Abstract
Endomesoderm specification by a maternal β-catenin signal and body axis patterning by interpreting a gradient of zygotic Wnt/β-catenin signalling was suggested to predate the split between Bilateria and their sister clade Cnidaria. However, in Cnidaria, the roles of β-catenin signalling in these processes have not been demonstrated directly. Here, by tagging the endogenous β-catenin in the cnidarian Nematostella vectensis, we confirm that its oral-aboral axis is indeed patterned by a gradient of β-catenin signalling. Strikingly, we show that, in contrast to bilaterians, Nematostella endomesoderm specification is repressed by β-catenin and takes place in the maternal nuclear β-catenin-negative part of the embryo. This completely changes the accepted paradigm and suggests that β-catenin-dependent endomesoderm specification was a bilaterian innovation linking endomesoderm specification to the subsequent posterior-anterior patterning.
Similar content being viewed by others
Introduction
Metazoan eggs are polarised along the ‘animal-vegetal’ axis, with the animal pole being the yolk-poor region where polar bodies are given off in meiosis and towards which the egg nucleus is shifted, and the vegetal hemisphere harbouring most of the yolk. During the early development of bilaterian embryos, β-catenin signalling is involved in two fundamental processes occurring sequentially (Fig. 1a). First, β-catenin accumulates in the cell nuclei at the vegetal side of the embryo to specify the endomesoderm. Then, it forms a nuclear gradient patterning the main, posterior-anterior (P-A) axis1,2,3,4,5,6,7,8,9,10,11, which is usually roughly colinear to the vegetal-animal axis of the egg. However, the central role of the “canonical” Wnt/ β-catenin (cWnt, Fig. 1b) signalling in the patterning of the main body axis is not restricted to Bilateria. Expression data indicate that cWnt signalling may regulate axial patterning in the earliest branching metazoan groups Ctenophora (comb jellies) and Porifera (sponges)12,13,14, and previous functional analyses showed the involvement of the cWnt signalling in the patterning of the main, oral-aboral (O-A) body axis in the bilaterian sister group Cnidaria (sea anemones, corals, jellyfish, Hydra)15,16,17,18,19,20,21. Moreover, nuclear localisation of β-catenin on one side of the sea anemone embryo at the early blastula stage and its failure to gastrulate upon β-catenin loss-of-function suggested that the involvement of β-catenin signalling in the endomesoderm specification was also an ancestral feature conserved at least since before the cnidarian-bilaterian split some 700 Mya11,15,22,23. However, despite convincing circumstantial evidence, there was no direct proof for either of these two functions of β-catenin signalling in a cnidarian. Here we set out to obtain such proof by tagging endogenous β-catenin in a model cnidarian—the sea anemone Nematostella vectensis—with superfolder GFP24 and detecting the localisation of nuclear β-catenin at the time of germ layer specification and in the axial patterning phase.
a The questions of this study. b Wnt/β-catenin pathway. Upon pathway activation, β-catenin translocates into the nucleus. Azakenpaullone (AZK) and alsterpaullone (ALP) are GSK3β inhibitors; iCRT14 is an inhibitor of the β-catenin/Tcf interaction. c Anti-GFP antibody staining detects sfGFP-β-catenin at the cell membranes and in the nuclei. d Overlay of the anti-GFP signal with the nuclear staining shows the positions, at which anti-GFP staining was quantified. The first nucleus, where anti-GFP staining intensity was measured is located at the relative position 0.00; the last nucleus – at the relative position 1.00. Yellow arrowheads on (c) and (d) point at the same example nuclei. e Axin in situ hybridisation staining intensity was measured along the pink line from the relative position 0.00 to the relative position 1.00. f LOESS smoothed curves show that nuclear sfGFP-β-catenin forms an oral-to-aboral gradient with two peaks (n = 6). These peaks correspond to the positions where β-catenin target Axin expression peaks as well (n = 10). Shaded areas show the 99% confidence interval for the mean. Black arrows on (c) and (e) point at the second, lower peak of nuclear sfGFP-β-catenin and the corresponding second peak of Axin expression.
Results
Nuclear β-catenin is involved in the O-A patterning
Previously we showed that genes expressed in distinct ectodermal domains along the O-A axis in Nematostella gastrula react dose-dependently to different levels of pharmacological upregulation of the β-catenin signalling (Fig. 1b15). This suggested that β-catenin signalling activity was graded along the O-A axis of the gastrula stage embryo. Downstream, β-catenin signalling activates a set of transcription factors, among which the more orally expressed ones act as transcriptional repressors of the more aborally expressed ones17. As a result of these regulatory interactions, the initially ubiquitous aboral identity of the embryo is restricted in a β-catenin-dependent manner to the future aboral domain as the oral and the midbody domains appear and become spatially resolved17. In this process, JNK signalling appears to act agonistically with β-catenin signalling: JNK inhibitor treatment aboralizes the embryo, and JNK inhibition is also capable of dose-dependently rescuing the oralization caused by pharmacological upregulation of β-catenin signalling with the GSK3β inhibitor azakenpaullone (Fig. 1b; Supplementary Fig. 1). The striking similarity of the regulatory logic of the β-catenin-dependent axial patterning and the complement of the downstream transcription factors between the sea anemone and the deuterostome bilaterians suggests the homology of the cnidarian O-A and the bilaterian P-A axis1,2,6,17,25,26.
To directly verify the presence of the oral-to-aboral β-catenin signalling, we used CRISPR/Cas9-mediated genome editing to generate a knock-in line, in which the nucleotides coding for the first 5 amino acids of β-catenin were replaced by the superfolder GFP (sfGFP) coding sequence. To test for the presence of the nuclear β-catenin gradient along the oral-aboral axis of the embryo, we incrossed heterozygous F1 polyps (wild type/sfGFP-β-catenin) and allowed the offspring to develop until late gastrula stage. As expected, approximately 3/4 of the embryos were fluorescent, however, fluorescent microscopy of live embryos only revealed strong signal at the cell boundaries—in line with the function of β-catenin in the cell contacts27. In order to detect nuclear sfGFP-β-catenin, we fixed the embryos and stained them with an anti-GFP antibody. Antibody staining revealed a comparatively weak but clear nuclear signal forming an oral-to-aboral gradient in the ectoderm (Fig. 1c, d). Quantification of the signal intensity in all ectodermal nuclei starting from the deepest cell of the pharyngeal ectoderm (relative position 0.00) and ending with the cell in the centre of the aboral ectoderm (relative position 1.00) showed a peak of nuclear sfGFP-β-catenin in the bend of the blastopore lip (approximately at relative position 0.20), and a second, smaller peak at the border between the midbody and the aboral domain (approximately at relative position 0.60). Both peaks coincide with the peaks of expression of the conserved and highly sensitive β-catenin signalling target Axin15,17,19 (Fig. 1e, f). We then upregulated β-catenin signalling with different concentrations of the GSK3β inhibitor alsterpaullone (Fig. 1b) and showed that changes in the expression domains of the β-catenin-dependent genes Axin, Brachyury (oral marker), Wnt2 (midbody marker), and Six3/6 (aboral marker) are consistent with the changes in the amount of nuclear sfGFP-β-catenin in different positions along the O-A axis (Supplementary Fig. 2). Thus, we conclude that the initial assumption that genes expressed in distinct domains along the O-A axis react to a graded β-catenin signal is indeed correct.
Nuclear β-catenin does not specify Nematostella endomesoderm
Our next goal was to verify the involvement of β-catenin signalling in the specification of the endomesoderm in Nematostella. It must be noted that the views on Nematostella germ layer identity are currently undergoing a transition. Traditionally, cnidarians, including sea anemones, are described as animals with an outer ectodermal layer, an invaginated ectodermal pharynx (absent in hydroids), and an inner cell layer termed endoderm or endomesoderm in different sources. Although cnidarians demonstrate a striking diversity of gastrulation modes28, the predominant gastrulation type characteristic for true jellyfish, corals, and sea anemones is invagination of the endomesoderm into a hollow blastula. However, recent studies show very convincingly that cnidarian endomesoderm resembles the bilaterian mesoderm, and cnidarian pharyngeal ectoderm resembles bilaterian endoderm both in terms of gene expression and in cell behaviour29,30. A new detailed expression analysis shows that the first zygotically expressed germ layer-specific markers appear at 6 h post-fertilisation in a contiguous patch of cells corresponding to the future endomesoderm31. These are mesodermal markers, and the onset of their expression is followed by the first endodermal markers around 8–10 hpf15,31. Some of the future key endodermal markers, such Brachyury, Wnt1, Wnt3, and WntA are initially transiently co-expressed with the mesodermal markers (Supplementary Fig. 3, see also ref. 15) making it possible to call this transient state a true “endomesoderm”. As development continues, the cells of the endomesoderm retain mesodermal marker expression, and eventually undergo apical constriction and invaginate, while endodermal marker expression progressively moves out of the mesoderm and into a ring of cells surrounding the mesoderm—the future blastopore lip/pharyngeal ectoderm (Supplementary Fig. 3). Recent analysis showed that this definitive endodermal domain is induced in the ectodermal cells in a Delta/Notch-dependent manner by ~12 hpf31. Based on marker gene expression, henceforth, we will use the terms “endoderm” and “mesoderm” for the pharyngeal ectoderm and endomesoderm, respectively, but we will continue using the term “endomesoderm” for the territory in the early embryo, which is permissive for the expression of the mesodermal and endodermal markers.
In line with the expression data31, our earlier pharmacological experiments showed that Nematostella endomesoderm specification is an early event happening at or prior to 6 hpf17,19, at which time nuclear sfGFP-β-catenin starts to be detectable in the developing embryos by fluorescent microscopy. Hence, in order to verify the involvement of β-catenin signalling in the specification of the endomesoderm, we immobilised sfGFP-β-catenin expressing embryos in low melting point agarose and performed live imaging from early cleavage until the onset of gastrulation (Fig. 2a-o, Supplementary Movies 1, 2, Supplementary Fig. 4). Faint nuclear sfGFP-β-catenin signal became detectable as early as 32-64-cell stage and increased in strength from the 128-cell stage on. From the very start, nuclear signal was confined to ~2/3 of the embryo. Nuclear sfGFP-β-catenin was visible during every cell cycle until shortly after the desynchronization of the mitotic divisions at ~2000-cell stage32, after which it became too weak to be detected by live imaging, while the sfGFP-β-catenin signal in the cell contacts remained strong. Strikingly, in all embryos we live-imaged (n = 10), the mesoderm invaginated on the side opposite to where nuclear sfGFP-β-catenin was detectable at earlier stages, i.e. early nuclear sfGFP-β-catenin was always observed on the future aboral side of the embryo. To make sure that we were indeed observing the nuclear sfGFP-β-catenin dynamics, we also live-imaged sfGFP-β-catenin expressing embryos, which were incubated in a 5 µM solution of the GSK3β inhibitor alsterpaullone from fertilisation on (Supplementary Movies 3, 4). In line with the previous publications11,15, upon GSK3β inhibition, fluorescent signal was observed in all nuclei from 16 to 32 cell stage on, and the embryos failed to gastrulate. In order to independently confirm that strong nuclear sfGFP-β-catenin signal we observed is indeed localised to the ectodermal rather than the endomesodermal domain, we combined hybridisation chain reaction with probes against an early endomesodermal marker ERG with anti-GFP antibody staining in 9 hpf and 12 hpf embryos. In 9 hpf embryos (~512-cell stage), strong GFP signal was detected in the interphase nuclei of all the cells except those expressing ERG, supporting our live imaging observations (Supplementary Fig. 5). In contrast, at 12 hpf (~1024-2000-cells), the strong aboral sfGFP-β-catenin staining started to progressively disappear and become replaced by weak staining, most prominent on both sides of the ERG expression boundary (Supplementary Fig. 5). This nascent nuclear sfGFP-β-catenin signal in the previously β-catenin-negative ERG-expressing cells is most likely caused by Wnt1, Wnt3 and WntA, which start to be co-expressed in the endomesoderm from 10 hpf on (ref. 15, Supplementary Fig. 3).
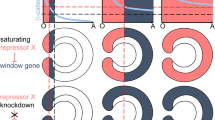
Individual frames from Supplementary Movie 1 showing the same embryo over the course of development. An – animal/oral/posterior pole, V – vegetal/aboral/anterior pole. From the time when mesodermal cells start to show signs of apical constriction, mesoderm is highlighted pink. The embryo is embedded in agarose and does not rotate between (a) and (o). sfGFP-β-catenin – black signal observed in the cell contacts (a–o) and in the interphase nuclei in the vegetal/aboral half of the embryo (c–h). Average developmental times on (a–o) are taken from ref. 32; time after fertilisation on (a–f) is shown in hh:mm format. See also Supplementary Fig. 4. Yellow line on (c–f) demarcates the sharp boundary between the nuclear sfGFP-β-catenin-positive and nuclear sfGFP-β-catenin-negative cells until the loss of synchronicity in cell division on (g). p–s Anti-GFP antibody staining shows that early vegetal nuclear sfGFP-β-catenin is maternal. Arrows on (q) show weak sfGFP signal in the cell-cell contacts. Arrowheads on (r, s) point at sfGFP-β-catenin-positive nuclei. Scale bars 50 µm. The experiment was replicated three times with similar results. t, u Comparison of the effect of the downregulation of β-catenin signalling in Nematostella and sea urchin. t1 Effect of the β-catenin morpholino injection. t2 Effect of early (<6 hpf) β-catenin signalling activation. u1 Effect of Axin mRNA injection. u2 Effect of constitutively active β-catenin mRNA injection. The cartoons showing the effects of the up- and downregulation of the β-catenin signalling are based on data from refs. 23,26,33.
Since endomesoderm specification by vegetal nuclear β-catenin has been shown to be controlled by the maternally deposited components in several bilaterians5,8,33,34,35,36, we next tested whether nuclear β-catenin observed in Nematostella embryos in the non-endomesodermal domain is maternal as well. To do so, we performed two reciprocal experiments: we crossed a homozygous sfGFP-β-catenin female to a wild-type male and a homozygous sfGFP-β-catenin male to a wild-type female. Then, we assayed the embryos from both crosses for the presence of the nuclear β-catenin by staining them with anti-GFP antibody at 9 hpf, when strong aboral signal is detectable in live imaging, and at 12 hpf, when nuclear β-catenin stops to be detectable by live imaging but β-catenin-dependent O-A patterning starts. We showed that in the embryos developing from the eggs laid by an sfGFP-β-catenin female, strong nuclear anti-GFP signal was observed at 9 hpf (Fig. 2p). In contrast, no nuclear anti-GFP signal was observed at 9 hpf in the embryos laid by the wild type female and fertilised by the sfGFP-β-catenin male (Fig. 2q). By 12 hpf, weak nuclear signal was observed in both crosses (Fig. 2r, s). Thus, we conclude that the strong nuclear sfGFP-β-catenin we observed in live imaging is maternal, while weak nuclear signal observed in the 12 hpf blastulae (Fig. 2r, s, Supplementary Fig. 5) as well as gastrulae (Fig. 1c, d) is zygotic.
Discussion
Previous analyses of the β-catenin-GFP mRNA injected Nematostella embryos showed nuclear β-catenin-GFP localisation on one side of the early blastula in the untreated embryos and in all blastoderm cells of the embryos upon GSK3β inhibition11,15. Moreover, Nematostella embryos with the nuclear localisation of β-catenin directly suppressed by various methods failed to gastrulate, remaining perfect blastula-like spheres11,23,37. Morphologically, this effect resembled the gastrulation block caused by functional suppression of β-catenin in sea urchin5 and led to the conclusion that the endomesoderm in Nematostella, just like the endomesoderm in a number of bilaterians, is specified by an early β-catenin signal at the future gastrulation pole of the embryo11. Although universally accepted in the field (also by us—see for example15,38), this hypothesis was contradicted by several important observations (Fig. 2t-u2, Supplementary Note 1, Supplementary Figs. 1, 5–7). First, unlike sea urchin embryos with suppressed β-catenin, Nematostella β-catenin morphants ubiquitously expressed mesodermal markers, rather than the zygotic markers of the aboral/anterior ectoderm corresponding to the low “β-catenin signalling” end of the O-A axis (compare Fig. 2t1 with Fig. 2u1, Supplementary Figs. 6, 723,31; Supplementary Note 1). Second, upon pharmacological activation of β-catenin signalling by early treatment with a GSK3β inhibitor, Nematostella embryos failed to segregate the mesoderm, form blastopore lips, and gastrulate. Treated embryos remained spherical and ubiquitously expressed endodermal markers such as Brachyury, FoxA, FoxB, while mesodermally expressed markers SnailA, ERG and many others were abolished (Fig. 2t2 19,23,39,40,41,42, Supplementary Figs. 6, 7; Supplementary Note 1). In contrast, in the sea urchin, activation of β-catenin signalling also strongly expands the endodermal portion of the embryo, however, the mesoderm is not lost (Fig. 2u2; Supplementary Fig. 633). Taken together, these data allow us to hypothesise that: i) similar to the situation in deuterostome Bilateria1,2,34,43, activation of β-catenin signalling can induce endodermal marker expression in the ectodermal territory of Nematostella, and ii) unlike in the sea urchin and other Bilateria, Nematostella endomesoderm specification is repressed by β-catenin signalling rather than activated by it. In line with this hypothesis, expression analysis clearly shows that mesodermal marker genes are repressed by β-catenin signalling (Supplementary Fig. 731). Moreover, the onset of the expression of the endodermal marker genes known to be under positive Wnt/β-catenin regulation only slightly later in development17,23, is also not activated by β-catenin (Supplementary Fig. 7). The presence of nuclear sfGFP-β-catenin on the aboral rather than on the oral side of the developing embryos (Fig. 2a-o, Supplementary Movies 1, 2) directly supports this hypothesis and explains the discrepancy between the sea urchin and Nematostella phenotypes mentioned above (Fig. 2t, u, Supplementary Fig. 6).
Our finding that an oral-to-aboral gradient of nuclear β-catenin exists in the Nematostella gastrula confirms a number of previous assumptions on the mode and logic of the oral-aboral patterning in this animal and is in line with the idea that the cnidarian O-A axis corresponds to the bilaterian P-A axis15,17,19. However, our second observation that Nematostella endomesoderm forms in the β-catenin-negative domain has a much greater importance for the understanding of the early evolution of the body axes and germ layers. In Bilateria, unless physically prevented by large amounts of yolk, endomesoderm specification and gastrulation take place at the vegetal pole, i.e. posteriorly. In contrast, in Cnidaria, gastrulation modes are highly variable. Some species gastrulate by invagination, unipolar ingression or epiboly, while others have multipolar modes of gastrulation such as cellular, morular or mixed delamination or multipolar ingression28. In case of multipolar gastrulation, germ layer specification and gastrulation movements are spatially uncoupled from the universally cWnt-dependent O-A patterning20,44,45,46,47. Importantly, in cnidarians with unipolar modes of gastrulation, endomesoderm specification, and gastrulation always takes place at the animal, rather than at the vegetal pole, and in all cnidarians analysed so far, the animal-vegetal axis of the egg exactly corresponds to the O-A axis of the embryo independent of whether they have a multipolar or a unipolar mode of gastrulation28,32,48,49,50. Previously, it has been proposed that the activation of the β-catenin-dependent endomesoderm specification at the vegetal rather than at the animal pole of a stem bilaterian resulted in the inversion of the position of the gastrulation site in Bilateria51 (Fig. 3a). Our new data suggest a different scenario (Fig. 3b). Both, in Nematostella and in Bilateria, maternal β-catenin accumulates in the vegetal pole nuclei, however, the specification of the endomesoderm by this signal appears to be a Bilateria-specific novelty, which linked germ layer specification, gastrulation movements and P-A patterning (Fig. 1a). In contrast, in Cnidaria, endomesoderm specification appears to be either negatively controlled by β-catenin (as in Nematostella) or not to be controlled by β-catenin at all (as in cnidarians with multipolar gastrulation modes), which provides a plausible explanation for the variety of gastrulation modes observed in this phylum. For example, in the unipolarly gastrulating hydroid Clytia, endomesoderm formation is delayed but not abolished by the knockdown of the maternally deposited Wnt3 or Frizzled1, which have been shown to be the central components of the cWnt-dependent O-A patterning in this animal, while O-A patterning is completely disrupted, and the embryo stays spherical45,46. Similarly, endomesoderm specification is not affected by the pharmacological suppression of the β-catenin signalling in the multipolarly gastrulating hydroid Dynamena47.
Traditional scenario of the β-catenin-dependent germ layer specification and main body axis patterning (a) and its modification based on our findings (b). An – animal pole; V – vegetal pole. c iCRT14 treatment shortens the pharynx and expands the gut (green staining) in the ctenophore Mnemiopsis. The experiment was replicated three times with similar results. Scale bar 50 µm. Asterisks denote the positions of the blastopore.
In order to verify that endomesoderm specification by β-catenin is indeed a bilaterian novelty it will be important to address the mechanisms of endomesoderm specification in the representatives of earlier branching animal clades. Among Placozoa, Porifera and Ctenophora (Fig. 1a), only representatives of the latter group have a morphologically distinct gut and clearly gastrulate. Notably, in ctenophores, the oral pole forms at the animal pole, like in Cnidaria52. Due to difficulties with microinjection, gene function studies in the model ctenophore Mnemiopsis leidyi are still in their infancy53,54. Nevertheless, the role of β-catenin has been addressed in a preprint by Salinas-Saavedra and colleagues where they showed that injection of the mRNA of the cytoplasmic domain of the sea urchin cadherin as well as CRISPR/Cas9-mediated β-catenin knockout resulted in the loss of gut and musculature55. However, our pharmacological tests show a different picture. Previous analyses demonstrated that LysoTracker is a robust marker of the forming gastrovascular system in Mnemiopsis53. Treatment of the developing Mnemiopsis embryos from 8 to 16 cell stage on with the inhibitor of the β-catenin/Tcf interaction iCRT14 (Fig. 1b) resulted in the formation of the cydippid stage juveniles with shortened pharynx and expanded gut (by analogy to Nematostella—a putative endodermal and mesodermal tissue, respectively; Fig. 3c), suggesting that, similar to the situation in cnidarians, β-catenin may play an “antiendomesodermal” role in this ctenophore.
In summary, our work resolves previous conflicting findings regarding the early functions of β-catenin signalling in the sea anemone Nematostella: we show that maternal β-catenin signalling inhibits (rather than activates) endomesoderm specification, while a gradient of zygotic β-catenin signalling patterns the endoderm and ectoderm along the O-A axis. We propose that the axial patterning function of Wnt/β-catenin is conserved between Cnidaria and Bilateria, however, endomesoderm specification by β-catenin appears to have evolved in Bilateria and possibly tethered gastrulation to the vegetal pole of the embryo making it posterior (Fig. 1a). Many questions arise based on our observations: i) what causes endomesoderm specification and subsequent gastrulation movements in the β-catenin-negative domain in Nematostella and in random positions in cnidarians with multipolar gastrulation modes, ii) what regulatory changes tethered bilaterian gastrulation to the ancestral site of the nuclear β-catenin accumulation at the vegetal pole, or iii) does β-catenin signalling really prevent endomesoderm formation in the earlier branching Ctenophora, which also gastrulate from the animal pole? In any case, our data suggest that the current view on the ancestral mode of endomesoderm specification in animals needs to be re-assessed.
Methods
Animal culture, generation of the sfGFP-β-catenin knock-in line
Nematostella vectensis polyps were maintained and spawning was induced as described56. Embryos were raised at 21 °C. To generate a sfGFP-β-catenin knock-in, a single gRNA with a protospacer 5′-ACCATGGAGACACACGGTAT-3′ recognising a sequence starting at the position 134602 on the scaffold_183 of the Nematostella genome v.157 was selected using CHOPCHOP58, and CRISPR/Cas9 genome editing was performed as described in ref. 59. For homologous recombination, we generated a fragment in which the first five triplets of the Nematostella β-catenin coding sequence were replaced with the Superfolder GFP coding sequence introduced in frame by Gibson assembly. The fragment containing the homology arms and sfGFP was amplified using the primers 5′-GTGGAATTCGCAGCATTTCTCA-3′ and 5′-TCAAGGATGGCTCAGCAAGC-3′, which were modified as described60. F0 animals with clear fluorescent patches were raised to sexual maturity and crossed to wild type to generate heterozygous F1. To confirm the knock-in, we clipped single tentacles from individual heterozygous F1 animals, extracted genomic DNA from them and performed PCR using the primers 5′-GGTCGTAGATGGTACCCTAAG-3′ and 5′-CAACTCTGGGATAGCACGTGTAG-3′ located in the β-catenin genomic locus upstream and downstream of the homology arms. This PCR resulted in two β-catenin locus fragments with and without the sfGFP insertion, which we confirmed by Sanger sequencing. Genotyped knock-in animals were raised to maturity, sexed, and intercrossed. The offspring of these genotyped F1 animals was used in the experiments. All inhibitor treatments were performed as described in ref. 17. For morpholino-mediated knockdown of Nematostella β-catenin, we microinjected a previously described23 translation-blocking morpholino TTCTTCGACTTTAAATCCAACTTCA. To knock down LRP5/6 by RNAi, we electroporated a previously described19 shRNA against the target sequence GAGAGCCTTCCACTTGTAA according to the published protocol37.
Sea urchin experiments were performed during the University of Vienna course ‘Developmental Biology of Marine Invertebrates in Villefranche-sur-Mer’. Mature male and female Paracentrotus lividus were kindly provided by the Croce and Schubert lab and handled as described by ref. 61. For documenting early development (Supplementary Fig. 6c, d), fertilisation envelopes were removed right after fertilisation. For later stages (Supplementary Fig. 6e, f), the embryos were allowed to develop inside the fertilisation envelopes. DMSO and 1 µM azakenpaullone treatments we started after fertilisation and continued until fixation.
Laboratory cultures of cydippid Mnemiopsis leidyi were kept in 3 L Kreisel tanks at 17–19 °C on a 17 h/7 h light/dark cycle in 27.5 ppt artificial seawater (ASW) and spawned ∼6–7 h post-darkness. Animals used to set up culture were originally collected at the Kristineberg Marine Research Station, Sweden. For embryo collection, sexually mature cydippids were transferred into 200–250 ml beakers in the evening and screened for mature eggs and early embryos next morning. To inhibit β-catenin signalling, 8–16 cell embryos were treated with 10 μM iCRT14 (SML0203, Sigma). At ∼30 hpf, the embryos were transferred into filtered ASW containing 1 μM LysoTracker Yellow HCK-123 (Molecular Probes) and 10 ng/µl Hoechst 33342 for at least 1 h at room temperature. Live embryos were then mounted on glass slides in ASW for imaging with a Zeiss Axioplan microscope equipped with a Zeiss AxioCam camera.
Gene expression analysis, antibody staining and staining intensity measurements
For qPCR, total RNA was extracted with Trizol (Invitrogen) from 9 hpf and 12 hpf treated and control embryos in biological triplicates and reverse transcribed using LunaScript RT SuperMix Kit (NEB). qPCR results were normalised to GAPDH. Primer sequences can be found in Supplementary Table 1.
For anti-GFP antibody staining, the embryos were fixed for 1 h in 4%PFA/PBS-TT (PBS-TT = 1x PBS containing 0.2% Tween20 and 0.2% TritonX100) at 4 °C, washed five times for 5 min in PBS-TT, incubated for 2 h in a blocking solution consisting of 95% BSA/PBS-TT and 5% heat inactivated sheep serum (BSA/PBS-TT = 1% BSA w/v in PBS-TT), and stained overnight at 4 °C in rabbit polyclonal anti-GFP (abcam290, RRID:AB_303395) diluted 1:500 in the blocking solution. Unbound antibody was removed by five 15 min washes in PBS-TT, then the embryos were blocked again and stained overnight at 4 °C with AlexaFluor488 donkey anti-rabbit IgG (ThermoFisher A32790, RRID:AB_2762833) diluted 1:1000 in the blocking solution. The unbound secondary antibody was removed by five washes with PBS-TT; DAPI was added to the first wash to counterstain the nuclei, then the embryos were gradually embedded in Vectashield (Vectorlabs). 16-bit images of the DAPI and anti-GFP staining were obtained using the Leica SP8 LSCM equipped with a 63x glycerol immersion objective (n = 6). Single and double in situ hybridisation with RNA probes against Nematostella Axin, Brachyury, Wnt1, Wnt2, Wnt3, WntA, ERG, and Six3/6 was performed as described previously17.
The anti-GFP staining intensity was measured over all ectodermal DAPI-positive nuclei starting from the deepest pharyngeal cell (relative position 0.00—see Fig. 1D) to the cell in the middle of the aboral ectodermal domain (relative position 1.00—see Fig. 1d) using FIJI62. Briefly, to identify the ROIs, polygonal selections were drawn to separate the pharynx ectoderm and the outer ectoderm based on DAPI signal. Masks were then generated separately for the outer ectoderm and the pharynx ectoderm parts of the image using the Convert to mask and the Watershed commands. To generate the ROIs for the nuclei, particle analysis with a minimum size of 1 µm2 was performed. The resulting ROIs were then manually checked and sorted such that they were arranged from the relative position 0.00 to the relative position 1.00. The mean intensities in the sfGFP channel were measured for all ROIs. The relative position of each nucleus was determined as a nucleus number divided by the total number of nuclei with measured anti-GFP staining intensity in this particular embryo (see Fig. 1D). To normalise the staining intensity across embryos, the relative staining intensity Y in the nucleus k was determined as:
Where \({y}_{k}\) is the measured nuclear anti-GFP staining intensity over the nucleus k, \({y}_{\min }\) is the minimal nuclear anti-GFP staining intensity measured in this particular embryo, and \({y}_{\max }\) is the maximal nuclear anti-GFP staining intensity measured in this particular embryo. The relative anti-GFP staining intensity measurements for all embryos can be found in the Supplementary Data File 1 in the Source Data. Axin in situ staining intensity was measured in FIJI62 on in situ images (n = 10) along a smoothened segmented line drawn from the deepest pharyngeal cell (relative position 0.00) to the cell in the middle of the aboral ectodermal domain (relative position 1.00). The relative position of each staining intensity measurement point was determined as a distance from the position 0.00 along the abovementioned smoothened segmented line divided by the total length of this line in the particular embryo (see Fig. 1e). The relative Axin staining intensity Y in the position k was determined using the formula [1] above. In this case, \({y}_{k}\) is the measured Axin in situ staining intensity at the position k, \({y}_{\min }\) is the minimal Axin in situ staining intensity measured in this particular embryo, and \({y}_{\max }\) is the maximal Axin in situ staining intensity measured in this particular embryo. The relative Axin in situ hybridisation staining intensity measurements for all embryos can be found in Supplementary Data File 2 in the Source Data. Since in Supplementary Fig. 2 we are comparing staining in different experimental conditions, no staining intensity normalisation has been performed, and raw data have been plotted. The measurements for all the conditions plotted in Supplementary Fig. 2 can be found in Supplementary Data File 3 in the Source Data.
An aliquot of the Endo1 antibody recognising sea urchin embryonic endoderm63 was a kind gift of the Croce/Schubert lab at Villefranche. For Endo1 staining, sea urchin embryos were fixed in 3.7% formaldehyde in sea water for 1 h at 4 °C, washed 5 times for 5 min in PTw (1x PBS, 0.1% Tween 20), blocked in 1%BSA/PTw for 2 h and stained overnight in Endo1 antibody (1:15) dissolved in 1%BSA/PTw. Next day, the embryos were washed 5 times 10 min with PTw, blocked as above and stained for 2 h at room temperature with goat anti-mouse AlexaFluor488 secondary antibody (ThermoFisher, A32723, RRID:AB_2633275) at 1:1000 dilution. AlexaFluor568-phalloidin and DAPI were added to the secondary antibody. After five washes, the embryos were embedded in glycerol and imaged using Leica SP8 LSCM.
HCR/antibody staining combination
HCR was performed according to ref. 64, with modifications. In brief, the HCR probe pool for the fluorescent in situ mRNA visualisation of Nematostella ERG was generated using the modified HCR 3.0 in situ probe generator65 (Supplementary Table 2). The sfGFP-β-catenin knock-in embryos at 9 hpf and 12 hpf were fixed with 4% PFA diluted in 1xPBS for 1 h at room temperature, washed three times for 5 min with PTw (1x PBS, 0.1% Tween 20) and stored in 100% methanol at −20 °C until further use. For rehydration, embryos were washed for 5 min in 70% methanol/30% PTw, then 30% methanol/70% PTw, followed by three washes in PTw. Rehydrated embryos were washed three times for 5 min in 5xSSCT (5x saline-sodium citrate buffer, 0.1% Tween-20). Prehybridization was performed by incubating embryos in Probe Hybridisation Buffer (Molecular Instruments) for 30 min at 37 °C. For hybridisation, embryos were transferred into hybridisation buffer containing the in situ probe set (0.8 pmol in 100 μL hybridisation buffer) and incubated overnight at 37 °C. Samples were then washed three times for 10 min and twice for 30 min in Probe Wash Buffer (Molecular Instruments) at 37 °C. After five 5-min washes in 5xSSCT at room temperature, embryos were transferred to Signal Amplification Buffer (Molecular Instruments) for 30 min. Hairpins h1 and h2 (12 pmol) were heated separately to 95 °C for 90 s, snap-cooled to room temperature in the dark, and added to 100 μL Probe Amplification Buffer. Embryos were then transferred into amplification buffer containing hairpin amplifiers and incubated overnight at room temperature. After amplification, samples were washed in 5xSSCT: three times for 5 min and twice for 30 min. Then, embryos were stained with anti-GFP antibody to detect sfGFP-β-catenin as described above, counterstained with 5 µg/ml DAPI, and gradually embedded in Vectashield (Vectorlabs) for subsequent imaging using Leica Stellaris 5 CLSM.
Live imaging
Embryos were embedded in 0.7% low-melting agarose in Nematostella medium (Nematostella medium = 16‰ artificial seawater, Red Sea Salt) in an optical bottom 35 mm Petri dish (D35-20-1.5-N, Cellvis, US) and imaged with a 20X CFI Plan Apo Lambda Objective (Nikon, Japan) using a Nikon Ti2-E/Yokogawa CSU W1 Spinning Disk Confocal Microscope. A 488 nm laser was used in conjunction with a 525/30 Emission Filter (BrightLine HC, Semrock, US) and a 25 µm pinhole size disk. Images were acquired every 5 min using automated imaging, over 25 Z-sections covering 120 µm depth. In the first experiment, the embryos were left to develop in 16‰ artificial seawater. In the second experiment, the embryos were developed in a 5 µM solution of the GSK3β inhibitor alsterpaullone (Sigma) from 1 hpf on. Live imaging was stopped after gastrulation was observed in the sample developing in the absence of alsterpaullone. Since embryos placed into a GSK3β inhibitor do not gastrulate, we continued the imaging of the alsterpaullone-treated embryos for an additional hour in comparison to the normal embryos. During imaging, the medium in the sample dish was continuously pumped through a tube submerged in a room temperature (~23 °C) water bath to offset heating from the microscope. This was achieved with a modified sample dish lid with two liquid connectors, and a peristaltic pump (Minipuls 3, Gilson, US). Ten embryos were imaged together in each experiment. After imaging, each Z-stack of images was converted into a maximum-intensity projection. The frames presented in Fig. 2a-o with average developmental times for each developmental stage are additionally presented in Supplementary Fig. 1 with actual time stamps from the confocal microscope.
Reporting summary
Further information on research design is available in Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available in the main text or the supplementary materials. Source data are provided with this paper.
Change history
31 March 2025
In the version of the article initially published, Supplementary Movies 1–4 were missing and have now been added to the online version of the article.
References
Darras, S., Gerhart, J., Terasaki, M., Kirschner, M. & Lowe, C. J. beta-catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development 138, 959–970 (2011).
Darras, S. et al. Anteroposterior axis patterning by early canonical Wnt signaling during hemichordate development. PLoS Biol. 16, e2003698 (2018).
Henry, J. Q., Perry, K. J., Wever, J., Seaver, E. & Martindale, M. Q. Beta-catenin is required for the establishment of vegetal embryonic fates in the nemertean, Cerebratulus lacteus. Dev. Biol. 317, 368–379 (2008).
Kiecker, C. & Niehrs, C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189–4201 (2001).
Logan, C. Y., Miller, J. R., Ferkowicz, M. J. & McClay, D. R. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126, 345–357 (1999).
McCauley, B. S., Akyar, E., Saad, H. R. & Hinman, V. F. Dose-dependent nuclear beta-catenin response segregates endomesoderm along the sea star primary axis. Development 142, 207–217 (2015).
Nordström, U., Jessell, T. M. & Edlund, T. Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 5, 525–532 (2002).
Oda-Ishii, I. et al. A maternal system initiating the zygotic developmental program through combinatorial repression in the Ascidian embryo. PLoS Genet. 12, e1006045 (2016).
Pruhs, R., Beermann, A. & Schroder, R. The roles of the Wnt-antagonists Axin and Lrp4 during embryogenesis of the red flour beetle Tribolium castaneum. J. Dev. Biol. 5, 10 (2017).
Sun, H., Peng, C. J., Wang, L., Feng, H. & Wikramanayake, A. H. An early global role for Axin is required for correct patterning of the anterior-posterior axis in the sea urchin embryo. Development 148, dev191197 (2021).
Wikramanayake, A. H. et al. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature 426, 446–450 (2003).
Adamska, M. et al. Wnt and TGF-beta expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS ONE 2, e1031 (2007).
Leininger, S. et al. Developmental gene expression provides clues to relationships between sponge and eumetazoan body plans. Nat. Commun. 5, 3905 (2014).
Pang, K., Ryan, J. F., Mullikin, J. C., Baxevanis, A. D. & Martindale, M. Q. Genomic insights into Wnt signaling in an early diverging metazoan, the ctenophore Mnemiopsis leidyi. Evodevo 1, 10 (2010).
Kraus, Y., Aman, A., Technau, U. & Genikhovich, G. Pre-bilaterian origin of the blastoporal axial organizer. Nat. Commun. 7, 11694 (2016).
Kusserow, A. et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433, 156–160 (2005).
Lebedeva, T. et al. Cnidarian-bilaterian comparison reveals the ancestral regulatory logic of the β-catenin dependent axial patterning. Nat. Commun. 12, 4032 (2021).
Marlow, H., Matus, D. Q. & Martindale, M. Q. Ectopic activation of the canonical wnt signaling pathway affects ectodermal patterning along the primary axis during larval development in the anthozoan Nematostella vectensis. Dev. Biol. 380, 324–334 (2013).
Niedermoser, I., Lebedeva, T. & Genikhovich, G. Sea anemone Frizzled receptors play partially redundant roles in the oral-aboral axis patterning. Development 149, dev200785 (2022).
Plickert, G., Jacoby, V., Frank, U., Muller, W. A. & Mokady, O. Wnt signaling in hydroid development: formation of the primary body axis in embryogenesis and its subsequent patterning. Dev. Biol. 298, 368–378 (2006).
Röttinger, E., Dahlin, P. & Martindale, M. Q. A framework for the establishment of a cnidarian gene regulatory network for ‘endomesoderm’ specification: the inputs of ss-Catenin/TCF signaling. PLoS Genet 8, e1003164 (2012).
Dohrmann, M. & Wörheide, G. Dating early animal evolution using phylogenomic data. Sci. Rep. 7, 3599 (2017).
Leclère, L., Bause, M., Sinigaglia, C., Steger, J. & Rentzsch, F. Development of the aboral domain in Nematostella requires beta-catenin and the opposing activities of Six3/6 and Frizzled5/8. Development 143, 1766–1777 (2016).
Pedelacq, J. D., Cabantous, S., Tran, T., Terwilliger, T. C. & Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24, 79–88 (2006).
Range, R. C. Canonical and non-canonical Wnt signaling pathways define the expression domains of Frizzled 5/8 and Frizzled 1/2/7 along the early anterior-posterior axis in sea urchin embryos. Dev. Biol. 444, 83–92 (2018).
Range, R. C., Angerer, R. C. & Angerer, L. M. Integration of canonical and noncanonical wnt signaling pathways patterns the neuroectoderm along the anterior–posterior axis of sea urchin embryos. PLOS Biol. 11, e1001467 (2013).
Brembeck, F. H., Rosário, M. & Birchmeier, W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet Dev. 16, 51–59 (2006).
Kraus, Y. & Markov, A. V. Gastrulation in Cnidaria: The key to an understanding of phylogeny or the chaos of secondary modifications? Biol. Bull. Rev. 7, 7–25 (2017).
Steinmetz, P. R., Aman, A., Kraus, J. E. M. & Technau, U. Gut-like ectodermal tissue in a sea anemone challenges germ layer homology. Nat. Ecol. Evol. 1, 1535–1542 (2017).
Kirillova, A. et al. Germ-layer commitment and axis formation in sea anemone embryonic cell aggregates. Proc. Natl Acad. Sci. 115, 1813–1818 (2018).
Haillot, E. et al. Notch, β-catenin and MAPK signaling segregate endoderm and mesoderm in the diploblast Nematostella vectensis. Preprint at https://doi.org/10.1101/2024.10.29.620801 (2024).
Fritzenwanker, J. H., Genikhovich, G., Kraus, Y. & Technau, U. Early development and axis specification in the sea anemone Nematostella vectensis. Dev. Biol. 310, 264–279 (2007).
Wikramanayake, A. H., Huang, L. & Klein, W. H. beta-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc. Natl Acad. Sci. USA 95, 9343–9348 (1998).
Croce, J. et al. Wnt6 activates endoderm in the sea urchin gene regulatory network. Development 138, 3297–3306 (2011).
Rothbächer, U., Bertrand, V., Lamy, C. & Lemaire, P. A combinatorial code of maternal GATA, Ets and beta-catenin-TCF transcription factors specifies and patterns the early ascidian ectoderm. Development 134, 4023–4032 (2007).
Wylie, C. et al. Maternal beta-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development 122, 2987–2996 (1996).
Karabulut, A., He, S., Chen, C. Y., McKinney, S. A. & Gibson, M. C. Electroporation of short hairpin RNAs for rapid and efficient gene knockdown in the starlet sea anemone, Nematostella vectensis. Dev. Biol. 448, 7–15 (2019).
Genikhovich, G. & Technau, U. On the evolution of bilaterality. Development 144, 3392–3404 (2017).
Martindale, M. Q., Pang, K. & Finnerty, J. R. Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development 131, 2463–2474 (2004).
Fritzenwanker, J. H., Saina, M. & Technau, U. Analysis of forkhead and snail expression reveals epithelial-mesenchymal transitions during embryonic and larval development of Nematostella vectensis. Dev. Biol. 275, 389–402 (2004).
Magie, C. R., Daly, M. & Martindale, M. Q. Gastrulation in the cnidarian Nematostella vectensis occurs via invagination not ingression. Dev. Biol. 305, 483–497 (2007).
Amiel, A. R. et al. A bipolar role of the transcription factor ERG for cnidarian germ layer formation and apical domain patterning. Dev. Biol. 430, 346–361 (2017).
Lhomond, G., Schubert, M. & Croce, J. Spatiotemporal requirements of nuclear β-catenin define early sea urchin embryogenesis. PLoS Biol. 22, e3002880 (2024).
Kraus, Y. et al. The embryonic development of the cnidarian Hydractinia echinata. Evol. Dev. 16, 323–338 (2014).
Momose, T. & Houliston, E. Two oppositely localised frizzled RNAs as axis determinants in a cnidarian embryo. PLoS Biol. 5, e70 (2007).
Momose, T., Derelle, R. & Houliston, E. A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development 135, 2105–2113 (2008).
Vetrova, A. A. et al. From apolar gastrula to polarized larva: embryonic development of a marine hydroid, Dynamena pumila. Dev. Dyn. 251, 795–825 (2022).
Byrum, C. A. & Martindale, M. Q. Gastrulation in the Cnidaria and Ctenophora. in Gastrulation. From Cells to Embryos. (ed. Stern, C.) 33–50 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2004).
Freeman, G. The cleavage initiation site establishes the posterior pole of the hydrozoan embryo. Roux’s. Arch. Dev. Biol. 190, 123–125 (1981).
Lee, P. N., Kumburegama, S., Marlow, H. Q., Martindale, M. Q. & Wikramanayake, A. H. Asymmetric developmental potential along the animal-vegetal axis in the anthozoan cnidarian, Nematostella vectensis, is mediated by Dishevelled. Dev. Biol. 310, 169–186 (2007).
Martindale, M. Q. & Hejnol, A. A developmental perspective: changes in the position of the blastopore during bilaterian evolution. Dev. Cell 17, 162–174 (2009).
Martindale, M. Q. & Henry, J. Q. Ctenophora. in Evolutionary Developmental Biology of Invertebrates 1: Introduction, Non-Bilateria, Acoelomorpha, Xenoturbellida, Chaetognatha (ed. Wanninger, A.) 179–201. https://doi.org/10.1007/978-3-7091-1862-7_8 (Springer, 2015).
Presnell, J. S. & Browne, W. E. Kruppel-like factor gene function in the ctenophore Mnemiopsis leidyi assessed by CRISPR/Cas9-mediated genome editing. Development 148, dev199771 (2021).
Presnell, J. S., Bubel, M., Knowles, T., Patry, W. & Browne, W. E. Multigenerational laboratory culture of pelagic ctenophores and CRISPR-Cas9 genome editing in the lobate Mnemiopsis leidyi. Nat. Protoc. 17, 1868–1900 (2022).
Salinas-Saavedra, M., Wikramanayake, A. H. & Martindale, M. Q. β-catenin has an ancestral role in cell fate specification but not cell adhesion. bioRxiv https://doi.org/10.1101/520957 (2019).
Genikhovich, G. & Technau, U. Induction of spawning in the starlet sea anemone Nematostella vectensis, in vitro fertilization of gametes, and dejellying of zygotes. CSH Protoc. 2009, pdb prot5281 (2009).
Putnam, N. H. et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization.Science 317, 86–94 (2007).
Labun, K. et al. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 47, W171–W174 (2019).
Ikmi, A., McKinney, S. A., Delventhal, K. M. & Gibson, M. C. TALEN and CRISPR/Cas9-mediated genome editing in the early-branching metazoan Nematostella vectensis. Nat. Commun. 5, 5486 (2014).
Gutierrez-Triana, J. A. et al. Efficient single-copy HDR by 5’ modified long dsDNA donors. eLife 7, e39468 (2018).
Formery, L. et al. Developmental atlas of the indirect-developing sea urchin Paracentrotus lividus: From fertilization to juvenile stages. Front Cell Dev. Biol. 10, 966408 (2022).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Nocente-McGrath, C., Brenner, C. A. & Ernst, S. G. Endo16, a lineage-specific protein of the sea urchin embryo, is first expressed just prior to gastrulation. Dev. Biol. 136, 264–272 (1989).
Choi, H. M. T. et al. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018).
Kuehn, E. et al. Segment number threshold determines juvenile onset of germline cluster expansion in Platynereis dumerilii. J. Exp. Zool. B Mol. Dev. Evol. 338, 225–240 (2022).
Acknowledgements
Sea urchins and Endo1 antibody were kindly provided by the Croce/Schubert lab at the Institut de la Mer de Villefranche. We thank Max Schwab for help with the double in situs in Nematostella, the students of the practical course Developmental Biology of Marine Invertebrates in Villefranche-sur-Mer for performing sea urchin inhibitor treatment experiments and the Core Facility for Cell Imaging and Ultrastructure Research of the University of Vienna for the access to the confocal microscopes. The work in Genikhovich group is funded by the Austrian Science Fund (FWF) grants DOI 10.55776/P30404, 10.55776/P32705, and 10.55776/P36080 (to GG). For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission. The work in the Adameyko group is funded by the ERC Synergy grant “KILL-OR-DIFFERENTIATE” 856529. T.L. was a recipient of the Ph.D. completion grant of the Vienna Doctoral School of Ecology and Evolution. D.M. was a recipient of the Lise-Meitner Fellowship M3291-B of the FWF. The work in the Hejnol group is funded by the Human Frontier Science Programme (HFSP) grant RGP0041/2022 (to A.H.).
Author information
Authors and Affiliations
Contributions
T.L. planned and performed experiments and generated the knock-in line; J.B. performed live imaging; S.K. performed iCRT14 treatment in Mnemiopsis; I.N. performed Axin in situ hybridisation; D.M. and E.G. measured and analysed the gradient data; A.H. provided funding for the Mnemiopsis part of the project; I.A. provided access to the spinning disk confocal microscope; G.G. conceived the project, planned and performed experiments, and wrote the paper. All authors edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lebedeva, T., Boström, J., Kremnyov, S. et al. β-catenin-driven endomesoderm specification is a Bilateria-specific novelty. Nat Commun 16, 2476 (2025). https://doi.org/10.1038/s41467-025-57109-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57109-w