Abstract
Radiotherapy using very-high-energy electron (VHEE) beams (50-300 MeV) has attracted considerable attention due to its advantageous dose deposition characteristics, enabling deep penetration and easy manipulation by magnetic components. One promising approach to compactly delivering these high energy electron beams in a cost-effective manner is laser wakefield acceleration (LWFA), which offers ultra-strong accelerating gradients. However, the transition from this concept to a functional machine intended for tumor treatment remains elusive. Here we present the self-developed pro- totype for LWFA-based VHEE radiotherapy, exhibiting compactness (occupying less than 5 m2) and long-term operational stability (validated over a period of one month). Subsequently, we employ this device to irradiate a tumor implanted in a mouse model. Following a dose delivery of 5.8 ± 0.2 Gy with precise tumor conformity, all irradiated mice exhibit pronounced control of tumor growth. For comparison, this tumor-control efficacy is similar to that achieved using commercial X-ray radiotherapy equipment operating at equivalent doses. These results demonstrate a compact and stable laser-driven VHEE system dedicated for preclinical studies involving small animal models and its promising prospects for future clinical translation in cancer therapy.
Similar content being viewed by others
Introduction
Nowadays, cancer curability remains a significant global challenge despite constant advances in early diagnosis and treatment options. Radiotherapy, applied to more than half of the patients, is an essential part of cancer treatment that relies on the use of ionizing radiation such as photons, electrons, protons and ions to locally deposit dose to damage cancer cells. Currently, the widely applied radiotherapy facilities still mainly rely on high-energy photon beams. With the development of new technology such as intensity-modulated radiotherapy1 or volumetric modulated arc therapy2, the doses can be delivered to tumors more precisely than a few decades ago, whereas the risk of exposure of the surrounding normal tissues remains a concern for patient outcomes. Proton therapy3,4, on the other hand, has been considered to exhibits more doseconformality due to the Bragg peak effect. However, the sychrotron/cyclotron machines to accelerate proton beams are large and expensive, and the dose distribution is sensitive to tissue density inhomogeneity, resulting in range uncertainties and potential over-irradiation of healthy tissues5,6.
Over the past two decades, the use of very high-energy electrons (VHEE) beams, ranging in energies from 50 to 300 MeV, has been considered as a promising alternative approach for treating deep-seated tumors7,8. Compared to photon and proton beams, VHEE beams offer several advantages, including the insensitivity to nonuniform density9,10, easy manipulation by magnetic components due to their charged and light particle nature11,12,13 and the ability to deposit a dose maximum deeply inside the body if strongly focused14,15. Previous numerical studies based on Monte-Carlo simulations have shown that even for a parallel (unfocused) VHEE beam, the dose into normal tissues is 20–70% lower than VMAT X-rays16,17,18. However, despite the significant benefits of VHEE, current mature technology for generating VHEE beams falls short of providing the desired cost-effectiveness. Conventional radio-frequency accelerators, due to the risk of material breakdown, can only reach an acceleration gradient of less than 10 MeV/m. Consequently, accelerating electron beams with energies exceeding 100 MeV requires several tens of meters of distance, rendering it impractical and prohibitively expensive for standard hospitals. Efforts have been made to reduce the size of VHEE accelerator through the use of high-gradient X-band radio-frequency waves, but the total length of the beamline still exceeds 10 m19.
A promising solution to this issue lies in a rapidly developing acceleration technology known as laser-wakefield acceleration (LWFA)20,21. In LWFA22, electrons are accelerated by the plasma wave driven by a short intense laser pulse. By directly using plasma as an acceleration medium, the risk of breakdown is eliminated, and the acceleration gradient can be as high as 100 GV/m, more than 1000 times stronger than the conventional accelerators, resulting in several hundreds MeV energy gain of electrons within only a few millimeters distance23,24,25,26,27. Many research groups worldwide have been dedicated to the LWFA-based VHEE field, and progress on dosimetry12,28,29, treatment planning12,30 and radiobiological effect31,32 have been made. However, these explorations are still in the scientific research stage, and a compact, stable, and conformal LWFA-based VHEE machine suitable for clinical applications has yet to be developed.
In this article, we introduce the prototype of an LWFA-based VHEE machine that addresses the clinical demands for VHEE therapy in the following aspects. Firstly, occupying less than 5 m2 in size, this machine consists of a self-developed industry-level 20 TW laser system, a laser-driven electron acceleration module, a beam transport module, a dose shaping module, and a final platform for dosimetry and small animal treatment. This compact size allows for easy integration within standard photon therapy rooms when equipped with more advanced positioning and conformal systems in the future. Secondly, this machine has demonstrated stable operation, with consistent performance over an entire month (from Monday to Friday excluding the weekends, in total 22 weekdays with 2000–3000 electron pulses per day), and continuous running for 10 h, ensuring its feasibility for clinical uses. Thirdly, a dedicated dose delivery with prescribed homogeneous dose distributions and sub-millmeter pointing fluctuation is achieved. We further apply this prototype to perform a small animal irradiation study. After 5.8 ± 0.2 Gy was delivered by the LWFA electron beams, significant tumor control is observed for all irradiated mice in the following few weeks, and the efficacy is shown comparable with the commercial photon radiotherapy devices using equivalent doses.
Results
Physical layout of the prototype
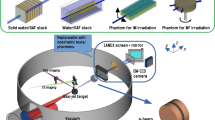
The general construction of the LWFA-based VHEE therapy machine is presented in Fig. 1. To meet the demand for potential industry-level medical applications, a self-developed compact 20 TW laser system was adopted. Through the implementation of independent modules and cooling systems, the stability and compactness are significantly improved. It is 1.4 m by 1.3 min size, much smaller than other similar-level commercial products. Additionally, since all key elements, including oscillators, pumps, amplifiers, and pocket cells, are self-manufactured, the cost of the laser system has been greatly reduced. These advancements will substantially reduce the cost and size of the entire VHEE machine. Meanwhile, the long-term stability also reaches veryhigh level, with energy stability less than 0.7% (RMS) and angular pointing less than 2 µrad (RMS) (8 h at 10 Hz), thanks to the usage of flexure mirror mounts for optical elements and an independent cooling system inside each module. More details about the laser system can be found in the Supplementary Information (Supplementary Table 1 and Supplementary Figs. 1, 2).
a General layout of the device, measuring 2.8 m by 1.4 m in size. b The detailed setup contained within the dashed circle in (a), where the 20 TW laser pulse was focused onto a gas jet to generate a high-energy electron beam with energy up to 175 MeV. The laser focus was assessed using a relay camera, and the plasma density information was examined using a synchronized, low-energy laser pulse directed to a Normasky interferometer. A PMQ triplet was employed to re-collimate the electron beam, reduce pointing variations and eliminate low-energy electrons, which was followed by a permanent dipole magnet to measure the electron spectrum. After the e− chamber’s exit, a scattering plate and a collimating aperture were introduced to shape the beam profile before it reached the sample stage. During the experiment, either animal samples or phantoms were placed on the stage.
Following the main laser chain, the 800 nm laser pulse was compressed to about 25 fs by a pair of gratings in the compressor chamber and then guided to the e− chamber for accelerating electron beams. There, it was focused by an off-axis parabolic mirror into a supersonic gas jet with an on-target energy of around 490 mJ. A laser probe beam was directed perpendicular to the main laser pulse, passing through the nozzle, and carrying the plasma density information which was then measured using a Nomarski interferometer. After exiting the gas jet, the electron beam propagated through a beam transport module consisting of three permanent magnet quadrupoles (PMQs) to reduce the pointing jitter. Before the electron beam exited the vacuum chamber, a movable permanent magnet dipole was installed for measuring the beam energy spectrum.
A thin film made of polyimide with a thickness of 100 microns was placed on the exit flange, which served as a barrier between the atmosphere and the vacuum while minimizing its impact on the electron beam. After the electron beam entered the atmosphere, it passed through a dose shaping module consisting of a scattering plate and a collimating aperture to control the dose spatial distribution and then irradiated on the samples. The entire treatment system’s dimensions were 2.8 m by 1.4 m.
Operation stability
By using a gas mixture of helium containing 1% nitrogen with a plasma density of 1 × 1019 cm−3, repetitive and high-charge (>100 pC) electron beams were obtained through ionization injection33,34. The beam properties including the energy spectra, beam profile, and dose distribution were routinely checked for an entire month (from Monday to Friday excluding the weekends, in total 22 weekdays) with 2000–3000 electron beam pulses each day and the operation repetition rate was 1 Hz.
Figure 2a presents the daily recorded electron beam energy spectra, with a data collectionof 100–130 electron beam pulses per day. We note that though ionization injection in a laser wakefield accelerator usually results in a continuous beam spectrum, in our prototype machine the low energy electrons (<40 MeV) were largely eliminated by a beam transport system, as will be discussed later. Figure 2b provides a statistical analysis of the charge and mean energy distribution for electrons based on Fig. 2a, revealing that the daily charge varied between 90 and 120 pC, with a maximum daily fluctuation (RMS) below 15%, and the mean energy between 60 and 70 MeV, with an RMS below 6.0%. Noting that significant variation in terms of energy spectrum could be observed in Fig. 2 between the 9th and 13th day. These fluctuations are attributed to the gradual accumulation of contaminants on the optical components over long-term operation. On the 9th and 13th day, element cleaning and optical alignment were conducted respectively to optimize accelerator performance.
a Sampled electron beam spectra throughout April of the year 2023. The total number of electron beam pulses recorded is 2522, with 100−130 beam pulses sampled each day. b Averaged charge (blue) and mean energy (yellow) of the spectrum sampled each day, only the portion of the electron beams with energy higher than 40 MeV were measured. It is noted that, on the 9th and 13th day, element cleaning and optical alignment were conducted respectively to optimize accelerator performance. Data are presented as mean values ± SD. The daily technical replicates counts are provided in Supplementary Table 3 of the Supplementary Information. Source data are provided as a Source Data file.
In parallel, another experimental test for 10 h, 0.3 Hz running was also performed and the preliminary results of obtained electron spectra can be found in Supplementary Fig. 3, which shows the stability over a continuous operation of the system.
Dose delivery
The pointing jitter of an LWFA electron beam is usually at several mrad level. In our case, as shown in Fig. 3a, the standard deviation of the beam center (yellow dots) at the sample position located 1 m away from the plasma source was measured to be 2.4 mm in horizontal (x) direction and 2.2 mm in vertical (y) direction, which is deficient for accurate dose delivery. To overcome this issue, a beam transport module consisting of three PMQs was employed, which improves the pointing stability of the beam center (blue dots) to 0.31 mm (horizontal) and 0.32 mm (vertical). Furthermore, the beam transport module also exhibits a selective energy effect, reducing the transmission efficiency of the low-energy portion to minimize the radiation dose to the sample/animal skin. Comparing the spectra between Fig. 3b and c, one can see that the electron beam after passing through the transport module, had a spectrum with a characteristic butterfly-shaped pattern (the focal point was at about 70 MeV) and the low-energy portion was largely divergent. From Fig. 3d, one can see electrons with energies above 40 MeV experienced only minimal loss (less than 10%) which was cross-verified by numerical simulations. Electrons with energies below 40 MeV, according to the simulations, experienced a loss more than 75%. More details about the simulations can be found in the “Methods” section and Supplementary Fig. 9.
a–d Beam pointing jitter and spectrum with (blue) and without (yellow) the transport module for 100 consecutive electron beam pulses. Data in Figure d are presented as mean values ± SD. b, c The images on the spectrometer screen without and with the transport module. e The focused beam profile at the sample position. f The diffused beam profile at the sample position. g Dose deposition after the collimator for 600 accumulated pulses. Source data are provided as a Source Data file.
To study the radiobiological effect, achieving a large and uniform dose distribution covering the entire space occupied by the tumor was essential. However, at the sample position, the laterally focused beam exhibits a Gaussian-like distribution with an FWHM size of ~4.1 mm (horizontal) and 4.9 mm (vertical) (see Fig. 3e). To achieve a larger lateral irradiation size with uniform dose deposition to cover the 5–8 mm mice tumor, we placed a 1.45 mm-thickness copper scatter plate in front of the sample, and the scattered electron beams form a Gaussian-like distribution with an FWHM size of 19 mm at the sample position (see Fig. 3f). To further localize the tumor position and minimize the radiation dose to surrounding normal tissues, we adopted a 3 cm-thickness collimating aperture (stainless steel) with a diameter of 15 mm in close proximity to the phantom or biological sample. As shown in Fig. 3g, after the accumulation of 600 electron beam pulses, a circular irradiation area with the same size as the aperture and a near-flattop distribution was realized, and the relative dose fluctuation in the flat-topped region was around 10%, ensuring the effectiveness of the biological experiments.
Mice in-vivo tumor irradiation
5-to-6-week-old male C57BL/6JNifdc mice total 24 were used for the tumor control experiment, which were subcutaneously injected with mouse-derived liver cell carcinoma H22 in their hind legs. The H22 cell line is radiosensitive, and significant changes in tumor volume can be clearly observed at a dose of 5–6 Gy while severe radiation-induced inflammation in healthy tissue is not likely to happen. These mice were equally divided into three groups: the mice in the control group were not subjected to any radiation; the mice in the LWFA irradiation group were exposed to radiation from the LWFA-based VHEE; the mice in X-ray irradiation group underwent irradiation using a medical X-ray radiotherapy machine (Varian Edge series). All mice were fed identically and received injections of cancer cells on the same day. After a period of 7 days, tumors had grown to ~5–8 mm in size. Mice from both the LWFA irradiation group and the X-ray irradiation group were subjected to radiation on the same day.
Prior to the tumor irradiation experiments, we assessed the dose deposition distribution of the LWFA-based VHEE beams by utilizing a solid water phantom equipped with EBT3 film dosimeters (see Supplementary Fig. 4). A comprehensive analysis of the depth dose and penumbra characteristics is provided in the Supplementary Information (Supplementary Fig. 5). Furthermore, to evaluate the VHEE dose deposition within a mouse, we initially performed a computed tomography (CT) scan on a selected mouse to create a tumor model. To conduct the CT scan, the mouse was anesthetized and placed inside a plastic tube. Subsequently, we conducted Monte Carlo simulations within the CT model, using the VHEE beam parameters measured from our prototype machine. The simulation outcomes are detailed in the Supplementary Information. As evidenced in Supplementary Figs. 6 and 7, the dose distribution covers the entire tumor region with relatively good uniformity.
During the experiment, the mice in both experimental groups were anesthetized and fixed onto an anatomical holder. For the LWFA-based VHEE irradiation group, to induce effective tumor control, the dose administered to each mouse was prescribed to be 5.8 ± 0.2 Gy, and the EBT3 films measuring the dose administrated (see Fig. 3g) were placed closely onto the mice skin. The dose administered consisted of 600 pulses of electron beams 1 Hz repetition rate and 1 cGy per pulse, the same as conducted in the stability testing experiments. The electron bunch length was estimated to be less than 10 fs according to our Particle-in-Cell simulation and previous experimental measurements for similar accelerators35,36. Therefore, the estimated peak dose rate could be over 1012 Gy/s, with an average dose rate of around 0.01 Gy/s. In comparison, the irradiation dose administered to the mice in X-ray group was 5.8 Gy, also measured by an EBT3 film at the surface of each mouse’s skin. Due to the characteristic of build-up region in X-ray dose deposition, a 1 cm compensation was placed above the mouse skin to ensure sufficient irradiation to the tumor. The dose measurement results for the LWFA experimental group and the X-ray experimental group are illustrated in Fig. 4a. Detailed configuration of the EBT3 film positioning can be found in “Methods”.
a The yellow solid line represents the dose received by 8 mice using the medical X-ray radiotherapy machine simultaneously (Varian Edge series) with the dashed line indicating the uncertainty of the EBT3 film measurements. The blue square represents the dose received by 8 mice using the laser-plasma accelerator system. Dose data are presented as measurement values ± the uncertainty which is estimated to be 10% of the measurement values. b The changes in tumor volume over a period of 29 days for three groups of mice(8 mice in each group). The gray line represents the tumor volume changes in the control group mice, the yellow line represents the tumor volume changes in mice treated with a medical X-ray machine, and the blue line represents the tumor volume changes in mice treated with a laser-plasma accelerator. Source data are provided as a Source Data file.
Over a period of 29 days following irradiation, we measured the tumor size in mice and calculated the tumor volume. Figure 4b shows the daily tumor volume evolution normalized by the volume on the irradiation day. The complete data of all 24 mice in the experiment is summarized in the Source Data in this paper. We observed that despite no significant decrease in the overall body weight of the mice, the tumor size of mice was effectively controlled by irradiation from the LWFA-based VHEE, with similar efficacy compared to commercial X-ray radiotherapy device.
Discussion
In conclusion, we have demonstrated a sustained and stable operation of an LWFA-based VHEE beam on a self-constructed compact prototype. Employing this prototype, we carried out in vivo experiments targeting live mice tumors and verified that the impact of the LWFA-VHEE beam on tumor growth inhibition is prominent and comparable to the effects induced by commercial medical X-ray radiotherapy machines. The presented device, characterized by its occupational compactness, operational stability, uniform dose delivery, and evident radiobiological effects, emerges as a platform for advancing translational research in the field of LWFA-based radiation oncology.
In the future, we are committed to conducting in-depth research on exploring the biophysical mechanisms of tumor suppression by LWFA-based VHEE, and on advancing the engineering of our system for clinical applications. For the biophysical studies, we will continue to study the effects on different cell lines under varying doses and compare these findings with those from more established methods, such as X-ray radiotherapy or hadron therapy. Moreover, given the ultrafast nature of LWFA electron beams, ongoing exploration into the potential for achieving the ultra-high dose rate effect is another key area of our research.
In terms of engineering clinical applications, we intend to incorporate machine learning algorithms into our system for optimizing beam quality and stability37,38,39. Furthermore, we also aim to explore innovative diagnostic methods, such as the femtosecond relativistic electron microscopy40,41,42,43, to achieve a more comprehensive understanding and monitoring of the electron acceleration process. Currently, the presented prototype delivers electron beams in a fixed direction. Our future developments will include the integration of multi-angle treatment capabilities, such as a rotating gantry, coupled with our self-developed beam delivery methods15 and treatment planning systems designed for VHEE. This strategic move is intended to further reduce radiation doses to healthy tissues while maximizing the inherent advantages of VHEE for treating deep-seated tumors.
Methods
Ethics statement
The animal facilities and the experiments were approved according to the regulations on the management of experimental animals in China and the local ethics committee (approval 2023- KY-0703-001, Ethics Review Committee for Scientific Research Projects of the First Affiliated Hospital of Zhengzhou University, Henan, China). All ethical regulations were complied during the experiment. According to ethical regulations, the size of tumors in mice is not permitted to exceed 14 mm. Throughout the experiment, any mice with tumors exceeding 13 mm in size or with tumors causing skin ulceration were euthanized.
Generation, transport, and diagnostic of the LWFA-VHEE beams
The 490 mJ, 25 fs laser pulses were focused to 12.3 µm and 12.6 µm (FWHM), corresponding to a peak normalized vector potential a0 of 2.5. A supersonic 2-mm nozzle was employed and filled with a gas mixture of 99% helium and 1% nitrogen. The working plasma density was ~1 × 1019 cm−3. This configuration of the laser pulse and gas source could efficiently generate plasma wakefield in the nonlinear blowout regime44,45 and induce ionization injection33,34, which resulted in a broad electron energy spectrum up to 175 MeV.
The beam transport module comprising three PMQs was designed to focus the electron beam into parallel beams for successive irradiation. The transport system was located 8.5 cm behind the source, with a distance of 4 cm between the first and second magnets, and a distance of 2.8 cm between the second and third magnets. The detailed parameters of the PMQs can be found in Supplementary Table 2 of the Supplementary Information.
For the energy spectrum diagnosis, a dipole magnet had a maximum magnetic field of 1 Tesla and was positioned 9 cm behind the beam transport system. A calibrated scintillation screen was positioned 9 cm behind the dipole magnet to collect the beam information, from which the electron beam spectrum as well as the beam charge can be deduced.
Numerical simulations
We have performed the beam transport simulation using the code TraceWin to calculate the electron beam envelope evolution (see Supplementary Fig. 8 in the Supplementary Information) and to estimate the spectra tailoring effect of the PMQs (see Supplementary Fig. 9 of the Supplementary Information). In the simulation, The electron beam was initialized at the gas jet with a RMS beam size of 1 µm (approximate point source), and a RMS divergence of θx = 5 mrad and θy = 6.8 mrad. A linear spectrum ranging from 0 to 150 MeV resembling the experimental spectrum (the yellow curve of Fig. 3d) was adopted. This system was capable of filtering 75% electrons with energies below 40 MeV, while achieving a near 100% transport efficiency for electrons with energies over 50 MeV.
In addition, a Monte Carlo simulation using the code TOPAS46 was carried out to simulate the beam size at the sample position for different configurations. From Supplementary Fig. 10 of the Supplementary Information, one can see that, without passing through the beam transport system, the FWHM beam spot sizes were 12 mm (horizontal) and 9 mm (vertical). After being focused by the triplet, they narrowed down to 4 mm (horizontal) and 3 mm (vertical). Subsequently, after being scattered by the scattering plate, the beam distribution became more uniform, with FWHM sizes of 20 mm (horizontal) and 18 mm (vertical). These simulation results were in good agreement with the experiment (see Fig. 3).
Dosimetry of the LWFA-VHEE beams
In the present study, we employed EBT3 film for measuring radiation dose. The principle of dose measurement of EBT3 film is establishing a relationship between the coloration of the irra- diated film and the received dose. Prior to the experiment, a medical X-ray radiotherapy machine (VarianEgde series) and an Epson 12000XL scanner were utilized to calibrate the film coloration, and the Film QA pro software was utilized for calibrating the dose-response curve and read the dose results. The EBT3 films were scanned 24 h after irradiation.
The schematic setup of the dose measurement in the in vivo experiment is shown in Fig. 5. In the VHEE radiation experiment, the EBT3 films were attached to the back side of the collimator by tapes. The mice were anesthetized and attached onto an anatomical holder with the tumor region directly exposed to the irradiation. In the X-ray irradiation experiment, the EBT3 films were attached beneath the 1-cm-thick bolus, and the mice, also anesthetized, were placed under the bolus. In both cases, the mice were placed very close to the EBT3 films with their skin almost touching the films.
a, for the VHEE radiation experiment, the EBT3 film was attached to the back side of the collimator by tapes. b, for the X-ray irradiation experiment, the EBT3 films were attached beneath the 1-cm-thick bolus. In both cases, the mice were placed very close to the EBT3 films with their skin almost touching the films.
Tumor model
The experiments were performed using 5-to-6-week-old male C57BL/6JNifdc mice purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. one week prior to the tumor injection. The animals were kept grouped with a maximum of four mice per cage at 12:12 h light-dark cycle, constant temperature of about 26 °C, and relative humidity of 70%. The mice were fed with a commercial laboratory animal diet and water ad libitum. Studies were carried out for the mouse-derived liver cell carcinoma H22, which is an established technique that easily develops into tumors with suitable volume with diameters of 5–8 mm. Tumor tissues constructed from H22 cells exhibit observable tumor growth inhibition with single-field irradiation doses of 5–6 Gy. At this dose level, even if the mice’s vital organs are irradiated, they will not develop fatal radiation-induced inflammation. Tumor growth was measured every day using a caliper. The corresponding tumor volumes were calculated as \(\frac{{\pi {ab}}^{2}}{6}\), where a is the longest tumor axis and b is the shortest tumor axis perpendicular to tumor-bearing animals that met the eligibility criteria were randomly assigned to different treatment groups in order to distinguish the temporal variations of the tumor model and non-radiation effects from the treatment outcomes. The artwork of the mouse model in this article is sourced from the SciDraw website and distributed under the CC-BY 4.0 license, created by Annie Park, and can be accessed via https://doi.org/10.5281/zenodo.10940480.
Irradiation using a clinical X-ray radiotherapy machine
In this experiment, we used high-energy X-rays generated by a medical linac Varian Edge to irradiate mice in order to compare the radiobiological influence between conventional medical radiotherapy and LWFA-VHEE radiotherapy. The X-ray is generated due to the bremsstrahlung effect of 6 MeV electron beams. Such high-energy X-rays can penetrate tens of centimeters into human tissue, enabling the treatment of deeply seated tumors, comparable to the proposed VHEE therapy. The medical X-ray linac can generate a uniform dose distribution with the SAXS data collected in Supplementary Table 4. In the X-ray experimental group, due to the characteristics of X-ray interactions in water, including the build-up effect and backscattering effect, mice were placed between a 1 cm thick tissue compensator and a 5 cm thick water phantom. This setup allowed for more uniform and stable dose delivery to the mice. EBT3 film was placed between the tissue compensator and the mice skin to monitor the dose received by the mice.
Statistics and reproducibility
The general information on the irradiation experimental design have been described in the tumor model section of the Methods. The sample sizes for animal studies were chosen based on preliminary experiments and existing literature47 to ensure sufficient statistical power for observing the effects of different treatments. No statistical method was used to predetermine the sample size. For this study, the aim was to have eight animals per group in analysis. Altogether, 24 animals were applied in this experiment split in 3 groups randomly. No data were excluded from the analyses. Blinding during allocation was not feasible because the groups were filled in parallel, and each group required different treatment protocols. However, during the follow-up phase, efforts were made to measure tumor growth as blindly as possible. This was achieved by separating the animal allocation records from the tumor growth data and assigning tumor volume measurements to caretakers who were not involved in the allocation or treatment processes.
Reporting summary
Further information on research design is available in Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting this work are included in the main article, supplementary information, or source data file. Data are provided with this article, or deposited in https://doi.org/10.5281/zenodo.14405492. Source data are provided with this paper.
Code availability
The source codes used in this study are publicly available at https://doi.org/10.5281/zenodo.14405492.
References
Bentzen, S. M. Radiation therapy: intensity-modulated, image-guided, biologically optimized and evidence-based. Radiother. Oncol. 77, 227–230 (2005).
Otto, K. Volumetric modulated arc therapy: Imrt in a single gantry arc. Med. Phys. 35, 310–317 (2008).
Wilson, R. R. Radiological use of fast protons. Radiology 47, 487–491 (1946).
Smith, A. R. Proton therapy. Phys. Med. Biol. 51, 491 (2006).
Urie, M., Goitein, M., Holley, W. R. & Chen, G. T. Y. Degradation of the bragg peak due to inho- mogeneities. Phys. Med. Biol. 31, 1 (1986).
Flatten, V., Baumann, K.-S., Weber, U., Engenhart-Cabillic, R. & Zink, K. Quantification of the dependencies of the Bragg peak degradation due to lung tissue in proton therapy on a ct-based lung tumor phantom. Phys. Med. Biol. 64, 155005 (2019).
DesRosiers, C., Moskvin, V., Bielajew, A. F. & Papiez, L. 150-250 MeV electron beams in radiation therapy. Phys. Med. Biol. 45, 1781 (2000).
Ronga, M. G. et al. Back to the future: very high-energy electrons (VHEEs) and their potential application in radiation therapy. Cancers 13 https://doi.org/10.3390/cancers13194942 (2021).
Papiez, L., DesRosiers, C. & Moskvin, V. Very high energy electrons (50–250 mev) and radiation therapy. Technol. Cancer Res. Treat. 1, 105–110 (2002).
Lagzda, A. et al. Influence of heterogeneous media on very high energy elec- tron (VHEE) dose penetration and a Monte Carlo-based comparison with existing radiotherapy modalities. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 482, 70–81 (2020).
Glinec, Y. et al. Radiotherapy with laser-plasma accelerators: Monte Carlo simulation of dose deposited by an experimental quasi monoenergetic electron beam. Med. Phys. 33, 155–162 (2006).
Svendsen, K. et al. A focused very high energy electron beam for fractionated stereotactic radiotherapy. Sci. Rep. 11, 5844 (2021).
Whitmore, L. et al. CERN-based experiments and Monte-Carlo studies on focused dose delivery with very high energy electron (VHEE) beams for radiotherapy applications. Sci. Rep. 14, 11120 (2024).
Whitmore, L., Mackay, R. I., Van Herk, M., Jones, J. & Jones, R. Focused VHEE (very high energy elec- tron) beams and dose delivery for radiotherapy applications. Sci. Rep. 11, 14013 (2021).
Zhou, B. et al. Angular scanning VHEE (very high energy electron) pencil beam delivery for radiotherapy. arXiv preprint arXiv:2401.01557 https://doi.org/10.48550/arXiv.2401.01557 (2024).
Bazalova-Carter, M. et al. Treatment planning for radiotherapy with very high-energy electron beams and comparison of VHEE and VMAT plans. Med. Phys. 42, 2615–2625 (2015).
Schüler, E. et al. Very high-energy electron (VHEE) beams in radiation therapy; treatment plan comparison between VHEE, VMAT, and PPBS. Med. Phys. 44, 2544–2555 (2017).
Sarti, A. et al. Deep seated tumour treatments with electrons of high energy delivered at flash rates: the example of prostate cancer. Front. Oncol. 11, 777852 (2021).
The ChUV-CERN collaboration to design and construct a high-energy electron flash therapy facility. In: Proc. CLIC Project Meeting #40 (2021).
Malka, V. et al. Principles and applications of compact laser–plasma accelerators. Nat. Phys. 4, 447–453 (2008).
Joshi, C., Corde, S. & Mori, W: Perspectives on the generation of electron beams from plasma-based accelerators and their near and long-term applications. Phys. Plasmas 27, 070602 (2020).
Tajima, T. & Dawson, J. M. Laser electron accelerator. Phys. Rev. Lett. 43, 267–270 (1979).
Mangles, S. P. et al. Monoenergetic beams of relativistic electrons from intense laser-plasma interactions. Nature 431, 535–538 (2004).
Geddes, C. et al. High-quality electron beams from a laser wakefield accelerator using plasma-channel guiding. Nature 431, 538–541 (2004).
Faure, J. et al. A laser-plasma accelerator producing monoenergetic electron beams. Nature 431, 541–544 (2004).
Gonsalves, A. et al. Petawatt laser guiding and electron beam acceleration to 8 GeV in a laser-heated capillary discharge waveguide. Phys. Rev. Lett. 122, 084801 (2019).
Liu, S. et al. A scalable, high-efficiency, low-energy-spread laser wakefield accelerator using a tri-plateau plasma channel. Research 7, 0396 (2024).
Lundh, O. et al. Comparison of measured with calculated dose distribution from a 120-MeV electron beam from a laser-plasma accelerator. Med. Phys. 39, 3501–3508 (2012).
Labate, L. et al. Toward an effective use of laser-driven very high energy electrons for radiotherapy: feasibility assessment of multi-field and intensity modulation irradiation schemes. Sci. Rep. 10, 17307 (2020).
Fuchs, T. et al. Treatment planning for laser-accelerated very-high-energy electrons. Phys. Med. Biol. 54, 3315 (2009).
Oppelt, M. et al. Comparison study of in vivo dose response to laser-driven versus conventional electron beam. Radiat. Environ. Biophys. 54, 155–166 (2015).
Small, K., et al. Evaluating very high energy electron rbe from nanodosimetric pbr322 plasmid DNA damage. Sci. Rep. 11 https://doi.org/10.1038/s41598-021-82772-6 (2021).
McGuffey, C. et al. Ionization-induced trapping in a laser wakefield accelerator. Phys. Rev. Lett. 104, 025004 (2010).
Pak, A. et al. Injection and trapping of tunnel-ionized electrons into laser-produced wakes. Phys. Rev. Lett. 104, 025003 (2010).
Lundh, O. et al. Few femtoseconds, few kiloampere electron bunch produced by a laser-plasma accelerator. Nat. Phys. 7, 219–222 (2011).
Zhang, C. et al. Temporal characterization of ultrashort linearly chirped electron bunches generated from a laser wakefield accelerator. Phys. Rev. Accel. Beams 19, 062802 (2016).
Kirchen, M. et al. Optimal beam loading in a laser-plasma accelerator. Phys. Rev. Lett. 126, 174801 (2021).
Jalas, S. et al. Bayesian optimization of a laser-plasma accelerator. Phys. Rev. Lett. 126, 104801 (2021).
Irshad, F. et al. Pareto optimization and tuning of a laser wakefield accelerator. Phys. Rev. Lett. 133, 085001 (2024).
Zhang, C. et al. Femtosecond probing of plasma wakefields and observation of the plasma wake reversal using a relativistic electron bunch. Phys. Rev. Lett. 119, 064801 (2017).
Wan, Y. et al. Direct observation of relativistic broken plasma waves. Nat. Phys. 18, 1186–1190 (2022).
Wan, Y. et al. Femtosecond electron microscopy of relativistic electron bunches. Light. Sci. Appl. 12, 116 (2023).
Wan, Y. et al. Real-time visualization of the laser-plasma wakefield dynamics. Sci. Adv. 10, 3595 (2024).
Lu, W., Huang, C., Zhou, M., Mori, W. & Katsouleas, T. Nonlinear theory for relativistic plasma wakefields in the blowout regime. Phys. Rev. Lett. 96, 165002 (2006).
Pukhov, A. & Meyer-ter-Vehn, J. Laser wakefield acceleration: the highly non-linear broken-wave regime. Appl. Phys. B 74, 355–361 (2002).
Perl, J., Shin, J., Schumann, J., Faddegon, B. & Paganetti, H: Topas: An innovative proton monte carlo platform for research and clinical applications. Med. Phys. 39, 6818–6837 (2012).
Kroll, F. et al. Tumour irradiation in mice with a laser-accelerated proton beam. Nat. Phys. 18, 316–322 (2022).
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grants nos. XDB0530000, XDB0530100 and XDB0530200) to W.L. and Y.W., National Natural Science Foundation of China (Grants No. 11991071 to W.L. and No. 12405169 to S.L.), Science Fund Program for Distinguished Young Scholars of the National Natural Science Foundation of China (Overseas) to Y.W., Discipline Construction Foundation of “Double World-class Project” to W.L. and J.H., Key Scientific Research Projects of Henan Provincial Colleges and Universities (No. 23B320004 to H.W. and No. 25ZX002 to Y.W.), Henan Province Medical Science and Technology Research Program Provincial and Ministry Co- constructed Youth Project (No. SBGJ202103073 to H.W.). Henan Province Medical Education Research Project (No. WJLX2024061 to H.W.). The simulation work is supported by the National Supercomputing Center in Zhengzhou and the Center of High-Performance Computing, Tsinghua University. The authors thank Dr. Kuo Men from Cancer Hospital Chinese Academy of Medical Sciences and Dr. Tie-Yan Liu, and Dr. Guoqing Liu from Microsoft Research for valuable discussions.
Author information
Authors and Affiliations
Contributions
Y.W. and W.L. conceived the idea, designed the prototype setup and supervised the project. The 20-TW laser system was developed by W.L. and his laser team at the Beijing Academy of Quantum Information Sciences. S.L., B.Z., Z.G., and Y.W. prepared and conducted the experiments with the support of J.L, H.W., Y.P, J.H., and B.G. J.L., Z.G., Y.W., X.W., and Y.M. contributed to animal handling and care. Z.G., H.W., and Y.P. contributed to the EBT3 film calibration and dosimetry. B.Z. performed the TraceWin simulations. Z.G. and B.Z. conducted Monte-Carlo simulations. S.L. and B.Z. contributed to laser operation and maintenance. S.L., Z.G, and B.Z. analyzed the experimental data. S.L., Z.G., and Y.W. wrote the manuscript. All the authors reviewed the manuscript and contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Leonida Gizzi, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, Z., Liu, S., Zhou, B. et al. Preclinical tumor control with a laser-accelerated high-energy electron radiotherapy prototype. Nat Commun 16, 1895 (2025). https://doi.org/10.1038/s41467-025-57122-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57122-z