAs that would be expected to generate truncated, likely nonfunctional NF-κB proteins (Supplementary Fig. S3a). These are predicted to be destroyed by the nonsense-mediated decay (NMD) mechanism since they all have premature termination codons located more than 55 nucleotides upstream of the last exon of NFKB125. As shown in Supplementary Fig. S3b, both (i.e., NFKB1-AS1 and NFKB1-AS3) exon skipping events were more frequently observed in the Aymara population compared to Europeans: although NFKB1-AS2 was detected in RNA-seq data, it was not significantly differentiated in Aymara since there were only a small number of the junction reads (data not shown). The integrative analysis of these Aymara transcriptomes and WGS data identified 11 SNPs that serve as sQTLs associated with skipping of exon 4 or 5 of NFKB1 and have alternative allele frequencies higher in Aymara samples compared to European samples (Supplementary Data 7). NF-kB signaling plays an important role in erythropoiesis, inflammation, and the HIF-1 pathway. NFKB1 is a transcription factor that contributes to NF-kB level and activity. We, therefore, hypothesized that these sQTLs might be important for Aymara high-altitude adaptations (i.e., Hb level and inflammation) due to their affecting exon 4 and 5 splicing patterns in NFKB1. To explore this hypothesis, we further conducted a comprehensive functional analysis of NFKB1 exon splicing (more frequent with the sQTLs in Aymara), linking it to inflammation, Hb levels, and HIF-1 and HIF-2 pathways.
The detected sQTLs could be categorized into three groups: those with consistent genotypes among the ten Aymara samples (Supplementary Fig. S3c): (1) rs230525, rs230527, rs230517, and rs230516; (2) rs230519; and (3) rs230509, rs230511, rs230504, rs230491, rs230495, and rs230493. We selected representative SNPs for each group: two in group 1 (rs230525 and rs230527), one in group 2 (rs230519), and two in group 3 (rs230511 and rs230504) to further experimentally validate our NFKB1 results. Associations of these five SNPs with AS-NFKB1 and RNA-seq and WGS data are depicted in Supplementary Fig. S3d. For example, two SNPs in high linkage disequilibrium (LD) (Supplementary Fig. S3e) were in the intron between exon 4 and 5; an additional three SNPs were located between exon 5 and 6 (Supplementary Fig. S3a).
Validation of NFKB1 exon skipping events in an extended sample
To validate the exon skipping events observed in our initial RNA-seq analysis, we analyzed larger number of Aymaras (n = 55), and Europeans (n = 18) living in La Paz (3650 m) and measured and compared expression levels of NFKB1-AS1, NFKB1-AS2, and NFKB1-AS3 by qRT-PCR. Canonical NFKB1 transcript expression was not significantly different, while transcript levels of NFKB1-AS1, NFKB1-AS2, and NFKB1-AS3 were significantly higher in Aymara than in Europeans (Fig. 2a). In addition, we genotyped the five sQTLs in these extended samples and found a significant association with these AS-NFKB1, but not with canonical NFKB1 transcript levels (Fig. 2b–e).
a Canonical NFKB1, NFKB1-AS1 (Exon 4 skipped AS-NFKB1), NFKB1-AS2 (Exon 5 skipped AS-NFKB1), NFKB1-AS3 (Exon 4 and 5 skipped AS-NFKB1) transcript levels in granulocytes of Aymara (n = 55) and European (n = 18) were measured and expressed as fold changes. b Canonical NFKB1, c NFKB1-AS1, d NFKB1-AS2, e NFKB1-AS3 transcript levels of genotypes of rs230527, rs230525, 230519, rs230511, rs230504 were measured. P values were calculated by two-tailed Mann–Whitney test. All the data shown here are mean ± standard deviation (SD).
Genetic variants of sQTLs enriched in the Aymara population
Among the five sQTL SNPs, two shared the highest allele frequencies (AFs): rs230511-T and rs230504-T, with AF = 0.878 (Fig. 3a, b). These two SNPs belong to an LD block26, and we selected rs230511 for further analysis.
a Allele frequencies in the Aymara population. b Genotype frequencies in the Aymara population. c Allele frequencies of rs230511 in Aymara, Peruvians from Lima, Peru (PEL), Tibetans, Han Chinese in Beijing, China (CHB), Europeans (EUR), and Yoruba in Ibadan, Nigeria (YRI). d Geography of rs230511 alleles provided from the Geography of Genetic Variants Browser28. e Selection signals of Aymara enriched NFKB1 SNPs, calculated from F12 values that were identified as Fst between Aymara and lowland Native American ancestry component and PBSn1 calculated by normalizing the PBS statistic for 10 Aymara-enriched SNPs10. f Allele frequency of rs230511 in Aymara living at three different altitudes (n = 43 from Santa Cruz at 400 m, n = 133 from La Paz at 3650 m, and n = 12 from Chorolque at 5000 m). g Allele frequencies of rs230511 in Aymara (n = 12) and Quechuas (n = 49) living at 5000 m. P values in f and g were calculated using the two-tailed Chi-squared (χ²) test.
The allele rs230511-T was observed to be enriched in Aymara compared to the 1000 genome populations27 (Fig. 3c and geographic distribution visualized28 in Fig. 3d). The T allele was also more common in Aymara (AF = 0.878) than Tibetans (n = 72), another population living at high altitude; the frequency in Tibetans (AF = 0.364) was similar to that in Europeans (AF = 0.334, Fig. 3c).
We next collected and genotyped 188 samples of Bolivian Aymara living at different altitudes (n = 43 from Santa Cruz at 400 m, n = 133 from La Paz at 3650 m, and n = 12 from Chorolque at 5000 m), and found that the T allele frequency increased with altitude (Fig. 3f). We also observed that the T allele was more common in Aymara (n = 12) from Chorolque than in Quechuas (n = 49) at the same location (p = 0.00427, Fig. 3g); Quechuas had higher allele frequencies than Europeans, which is compatible with their distant common ancestry with Aymara. In our previous WGS analysis of Andeans, we showed that windows in the NFKB1 region were highly differentiated relative to lowland Native South Americans consistent with positive selection10 with a peak PBSn1 value of 0.3107 ranked in the top 0.1 percentile genome-wide, which suggests the broader genomic region including rs230511 is significantly differentiated in Aymara from Native South American lowlanders (Fig. 3e). Our previous dataset included allele frequencies for three of the five sQTL intronic SNPs, with rs230511 being the most differentiated SNP in this region (Fig. 3e), suggesting that this SNP may be the target of selection. Therefore, we observed rs230511-T to be enriched in the Aymaras compared to general populations, Tibetans, and a different population living in the same region and used it for further analyses.
Increased inflammatory protein and transcript levels correlate with alternative splicing of NFKB1
To evaluate the hypothesis that AS-NFKB1 variants may affect the inflammatory response, we next measured canonical transcript levels of the inflammatory genes IL6 and IFNG (i.e., two target genes of NF-kB) in granulocytes, along with their plasma protein levels (i.e., IL6 and IFN-γ). Both transcripts showed significant association with the rs230511 T allele (i.e., the Aymara enriched allele) (Fig. 4a, d), and their plasma protein levels were also significantly associated in the same direction (Fig. 4c, f). The transcript levels of these target genes were positively correlated with AS-NFKB1 transcript levels, but weakly and inversely correlated with canonical NFKB1 transcript levels, though the correlation was not statistically significant (Fig. 4b, e).
a IL6 transcript levels in granulocytes and b their correlation with canonical NFKB1, NFKB1-AS1 (Exon 4 skipped AS-NFKB1), NFKB1-AS2 (Exon 5 skipped AS-NFKB1), and NFKB1-AS3 (Exon 4 and 5 skipped AS-NFKB1). c Plasma IL6 protein levels according to rs230511 genotype. d IFNG transcript levels in granulocytes and (e) their correlation with canonical NFKB1, NFKB1-AS1, NFKB1-AS2, and NFKB1-AS3. f Plasma IFN-γ protein levels according to rs230511 genotype. C/C (n = 13), C/T (n = 19), T/T (n = 35), All data are expressed as mean ± standard deviation (SD). P values were calculated by two-tailed Mann–Whitney test. Spearman’s r and p values of correlation analyses were calculated by GraphPad Prism 10. The p value was determined using a two-tailed test, and the confidence interval was set at 95%.
We then searched for correlations between dysregulated expression of inflammatory genes and AS-NFKB1 transcripts. Among the 60 inflammatory genes upregulated in Aymara, 33, 58, and 59 genes were positively correlated with NFKB1-AS1, NFKB1-AS2, and NFKB1-AS3, respectively. Ten genes of these 60 genes were inversely correlated with canonical NFKB1, but positively correlated with NFKB1-AS2 and NFKB1-AS3. Among 16 genes downregulated in Aymara, only 1 and 2 genes were inversely correlated with NFKB1-AS2 and NFKB1-AS3, respectively. Ten genes of these 16 genes were positively correlated with canonical NFKB1 transcript levels. Among these downregulated genes, only two genes (CORO1A and PYCARD) were inversely correlated with NFKB1-AS3 but positively correlated with canonical NFKB1. Only 16 genes among the 76 dysregulated inflammatory genes were correlated in opposite directions by NFKB1-AS2, NFKB1-AS3, and canonical NFKB1 (Supplementary Data 8).
Adaptive targets of NF-kB-regulated genes
We explored the possibility that AS-NFKB1 may directly modulate the transcript levels of genes regulated by NF-kB. Ingenuity Pathway Analysis (IPA) analysis indicated activation of the NF-kB complex as an upstream regulator (Supplementary Fig. S4), suggesting that NF-kB target genes are likely to be upregulated in Aymara. Using RNA-seq data, we examined the expression of NF-kB target genes in Aymara granulocytes. Among 60 dysregulated NF-kB target genes, transcripts of 51 genes were upregulated and those of 9 genes were downregulated (Adj. p < 0.05 and Log2 fold change >0.5).
Among the 51 upregulated genes in Aymara, 38 had expression levels that positively correlated with NFKB1-AS1. NFKB1-AS2 and NFKB1-AS3 were positively correlated with 53 and 52 genes, respectively. Among these 51 genes, 6 genes (CCR7, MYC, CDC25B, CD40LG, IFNG, CLU) were inversely correlated with canonical NFKB1.
Among the 9 downregulated genes in Aymara, none of these genes were correlated with AS-NFKB1 transcripts but P2RY2, FCGRT, CXCL6 were positively correlated with canonical (Supplementary Data 9).
Adaptive changes of HIF target genes
Since NF-kB also regulates HIFs29,30, our transcriptome analysis also predicted that NF-kB increases HIF-1α expression (Supplementary Fig. S4), and HIF-1 and HIF-2 signaling pathways were upregulated in Aymara (Supplementary Fig. S5), we next analyzed changes in HIF-regulated gene expression using whole transcriptome data. Among 46 dysregulated genes, 41 were upregulated and 5 downregulated in Aymara compared to Europeans (Adj. p < 0.05 and Log2 fold change >0.5). Out of the 41 upregulated genes, 21, 40, and 40 were positively correlated with NFKB1-AS1, NFKB1-AS2, and NFKB1-AS3, respectively, while 7 genes were inversely correlated with canonical NFKB1. Among 4 downregulated genes in Aymara, CORO1A, and NCOA4 were inversely correlated with NFKB1-AS2 and NFKB1-AS3 but positively correlated with canonical NFKB1. BID showed a positive correlation with canonical NFKB1 but no correlation with AS-NFKB1 transcripts (Supplementary Data 10).
Our analysis identified 14 genes (CD44, IFNG, TNFAIP3, F3, SNAI1, CCL5, SLC7A5, TNF, MYC, AHR, CDKN1A, SMAD7, BACH2, and BIRC2) regulated by both HIFs and NF-kB (Adj. p < 0.05 and Log2 fold change >0.5) and all genes’ transcripts except BIRC2 were upregulated in Aymara. These genes were positively correlated with NFKB1-AS2 and NFKB-AS3. Except IFNG and CCL5, other genes displayed positive correlations with NFKB1-AS1. IFNG and MYC were negatively correlated with canonical NFKB1 (Supplementary Data 11).
We further confirmed the changed expression of CDKN1A, TNF, BIRC2 (three genes regulated by both HIF and NF-kB), BCL2 (NF-kB target gene), EDN1, PTGS2, SOD2 (HIF target gene) transcripts by qRT-PCR (Supplementary Fig. S6).
Correlation of Aymara-enriched NFKB1 SNPs with alternative spliced NFKB1 transcripts and high Hb
We then evaluated the presence of correlations between Aymara-enriched NFKB1 SNPs and Hb levels. Individuals with C/T and T/T genotypes at rs230511 had significantly higher Hb levels relative to C/C controls (Fig. 5a) and canonical NFKB1 transcript levels inversely correlated with high Hb. Hb levels correlated positively with NFKB1-AS2 and NFKB1-AS3: NFKB1-AS3 was observed to have the strongest association with Hct (r = 0.4125, p = 0.0291); (Fig. 5d). Among the other four SNPs less enriched in Aymara, only those heterozygous at any of three SNPs (rs230527, rs230525, and rs230519) had high correlation with high Hb compared to controls (data not shown).
a Hemoglobin (Hb) (n = 8 for C/C, n = 28 for C/T, n = 49 for T/T), b white blood cell count (n = 9 for C/C, n = 18 for C/T, n = 39 for T/T), and c platelet count (n = 9 for C/C, n = 19 for C/T, n = 37 for T/T) according to rs230511 genotype. Correlation of canonical NFKB1, NFKB1-AS1 (Exon 4 skipped AS-NFKB1), NFKB1-AS2 (Exon 5 skipped AS-NFKB1), and NFKB1-AS3 (Exon 4 and 5 skipped AS-NFKB1) with d Hb, e white blood cell count, and f platelet count. All data are expressed as mean ± standard deviation (SD). P value was calculated by two-tailed unpaired t-test. Spearman’s r-values of correlation analyses were calculated by GraphPad Prism 10. The p-value of correlation analyses was determined using a two-tailed test, and the confidence interval was set at 95%.
Aymara-enriched NFKB1 SNPs and alternatively spliced NFKB1 transcripts correlate with white blood cell and platelet counts
Individuals homozygous for the Aymara enriched allele (T/T) at rs230511 had higher white blood cell (WBC) and platelet count compared to those with C/T genotype (Fig. 5b, c). Furthermore, these cell counts positively correlated with all AS-NFKB1 transcript levels: NFKB1-AS2 was observed to have the strongest association (Fig. 5e, f). However, canonical NFKB1 transcript levels were inversely correlated with platelet counts but not with WBC counts (Fig. 5e, f).
AS-NFKB1 variants cause disrupted protein translation and processing
To functionally characterize AS-NFKB1 variants, we overexpressed N-terminal EGFP-tagged fusion constructs of canonical NFKB1/p105 and p50 proteins and corresponding AS-NFKB1 variants in HEK293 cells. Both variants, where stop codon is generated due to deletion of exon 4 or exon 4 and 5 (AS1 and AS3), fail to translate into proteins, as evidenced by the detection of only N-terminally expressed GFP in western blots (Fig. 6a). For the AS2 variant, we consistently observed reduced expression levels of p105 and p50, along with impaired p50 processing (Fig. 6a, red arrow indicating the expected position of processed NFKB1/p50 protein). Subsequently, we analyzed cytoplasmic and nuclear extracts from HEK293 cells transfected with NFKB1/p105 canonical (WT) and AS2 variants. While canonical endogenously processed NFKB1/p50 was detected in both fractions (Fig. 6b), NFKB1/p50 AS2 protein was only evident in the cytoplasmic lysate after prolonged exposure (Supplementary Fig. S7). Despite these observations, the reporter gene transcription assay revealed that processed NFKB1/p50 AS2 exhibited a comparable inhibitory effect on RelA-dependent promoter activation as the WT, whereas NFKB1/p50 AS1 and AS3 variants showed no impact on promoter activity (Fig. 6c). Overall, AS-NFKB1 transcripts cause complete (AS1 and AS3) and partial (AS2) loss of canonical NFKB1 function.
HEK293 cells were transiently transfected with EGFP-fusion constructs either encoding canonical NFKB1/p105 and p50 forms (WT) or AS-NFKB1 transcripts (AS1, 2, 3). a Whole cell lysates were analyzed by western blotting using antibodies against GFP (left) and NFKB1 (right), β-actin was used as loading control. b Cytoplasmic and nuclear extracts of HEK293 cells transiently transfected with EGFP-fusion constructs of canonical NFKB1/p105 and NFKB1-AS2 (GFP antibody was used and β-actin as loading control). c The inhibitory effect on RelA-dependent promoter activation is not mediated by NFKB1/p50 AS1 and AS3 variants (left), whereas NFKB1/p50 AS2 has comparable inhibitory effect as WT (right). HEK293 were co-transfected with synthetic reporter gene composed of an NF-κB responsive promoter driving luciferase expression. Reporter expression is switched on to maximum by co-expression of RelA (p65, 50 ng). The different concentrations of NFKB1/p50 WT and AS2 were added to inhibit the RelA-mediated reporter activation, maximum concentration (200 ng) was used for NFKB1/p50 AS1 and AS3. We performed at least three independent experiments. Abbreviations: B—background (not transfected HEK293 cells), red arrow indicates expected position of processed NFKB1 protein. All data are expressed as mean ± standard deviation (SD).
AS-NFKB1 overexpression induces NF-kB protein but reduces it under inflammatory stress
We investigated whether AS-NFKB1 overexpression affects NF-κB protein levels in granulocytes and its targeted inflammatory gene expression levels. We overexpressed three AS-NFKB1 isoforms in HL60 cells, a promyelocytic leukemia cell line that serves as a model for granulocytes. Cytoplasmic and nucleus NF-κB protein levels were measured both with and without TNF treatment in conditions of AS-NFKB1 overexpression. Cytoplasmic NF-κB protein levels did not differ significantly in response to AS-NFKB1 overexpression or TNF treatment (Fig. 7a and Supplementary Fig. S8). However, TNF treatment promoted NF-κB translocation into the nucleus. Compared to WT-NFKB1 overexpression, AS3 overexpression increased NF-κB protein levels. However, with TNF treatment, AS3 overexpression led to a reduction in NF-κB protein levels in the nucleus (Fig. 7b and Supplementary Fig. S8). We then measured the expression levels of inflammatory genes regulated by NF-κB. Overexpression of AS3 induced the expression of inflammatory genes such as IL1B, TNF, and CDKN1A (Fig. 7c and Supplementary Fig. S9). HIF-target genes, including SLC2A1, VEGFA, and EPAS1 were also upregulated compared to cells overexpressing WT-NFKB1. However, under conditions of heightened inflammatory stress (induced by TNF treatment), the expression levels of SLC2A1, VEGFA, LDHA, and BCL2 with AS3 overexpression were reduced (Fig. 7d and Supplementary Fig. S9). These results correlated with decreased expression of NF-κB protein levels in the nucleus.
a Cytoplasmic NF-κB and b nuclear NF-κB protein levels were measured in three AS-NFKB1 or canonical NFKB1-overexpressed HL60 with and without TNF treatment. Measured cytoplasmic or nuclear NF-κB protein levels were normalized against GAPDH or Histone H3, respectively. We conducted five independent Western blot experiments under identical experimental conditions. P values were calculated using a two-tailed paired t-test. The expression levels of c Inflammatory (IL1B, CDKN1A, TNF) and d HIF-targeted genes (SLC2A1, VEGFA, LDHA) were measured in the same cells and normalized against RPL13A transcript levels. We conducted four independent overexpression experiments under identical experimental conditions. P values were calculated using a two-tailed paired t-test (for comparisons to NT or between WT and AS) or an unpaired t-test (for comparisons to TNF). Black P value compared to NT; Red P value compared to TNF; All data are expressed as mean ± standard deviation (SD). Abbreviations: NT; no-treatment, WT; canonical NFKB1, AS1; NFKB1-AS1, AS2; NFKB1-AS2, AS3; NFKB1-AS3, TNF; TNF treatment.
NFKB1 SNP correlated with lower inflammatory gene expression in patients with high baseline inflammation
Overexpression of AS-NFKB1 experiment showed decreased or no changes of inflammatory and HIF-targeted gene expression under high inflammatory stress. We tested if inflammatory gene expression levels in the patients with high inflammation correlate with NFKB1 SNP rs230511. We collected granulocytes and platelets of polycythemia vera (PV) and essential thrombocythemia (ET), which are acquired blood diseases with high baseline inflammation compared to healthy controls31. We genotyped 30 PV and 15 ET patients and assessed the expression of inflammatory genes (IL1B, CXCL8, IL6, IL15, and TNF) in granulocytes and platelets. In platelets, individuals with the C/T genotype showed lower transcript levels of these inflammatory genes compared to those with the C/C genotype, while IL1B, CXCL8, and TNF were significantly lower in the T/T genotype. In granulocytes, IL1B and CXCL8 expressions were reduced in the T/T genotype, whereas IL15 and TNF were decreased in the C/T genotype. We then analyzed HIF-target genes (LDHA, SLC2A1, and VEGFA), which were significantly lower in C/T genotypes in both PV and ET granulocytes and platelets (Supplementary Fig. S10).
Discussion
In this study, we conducted integrative analysis of WGS and RNA-seq data measured from granulocytes of Andean Aymara and European samples to explore a molecular signature of Aymara adaptation to high-altitude. Since granulocytes are abundant in whole blood and release pro-inflammatory cytokines, chemokines, and other mediators, they play a key role in initiating and sustaining the inflammatory process, leading to our decision to study these cells. Although high Hb levels can be detrimental, Aymara exhibits a distinct evolutionary adaptation with high Hb, unlike high-altitude Tibetans and Ethiopians, which maintain Hb levels similar to those of lowlanders. Our previous WGS analysis found evidence for natural selection at a number of genomic regions, but the strongest signals did not include genes that would explain high Hb in Aymara as an evolutionary strategy to improve oxygen delivery through induced erythropoiesis10. Therefore, we employed a strategy integrating genetic and transcriptional regulation to investigate the genetic underpinnings of Aymara phenotypes, positing that WGS analysis may not provide a complete picture of the genetic signature of this adaptation. We have also used a higher resolution than has been performed in other integrative studies32,33.
Our results revealed transcriptional perturbations (i.e., DEGs and DSGs) and associated cis-genetic regulators (eQTLs and sQTLs) of immune-, inflammatory-, and hypoxic-related pathways and thereby potentially contribute to distinct erythropoiesis regulation in the Aymara. We discovered AS-NFKB1 transcripts that skip exon 4 and/or exon 5 encoding the NFKB1 DNA-binding domain. NFKB1 was considered a strong candidate gene to explain Aymara adaptation since (1) it is a hub gene for inflammation and hypoxia pathways in our PPI network and (2) the AS-associated SNP sQTL, rs230511-T was enriched in the Aymara population relative to other populations.
NFKB1 is well-documented to play the regulatory role in functional pathways of inflammation, hypoxia, and erythropoiesis. It encodes the protein p105, which is processed into the shorter protein p50. P50 generates heterodimeric NF-kB complexes by interacting with p65 (RELA), c-Rel, or RelB. Inhibitors of NF-kB (IkBs) bind to these heterodimers. In the presence of appropriate stimuli, IkBs are phosphorylated by IkB kinase (IKK) and then degraded by the proteasome. Uninhibited, the NF-kB heterodimer complex is translocated into the nucleus and induces expression of genes encoding proinflammatory cytokines, chemokines, adhesion molecules, and anti-apoptotic factors that are associated with inflammation34,35. NFKB1 functions as a potent inflammation suppressor allowing p50 to form a homodimer that acts as a transcriptional repressor36. Nfkb1 knockout mice exhibit increased inflammation and tissue neutrophil infiltration associated with elevated chemokine and cytokine production37. Non-functional AS-NFKB1 transcripts correlate with elevated levels of inflammatory transcripts and proteins, whereas canonical NFKB1 transcript levels exhibit an inverse relationship with these measurements (Fig. 4b, e), providing further evidence for the inhibitory role of NFKB1 in inflammation, thus its loss-of-function transcripts have opposite function.
Additionally, NF-kB is known as an inducer of HIF1A (encoding HIF-1α) gene transcription; HIFs α subunits determine the levels of HIFs dimers and HIFs transcriptional activity. HIF-1 also regulates NF-kB38. Under hypoxia, HIF-1 induces cell proliferation via activation of NF-kB39. Decrease of HIF1A transcripts with siRNA suppresses NF-kB activity by reducing its DNA binding activity40. In the presence of external stimuli such as lipopolysaccharide and IFN-γ, the NF-kB complex increases HIF-1α protein levels41. In addition, inhibition of NF-kB complex activity by pyrrolidine dithiocarbonate decreases HIF1A transcript levels in fibroblasts42. Overexpression of two subunits of NF-kB (p50 and p65) has been shown to enhance HIF1A transcription, while inhibition of NF-kB attenuates HIF1A transcription induced by hypoxia43 and also increases expression of EPAS1, which encodes HIF-2α, principal inducer of EPO transcription44. An EPAS1 promoter binding assay identified RELA, a NF-kB family member, as a potent inducer of EPAS1 expression45. As expected, the activated NF-kB complexes induced HIF1A transcription (Supplementary Fig. S4), and HIF-1 signaling pathway was upregulated in Aymara (Supplementary Fig. S5).
Finally, NF-kB signaling also regulates erythropoiesis. NF-kB activity is higher in early erythroid progenitors, leading to downregulation of genes required for differentiation. During differentiation, NF-kB activity decreases, allowing the expression of NF-E2, an erythroid transcriptional factor46,47. Mice lacking RelB, a member of NF-kB complexes, have impaired erythropoiesis in the bone marrow, which is compensated by increased hematopoiesis in the liver and spleen, resulting in normal Hb levels48. Using in vitro erythroid expansion of erythroid progenitor cells from peripheral blood, we confirmed that NFKB1 and AS-NFKB1 transcript levels also decreased during erythroid differentiation (data not shown). However, due to the paucity of material, we could not study the changes in differentiation, proliferation, and apoptosis of erythroid cells with and without the AS-NFKB1 transcripts.
We set out to validate the results from our integrative analysis by conducting a comprehensive functional analysis. We validated the splicing events in NFKB1 and their genetic regulator, rs230511, as a sQTL in a region that shows high levels of genetic differentiation between the Aymara population and lowland Native Americans, consistent with recent positive selection of this genomic region (Fig. 3e). We then evaluated gene frequency of rs230511 SNP in our Tibetan and European genomes database14,49 and found rs230511-T at lower allele frequencies in these two populations than in Aymaras. We also genotyped Quechuas, another high-altitude native Andean population distantly related to Aymaras, living at Chorolque (~5000 m) and found lower rs230511 allele frequency than Aymaras (Fig. 3g). Moreover, genotyping of Aymaras living at different altitudes revealed that this variant allele is more frequent with increasing altitude, consistent with a selective advantage at high altitude. Our findings indicated that AS-NFKB1 decreased inflammatory responses under inflammatory stress although it is positively correlated with inflammation in the normal state. The decreased inflammatory responses in Aymara population might help in adaptation to high altitude that could contribute to alleviation of chronic inflammation50,51,52. Taken together, these results suggest that rs230511 is the target of natural selection in this genomic region and underlies, at least in part, the genetic and physiological adaptations to the high altitude in the Aymaras.
Our functional study identified AS-NFKB1 transcripts as positively correlating with not only transcript and protein levels of the inflammatory genes IL6 and IFNG (encoding interferon γ), but also with Hb levels, white blood cells, and platelets, demonstrating that these evolutionary selected genetic variants account for Aymara high Hb. Notably, although inflammation suppresses erythropoiesis through increasing hepcidin, the master regulator of iron metabolism53, we found a strong correlation of Hb level in Aymara with NFKB1 Aymaras’ evolutionary selected SNPs and AS-NFKB1 transcripts. However, AS-NFKB1 also positively correlated with HIF-regulated genes. This indicates that the upregulation of HIFs augments erythropoiesis and overcomes the inhibitory effects of inflammation on erythropoiesis. Other evidence suggests that high-altitude-induced hypoxia may also prolong the lifespan of red blood cells54,55, which might be an additional causative mechanism of erythrocytosis in Aymara56 independent of the NF-kB pathway.
We also tested whether AS-NFKB1 was translated into protein and altered NF-κB protein levels (Figs. 6a–c, and 7). Only AS2 generated p50 (an NF-κB protein) but had a defect in nuclear translocation. Overexpression of AS-NFKB1 did not affect cytoplasmic p50 protein levels; however, nuclear NF-κB protein levels were altered with AS-NFKB1 overexpression or inflammatory stress induced by TNF treatment. These data suggest that the nuclear translocation of NF-κB protein may be influenced by AS-NFKB1. Inflammatory gene expression under these conditions correlated with these protein levels. We also confirmed these changes in disease models, including PV and ET, which are characterized by increased baseline inflammation. In these patients, the NFKB1 rs230511 (C/T or T/T genotype) was associated with lower inflammatory gene expression. These findings suggest that AS-NFKB1 may be beneficial under inflammatory stress by preventing excessive expression of inflammatory genes.
Higher WBC counts also correlate with increased inflammation57, as well as platelet counts, that positively correlate with inflammatory proteins plasma levels including C-reactive protein (CRP) and cytokines58. We also found a positive correlation of AS-NFKB1 transcript levels with WBC and platelet counts, which suggests a possible role for these transcripts in Aymara by upregulating the inflammatory response under hypoxic conditions.
Although we found only a small number of genes that were simultaneously DEGs and DSGs, enriched pathways were largely shared between the two differential gene sets for immune-related functions. This suggests that a complex interconnection of gene expression and splicing may contribute to the genomic and transcriptomic regulation that results in the distinct Aymara molecular signatures resulting in their beneficial physiological adaptation to extreme environmental hypoxia. We present one such mechanism here: NFKB1 alternative splicing. Differential splicing of its exons 4 and 5 could affect the functional levels of NFKB1, HIF-1, and immune-related genes. The altered splicing of this upstream regulator changes the expression of its downstream target genes that contribute to Aymara adaptations. This observation could explain why there is a significant overlap in gene sets between DEGs and DSGs, but less similarity at the level of individual genes. We then found the genetic signature of cis-SNPs that act as sQTLs and regulate these splicing events. Notably, these SNPs are considered both cis-sQTLs and trans-eQTLs since the associated splicing status could also affect downstream target gene expression.
Firstly, there are some limitations of our study that could lead to potential overinterpretation of these findings. In our pilot studies, we started with a relatively small sample set that may result in a possibility of missing some genes that also contribute to Aymara adaptation. Therefore, further study with more samples is required to obtain a more comprehensive perspective and replicate our computational analysis results. Additionally, gene regulation can differ across various cell types, so while focusing on granulocytes provided specific insights, this approach may not capture the full complexity of gene regulation across other blood cell types. The challenges of separating cells from whole blood in Bolivia further restricted our ability to obtain pure populations of platelets and other blood cells, limiting the scope of our analysis to granulocytes. Secondly, AS-NFKB1 variants showed loss of function as repressors of NF-κB pathway, nevertheless, NFKB1/p105 have also non-canonical function59, which remains to be determined. Also, in our study, the increased AS-NFKB1 transcript level was associated with high NF-kB transcriptional activity and the possible role of the non-coding transcript variants of protein-coding genes needs to be further explored. While we demonstrated that AS-NFKB1 reduced NF-kB translocation to the nucleus and downregulated inflammatory and HIF-targeted gene expression under inflammatory stress, the underlying mechanisms remain to be fully elucidated. Thirdly, although our findings indicate relationships between AS-NFKB1 and inflammation, as well as HIF-targeted genes, suggesting that high Hb levels in Aymara individuals may be, at least in part, mediated by upregulation of HIFs via the loss of function of AS-NFKB1 transcripts. However, the complete mechanism underlying AS-NFKB1’s regulation of erythropoiesis in this population requires further characterization in future studies.
It will be necessary for follow-up experimental studies to confirm our other computational results and elucidate other Aymara-specific molecular mechanisms, as the scope of the deep analysis in this work was largely limited to NFKB1. However, our data provides the first candidate for Aymara increased Hb in high altitude and their possible protective mechanisms against excessive inflammation.
Methods
Study subjects
The initial ten Aymara and four European samples were collected at La Paz-Bolivia (3650 m) its suburb El Alto is at 4150 m. This was followed by validation samples of 55 Aymara and 18 Europeans living in La Paz or its higher-located suburb, El Alto. Subsequent samples were collected from Bolivian Aymara living at different altitudes: n = 43 from Santa Cruz (400 m), n = 133 from La Paz (3650 m), and n = 12 from Chorolque (5000 m). Additionally, n = 49 Quechua individuals from Chorolque were recruited by Dr. Ricardo Amaru. The study was approved by the Institutional Review Board of San Andres University Medical School, La Paz, Bolivia, and all participants provided informed consent. Peripheral blood was collected using ethylene diamine tetra acetic acid (EDTA) tubes and complete blood count was performed by automated hematological counter (Horiba ABX Micros ES 60, France). Since hemoglobin levels differ between males and females, we included only male participants in this manuscript to reduce variation related to menstrual cycle-caused iron deficiency and pregnancies in young otherwise healthy women.
WGS and RNA-seq data processing
RNA-seq data was generated using the same methods as we described in Gangaraju, et al.31, and detailed methods were as follows. mRNA sample preparation utilized the standard Illumina protocols. The Illumina TruSeq stranded RNA sample preparation kit was used for the total RNA sample libraries prepared from granulocytes. The mRNA libraries were constructed by removing ribosomal RNA from the total RNA samples with Ribo-Zero Gold oligos including beads that are complementary to cytoplasmic rRNA. The quantity and quality of RNA samples were measured on an Agilent Technologies 2200 TapeStation using the D1000 ScreenTape assay. For each of the 14 independent biological samples, RNA-seq data were generated from a HiSeq using 125 Cycle Paired-End Sequencing v4. The raw sequencing file (fastq format) contained about 30–35 million reads of 125 nucleotides in length per sample.
For WGS data, we obtained WGS data (NCBI BioProject accession number PRJNA393593) from the same samples used for RNA-seq, which were generated and analyzed in our previous study10. We conducted quality control (QC) for all paired-end WGS data using FastQC60 and trimmomatic 0.3261 with the following options: TruSeq3-PE-2.fa:2:20:10, LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15, and MINLEN:75. Contaminant sequences (i.e., adaptor or linker sequences) were removed during QC. Clean reads were then mapped to the human genome reference sequence (GRCh37.75 based on hg19) using HISAT2. Next, we added read-group identifier (ID) information and marked duplicate reads in the mapped bam files using AddOrReplaceReadGroups and MarkDuplicates of the Picard v2.1.1 package. Finally, the reads in regions close to putative indels were locally re-aligned using IndelRealigner from the Genome Analysis Toolkit (GATK)62 based on known indels reported in dbSNP based on hg19 reference, obtained from 1000 Genomes Project (TGP)63. Base quality scores were then recalibrated using BaseRecalibrator, also in GATK. From the pre-processed bam files, we then generated one single pileup result including all bam information (i.e., Aymara and European samples) using the mpileup function in SAMtools. Finally, we transformed the mapped information saved in the pileup result file into the vcf file format, including genotypes for each individual (i.e., SNPs), using VarScan v2.3.9.
For RNA-seq data, we performed quality control on each read from our raw data (fastq file) using the FastQC tool60 the same as in the WGS analysis. We utilized RSEM to estimate gene expression based on gene annotations in GTF format (GRCh37.75) as transcripts per million (TPM) values using the STAR v2.5 aligner. We used mapped bam files (i.e., output from STAR) as input for rMATS64, which is a tool for investigating alternative splicing of exons based on junction reads; this yielded differentially spliced exons between Aymara and Europeans. We identified all possible AS events: exon skipping (ES), alternative 5’ splicing site (A5SS), alternative 3’ splice site (A3SS), intron retention (IR), and mutually exclusive exon splicing (MXE). rMATS calculates the expression of each AS exon, represented as the percent spliced in (PSI, range zero to one) based on the reads mapped onto a boundary between consecutive exons (called junction reads). The PSI indicates the fraction of alternatively spliced exons. For example, with an exon-skipping event, a PSI of zero means that all mRNAs from the gene skipped the AS exon, while the value of one indicates that all mRNAs have the AS exon.
Differentially expressed and alternatively spliced (AS) genes in Aymara
After estimating the gene expression and PSI level of each AS exon, we compared them between Aymara and European samples. DESeq2 was employed to compare gene expressions, and the rMATs comparison function was used to compare PSI levels. In both comparisons, P values were corrected according to the false discovery rate (FDR). Differentially expressed genes (DEGs) and spliced exons (DSEs) were considered significant at FDR < 0.05, in conjunction with an absolute fold change >1.5 for gene expression levels and a difference of PSI > 0.1 (10% difference in PSI between the two populations) for AS PSI levels.
Gene set enrichment and network analysis
For functional interpretation of our identified DEGs and DSEs, we performed gene set enrichment analysis for canonical pathways using ConsensusPathDB (CPDB: http://cpdb.molgen.mpg.de/)65. The cutoff value for significance was FDR < 0.05. We also constructed a protein-protein interaction network from the identified DEGs and DSEs using the StringDB web tool66 to further gain insight into their molecular functions at the systems level. We selected only the first interaction relationship between nodes (i.e., genes) with two high confidence levels at >0.7 and excluded disconnected nodes from the network.
Cis regulators for DEGs and DSEs
For each DEG and DSE, we performed association tests with all possible genotypes of a given SNP using a linear regression model; SNPs identified as associated are referred to as expression quantitative trait loci (eQTLs) and splicing quantitative trait loci (sQTLs), respectively. To identify eQTLs, gene expression as TPM was tested for association with SNPs located in promoter regions (upstream 1500 and downstream 200 from each transcript start site), and to identify sQTLs, spliced exon levels as PSI were tested for association with SNPs within AS exons and their flanking introns. To calculate the empirical p value for naïve eQTLs and sQTLs, we generated 1000 random sets of shuffled populations in which data were randomly assigned to SNPs. For each set, we performed the same analysis of eQTLs and sQTLs (i.e., linear regression) and applied the same cutoff criterion of p < 0.05, but with randomized genotypes for each population and PSI. Finally, we estimated 1000 p values for the statistically significant eQTLs and sQTLs.
Determination of Aymara adapted sQTLs
To evaluate adapted sQTLs in the Aymara, we compared the allele frequencies of eQTL and sQTL genotypes between our Aymara samples and non-Finnish European ancestry samples from the Genome Aggregation Database (gnomAD; v3.1.2)67. This database allowed us to secure reliable differences with a large number of European samples (n = 76,156). Differences (that is, eQTLs and sQTLs that are genetic regulators for DEGs and DSEs in the Aymara) were identified using the Chi-square test. Aymara-adapted eQTLs and sQTLs were defined with a cutoff value of FDR < 0.05 and |different minor allele frequency > 0.3.
Calculation of genetic differentiation using PBSn1
Population genetic differentiation statistics (FST and PBSn1) were extracted from a previously published dataset10. Briefly, low-coverage whole genome sequencing data were collected using the Illumina HiSeq 2500 platform from a sample of 42 people living at high altitude with Aymara or Andean ancestry (NCBI BioProject accession number PRJNA393593). Genotype likelihoods were calculated for each individual and then merged with genotype likelihood data from a subset of the Human 1000 Genomes panel. Admixture-corrected allele frequencies were used to calculate PBSn1 at SNPs and in windows across the genome with the Andean population component as the target population. For the analysis in this work, we identified the window centered on the target sQTL SNP and present both the window and SNP-based statistics extracted from the broader, genome-wide dataset.
NFKB1 SNP genotyping
Genomic DNA (gDNA) was isolated from granulocytes using the Gentra Puregene Blood Kit (Qiagen). Five NFKB1 SNPs were genotyped using TaqMan SNP genotyping assays (Thermofisher) (Assay IDs: C____804242_30 for rs230525, C___3066468_10 for rs230511, C____804227_10 for rs230504, C____804244_20 for rs230527, and C____804237_20 for rs230519).
Measurement of AS-NFKB1, NFKB1, and inflammatory gene transcript levels
RNA was isolated from granulocytes using Tri-reagents according to the manufacturer’s protocol (Molecular Research Center). cDNA was synthesized from RNA using the SuperScript™ VILO™ cDNA Synthesis Kit (Invitrogen). Primers were designed to span exon-exon boundaries: For NFKB1-AS1, F-5′ ACTGCCAACAGGAGAGGATTTC 3′and R-5′ TAGTTGCAGATCTTTGACCTGA 3′; For NFKB1-AS2, F-5′ GCACTGCCAACAGGCAGATGGC 3′ and R-5′ TAGTTGCAGATCCTGTTTAGGTT 3′; For NFKB1-AS3, F-5′ GGAAGGCCTGAACAAGATGTTT 3′ and R-5′ AGTTGCAGATCCTGTTGGCAGT 3′; For canonical NFKB1, F-5′ GCACTGCCAACAGCAGATGGC 3′ and R-5′ TAGTTGCAGATCTTTGACCTGA 3′. AS-NFKB1 and canonical NFKB1 transcript levels were measured by qRT-PCR using TaqMan SYBR Expression Assays (Applied Biosystems). Transcript levels were measured by TaqMan expression assay (Thermofisher) with the following assay IDs: IFNG (Hs00989291_m1), IL6 (Hs00174131_m1), CDKN1A (Hs00355782_m1), EDN1 (Hs00174961_m1), BCL2 (Hs04986394_s1), TNF (Hs00174128_m1), PTGS2 (Hs00153133_m1), SOD2 (Hs00167309_m1), and BIRC2 (Hs01112284_m1). Expression levels were normalized against HPRT1 (Hs02800695_m1) expression, calculated using 2−ΔΔCt method, and expressed as fold change.
Measurement of inflammatory protein levels in plasma
IL-6 and interferon-γ protein levels were measured in frozen plasma using the ProQuantum Immunoassay Kit (Thermofisher) according to the manufacturer’s instructions.
AS-NFKB1 cloning, western blot, and reporter assay
The AS-NFKB1 variants were constructed using cDNA encoding N-terminally EGFP-tagged canonical NFKB1/p105 and NFKB1/p50 (kind gift of M. Fliegauf and B. Grimbacher, University of Freiburg). The DNA fragments spanning deletion of exon 4, and exon 5 and both were commercially synthetized by Integrated DNA Technologies, Inc. (IDT), Coralville, Iowa. With unique restriction sites (BglII and Bst1107I), the wild-type sequence was replaced. Final constructs were subjected to whole plasmid sequencing (Eurofins Genomics). HEK293 (human embryonic kidney, Cat. ATCC-CRL-3249) cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The HEK293 cells were transiently transfected with NFKB1/p105 (or p50) WT, AS1, AS2, and AS3 plasmids using Lipofectamine 2000 (Thermo Fisher Scientific). Twenty-four hours post-transfection, 1. crude cell lysates were prepared and separated on gradient 4–20% SurePage gels (GenScript) for western blots using rabbit anti-GFP (#ab290, Abcam, dilution 1:1000), rabbit anti-NFKB1 (#13586, Cell Signaling, dilution 1:500) and rabbit anti-β-actin (#ab8227, Abcam, dilution 1:10000) antibodies; 2. cytoplasmic and nuclear fractions were prepared using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) and separated on gradient 4-20% SurePage gels (GenScript) with antibodies specified above. For luciferase-based reporter assay, HEK293 cells were transiently transfected with NFKB responsive reporter, RelA (p65), Renilla, and NFKB1 variants, at a 1:2:1:8 ratio, using Lipofectamine 2000 (Thermo Fisher Scientific). Twenty-four hours post-transfection, the luciferase production was quantified in cell lysates with Dual-Luciferase® Reporter Assay kit (Promega) on a Glomax 20/20 luminometer (Promega). Firefly:Renilla luciferase ratios were calculated for each condition in order to normalize the NFKB1 inhibitory effect for different constructs.
AS-NFKB1 overexpression and western blot experiment
HL60 cells (Cat. CCL-240), a promyelocytic leukemia cell line, were purchased from ATCC and cultured in RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 20% FBS at 37 °C in a humidified atmosphere with 5% CO2. AS-NFKB1 constructs were used for overexpression experiments. A total of 200 ng of each construct, including canonical NFKB1 p50, NFKB1-AS1, NFKB1-AS2, and NFKB1-AS3, were transfected into 0.5 × 106 HL60 cells using Lipofectamine 2000 (Thermo Fisher Scientific). After 24 h of incubation, cytoplasmic and nuclear proteins were extracted using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) in the presence of complete, EDTA-free Protease Inhibitor (Roche). Proteins were separated on Any kD™ Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-Rad) and transferred to Immun-Blot® PVDF membranes (Bio-Rad). NF-kB, GAPDH, and Histone H3 were detected using antibodies specific to NF-kB (Cat. NBP2-22178, Novus, dilution 1:1000), GAPDH (Cat. MA5-15738-HRP, Thermo Fisher Scientific, dilution 1:10,000), and Histone H3 (Cat. ab1791, Abcam, 1:10,000 dilution), respectively. Protein bands were visualized using Immobilon® ECL UltraPlus Western HRP Substrate (Sigma) and quantified using ImageJ software. Total RNA was isolated 24 h post-transfection and reverse-transcribed into cDNA following the same method described above. Transcript levels of inflammatory and HIF-targeted genes were quantified using TaqMan expression assays (Thermo Fisher Scientific) with the following assay IDs: IL1B (Hs01555410_m1), CDKN1A (Hs00355782_m1), TNF (Hs00174128_m1), SLC2A1 (Hs00892681_m1), VEGFA (Hs00900055_m1), LDHA (Hs01378790_g1), EPAS1 (Hs01026149_m1), and BCL2 (Hs04986394_s1). Expression levels were normalized to RPL13A (Hs04194366_g1) using the 2-ΔΔCt method and expressed as fold change.
Correlation of NFKB1 SNP rs230511 with inflammatory and HIF-targeted genes in PV and ET
Patients were recruited from clinics at the Huntsman Cancer Center and the Veterans Affairs Hospital in Salt Lake City, Utah. The study was approved by the University of Utah’s Institutional Review Board, and all participants provided informed consent. Granulocytes and platelets were isolated from whole blood using Ficoll-Paque density gradient centrifugation31. Total RNA was extracted and reverse-transcribed into cDNA following the same method as described above. Transcript levels of inflammatory and HIF-targeted genes were quantified using TaqMan expression assays (Thermo Fisher Scientific) with the following assay IDs: IL1B (Hs01555410_m1), CXCL8 (Hs00174103_m1), IL6 (Hs00174131_m1), IL15 (Hs01003716_m1), TNF (Hs00174128_m1), SLC2A1 (Hs00892681_m1), VEGFA (Hs00900055_m1), and LDHA (Hs01378790_g1). Expression levels were normalized to RPL13A (Hs04194366_g1) using the 2−ΔΔCt method and expressed as fold change. The NFKB1 SNP rs230511 genotype was determined using genomic DNA extracted from granulocytes, following the same method as described above.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The transcriptome data of granulocytes from Aymara and European individuals generated in this study have been deposited in the NCBI database under accession code PRJNA1204927. The WGS data of 42 people living at high altitude with Aymara or Andean ancestry is available in NCBI database under accession code PRJNA393593. The data that support the findings of this study are available from the corresponding author, Josef T. Prchal (josef.prchal@hsc.utah.edu), upon request.
Code availability
Analysis codes used in this study are available on GitHub (https://github.com/hangost/AymaraSplicing).
References
Tashi, T., Song, J. & Prchal, J. T. Congenital and evolutionary modulations of hypoxia sensing and their erythroid phenotype. Curr. Opin. Physiol. 7, 27–32 (2019).
Vizcardo-Galindo, G., Leon-Velarde, F. & Villafuerte, F. C. High-altitude hypoxia decreases plasma erythropoietin soluble receptor concentration in lowlanders. High. Alt. Med. Biol. 21, 92–98 (2020).
Stauffer, E. et al. Blood viscosity and its determinants in the highest city in the world. J. Physiol. 598, 4121–4130 (2020).
Huerta-Sanchez, E. et al. Genetic signatures reveal high-altitude adaptation in a set of Ethiopian populations. Mol. Biol. Evol. 30, 1877–1888 (2013).
Fan, F. C., Chen, R. Y., Schuessler, G. B. & Chien, S. Effects of hematocrit variations on regional hemodynamics and oxygen transport in the dog. Am. J. Physiol. 238, H545–22 (1980).
Brutsaert, T. D., Araoz, M., Soria, R., Spielvogel, H. & Haas, J. D. Higher arterial oxygen saturation during submaximal exercise in Bolivian Aymara compared to European sojourners and Europeans born and raised at high altitude. Am. J. Phys. Anthropol. 113, 169–181 (2000).
Stembridge, M. et al. The overlooked significance of plasma volume for successful adaptation to high altitude in Sherpa and Andean natives. Proc. Natl. Acad. Sci. USA 116, 16177–16179 (2019).
Amaru, R. et al. Bolivian Aymara natives with chronic mountain sickness have autonomous BFU-E Growth. Blood 126, 5206–5206 (2015).
Sandoval, J. R. et al. The genetic history of indigenous populations of the Peruvian and Bolivian altiplano: the legacy of the Uros. PLoS ONE 8, e73006 (2013).
Crawford, J. E. et al. Natural selection on genes related to cardiovascular health in high-altitude adapted andeans. Am. J. Hum. Genet 101, 752–767 (2017).
Prchal, J. T. Primary and Secondary Erythrocytoses/Polycythemias. In Williams Hematology, 10e (eds. Kaushansky, K. et al.) (McGraw-Hill Education, New York, NY, 2021).
Yi, X. et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78 (2010).
Simonson, T. S. et al. Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75 (2010).
Tashi, T. et al. Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E:C127S) in combination with EPAS1 (HIF-2alpha) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J. Mol. Med. 95, 665–670 (2017).
Zhou, D. et al. Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am. J. Hum. Genet. 93, 452–462 (2013).
Amaru, R., Song, J., Reading, N. S., Gordeuk, V. R. & Prchal, J. T. “What we know and what we do not know about evolutionary genetic adaptation to high altitude hypoxia in andean aymaras&rdquo. Genes 14, 640 (2023).
Sato, J. et al. Family with sequence similarity 5, member C (FAM5C) increases leukocyte adhesion molecules in vascular endothelial cells: implication in vascular inflammation. PLoS ONE 9, e107236 (2014).
Weigand, J. E., Boeckel, J. N., Gellert, P. & Dimmeler, S. Hypoxia-induced alternative splicing in endothelial cells. PLoS ONE 7, e42697 (2012).
Wang, Y. et al. Down-regulation of CASK in glucotoxicity-induced insulin dysfunction in pancreatic beta cells. Acta Biochim. Biophys. Sin. 50, 281–287 (2018).
Nakayama, K. & Kataoka, N. Regulation of gene expression under hypoxic conditions. Int. J. Mol. Sci. 20, 3278 (2019).
Farina, A. R. et al. Hypoxia-induced alternative splicing: the 11th Hallmark of Cancer. J. Exp. Clin. Cancer Res. 39, 110 (2020).
Boyle, A. P. et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 22, 1790–1797 (2012).
Szklarczyk, D. et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Mistry, J. et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 49, D412–D419 (2021).
Popp, M. W. & Maquat, L. E. Leveraging rules of nonsense-mediated mRNA decay for genome engineering and personalized medicine. Cell 165, 1319–1322 (2016).
Wall, J. D. & Pritchard, J. K. Haplotype blocks and linkage disequilibrium in the human genome. Nat. Rev. Genet. 4, 587–597 (2003).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Marcus, J. H. & Novembre, J. Visualizing the geography of genetic variants. Bioinformatics 33, 594–595 (2017).
Zhou, J., Schmid, T. & Brune, B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol. Biol. Cell 14, 2216–2225 (2003).
Arora, S. et al. An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor kappaB- and hypoxia-inducible factor 1alpha-mediated up-regulation of CXCR4. J. Biol. Chem. 288, 21197–21207 (2013).
Gangaraju, R. et al. Thrombotic, inflammatory, and HIF-regulated genes and thrombosis risk in polycythemia vera and essential thrombocythemia. Blood Adv. 4, 1115–1130 (2020).
van Schouwenburg, P. A. et al. Application of whole genome and RNA sequencing to investigate the genomic landscape of common variable immunodeficiency disorders. Clin. Immunol. 160, 301–314 (2015).
Werling, D. M. et al. Whole-genome and RNA sequencing reveal variation and transcriptomic coordination in the developing human prefrontal cortex. Cell Rep. 31, 107489 (2020).
West, A. P., Koblansky, A. A. & Ghosh, S. Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 22, 409–437 (2006).
Mussbacher, M. et al. Cell type-specific roles of NF-kappaB linking inflammation and thrombosis. Front. Immunol. 10, 85 (2019).
Cao, S., Zhang, X., Edwards, J. P. & Mosser, D. M. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 281, 26041–26050 (2006).
Mizgerd, J. P., Lupa, M. M. & Spieker, M. S. NF-kappaB p50 facilitates neutrophil accumulation during LPS-induced pulmonary inflammation. BMC Immunol. 5, 10 (2004).
D’Ignazio, L., Bandarra, D. & Rocha, S. NF-kappaB and HIF crosstalk in immune responses. FEBS J. 283, 413–424 (2016).
Walmsley, S. R. et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med. 201, 105–115 (2005).
Rohwer, N. et al. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS ONE 5, e12038 (2010).
Tacchini, L., Gammella, E., De Ponti, C., Recalcati, S. & Cairo, G. Role of HIF-1 and NF-kappaB transcription factors in the modulation of transferrin receptor by inflammatory and anti-inflammatory signals. J. Biol. Chem. 283, 20674–20686 (2008).
Park, S. Y. et al. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1alpha activation. Eur. J. Immunol. 45, 1216–1227 (2015).
Belaiba, R. S. et al. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol. Biol. Cell 18, 4691–4697 (2007).
Olivotto, E., Otero, M., Marcu, K. B. & Goldring, M. B. Pathophysiology of osteoarthritis: canonical NF-kappaB/IKKbeta-dependent and kinase-independent effects of IKKalpha in cartilage degradation and chondrocyte differentiation. RMD Open 1, e000061 (2015).
Saito, T. et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat. Med. 16, 678–686 (2010).
Liu, J. J., Hou, S. C. & Shen, C. K. Erythroid gene suppression by NF-kappa B. J. Biol. Chem. 278, 19534–19540 (2003).
Zhang, M. Y., Sun, S. C., Bell, L. & Miller, B. A. NF-kappaB transcription factors are involved in normal erythropoiesis. Blood 91, 4136–4144 (1998).
Weih, F. et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell 80, 331–340 (1995).
Hu, H. et al. Evolutionary history of Tibetans inferred from whole-genome sequencing. PLoS Genet. 13, e1006675 (2017).
Pham, K., Parikh, K. & Heinrich, E. C. Hypoxia and inflammation: insights from high-altitude physiology. Front. Physiol. 12, 676782 (2021).
Mishra, K. P. et al. Hypoxia-induced inflammatory chemokines in subjects with a history of high-altitude pulmonary edema. Indian J. Clin. Biochem. 31, 81–86 (2016).
Nguyen, K. T. et al. Evaluation of the effects of altitude on biological signatures of inflammation and anxiety- and depressive-like behavioral responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 111, 110331 (2021).
Ganz, T. & Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 1823, 1434–1443 (2012).
Tang, F. et al. Inhibition of suicidal erythrocyte death by chronic hypoxia. High. Alt. Med. Biol. 20, 112–119 (2019).
Song, J., Tashi, T. & Prchal, J. T. Editorial comment on: inhibition of suicidal erythrocyte death by chronic hypoxia by Tang et al. (From: Tang F, Feng L, Li R, Wang W, Liu H, Yang Q, Ge R-L. High Alt Med Biol 2019;20:112-119; DOI: 10.1089/ham.2017.0159). High. Alt. Med. Biol. 20, 120–121 (2019).
Amaru, R. et al. Hematopoietic progenitor cells from patients with chronic mountain sickness lack the JAK2(V617F) mutation, show hypersensitivity to erythropoietin and are inhibited by statins. Blood 114, 752–752 (2009).
Labrecque, N. & Cermakian, N. Circadian clocks in the immune system. J. Biol. Rhythms 30, 277–290 (2015).
Vayrynen, J. P. et al. Platelet count, aspirin use, and characteristics of host inflammatory responses in colorectal cancer. J. Transl. Med. 17, 199 (2019).
Cartwright, T., Perkins, N. D. & C, L. W. NFKB1: a suppressor of inflammation, ageing and cancer. FEBS J. 283, 1812–1822 (2016).
S, A. FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
McKenna, A. et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Siva, N. 1000 genomes project. Nat. Biotechnol. 26, 256 (2008).
Shen, S. H. et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 111, E5593–E5601 (2014).
Kamburov, A., Wierling, C., Lehrach, H. & Herwig, R. ConsensusPathDB-a database for integrating human functional interaction networks. Nucleic Acids Res. 37, D623–D628 (2009).
Szklarczyk, D. et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Acknowledgements
This research was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 2T32HL007576-31 from the National Heart, Lung, and Blood Institute (JS), Dorothy Brown Innovation in Science (JS), and a VA merit grant (JTP). This study was also partially supported by development funding for YL from the College of Veterinary Medicine, Seoul National University. The support and resources from the Center for High-Performance Computing (CHPC) at the University of Utah are gratefully acknowledged. The support from the Cell Biology Unit, School of Medicine at University of San Andres, as well as from Julieta Luna, Silvia Mancilla, Daniela Paton, Juan Carlos Valencia, Felipe Mamani, and Emerson Cayo, is also gratefully acknowledged. This research was also supported by Next Generation EU project LX22NPO5102 and Czech Science Foundation projects 24-11730S (LL). The authors would like to thank Vladimir Korinek for help with cloning strategy and Manfred Fliegauf and Bodo Grimbacher for providing NFKB1 and RelA constructs.
Author information
Authors and Affiliations
Contributions
RA, JS, and JTP conceived the study. JS and JTP drafted the first version of the manuscript. JS conducted genotyping and measured AS-NFKB1, inflammatory gene, and protein levels in Aymara samples. JS designed and carried out AS-NFKB1 overexpression experiments in vitro and assessed inflammatory gene expression levels in PV and ET samples. RA recruited the study subjects. SH and YL contributed to the conception and design of the bioinformatics analysis and discovered the alternative splicing events in NFKB1. SH and DK were involved in the interpretation of computational results that YL supervised. LL prepared the NFKB1 constructs, conceived the in vitro study, and edited the manuscript. TQ processed blood samples. JC analyzed selection signal of NFKB1 SNPs. SJK performed gene expression assay for AS-NFKB1 overexpression experiments in vitro. All authors wrote and reviewed the manuscript. YL and JTP supervised the generation and interpretation of all data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Niroj Sethy, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, J., Han, S., Amaru, R. et al. Alternatively spliced NFKB1 transcripts enriched in Andean Aymara modulate inflammation, HIF and hemoglobin. Nat Commun 16, 1766 (2025). https://doi.org/10.1038/s41467-025-56848-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56848-0