Abstract
Extracellular plant small RNAs (sRNAs) and/or double-stranded RNA (dsRNA) precursors act as triggers of RNAi in interacting filamentous pathogens. However, whether any of these extracellular RNA species direct gene silencing in plant-interacting bacteria remains unknown. Here, we show that Arabidopsis transgenic plants expressing sRNAs directed against virulence factors of a Pseudomonas syringae strain, reduce its pathogenesis. This Antibacterial Gene Silencing (AGS) phenomenon is directed by Dicer-Like (DCL)-dependent antibacterial sRNAs, but not cognate dsRNA precursors. Three populations of active extracellular sRNAs were recovered in the apoplast of these transgenic plants. The first one is mainly non-vesicular and associated with proteins, whereas the second one is located inside Extracellular Vesicles (EVs). Intriguingly, the third population is unbound to proteins and in a dsRNA form, unraveling functional extracellular free sRNAs (efsRNAs). Both Arabidopsis transgene- and genome-derived efsRNAs were retrieved inside bacterial cells. Finally, we show that salicylic acid (SA) promotes AGS, and that a substantial set of endogenous efsRNAs exhibits predicted bacterial targets that are down-regulated by SA biogenesis and/or signaling during infection. This study thus unveils an unexpected AGS phenomenon, which may have wider implications in the understanding of how plants regulate microbial transcriptome, microbial community composition and genome evolution of associated bacteria.
Similar content being viewed by others
Introduction
RNAi is a conserved gene regulatory mechanism that promotes antiviral defense by repressing translation, accumulation and replication of viral RNAs1. In plants, RNAi also orchestrates resistance against bacterial, fungal and oomycetal pathogens, partly by fine-tuning the expression of immune-responsive genes2,3. The core mechanism of RNAi involves the processing of double-stranded RNAs (dsRNAs) by DCL proteins, leading to the production of 20–25 nt long small RNAs (sRNAs). Small RNAs are then loaded into Argonaute (AGO) proteins to direct post-transcriptional silencing of sequence-complementary mRNA targets, through endonucleolytic cleavage and/or translational inhibition4.
An important feature of plant sRNAs is their ability to trigger non-cell autonomous silencing in adjacent cells as well as in distal tissues5,6. This phenomenon is essential to prime antiviral response ahead of the infection front, but also to translocate silencing signals between plant cells and their non-viral eukaryotic interacting (micro)organisms7,8. For example, plant sRNAs are exported in the fungal pathogens Verticillium dahliae9 and Botrytis cinerea10, as well as in the oomycetal pathogen Phytophthora capsici11, to silence pathogenicity factors. The transfer of sRNAs from plant cells towards B. cinerea and P. capsici cells is in part mediated by extracellular vesicles (EVs)7,11,12. Conversely, fungal sRNAs from B. cinerea, and oomycetal sRNAs from Hyaloperonospora arabidopsidis, are translocated into plant cells to silence defense genes13,14. In addition, some transfer RNA-derived fragments (tRFs), produced by the rhizobium Bradyrhizobium japonicum, can silence genes in the soybean Glycine max to promote nodulation15. However, whether plant sRNAs could be transferred in interacting bacteria, and in turn directly regulate bacterial gene expression, remains elusive.
Artificial cross-kingdom RNAi has long been employed to direct Host-Induced Gene Silencing (HIGS), a technology used to characterize the function of virulence genes or to engineer disease resistance in plants3. HIGS notably relies on in planta expression of dsRNAs bearing homologies to essential and/or virulence genes, and can operate in interacting insects, nematodes, parasitic plants, oomycetes, and fungi16. For example, HIGS confers full protection against Fusarium graminearum17 and B. cinerea10, a phenotype that can be recapitulated by spraying antifungal dsRNAs and/or sRNAs onto wild-type plants prior to infection. The latter Spray-Induced Gene Silencing (SIGS) phenomenon is reminiscent of environmental RNAi, a process that involves the uptake of RNAs from the environment to trigger RNAi. However, so far, HIGS and SIGS have been shown to be effective in eukaryotic (micro)organisms possessing canonical RNAi factors. Indeed, there is no evidence indicating that the stable in planta expression, or the external application, of artificial sRNAs and/or dsRNAs could trigger gene silencing in a plant-interacting bacterium, which does not possess a canonical RNAi machinery, at least from what we know as of today.
Here, we used the Gram-negative pathogenic bacterium Pseudomonas syringae pv. tomato strain DC3000 (Pto DC3000), and the plant Arabidopsis thaliana, as a model interaction system to address these questions. We show that the stable in planta expression of an inverted repeat transgene generating sRNAs directed against two Pto DC3000 virulence factors can dampen bacterial pathogenesis. Three populations of active extracellular sRNAs were recovered from the apoplastic fluid. The first one is mostly associated with proteins that are located outside EVs, while the second one is located inside EVs. Intriguingly, the third one is unbound to proteins and exists in a dsRNA form. Importantly, a subset of these artificial extracellular free sRNAs (efsRNAs) was found internalized by Pto DC3000 cells. This was also true for endogenous efsRNAs produced from various Arabidopsis genomic origins. Finally, we show that SA promotes AGS activity, and that a substantial set of internalized endogenous sRNAs exhibits sequence-complementarity to Pto DC3000 mRNAs, whose cognate protein levels were found down-regulated by SA biogenesis and/or signaling during infection. Overall, this work unveils the vesicular and non-vesicular extracellular sRNAs that are causal for AGS. It also highlights a potential for endogenous plant sRNAs in directly reprogramming gene expression in plant-associated bacteria.
Results
Stable expression of anti-cfa6/hrpL sRNAs in Arabidopsis reduces Pto DC3000 virulence
Pto DC3000 is the causal agent of bacterial speck in tomato and can also infect Arabidopsis thaliana18. This bacterium enters Arabidopsis leaf tissues through stomata, and subsequently reaches the apoplast, where it multiplies at high population levels18. Upon detection of Pto DC3000, Arabidopsis triggers stomatal closure within an hour of infection, which limits the access of this bacterium into inner leaf tissues19,20. As a counter-defense, Pto DC3000 actively reopens stomata at 3 h post-inoculation (hpi), in part, by secreting the phytotoxin coronatine (COR)18,21. Accordingly, a Pto DC3000 Δcfa6 (PtoΔcfa6) strain that is deleted in Cfa6, a gene encoding a structural component of COR22, does not trigger stomatal reopening on wild-type Columbia-0 (Col-0) leaf sections at 3 hpi (Fig. 1a). A similar phenotype is observed in response to a Pto DC3000 ΔhrpL (PtoΔhrpL) strain that is deleted in HrpL (Fig. 1b). The latter gene encodes an alternative sigma factor that directly controls the expression of type III secretion system (TTSS)-associated genes, and indirectly the expression of COR biosynthesis genes23,24. Both phenotypes are rescued upon exogenous application of COR (Fig. 1a, b), indicating that they are caused by the inability of these mutant strains to produce COR23,24. Conversely, and as shown previously19, a normal stomatal reopening phenotype occurs during infection with a type III secretion-defective Pto DC3000 ΔhrcC mutant (PtoΔhrcC) (Fig. 1b), indicating that in a physiological context of infection, type III effectors appear to be dispensable for this response.
a The coronatine (COR)-defective PtoΔcfa6 strain is impaired in its ability to reopen stomata and this phenotype is rescued upon exogenous application of COR. Leaf sections of Col-0 plants were treated with mock (water), WT Pto DC3000 (Pto WT) or PtoΔcfa6—with or without COR—and the stomatal aperture response was analyzed at 3 h post-inoculation (hpi). The concentrations of bacterial strains and of COR were of 108 cfu mL−1 and 1 μM, respectively. The number of stomata analyzed per condition is written underneath each condition and statistical significance was assessed using a one-way ANOVA test (ns: p-value ≥ 0.05; ****: p-value < 0.0001). Similar results were obtained in three independent experiments and are pooled in a single plot. b The PtoΔhrpL strain, but not the PtoΔhrcC strain, is impaired in its ability to reopen stomata and this phenotype is rescued upon exogenous application of COR. The stomatal aperture assay was conducted as in (a). with leaf sections of Col-0 inoculated with Pto WT, ΔhrpL—with or without COR—and ΔhrcC strains. c Schematic representations of the IR-CFA6/HRPL chimeric hairpin construct stably expressed in Arabidopsis transgenic plants (upper panel), and of the 250 bp regions of the Cfa6 (1–250 nt, blue box) and HrpL (99–349 nt, green box) genes that are targeted by sRNAs in Pto DC3000 (bottom panel). d Size distribution and abundance of anti-cfa6/hrpL sRNA reads along the CFA6/HRPL inverted repeat, sequenced from the IR-CFA6/HRPL#4 plants. e Total sRNA reads that map to the IR-CFA6/HRPL inverted repeat are depicted. Anti-cfa6 and anti-hrpL sRNAs are shown in blue and green, respectively. f Pto DC3000 WT no longer induces stomatal reopening in Arabidopsis IR-CFA6/HRPL transgenic lines. Stomatal aperture measurements were conducted on leaf sections of IR-CFA6/HRPL#4, IR-CYP51 and IR-LUC lines infected with Pto WT, as described in (a). g The impaired stomatal reopening phenotypes observed in IR-CFA6/HRPL lines are rescued upon application of exogenous COR. Stomatal aperture measurements were conducted on leaf sections of IR-CFA6/HRPL#4, #5, #10 lines infected with Pto WT, as described in (a), with or without COR. h Arabidopsis IR-CFA6/HRPL lines exhibit reduced Pto WT titers when compared to Col-0 and IR-CYP51-infected plants at 2 days post-infection (dpi). Five to six-week-old Col-0, IR-CYP51 and IR-CFA6/HRPL#4, #5 and #10 plants were dip-inoculated, at a concentration of 5 × 107 cfu mL−1, with Pto WT-GFP. As a control condition, Col-0 plants were treated at the same concentration with PtoΔcfa6-GFP. Number of leaves analyzed is written underneath each condition. Data from three independent experiments are pooled in a single plot. Statistical significance was assessed using a two-way ANOVA test (ns: p-value > 0.05; ****: p-value < 0.0001).
Because both Cfa6 and HrpL genes are (i) critical for Pto DC3000 pathogenesis, notably by promoting bacterial entry into the leaf apoplast and thus ensuring the transition from an epiphytic to an endophytic lifestyle, and (ii) only expressed during infection22,23,24, we reasoned that they represent suitable targets to test the possible occurrence and relevance of AGS. We thus decided to stably express anti-cfa6 and anti-hrpL sRNAs from Arabidopsis and first monitored their possible anti-virulence effects through a stomatal reopening assay. More specifically, we generated Arabidopsis transgenic lines expressing a chimeric inverted repeat, bearing homologies with the coding regions of both Cfa6 and HrpL genes (Fig. 1c). As a negative control, we generated Arabidopsis transgenic lines expressing inverted repeats targeting three cytochrome P450 lanosterol C-14α-demethylase (CYP51) genes of the fungus F. graminearum17,25, or the luciferase (LUC) reporter gene. All the engineered inverted repeat transgenes were driven by the Cauliflower Mosaic Virus (CaMV) 35S promoter. The resulting stable transgenic lines, respectively, referred to as IR-CFA6/HRPL, IR-CYP51, or IR-LUC, expressed the cognate engineered artificial sRNAs, and did not exhibit developmental defects compared to Col-0 plants (Supplementary Fig. 1a–c). Small RNA sequencing (sRNA-seq) from the IR-CFA6/HRPL#4 reference line revealed typical peaks of 21 and 24 nt endogenous sRNAs mapping to the Col-0 genome (Supplementary Fig. 1d), and a high accumulation of 21 nt anti-cfa6/hrpL sRNAs (Fig. 1d), which were produced along the CFA6/HRPL regions of the hairpin (Fig. 1e). Further target prediction analysis of anti-cfa6/hrpL sRNAs against the Col-0 and Pto DC3000 annotated genes, coupled with the analysis of free energy of corresponding sRNA-target pairings, indicated that off-target effects are unlikely (Supplementary Fig. 1e, Supplementary Data 1 and 2).
We further monitored Pto DC3000-induced stomatal reopening response in IR-CFA6/HRPL transgenic lines. Importantly, these transgenic lines were found insensitive to the stomatal reopening response triggered by Pto DC3000 at 3 hpi (Fig. 1f), thereby mimicking the phenotypes observed during infection of Col-0 with the PtoΔcfa6 or PtoΔhrpL strains (Fig. 1a, b). By contrast, the Pto DC3000-induced stomatal reopening phenotype was unaltered in the infected IR-CYP51 and IR-LUC lines, compared to Col-0-infected plants (Fig. 1f), supporting a sequence-specific effect. Furthermore, the compromised stomatal reopening phenotypes detected in three independent IR-CFA6/HRPL-infected lines were rescued upon exogenous application of COR (Fig. 1g). Therefore, these phenotypes are presumably caused by an altered ability of the leaf-associated and/or surrounding Pto DC3000 cells to produce COR.
We next reasoned that, by suppressing stomatal reopening, anti-cfa6/hrpL sRNAs would limit the entry of Pto DC3000 into leaf tissues, and consequently reduce its apoplastic colonization. To test this, we dip-inoculated Col-0, IR-CYP51 and three independent IR-CFA6/HRPL lines with Pto DC3000 and monitored bacterial titers in those plants. It is noteworthy that Pto DC3000 infection did not alter the accumulation of anti-cfa6/hrpL sRNAs in these conditions (Supplementary Fig. 1f). We found that Pto DC3000 was less effective in colonizing the apoplast of IR-CFA6/HRPL lines compared to Col-0- and IR-CYP51-infected plants, thereby mimicking the growth defect of PtoΔcfa6 on Col-0 plants (Fig. 1h). Altogether, these data indicate that the expression of sRNAs directed against Cfa6 and HrpL genes in Arabidopsis plants can reduce the virulence functions of Pto DC3000 during infection.
Antibacterial sRNAs, but not cognate dsRNA precursors, are biologically active
To get a first insight into the active RNA entities responsible for AGS, we introduced the dcl2-1, dcl3-1, and dcl4-2 mutations in a reference line expressing an IR-HRPL inverted repeat under the control of the constitutive Ubi.U4 promoter26. It is noteworthy that this promoter was used here, instead of the 35S promoter, to prevent co-suppression effect caused by the presence of 35S copies in the T-DNA insertional mutant lines27,28. A drastic reduction in the accumulation of anti-hrpL sRNAs was observed in Ubi.U4::IR-HRPL dcl234 compared to Ubi.U4::IR-HRPL control plants, as revealed by northern blot analysis (Fig. 2a, left panel). Furthermore, unprocessed forms of IR-HRPL precursors accumulated in Ubi.U4::IR-HRPL dcl234 plants, while they were barely detectable in the Ubi.U4::IR-HRPL parental line (Fig. 2a, right panel). Collectively, these data support a DCL-dependent processing of HRPL dsRNAs into anti-hrpL siRNAs. Importantly, when the Ubi.U4::IR-HRPL dcl234 plants were used in a stomatal reopening assay, we found that Pto DC3000 triggered a normal stomatal reopening phenotype, similar to the effect observed in Col-0 and dcl234 control plants (Fig. 2b). By contrast, a full suppression of stomatal reopening events was achieved in the Ubi.U4::IR-HRPL reference line (Fig. 2b), which produces abundant anti-hrpL sRNAs (Fig. 2a). Collectively, these data provided evidence that long HRPL dsRNAs are unlikely responsible for the anti-virulence effect, and rather suggested that sRNA species are the RNA entities responsible for AGS. To further test this assumption, we decided to establish a semi-in vitro assay. Col-0 leaf sections were pretreated with IR-CFA6/HRPL#4 total RNA extracts for one hour and were subsequently challenged with Pto DC3000, following which the stomatal aperture events were monitored at 3 hpi. A full suppression of the Pto DC3000-induced stomatal aperture was detected in response to these RNA extracts (Fig. 2c). However, the stomatal reopening phenotype remained unaltered in response to RNA extracts derived from Col-0, IR-CYP51 and IR-LUC plants, supporting sequence-specificity (Fig. 2c). Therefore, the pretreatment of IR-CFA6/HRPL#4 RNA extracts on Col-0 leaf sections recapitulates the anti-virulence effect achieved by IR-CFA6/HRPL#4 leaf sections (Fig. 1f, g). We further conducted size separation of the IR-CFA6/HRPL#4 total RNA extracts and tested the activities of the resulting RNA fractions. The long RNA fraction (>200 nt), which presumably contains long CFA6/HRPL dsRNAs, did not suppress Pto DC3000-triggered stomatal reopening (Fig. 2d; Supplementary Fig. 2a, b). In contrast, the small RNA fraction (<200 nt), likely containing anti-cfa6/hrpL sRNAs, was as active as IR-CFA6/HRPL#4 total RNA extracts (Fig. 2d). Collectively, these data indicate that anti-cfa6/hrpL sRNAs must be the active RNA entities from the IR-CFA6/HRPL#4 total RNA extracts. To confirm this, we made use of the viral protein p19, whose property is to selectively bind sRNA duplexes29,30. Chitin magnetic beads coated with p19 proteins were incubated with total RNA extracts from IR-LUC and IR-CFA6/HRPL#4 plants. The eluted bound sRNA duplexes were subsequently analyzed by northern blot. Large amounts of pulled-down anti-cfa6/hrpL sRNAs, and anti-luc control sRNAs, were detected through this approach (Fig. 2e). Bound sRNA species were mostly 21 nt in length, which is consistent with the high affinity of p19 for 21 nt sRNA duplexes29. Importantly, pulled-down anti-cfa6/hrpL sRNAs were fully capable of suppressing stomatal reopening, such as IR-CFA6/HRPL#4 total RNA extracts (Fig. 2f). In contrast, the pulled-down anti-luc sRNAs were found inactive (Fig. 2f). These data further support that anti-cfa6/hrpL sRNA duplexes, but not anti-luc sRNAs, harbour anti-virulence activity against Pto DC3000. We finally synthesized CFA6/HRPL dsRNAs and anti-cfa6/hrpL sRNA duplexes in vitro (Fig. 2g), and tested their activities. In vitro-synthesized CFA6/HRPL dsRNAs did not alter the ability of Pto DC3000 to reopen stomata, nor did CYP51 control dsRNAs (Fig. 2h). In contrast, in vitro-synthesized anti-cfa6/hrpL sRNAs suppressed stomatal reopening events, which was not the case with anti-cyp51 sRNAs (Fig. 2h). Altogether, these data provide solid evidence that sRNAs, but not their unprocessed dsRNA precursors, are biologically active.
a Accumulation level of anti-hrpL sRNAs in Col-0, Ubi.U4 IR-HRPL, dcl2-1 dcl3-1 dcl4-2 (dcl234), and Ubi.U4 IR-HRPL in dcl234 mutant background (Ubi.U4 IR-HRPL dcl234) was assessed by low molecular weight northern blot analysis. The northern blot results, obtained from the same membrane, are shown at two different expositions: short (left) and long (right). The long exposition was depicted to better visualize unprocessed forms of dsRNA precursors, referred to as “sRNA precursors” in the panel. Because the same membrane was used to obtain the results shown in panel a, the same U6 loading control is pictured under each exposure. This is one representative experiment out of the three independent experiments performed. b Ubi.U4 IR-HRPL dcl234 plants do not suppress Pto DC3000-induced stomatal reopening response. The stomatal reopening assay was conducted on leaf sections of the same genotypes detailed in a. and as described in Fig. 1a. Similar results were obtained in three independent experiments and are pooled in a single plot. The number of stomata analyzed per condition is written underneath each condition and statistical significance was assessed using a one-way ANOVA test (ns: p-value ≥ 0.05; ****: p-value < 0.0001). c Twenty ng μl−1 of total RNA from IR-CFA6/HRPL#4 but not IR-LUC and IR-CYP51 prevents stomatal reopening. Col-0 leaf sections were incubated with total RNA from Col-0, IR-CFA6/HRPL#4, IR-LUC or IR-CYP51 and stomatal reopening assay was performed as in (b). Similar results were obtained in two independent experiments and are pooled in a single plot. d Small RNA species, but not corresponding long RNA species, from IR-CFA6/HRPL#4 plants suppress Pto DC3000-induced stomatal reopening. The stomatal reopening assay was conducted with Pto DC3000 and by incubating Col-0 leaf sections with total, long (>200 nt) or small (<200 nt) RNA fractions, separated from total RNAs of IR-CFA6/HRPL#4 plants. Similar results were obtained in three independent experiments and are pooled in a single plot. (ns: p-value ≥ 0.05; ****: p-value < 0.0001). e Accumulation of anti-cfa6/hrpL sRNAs in IR-CFA6/HRPL#4 and IR-LUC total RNAs before or after p19 pull-down was detected by low molecular weight northern blot analysis. U6 was used as a control of intracellular RNAs. This is one representative experiment out of the three independent experiments performed. f P19 pulled-down sRNAs from the IR-CFA6/HRPL#4 total RNAs suppress the ability of Pto DC3000 to reopen stomata. Stomatal aperture assay was conducted as in (b). Statistical significance was assessed using Student’s t-test (****: p-value < 0.0001). Similar results were obtained in three independent experiments and are pooled in a single plot. g Agarose gel picture of ethidium bromide-stained in vitro synthesized (IVS) long dsRNAs and sRNA duplexes. h In vitro synthesized anti-cfa6/hrpL sRNAs, but not cognate unprocessed dsRNA precursors, suppress Pto DC3000-triggered stomatal reopening. The stomatal reopening assay was conducted as in (b). Similar results were obtained in three independent experiments and are pooled in a single plot.
Anti-hrpL sRNAs are causal for the suppression of stomatal reopening
Although the above findings indicate that anti-cfa6/hrpL sRNAs are effective against Pto DC3000, they do not firmly demonstrate that they are causal for this phenomenon. To address this, we expressed, in the PtoΔhrpL mutant, either a WT HrpL transgene or a mutated (mut HrpL) version that contains as many silent mutations as possible in the sRNA targeted region (Fig. 3a, b). These mutations are expected to alter the binding of anti-hrpL sRNAs, as revealed by just a few predicted sRNA-mut HrpL interactions, whose free energy mean was higher compared to the one of the numerous sRNA-HrpL interactions (Fig. 3c, Supplementary Data 3). Both transgenes were expressed under the constitutive neomycin phosphotransferase II (NPTII) promoter (Fig. 3a). The resulting recombinant bacteria, referred to here as PtoΔhrpL WT HrpL and PtoΔhrpL mut HrpL, restored the ability of PtoΔhrpL strain to trigger the reopening of stomata (Fig. 3d), indicating that both transgenes are functional in a stomatal reopening assay. To assess the specific effect of sRNAs towards suppression of HrpL-mediated stomatal aperture, we generated two independent Arabidopsis 35S::IR-HRPL transgenic lines, referred to as IR-HRPL#1 and #4. These lines overexpressed anti-hrpL sRNAs (Supplementary Fig. 3), and, expectedly, suppressed the ability of Pto DC3000 to reopen stomata (Fig. 3e). Similar results were obtained in response to PtoΔhrpL WT HrpL (Fig. 3e), supporting a sensitivity of this bacterial strain to sRNA action. By contrast, the PtoΔhrpL mut HrpL strain was fully competent in reopening the stomata (Fig. 3e), indicating that anti-hrpL sRNAs no longer exert their anti-virulence effects towards this bacterium. Altogether, these data demonstrate that anti-hrpL sRNAs are causal for the suppression of HrpL-mediated stomatal reopening function.
a Schematic representation of PtoΔhrpL and of the recombinant strains expressing either the WT HrpL or mut HrpL constructs, under the control of the constitutive NPTII promoter. b The sequence of WT HrpL (99–349 nt) selected to generate the inverted repeat transgene (highlighted in green) was aligned with the sequence of mutated HrpL (mut HrpL) designed to contain as many silent mutations as possible in the sRNA targeted region (highlighted in purple). c Thermodynamic energy analysis of sRNA-target interactions between anti-hrpL sRNAs and the WT or mutated HrpL sequence versions. Centre line indicates the median, bounds of the box correspond to the lower (25%) and upper (75%) quartile, the minimum and maximum lines extend to the lowest and highest value, respectively, up to 1.5 the inner-quantile range. WT hrpL n = 452, mut hrpL n = 30. d Both the WT HrpL and mut HrpL constructs fully complemented PtoΔhrpL for its ability to reopen stomata. Col-0 leaves were incubated with PtoΔhrpL, PtoΔhrpL WT HrpL and PtoΔhrpL mut HrpL bacterial strains. Stomatal reopening response was assessed as described previously. Statistical significance was assessed using a one-way ANOVA test (ns: p-value ≥ 0.05; ****: p-value < 0.0001). Similar results were obtained from three independent experiments and the data are pooled in a single plot. e The PtoΔhrpL mut HrpL strain is refractory to anti-hrpL sRNAs action at 3 hpi. The stomatal reopening assay was conducted as in (d), IR-HRPL#1 and #4 leaf sections were treated with mock (water) or the indicated bacteria at a concentration of 108 cfu mL−1. Results of two independent experiments are pooled in a single plot. Statistical significance was assessed using a one-way ANOVA test (ns: p-value > 0.05; ****: p-value < 0.0001).
Active anti-cfa6/hrpL sRNAs from the apoplastic fraction that co-purifies with PEN1-positive EVs are mainly located outside EVs and likely associated with proteins
Since apoplastic EVs were previously shown to contribute to the trafficking of plant sRNAs towards fungal and oomycetal pathogens7,11, we investigated whether they could similarly participate in the transfer of active sRNAs from Arabidopsis towards Pto DC3000. Two main categories of plant EVs have been characterized so far. The first one is composed of vesicles that are heterogenous in size, and that are notably marked by PENETRATION 1 (PEN1)31. The second one, which is required for the transfer of active sRNAs from Arabidopsis towards B. cinerea7,12, is composed of smaller EVs, which are less heterogenous in size, and that are marked by TETRASPANIN8 (TET8)7. We first characterized the apoplastic fluid (APF) fraction from IR-CFA6/HRPL#4 plants co-purified with PEN1-positive EVs. To do so, we vacuum-infiltrated IR-CFA6/HRPL#4 plants with Vesicle-Isolation Buffer (VIB, see methods), and further collected APFs by low-speed centrifugation (at 900 × g for 15 min, at 4 °C). To remove dead cells and cell debris, these APFs were filtered (using a 0.2 µm filter), and the resulting filtered APFs further subjected to ultracentrifugation (at 40,000 × g for 60 min, at 4 °C), using a swing-out rotor (TST41.14). Nanoparticle tracking analysis (NTA) from the recovered “P40” pellets revealed the presence of particles in a size range between 50 to 400 nm (Supplementary Fig. 4a). Transmission Electron Microscopy (TEM) further unveiled particles exhibiting a cup-shaped structure and/or a round vesicle-like morphology, as previously described32, and their diameters ranged between 16 to 409 nm with a mean of 90 nm +/− 4 nm (Supplementary Fig. 4b, c). Furthermore, and as previously shown31, the EV markers PEN1 and PEN3 were readily detected in these P40 pellets (Supplementary Fig. 4d). Endogenous sRNAs sequenced from these APF fractions were mostly composed of tiny RNAs (tyRNAs; 10–17 nt), and were deprived of typical 21 and 24 nt peaks (Supplementary Fig. 4e)33,34. The sRNAs that mapped to the IR-CFA6/HRPL hairpin were composed of both 21 nt sRNAs and tyRNAs (Fig. 4a), and their accumulation profiles along the CFA6/HRPL regions of the hairpin were distinct from the ones of total sRNAs (Figs. 1e and 4b). The latter observation suggests that extracellular sRNAs are either selectively released in this apoplastic fraction, and/or stabilized by specific RNA-binding proteins, as previously proposed12,34. Furthermore, these anti-cfa6/hrpL sRNAs are unlikely derived from degraded cells and/or debris from IR-CFA6/HRPL#4 leaves, because we were unable to detect the U6 small nuclear RNA (snRNA) in the P40 fractions (Fig. 4c), which is readily detectable from total RNAs (Fig. 2a, e; Supplementary Fig. 1b, c, f; Supplementary Fig. 3).
a Size distribution and abundance of anti-cfa6/hrpL sRNA reads from MNase-treated P40 pellets of IR-CFA6/HRPL#4 plants. b Small RNA reads from the P40 pellets of IR-CFA6/HRPL#4 plants that map to the CFA6/HRPL inverted repeat are depicted. Anti-cfa6 and anti-hrpL sRNAs are shown in blue and green, respectively. c Anti-cfa6/hrpL sRNAs accumulation is reduced when the P40 pellets of IR-CFA6/HRPL#4 plants were treated with MNase and ProtK. The P40 pellets from IR-CFA6/HRPL#4 plants were treated with mock, MNase or MNase plus ProtK, and the accumulation of anti-cfa6/hrpL sRNAs was analyzed by northern blot analysis. P40 pellets from untreated IR-CYP51 plants were used as a negative control. The membrane was also probed with U6 to make sure that the P40 fractions were not contaminated by intracellular RNAs. The band intensities of cfa6 /hrpL siRNAs were quantified using ImageJ. Band intensities were normalized to the expression level of the mock condition. The results are shown above each blot. This is one representative experiment out of the three independent experiments performed. d The ability of Pto DC3000 to reopen stomata is impaired in response to P40 pellets from IR-CFA6/HRPL#4 plants, and this effect is compromised in the presence of P40 pellets treated with MNase and Proteinase K (ProtK). The stomatal reopening assay was performed on Col-0 leaf sections incubated with IR-CFA6/HRPL#4 or IR-CYP51-derived P40 pellets that were subjected to different enzymatic treatments. Statistical significance was analyzed using a one-way ANOVA test (ns: p-value ≥ 0.05; *: 0.05 > p-value ≥ 0.01; ****: p-value < 0.0001). Similar results were obtained in three independent experiments and are pooled in a single plot. e Anti-cfa6 and anti-hrpL sRNAs accumulation is significantly reduced after MNase, ProtK and Triton X100 (TX100) treatments. Accumulation of sRNAs in IR-CFA6/HRPL#4- and IR-CYP51-derived P40 pellets was detected by northern blot analysis. The band intensities of cfa6/hrpL siRNAs were quantified using ImageJ. Band intensities were normalized to the expression level of the mock condition. The results are shown above each blot. This is one representative experiment out of the two independent experiments performed.
We next investigated whether the anti-cfa6/hrpL sRNAs from the P40 pellets could be functional. To this end, we made use of the previously described semi-in vitro stomatal reopening assay (Fig. 2), performed here with P40 pellets instead of RNAs. The P40 pellets from IR-CFA6/HRPL#4 APFs suppressed stomatal reopening triggered by Pto DC3000, compared to the P40 pellets from IR-CYP51 APFs (Fig. 4d). This response was also detected upon incubation with the P40 pellets of IR-HRPL#4 APFs, but was almost fully abolished in the presence of PtoΔhrpL mut HrpL, but not PtoΔhrpL WT HrpL (Supplementary Fig. 4f). Collectively, these data not only provide evidence that anti-cfa6/hrpL sRNAs from the P40 pellets are biologically active, but also that the population of anti-hrpL sRNAs operates in a sequence-specific manner in Pto DC3000 cells. Nevertheless, the residual biological activity detected with the PtoΔhrpL mut HrpL strain treated with the P40 fraction of IR-CFA6/HRPL#4 plants, suggests that sRNA-independent entities from this APF fraction might additionally contribute to the stomatal reopening phenotype.
The Arabidopsis apoplastic P40 pellets are composed of a mixture of particles, including PEN1-positive EVs34. To determine in which particles the active anti-cfa6/hrpL sRNAs could be present, we subjected the P40 pellets from IR-CYP51 and IR-CFA6/HRPL#4 APFs to different enzymatic treatments and subsequently monitored their activities. It is noteworthy that none of these enzymatic treatments alters the morphology, the concentration nor the size distribution of apoplastic EVs (Supplementary Fig. 4a–c). The micrococcal nuclease (MNase) treatment, which degrades unprotected RNAs that are outside EVs and/or in contact with EVs, did not affect the ability of the P40 pellets to suppress stomatal reopening, nor the accumulation of anti-cfa6/hrpL sRNAs, as revealed by northern blot analysis (Fig. 4c, d). These data suggested that the active anti-cfa6/hrpL sRNAs from the P40 pellets are either inside EVs and/or outside EVs but associated with proteins, and thus protected from MNase-mediated degradation. Consistent with the latter hypothesis, we found that the combined MNase and Proteinase K treatment led to a decrease in the accumulation of anti-cfa6/hrpL sRNAs, and almost fully suppressed Pto DC3000-induced stomatal reopening (Fig. 4c, d; Supplementary Fig. 4g). An extra Triton X-100 (TX100) treatment, which disrupts EVs integrity31, led to an enhanced reduction in anti-cfa6/hrpL sRNA levels (Fig. 4e). This suggests the additional presence of intravesicular anti-cfa6/hrpL sRNAs that might contribute to the residual biological activity detected upon MNase plus Proteinase K treatments (Fig. 4d). Altogether, these data indicate that active anti-cfa6/hrpL sRNAs from the P40 pellets are mainly located outside EVs and presumably associated with proteins.
Active anti-cfa6/hrpL sRNAs from the apoplastic fraction that co-purifies with TET8-positive EVs are located inside EVs
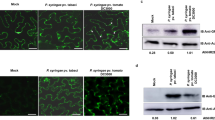
We next characterized the APF fraction from IR-CFA6/HRPL#4 plants co-purifying with TET8-positive EVs. To this end, we collected the supernatants from the above P40 pellets and subjected them to ultracentrifugation (at 100,000 × g for 60 min, at 4 °C), using the same swing-out rotor. The resulting “P100-P40” pellets were subsequently washed and analyzed microscopically, molecularly and functionally. An immunogold labeling approach, using native antibody recognizing the large extravesicular loop (the EC2 domain) of TET8 and a secondary antibody coating gold beads, confirmed the presence of TET8-positive EVs in these P100-P40 pellets (Fig. 5a). It is noteworthy that this subclass of EVs represented 22% of the whole vesicles analyzed from these APF fractions (Supplementary Fig. 5a). However, the proportion of TET8-positive EVs from P100-P40 fractions might be underestimated as TET8 molecules might not be adequately exposed during the drying and dehydration process, thereby preventing antibody binding. By contrast, none of the EVs from the P100-P40 fractions analyzed were labeled with an anti-UGPase antibody (Fig. 5a), indicating that the immunogold staining of TET8-positive EVs is specific. We next sequenced sRNAs from the P100-P40 pellets of IR-CFA6/HRPL#4 APFs. As observed with P40 pellets, endogenous sRNAs sequenced from P100-P40 pellets were mostly composed of tyRNAs (Supplementary Fig. 5b). Furthermore, the sRNAs that mapped to the CFA6/HRPL inverted repeat were composed of 21 nt sRNAs and tyRNAs (Fig. 5b), and their accumulation profiles along the CFA6/HRPL regions of the hairpin were distinct from the ones of total sRNAs (Figs. 1e and 5c). As previously observed with P40 fractions (Fig. 4c), we were unable to detect U6 snRNA from P100-P40 fractions (Fig. 5d), indicating that the detected anti-cfa6/hrpL sRNAs are unlikely derived from degraded cells and/or debris from IR-CFA6/HRPL#4 leaves.
a P100-P40 pellets contain TET8-positive EVs. EVs were visualized through TEM analysis, using native anti-TET8 (PhytoAB, PHY2750A) or UGPase (Agrisera) primary antibodies, followed by detection with a gold-conjugated secondary antibody. White arrows indicate the presence of gold beads. Scale bar: 50 nm. b Size distribution and abundance of anti-cfa6/hrpL sRNA reads from MNase-treated P100-P40 pellets of IR-CFA6/HRPL#4 plants. c Small RNA reads from the P100-P40 pellets of IR-CFA6/HRPL#4 plants that map to the CFA6/HRPL inverted repeat are depicted. Anti-cfa6 and anti-hrpL sRNAs are shown in blue and green, respectively. d Anti-cfa6/hrpL sRNAs accumulation is reduced when P100-P40 pellets of IR-CFA6/HRPL#4 plants were treated with TX100, MNase and ProtK. The P100-P40 pellets from IR-CFA6/HRPL#4 plants were treated with mock, MNase plus ProtK, or TX100 plus MNase plus ProtK and the accumulation of anti-cfa6/hrpL sRNAs was detected by northern blot analysis. P100-40 pellets from untreated IR-CYP51 plants were used as a negative control. The membranes used were hybridized with either the cfa6 probe (upper panel) or the hrpL probe (bottom panel). Of note, it was not possible to sequentially probe the same membrane with the cfa6 and hrpL probes because of low abundance of these sRNAs from P100-P40 pellets. The band intensities of cfa6/hrpL sRNAs were quantified using ImageJ. Band intensities were normalized to the expression level of the mock condition. The results are shown above each blot. The membranes were also probed with U6 to make sure that the P100-P40 fractions were not contaminated by intracellular RNAs. e The ability of Pto DC3000 to reopen stomata is impaired in response to P100-P40 pellets from IR-CFA6/HRPL#4 plants, and this effect is compromised when these P100-P40 pellets were treated with MNase, ProtK, and TX100. Stomatal aperture measurements were performed on Col-0 leaf sections incubated with IR-CFA6/HRPL#4 or IR-CYP51-derived P100-P40 pellets that were subjected to different enzymatic treatments. Statistical significance was analyzed using a one-way ANOVA test (ns: p-value ≥ 0.05; ****: p-value < 0.0001). Similar results were obtained in three independent experiments and are pooled in a single plot.
We further subjected the P100-P40 pellets from IR-CFA6/HRPL#4 APFs to different enzymatic treatments and monitored their activities. Of note, none of these enzymatic treatments markedly alter the size distribution of EVs from these APF fractions, as revealed by TEM analysis (Supplementary Fig. 5c). Interestingly, in contrast with the results obtained with P40 pellets, the combined MNase and Proteinase K treatment did not alter the biological activity of P100-P40 pellets (Figs. 4d and 5e), nor the treatments with MNase or Proteinase K alone (Supplementary Fig. 5d, e). The former combined treatment did not markedly alter the accumulation of anti-cfa6/hrpL sRNAs either (Fig. 5d). These data indicate that anti-virulence sRNAs from the P100-P40 pellets are protected from enzymatic degradation. They also suggest that these active sRNAs are possibly protected inside the lumen of EVs from this APF fraction. Consistent with this hypothesis, we found that a triple treatment with MNase, Proteinase K and TX100, strongly altered the accumulation of anti-cfa6/hrpL sRNAs (Fig. 5d). In addition, it fully abolished the ability of the IR-CFA6/HRPL#4 P100-P40 pellets to suppress Pto DC3000-induced stomatal reopening (Fig. 5e). Altogether, these data demonstrate that the APF fraction co-purifying with TET8-positive EVs contains active antibacterial sRNAs that are located inside EVs.
Active anti-cfa6/hrpL sRNAs from the apoplastic fraction depleted in canonical EVs are likely unbound to protein and in a free form
We next investigated whether active anti-cfa6/hrpL sRNAs could additionally be present in the APF fraction that does not co-purify with EVs. For this purpose, we collected the supernatants from the above P100-P40 pellets. The recovered SN fractions exhibited a substantial reduction in extracellular particles, as observed by NTA (Supplementary Fig. 6a), and were notably deprived of the PEN1 and PEN3 markers (Supplementary Fig. 4d). RNAs from the SN fraction of IR-CFA6/HRPL#4 APFs were further precipitated and subjected to sRNA-seq. Small RNAs that mapped to the Col-0 genome were almost exclusively composed of tyRNAs (Supplementary Fig. 6b). Anti-cfa6/hrpL sRNAs were represented by small amounts of 21 nt sRNAs and large amounts of tyRNAs (Fig. 6a). However, a more pronounced accumulation of 21 nt anti-cfa6/hrpL sRNAs, compared to cognate tyRNAs, was detected by northern blot analysis (Fig. 6b, Supplementary Fig. 6c). This suggests that biases occurring during sRNA library preparation (e.g. adapter ligation), coupled with yet-unknown post-transcriptional sRNA modifications, might hamper the retrieval of all 21 nt long anti-cfa6/hrpL sRNAs from these SN fractions. As observed for P40 and P100-P40 fractions, we also found that the accumulation profiles of SN-derived anti-cfa6/hrpL along the CFA6/HRPL regions of the hairpin were different from the ones of total sRNAs (Figs. 1e and 6b). Furthermore, we were unable to detect U6 snRNA from SN fractions (Fig. 6b), indicating that the detected anti-cfa6/hrpL sRNAs are unlikely derived from degraded cells and/or debris from IR-CFA6/HRPL#4 leaves.
a Size distribution and abundance of anti-cfa6/hrpL sRNA reads from the SN fraction of IR-CFA6/HRPL#4 plants. b Small RNA reads from the SN fraction of IR-CFA6/HRPL#4 plants that map to the CFA6/HRPL inverted repeat are depicted. Anti-cfa6 and anti-hrpL sRNAs are shown in blue and green, respectively. c Accumulation of anti-cfa6/hrpL sRNAs in IR-CFA6/HRPL#4 and IR-LUC-derived SN fractions before or after p19 pull-down assay was detected by northern blot analysis. U6 was used as a control of intracellular RNA. The membrane was also probed with U6 to make sure that the SN fractions were not contaminated with intracellular RNAs. This is one representative experiment out of the two independent experiments performed. d The SN fraction from IR-CFA6/HRPL#4 plants prevents Pto DC3000-induced stomatal reopening, and this effect is abolished upon treatment of this APF fraction with MNase. Statistical significance was assessed using a one-way ANOVA test (ns: p-value ≥ 0.05; ****: p-value < 0.0001). Similar results were obtained in three independent experiments and are pooled in a single plot. e P19 pulled-down sRNAs from the IR-CFA6/HRPL#4 SN fraction suppress Pto DC3000-induced stomatal reopening. The stomatal reopening assay was conducted as in (c). Statistical significance was assessed using Student’s t-test (****: p-value < 0.0001). Similar results were obtained in three independent experiments and are pooled in a single plot.
We further monitored the activity of the SN fraction from IR-CFA6/HRPL#4 APFs. This APF fraction was found to suppress stomatal aperture triggered by Pto DC3000, compared to the one derived from IR-CYP51 plants (Fig. 6d). The same phenotype was detected in response to the SN fraction from IR-HRPL#4 APFs, but was impaired in the presence of PtoΔhrpL mut HrpL, but not PtoΔhrpL WT HrpL (Supplementary Fig. 6d). Collectively, these data indicate that anti-cfa6/hrpL sRNAs in the SN fraction are biologically active and that anti-hrpL sRNAs function in a sequence-specific manner in Pto DC3000 cells. To characterize in more depth the active sRNAs from the IR-CFA6/HRPL#4 SN fraction, we treated it with MNase or Proteinase K, and subsequently monitored its activity. The Proteinase K treatment did not alter the Pto DC3000-induced stomatal reopening phenotype (Fig. 6d), despite a substantial decrease in the accumulation of ~21 nt anti-cfa6/hrpL sRNAs, and of the control microRNA miR167-5p (Supplementary Fig. 6c). Collectively, these results indicate that the majority of ~21 nt anti-cfa6/hrpL sRNAs from the SN fraction is associated with proteins and not functional. To our surprise, we found that the MNase treatment alone fully abolished the ability of the IR-CFA6/HRPL#4 SN fraction to suppress Pto DC3000-induced stomatal reopening (Fig. 6d). Nevertheless, we were unable to detect, at the resolution of northern blot analysis, a significant decrease in the accumulation of anti-cfa6/hrpL sRNAs upon MNase treatment (Supplementary Fig. 6c). This effect was not due to a partial efficacy of the MNase treatment on sRNAs, because a full degradation of anti-cfa6/hrpL sRNAs was observed when RNA extracts from IR-CFA6/HRPL#4 plants were treated with this enzyme (Supplementary Fig. 6e). Collectively, these data suggest that a low-abundant pool of active anti-cfa6/hrpL sRNAs, and/or discrete sRNA species from the IR-CFA6/HRPL hairpin, must be unbound to proteins in the SN fraction, and thus sensitive to MNase action. We named these novel and functional extracellular sRNA species “extracellular free sRNAs” or efsRNAs.
Anti-cfa6/hrpL efsRNA duplexes are recovered in the apoplast and are biologically active
The fact that (i) anti-cfa6/hrpL duplexes, purified from IR-CFA6/HRPL#4 plants or in vitro-synthesized, were found functional (Fig. 2e–h), and (ii) sRNA duplexes are typically more stable than single-stranded RNAs35, prompted us to investigate whether active anti-cfa6/hrpL efsRNA duplexes could be present in the apoplast. To purify sRNA duplexes from SN fractions, p19-chitin magnetic beads were incubated with the SN fractions from IR-LUC and IR-CFA6/HRPL#4 APFs, and the eluted bound sRNA duplexes were further analyzed by northern blot. We consistently retrieved comparable amounts of anti-cfa6/hrpL and anti-luc sRNAs in the p19 pulled-down RNAs from IR-CFA6/HRPL#4 and IR-LUC SN fractions, respectively (Fig. 6b). These data demonstrate the presence of both anti-cfa6/hrpL and anti-luc efsRNA duplexes in these APF fractions. Importantly, the p19 pulled-down sRNAs from the IR-CFA6/HRPL#4 SN fraction specifically suppressed the ability of Pto DC3000 to reopen stomata, to the same extent as total sRNAs from this apoplastic fraction (Fig. 6e). Collectively, these data provide evidence that the SN fraction of IR-CFA6/HRPL#4 APFs contains active efsRNAs, which are, at least in part, in a free and dsRNA form.
A subset of SN-derived anti-hrpL sRNAs, including a functional 21 nt sRNA, is internalized by Pto DC3000 cells
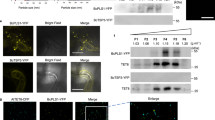
Although anti-hrpL efsRNAs were found to be causal for AGS (Supplementary Fig. 6d), it was not experimentally shown whether they could be transferred in the cytoplasm of Pto DC3000 cells. To test this, we incubated Pto DC3000 cells with SN fractions from IR-CFA6/HRPL#4 APFs for 2 h. Bacterial cells were further collected, and their outer membrane lysed using a buffer containing Ethylenediaminetetraacetic acid (EDTA) prior to washing and RNA isolation (Fig. 7a)36. This stringent condition ensured that sRNAs adhering onto the bacterial surface were removed from our samples. Consistent with an effective degradation of the bacterial outer membrane, the EDTA treatment substantially increased the uptake of propidium iodide in Pto DC3000 cells (Supplementary Fig. 7a). Nevertheless, it also altered Pto DC3000 survival (Supplementary Fig. 7b), which could account for an under-representation of sequenced sRNAs inside bacterial cells. We then prepared sRNA sequencing libraries corresponding to mock- and SN-treated conditions. To retrieve anti-hrpL sRNAs that were specifically derived from these SN fractions, rather than from bacterial hrpL transcripts (e.g., RNA degradation products), we established a first bioinformatic filter. Briefly, this filter only keeps reads that were not present in the control samples, which were untreated with SN fractions. In comparison to anti-hrpL sRNAs derived from the SN fractions of IR-CFA6/HRPL#4 APFs (Fig. 6a), an over-representation of tyRNAs, in a 10–12 nt size window, was recovered inside Pto DC3000 (Fig. 7b, c). This suggests that Pto DC3000 either preferentially takes-up tyRNAs and/or that longer sRNAs are internalized by Pto DC3000 and further processed inside this bacterium. Nevertheless, we still detected a small peak of 21 nt long sRNAs that was constantly observed across the biological replicates (Fig. 7c). We further isolated the most represented 21 nt anti-hrpL internalized sequence in both replicates, and chemically-synthesized the corresponding sRNA duplex labeled with the fluorescent dye ATT0 565, positioned on the 5’ end of the sRNA strand that is complementary to the HrpL mRNA sequence (Fig. 7d, Supplementary Fig. 7c). Furthermore, because active anti-hrpL sRNAs were found to be dependent on DCLs for their biogenesis (Fig. 2a, b), we designed this synthetic sRNA duplex with 2 nt-3’ overhangs, to mimic a DCL product (Fig. 7d, Supplementary Fig. 7c). Confocal imaging of Pto DC3000-GFP cells incubated with this ATTO 565-labeled anti-hrpL sRNA duplex, confirmed its internalization by, and/or association with, bacterial cells (Fig. 7e, white arrows). Importantly, the cognate unlabeled sRNA duplex (WT hrpL) exhibited full functionality in a stomatal reopening assay, which was not the case of its cognate mutated version (mut hrpL), which behaved as a non-functional anti-cyp51 sRNA duplex control (Fig. 7f). Altogether, these data demonstrate that anti-hrpL sRNAs are transferred from the SN fractions of IR-CFA6/HRPL#4 APFs to the cytoplasm of Pto DC3000 cells. They also show that a chemically-synthesized 21 nt sRNA duplex, corresponding to a bona fide internalized anti-hrpL sRNA, is functional inside Pto DC3000.
a Schematic representation of the experimental set-up employed to sequence sRNAs that are internalized by Pto DC3000. Bacterial cells were either incubated in VIB or in SN, derived from SA-treated IR-CFA6/HRPL#4 plants (see methods), for 2 h before collection of the cells and washing with EDTA. Total RNAs were further extracted and used for sRNA-seq. b Internalized sRNA reads that map to the CFA6/HRPL inverted repeat are depicted. Anti-cfa6 and anti-hrpL sRNAs are shown in blue and green, respectively. c Size distribution and abundance of anti-cfa6/hrpL sRNA reads. d The most represented 21 nt anti-hrpL sequence is framed in blue on top of the panel, with occurrence numbers for both replicates shown above it. Below is depicted the corresponding duplex version labeled with ATTO 565 (red star) in the 5’ end of the anti-hrpL sRNA strand that is complementary to HrpL mRNAs. e Imaging of Pto DC3000-GFP (green) incubated with the hrpL duplex labeled with ATTO 565 (red). White arrows highlight examples of signals co-localization between ATTO 565 and GFP. Scale bar: 5 µm. f WT hrpL duplexes but not CYP51, nor mut hrpL duplexes, suppress Pto DC3000-induced stomatal reopening. The stomatal aperture measurements were performed on Col-0 leaf sections incubated with sRNA duplexes. Statistical significance was analyzed using a one-way ANOVA test (ns: p-value ≥ 0.05; ****: p-value < 0.0001). Similar results were obtained in three independent experiments and are pooled in a single plot.
Salicylic acid-treated IR-CFA6/HRPL plants trigger silencing of HrpL in Pto DC3000 cells
To monitor the silencing effect of anti-hrpL sRNAs on the HrpL target, we developed an experimental set-up, whereby Pto DC3000 cells expressing an epitope-tagged version of hrpL were exposed to extra-cellular/organismal sRNAs from IR-CFA6/HRPL#4 leaf sections (Fig. 8a). These conditions are reminiscent of the ones used in the stomatal reopening assay (Fig. 1f), with the exception that IR-CFA6/HRPL#4 plants were pretreated with SA. It is noteworthy that SA promotes the expression and/or activity of RNAi factors37,38,39,40,41,42,43, as well as the release of Arabidopsis EVs, and of silencing factors, in the apoplast31, and was thus included here to eventually optimize the silencing effect mediated by anti-hrpL sRNAs. More specifically, IR-CYP51 and IR-CFA6/HRPL#4 plants were pretreated 48 h before incubation with either mock or SA, and the corresponding leaf sections were further challenged for 2 h with the Pto DC3000 strain expressing a functional C-terminal FLAG-tagged version of hrpL under its native promoter (Fig. 8a)44. The mRNA and protein levels produced by HrpL were subsequently monitored by RT-qPCR and western blot analyses, respectively. With this assay, we did not observe a consistent and significant decrease in the accumulation of HrpL mRNAs across different biological replicates, upon incubation of bacterial cells with mock or SA-pretreated IR-CFA6/HRPL#4 leaves (Supplementary Fig. 8). On the contrary, although leaf sections from IR-CYP51 plants pretreated with SA exhibited a negative effect on HrpL protein accumulation, a more substantial reduction was always detected in the presence of IR-CFA6/HRPL#4 leaves treated in the same condition (Fig. 8b, c). Altogether, these data demonstrate that leaf sections of IR-CFA6/HRPL#4 plants pretreated with SA trigger the silencing of HrpL in Pto DC3000 cells. They also indicate that SA enhances HIGS efficacy from stable transgenic plants expressing antibacterial sRNAs.
a Schematic representation of the experimental set-up employed to expose Pto DC3000 cells to plant-derived extra-cellular/organismal sRNAs. IR-CFA6/HRPL#4 or IR-CYP51 plants were sprayed with SA (2 mM) for 48 h prior to the experiment, and leaf sections from those plants were further incubated with a Pto DC3000 strain expressing a Flag-tagged version of HrpL, namely the Pto DC3000 hrpL-FLAG strain44. Some possible extra-cellular/organismal sRNA populations are illustrated in the circle. RBPs, EVs, dsRNA, and ssRNA, stand for RNA-binding proteins, extracellular vesicles, double-stranded RNA and single-stranded RNA, respectively. b Western blot analysis showing the reduction in the accumulation of HrpL proteins upon incubation of the Pto DC3000 hrpL-FLAG strain with leaf sections of SA-treated IR-CFA6/HRPL#4 plants, compared to leaf sections of IR-CYP51 plants treated in the same condition. The accumulation of the elongation factor EF-Tu served as loading control. Pto DC3000 pBS181 contains the empty expression vector pBS181 and served as a negative control. The band intensities of hrpL-FLAG protein were quantified using ImageJ. They were normalized to corresponding EF-Tu bands. Ratio is pictured above the blot (FLAG/EF-Tu). IR-CYP51 or IR-CFA6/HRPL#4 SA-treated ratios were then further normalized to IR-CYP51 and IR-CFA6/HRPL#4 mock condition ratios, respectively. This ratio is also visible above the blot (SA/mock). This is one representative experiment out of the six independent experiments performed. c Plot depicting the band intensity of Pto DC3000 hrpL-FLAG protein levels normalized to corresponding EF-Tu bands. IR-CYP51 or IR-CFA6/HRPL#4 SA-treated ratios were then further normalized to IR-CYP51 and IR-CFA6/HRPL#4 mock condition ratios, respectively. Similar results were obtained in six independent experiments and the data are pooled in a single plot. Statistical significance was assessed using Student’s paired t-test (ns: p-value ≥ 0.05).
Endogenous sRNAs, from various genomic origins, are internalized by Pto DC3000 and exhibit predicted targets, some of which being repressed by SA biogenesis/signaling
We next assessed the potential contribution of endogenous Arabidopsis extracellular sRNAs on the targeting, and possibly silencing, of Pto DC3000 genes. To identify the putative Pto DC3000 targets of Arabidopsis sRNAs, we focused our analysis on SN-derived sRNAs that were found internalized by Pto DC3000 cells. For this purpose, we established a second bioinformatic filter, designed to retrieve, exclusively, Arabidopsis sRNAs inside Pto DC3000. Briefly, the sRNA sequences recovered from our first filter were selected, and those exhibiting multimapping between the Arabidopsis TAIR10 reference genome and the Pto DC3000 genome, further discarded. The size distribution analysis of these stringently filtered sRNAs unveils a majority of tyRNAs, which were derived from various genomic origins (Fig. 9a, b). This observation is consistent with an over-representation of this sRNA category from the SN-derived sRNAs mapping to the Arabidopsis genome (Supplementary Fig. 6b). It might additionally involve a further processing of SN-derived sRNAs inside bacterial cells. In agreement with the results obtained on transgenic lines expressing the artificial inverted repeat CFA6/HRPL, sRNAs produced from two well-described endogenous inverted repeats, namely IR71 and IR203945, were also retrieved inside Pto DC3000 (Fig. 9b–f). However, in this case, only tyRNAs were recovered (Fig. 9c, d). The remaining internalized endogenous sRNAs were produced from other genomic regions, including mRNAs, transposable elements (TEs), rRNAs and tRNAs (Fig. 9b). In addition, because plant and mammalian tRNA-derived sRNAs (tsRNAs) (i) are commonly retrieved in extracellular compartments and biofluids33,34,46,47, and (ii) have recently been shown to be transferred bidirectionally between host and microbes15,48,49,50,51,52, we investigated whether Arabidopsis tsRNAs could be part of the most represented sRNAs inside Pto DC3000. To do so, we extracted the top 100 most represented internalized sRNA sequences that are over 17 nt long in both replicates and examined the Arabidopsis tRNA database tRex53, for the presence of tsRNAs. We found that some of the top over-represented sRNA sequences are tRFs, derived from various tRNA precursors, including ProAGG.17 (Fig. 9g). Furthermore, we noticed that the corresponding tRF sequence displays plausible sequence-complementary targets in Pto DC3000 (Fig. 9h). Therefore, some Arabidopsis tRFs are taken-up by Pto DC3000 and exhibit a potential for targeting transcripts in this bacterium.
a Size distribution of all the endogenous sRNAs that were recovered in Pto DC3000 cells. SN fractions from SA-treated IR-CFA6/HRPL#4 plants were incubated for 2 h with Pto DC3000 cells (Pto DC3000 + SN 2 h), and the internalized endogenous sRNAs analyzed using the second stringent filter (see methods). b Class repartition of reads after the second filtering step. c Size distribution of reads mapping to IR71 after the second filtering step. d Size distribution of reads mapping to IR2039 after the second filtering step. e, f Coverage of sRNAs on IR71 (e) and IR2039 (f) after the second filtering step. g RNA-fold structure of ProAGG.17. The most represented tRNA sequence from our filtered samples is highlighted in orange. h Examples of predicted Pto DC3000 targets of the tRF highlighted in (g). The three best predictions according to the minimal free energy value are represented. i Three hundred seven Pto DC3000 SA-responsive proteins were predicted to be targets of internalized sRNAs. The heatmap depicts their accumulation in Col-0, sid2, pad4 and sid2 pad4 that were infected for 48 h with Pto DC300054. Z-score normalized (by row) values based on protein accumulation values reported in Nobori et al.54. j Twenty-two of the SA-responsive proteins corresponded to targets of the top 100 most represented internalized sRNA reads. Their accumulation in Col-0-, sid2-, pad4- and sid2 pad4-infected plants is shown in the heatmap. k Representation of some candidate sRNA-Target pairings corresponding to internalized sRNAs predicted to target mRNAs, whose protein levels are down-regulated in Col-0-infected plants but no longer repressed in sid2-, pad4-, and/or pad4/sid2-infected plants (from the 5 purple framed candidates depicted in j).
Finally, because SA was found here to promote AGS (Fig. 8), we analyzed whether SA biogenesis and/or signaling contribute to the possible silencing of sRNA predicted targets in Pto DC3000. To do so, we made use of Pto DC3000 proteomic datasets generated from infected Col-0, the SA biogenesis mutant sid2, the SA signaling mutant pad4, and the double sid2 pad4 mutant54. By cross-referencing the list of predicted Pto DC3000 targets from the whole set of internalized sRNAs, with the one of SA-responsive proteins, we found that 209 (68.1%), 213 (69.4%), 214 (69.7%), out of the 307 SA-responsive predicated sRNA targets, were less expressed in Col-infected plants compared to sid2-, pad4- and sid2 pad4-infected plants, respectively (Fig. 9i, Supplementary Data 4). A similar proportion of under-expressed targets, represented by 63.6% in Col-0 vs. sid2, 68.2% in Col-0 vs. pad4 and 68.2% in Col-0 vs. sid2 pad4, was observed when the same analysis was performed on the predicted targets of the top 100 most represented internalized sRNAs, which are above 17 nt in size (Fig. 9j). Among those, we recovered functionally relevant Pto DC3000 genes, whose cognate mRNAs exhibit plausible Watson-Crick base-pairing with specific internalized sRNAs, including the type III effector HopE1, TonB dependent siderophore receptors, a type I restriction-modifying enzyme as well as the cell division-related gene ftsk (Fig. 9k). Altogether, these data indicate that the uptake of sRNAs by Pto DC3000 is not restricted to artificial sRNAs, but also occurs with endogenous sRNAs. They also highlight a potential for Arabidopsis endogenous sRNAs in targeting, and possibly silencing, Pto DC3000 genes, in part through SA biogenesis and/or signaling.
Discussion
Arabidopsis-encoded anti-cfa6/hrpL sRNAs are externalized from plant cells and dampen Pto DC3000 pathogenesis
We show here that Arabidopsis-encoded artificial sRNAs can target Pto DC3000 virulence-associated genes, resulting in the dampening of bacterial pathogenesis. In particular, we found that host-encoded sRNAs directed against the Pto DC3000 Cfa6 and HrpL genes, fully suppressed bacterial-induced stomatal reopening (Fig. 1). Furthermore, we showed that, in Arabidopsis plants expressing anti-hrpL sRNAs, this phenotype no longer occurred in response to the PtoΔhrpL mut HrpL strain (Fig. 3). Altogether, these findings indicate that antibacterial sRNAs act in a sequence-specific manner and can operate at the pre-invasive stage of infection, presumably by limiting COR biosynthesis in Pto DC3000 cells that come in contact with the leaf surface. In addition, we found that anti-cfa6/hrpL sRNAs reduced the ability of Pto DC3000 to multiply in the leaf apoplast of Arabidopsis upon dip-inoculation (Fig. 1). This phenotype might be caused by the inability of Pto DC3000 to reopen stomata in IR-CFA6/HRPL lines, thereby limiting its access to inner leaf tissues and thus to the apoplast, which is its main replicative niche. Collectively, these data imply that antibacterial sRNAs must be externalized from plant cells towards the surface of Arabidopsis leaves to reach epiphytic Pto DC3000 populations.
Anti-cfa6/hrpL sRNA duplexes, but not their dsRNA precursors, are biologically active
By using genetic and molecular biology approaches (Fig. 2), we demonstrated that DCL-dependent antibacterial sRNAs, but not cognate dsRNA precursors, are the RNA entities responsible for AGS (Fig. 10). These data imply that Pto DC3000 must be capable of taking-up—passively and/or actively—anti-cfa6/hrpL sRNAs, despite the presence of a cell wall and an intricate double membrane structure. This assumption was confirmed by showing that artificial anti-hrpL sRNAs from the non-vesicular apoplastic fraction of IR-CFA6/HRPL#4 plants, but also a chemically synthesized 21 nt anti-hrpL sRNA duplex with 2-nt 3’ overhangs, were transferred into the cytoplasm of Pto DC3000 cells (Fig. 7). These results thus unveil major differences with environmental RNAi in eukaryotic organisms, such as C. elegans and plant herbivores, which specifically rely on long dsRNAs, or filamentous pathogens, which are dependent on both dsRNAs and sRNAs55. They also suggest that the industrial dsRNA production platforms, currently designed to produce long dsRNAs against pathogens and parasites, are not adapted to produce RNA-based biocontrol agents against bacterial pathogens.
Model depicting the characterized Arabidopsis-encoded artificial sRNA species directing gene silencing in interacting Pto DC3000 cells. The Arabidopsis IR-CFA6/HRPL#4 transgenic plants produce an inverted repeat transcript that is processed by DCL proteins into anti-cfa6/hrpL sRNAs (in green). The cytoplasmic anti-cfa6/hrpL sRNA species are further incorporated into protein complexes containing RNA binding proteins (RBPs), or potentially present in a free and dsRNA form in the cytosol, or in EVs. Both sRNA species are then exported in the apoplast through unknown mechanisms (dashed arrows). Three populations of active anti-cfa6/hrpL sRNAs have been characterized in this study: a first pool that is bound to proteins, which are either non-associated to PEN1-positive EVs, or potentially located on their surface. A second pool of sRNAs that is located inside TET8-positive EVs, and a third pool that is unbound to proteins and in a free and dsRNA form, named efsRNAs. Active plant extracellular-sRNA species (e.g. anti-hrpL sRNAs), are subsequently transferred towards Pto DC3000 cells (dashed arrows), to direct sequence-specific gene silencing of hrpL through as-yet unknown mechanisms. Finally, the model depicts the endogenous sRNAs (in red), produced from various Arabidopsis endogenous genomic origins, including specific tRFs, which are internalized by Pto DC3000 cells. Although not tested in this study, we do not exclude the possibility that endogenous sRNAs can additionally be taken-up by Pto DC3000 in an EV-dependent manner.
A pool of active apoplastic protein-bound sRNAs is located outside EVs
We found here that a large proportion of apoplastic anti-cfa6/hrpL sRNAs is presumably associated with proteins, and thus protected from MNase-mediated degradation (Fig. 4). This feature was notably observed for the apoplastic fraction that co-purified with PEN1-positive EVs (i.e. the P40 pellet), as well as the fraction exhibiting a substantial reduction in EVs and a depletion in PEN1 (i.e. the SN fraction). Nevertheless, only the protein-bound anti-cfa6/hrpL sRNAs from the P40 pellet, but not those from the SN fraction, were found active (Figs. 4 and 6). In addition, protease and RNase protection assays from the P40 pellet revealed that these protein-bound antibacterial sRNAs are likely located outside EVs. Based on these observations, we propose two possible non-mutually exclusive scenarios by which extravesicular protein-bound sRNAs from the P40 fraction could be transferred to, and/or effective in, Pto DC3000 cells (Fig. 10). The first one, which does not implicate EVs, would involve yet-unknown plant factor(s) from the protein-bound sRNAs complex(es) that would be specifically present in the P40 pellet, but not in the SN fraction. Potential candidates include the RNA-binding protein GRP7, as well as AGO2, which are both known to bind sRNAs56,57,58,59, and that have recently been recovered in the Arabidopsis apoplastic P40 fractions, in extravesicular forms34. The second one would involve PEN1-positive EVs, decorated with these protein-bound sRNAs complexes at their surface, which would act as carriers of these RNA-associated particles. The latter hypothesis is supported by the relatively large surface of EVs compared to their volume, which notably contains exposed phospholipids that can bind specific proteins from the extracellular environment60.
A pool of active apoplastic sRNAs is likely located inside EVs
By characterizing the apoplastic fraction from IR-CFA6/HRPL#4 plants co-purifying with TET8-positive EVs (i.e. the P100-P40 fraction), we found that the MNase and Proteinase K combined treatment did not alter its antibacterial activity (Fig. 5). In contrast, an extra detergent treatment led to a substantial reduction in the accumulation of anti-cfa6/hrpL sRNAs, as well as a loss in AGS activity (Fig. 5). Collectively, these data indicate that a pool of active anti-cfa6/hrpL sRNAs is likely located inside TET8-positive EVs (Fig. 10). Similarly, the remaining—albeit mild—activity detected in protease and RNase protection assays of the P40 pellets (Fig. 4), might be due to active anti-cfa6/hrpL sRNAs located either inside PEN1-positive EVs and/or TET8-positive EVs, the latter being underrepresented, yet still detectable in this apoplastic fraction34. Collectively, these results are thus congruent with former studies showing that intravesicular plant antifungal sRNAs are delivered into B. cinerea through TET8-positive EVs7,12. They are also consistent with recent studies showing that i) antibacterial miRNAs, located inside ginger exosome-like nanoparticles (GELNs), can be delivered and active in the periodontal pathogen Porphyromonas gingivalis and the commensal intestinal bacterium Lactobacillus rhamnosus61,62, and (ii), let-7b-5p, embedded in EVs from human airway epithelial cells, is translocated in the opportunistic human pathogen Pseudomonas aeruginosa, to reduce its ability to form biofilm and to enhance its antibiotic sensitivity36. These findings also pave the way for the development of novel EV-based delivery systems for antibacterial sRNAs.
A pool of active apoplastic sRNAs is unbound to protein and in a dsRNA form
Intriguingly, we found that anti-cfa6/hrpL sRNAs from the SN fraction harbour anti-virulence activity, which was found abolished upon MNase treatment alone (Fig. 6). Furthermore, by pulling-down anti-cfa6/hrpL sRNA duplexes from these apoplastic fractions, we showed that these extracellular sRNA species were functional in a stomatal aperture assay (Fig. 6). These data indicate that a third pool of anti-cfa6/hrpL efsRNAs, which is unbound to protein, and in a free and dsRNA form, is biologically active (Fig. 10). Although our findings do not exclude the possibility that extracellular single-stranded RNA species (ssRNAs) could also be present and functional in the apoplast, they suggest that the dsRNA structure of the above characterized efsRNAs might represent a structural feature that favors their stability in the apoplast. This hypothesis would provide an explanation for why artificial sRNA duplexes, exogenously applied on different plant species, were transmitted through, and stable in, xylem vessels, which are part of the apoplast63. It is also possible that yet-unknown post-transcriptional modifications of these functional efsRNAs contribute to their stability in the Arabidopsis apoplast.
Salicylic acid-treated IR-CFA6/HRPL plants trigger hrpL silencing in Pto DC3000
To monitor the potential effect of anti-hrpL sRNAs on the silencing of the HrpL gene, we incubated the Pto DC3000 strain expressing an epitope-tagged version of HrpL, with leaf sections of IR-CFA6/HRPL#4 plants. Using this assay, we found a substantial reduction in the accumulation of HrpL proteins in Pto DC3000, which was specifically detected when the above transgenic plants were challenged with SA (Fig. 8). Our findings thus provide insights into strategies that can be further developed to improve HIGS efficacy. In addition, we observed that the levels of HrpL mRNAs were not consistently and significantly reduced in the presence of SA-pretreated IR-CFA6/HRPL#4 leaf sections across different biological replicates (Supplementary Fig. 8). Although these findings do not exclude the possibility that internalized anti-hrpL RNAs direct mRNA cleavage and/or degradation of HrpL mRNAs in Pto DC3000, they suggest that extra-cellular/organismal anti-hrpL sRNAs, produced from SA-challenged IR-CFA6/HRPL plants, predominantly dampen the translation of HrpL mRNAs in these experimental settings (Fig. 10). This regulatory process might involve the internalization by Pto DC3000 cells of vesicular and/or non-vesicular plant factors that are secreted in the apoplast in response to SA34,64, and/or SA-induced plant signals, which would enhance anti-hrpL sRNA-directed translational repression of HrpL mRNAs in Pto DC3000. Although the detailed mechanism remains unknown, various sRNA-directed translational inhibition processes are known to operate in animals, plants, and bacteria65,66,67,68,69,70,71,72,73. For instance, some RNA-based antisense oligonucleotides (ASOs), which are in the same size range as tyRNAs, siRNAs and miRNAs, but also longer bacterial small non-coding RNAs, which are typically between 50 to 500 nt in size, have been shown to trigger translational inhibition of their mRNA targets through different mechanisms in prokaryotic cells68,73,74,75. Altogether, these data suggest that Pto DC3000 must have evolved a machinery to take charge of the internalized plant sRNAs and direct gene silencing. It will thus be appealing to identify such machinery and elucidate the principles of plant sRNA target recognition and mode of action in bacterial cells.
Endogenous efsRNAs, including tRNA-derived sRNAs, are taken-up by Pto DC3000 cells
By sequencing SN-derived sRNAs from IR-CFA6/HRPL plants that are transferred into the cytoplasm of Pto DC3000 cells, we did not only retrieve artificial sRNAs from the CFA6/HRPL hairpin but also Arabidopsis endogenous sRNAs. The latter sRNAs were derived from various genomic origins, including endogenous inverted repeats, mRNAs, TEs, rRNAs, and tRNAs (Figs. 9 and 10). Interestingly, among the most abundant sRNAs internalized by Pto DC3000 cells, we recovered tRFs, some of which exhibiting plausible bacterial mRNA targets (Fig. 9). Transfer RNA-derived sRNAs have previously been shown to be transferred bidirectionally in host-bacteria interactions15,48,49,50,51,52. More specifically, an abundant tRF was recovered inside outer membrane vesicles (OMVs) from P. aeruginosa, and translocated into human airway cells48. Furthermore, this tRF displays predicted targets encoding kinases from the lipopolysaccharide (LPS)-stimulated MAPK signaling pathway, and was shown to dampen the LPS-mediated induction of interleukin-8 (IL-8) secretion in primary human airway epithelial cells, as well as keratinocyte-derived chemokine production in mouse lung. Interestingly, tRFs produced from the rhizobium strain B. japonicum have also more recently been shown to be transferred inside soybean cells and further loaded into host AGO1, thereby silencing negative regulators of nodulation15. Inversely, two human 5’ tRNA halves from oral keratinocytes, were shown to be secreted outside these cells and further taken-up from the saliva by the Gram-negative pathobiont Fusobacterium nucleatum49,50. Importantly, these tsRNAs were able to reduce the growth of different F. nucleatum strains in vitro, presumably by interfering with the ribosome-related function of endogenous bacterial tRNAs50. Based on these collective findings, we speculate that the abundant Arabidopsis tRFs recovered inside Pto DC3000 might either silence sequence-complementary mRNA bacterial targets, and/or dampen protein biogenesis by acting as decoys of bacterial tRNAs. In addition, we found that a large set of Arabidopsis sRNAs recovered inside Pto DC3000 exhibits predicted bacterial mRNA targets, whose protein levels were reduced by SA signaling and/or biogenesis during infection (Fig. 9). Although these analyses have not been conducted in the natural host of Pto DC3000, they highlight a potential for endogenous plant sRNAs in directly reprogramming bacterial gene expression during infection. However, further studies are necessary to test whether plant sRNAs can indeed regulate gene expression in natural host-bacteria interactions, and also shape plant microbiomes composition as well as bacterial genomes evolution in these contexts.
Methods
Plasmid construction
The IR-CFA6/HRPL construct is composed of 250 bp regions of Pto DC3000 genes, cfa6 (1–250 nt) and hrpL (99–349 nt), aligned in sense and antisense directions, with the intron of the petunia chalcone synthase gene (CHSA) in between. The control vector construct IR-CYP51 is designed to target conserved regions from F. graminearum that encompass CYP51A, CYP51B and CYP51C genes17, with the same CHSA intron. The IR-CFA6/HRPL and IR-CYP51 constructs containing EcoRI and SalI sites at both extremities were synthesized by GenScript®, and inserted by restriction enzyme digestion into a modified pDONR221-P5-P2 vector carrying additional EcoRI and SalI sites to facilitate the insertion of these long-inverted repeats. The plasmids containing the 35S::IR-CFA6/HRPL and 35S::IR-CYP51 constructs were obtained by a double recombination between pDONR221-P5-P2 carrying the inverted repeat sequences and pDONR221-P1-P5r carrying the 35S promoter sequence, in the pB7WG Gateway destination vector using LR Clonase Plus (Life Technologies). These plasmids were then introduced into the Agrobacterium tumefaciens C58C1 strain. The IR-HRPL construct, which is composed of the same 250 bp region of hrpL as in the IR-CFA6/HRPL construct, was recombined using GreenGate technology76 to generate the 35Spro:IR-HRPL plasmid, which was then transformed in the Agrobacterium C58C1 strain. To generate the WT hrpL and the mut hrpL plasmids, the wild type (WT) hrpL sequence was amplified from the genomic DNA isolated from Pto DC3000, while the mutant hrpL sequence was amplified from a mutated sequence synthesized by GenScript®. These two sequences were further cloned into pDONR207 vector using BP Clonase (Life Technologies) and then introduced by recombination using LR Clonase (Life Technologies) into the pBS0046 destination vector, which carries a constitutive NPTII promoter. Specific primers used for the purpose of cloning are listed in Supplementary Data 5. The Ubi.U4::IR-HRPL construct, was generated the same way as 35S::IR-HRPL but using Ubi.U4 promoter instead of 35S promoter. The second control vector IR-LUC construct was also generated using the GreenGate technology76, and is designed to express an inverted repeat targeting a 250 bp region of the firefly luciferase, under the control of 35S promoter.
Plant material and growth conditions
Stable transgenic lines expressing 35S::IR-CFA6/HRPL, 35S::IR-CYP51, 35S::IR-LUC and 35S::IR-HRPL, and UbiU4::IR-HRPL constructs were generated by transforming Arabidopsis (Columbia-0 accession, Col-0) plants using the Agrobacterium-mediated-floral dip method77. Three independent Arabidopsis T4 transgenic lines expressing the IR-CFA6/HRPL hairpin, #4, #5 and #10, two independent Arabidopsis T2 transgenic lines expressing the 35S::IR-HRPL hairpin, #1 and #4, and one reference Arabidopsis T4 transgenic line expressing the IR-CYP51 hairpin #2, one T3 IR-LUC line, one T3 Ubi.U4::IR-HRPL line and one Ubi.U4::IR-HRPL dcl234 line, generated by crossing the Ubi.U4::IR-HRPL line with dcl234 mutant were generated and used in our experiments. Sterilized seeds of Arabidopsis Col-0 and the selected homozygous transgenic lines were first grown for 12–14 days at 22 °C/19 °C (day/night) on plates containing ½ x MS medium (Duchefa), 1% sucrose and 0.8% agar (with or without antibiotic selection), or in soil, in an 8 h photoperiod under a light intensity of 100 μE/m2/s. Seedlings were then pricked out to individual soil pots and grown in the same environmentally controlled conditions described above. Four- to six-week-old plants were used for all the experiments.
Bacterial strains
The PtoΔcfa6-GFP (Pto DC3118-GFP) strain is a gift from S. Y. He, while the PtoΔhrpL strain is a gift from C. Ramos78. The Pto hrpL-FLAG and Pto pBS181 strains are gifts from M. J. Filiatrault and S. Cartinhour, respectively44. The PtoΔhrpL strain expressing the GFP reporter gene was generated by transforming the bacterial mutant strain with the GFP-pPNpt Green plasmid by electroporation and then plating at 28 °C on NYGA medium (5 g L−1 bactopeptone, 3 g L−1 yeast extract, 20 ml L−1 glycerol, 15 g L−1 agar) containing gentamycin (1 µg mL−1) for selection. To generate the PtoΔhrpL expressing WT HrpL or mut HrpL constructs, the PtoΔhrpL strain was transformed by electroporation with the NPTII::WT-HrpL or NPTII::mut-HrpL plasmids. The bacteria were then plated at 28 °C on NYGA medium with gentamycin (1 µg mL−1), to select the transformants that were confirmed by PCR using primers specific to HrpL gene (Supplementary Data 5).
Stomatal aperture measurements
For each indicated genotype, 0.5 cm2 square sections were dissected from unpeeled leaves of 3 plants and immersed in mock solution (water) or in bacterial suspension at 108 cfu mL−1. After 3 h, unpeeled leaf sections were stained with 10 μg mL−1 propidium iodide (Sigma) and the abaxial surface was observed with a SP5 or SP8 laser scanning confocal microscope. The stomatal aperture (width relative to length ratio) was measured using ImageJ software for at least 90–110 stomata per condition. For RNA and vesicle treatments, the leaf sections were incubated for 1 h with total RNAs (20 ng μl−1), P40 pellets, P100-P40 pellets and SN fractions extracted from specified genotypes, before incubation with the bacteria. In specified experiments, 1 μM of Coronatine (COR; Sigma) or in vitro synthesized sRNAs or long dsRNAs were supplemented to the bacterial suspension. Two to 5 independent experiments were pooled to generate each plot.
Bacterial dipping assay
Three hours after the beginning of the night cycle in a growth chamber, three plants per condition were dip-inoculated with Pto DC3000 at 5 × 107 cfu mL−1 supplemented with 0.02% Silwet L-77 (Lehle seeds). Plants were then immediately placed in chambers with high humidity. At 2 days post-inoculation, bacterial titers were measured for individual infected leaves (n = 8), using a classical serial dilution method79. Three independent experiments were pooled in the same plot.
Purification of apoplastic fluid (APF), extracellular vesicles (EVs), and supernatant fraction (SN)
Apoplastic fluid (APF) extraction was adapted from Rutter and Innes, 201731. Briefly, 12 to 24 rosettes from five to seven-week-old IR-CYP51, IR-LUC, IR-CFA6/HRPL#4 or IR-HRPL#4 plants were vacuum-infiltrated with vesicle isolation buffer (VIB; 20 mM MES, 2 mM CaCl2, 0.1 M NaCl, pH 6.0). Rosettes were then placed inside a 20 mL needleless syringe that was inserted in a 50 mL Falcon tube and centrifuged at 900 × g for 15 min at 4 °C. APF was collected and filtered through a 0.2 µm filter to discard cell debris. The APF was subjected to an ultracentrifugation step at 40,000 × g for 60 min at 4 °C giving rise to P40 pellets containing EVs. The supernatant from P40 pellets was subjected to another ultracentrifugation step at 100,000 × g for 60 min at 4 °C to collect the P100 pellet. At this step the supernatant contains the lowest amount of canonical EVs and is named the SN fraction. The P100 pellet, referred to as P100-P40, was resuspended in VIB for washing and then ultracentrifuged at 100,000 × g for 60 min at 4 °C, and used for further analyses. The size and concentration of EVs from P40 or P100-P40 pellets and SN fractions were analyzed using the ZetaView NTA instrument (Particle Metrix). All ultracentrifugations were performed using a swing-out rotor (TST41.14).
Treatments of P40, P100-P40 pellets and SN fractions
When indicated, the P40 pellets, the P100-P40 pellets or the SN fractions were treated with enzymes to digest either RNAs and/or proteins that were not protected by EVs. When enzymatic treatments were performed, the P40 pellets, P100-P40 pellets, or SN fractions, were divided into equal volumes according to the number of conditions presented. Each volume was treated identically and heated for the same duration regardless of whether enzyme were added or not to the tube. To eliminate RNAs, Micrococcal Nuclease (MNase) (Thermo Fisher Scientific) was used at 0.2 U μL−1 at 37 °C for 15 min. The reaction was stopped with 20 mM EGTA. To digest proteins, Proteinase K (Thermo Fisher Scientific) was used at 2 U μL−1 at 37 °C for 15 min. To digest both RNAs and proteins, MNase was first added to the sample for 15 min at 37 °C, inactivated by addition of EGTA, and then Proteinase K was added for 15 min at 37 °C. To disrupt EVs, they were incubated with 1% of Triton X-100 at room temperature for 1 h, prior to enzymatic treatments. For all experiments, a negative control was performed by incubating samples at 37 °C without enzyme during the same timeframe as for the enzymatic treatments.
Extracellular vesicles observation by transmission electron microscopy (TEM)
Five μL of P40, P100-P40 pellets or SN fraction were deposited on copper formvar coated, carbonated grids (Electron Microscopy Sciences - EM-FCF200-CU-SB) and incubated for 20 min at room temperature. Grids were fixed afterwards in 2% paraformaldehyde/1% glutaraldehyde in 100 mM phosphate buffer pH 7.4 for 20 min at room temperature. The grids were washed 6 times in distilled water for 2 min. For embedding and contrast, grids were next incubated in a solution of 2% methylcellulose/1% uranyl acetate mixed at a 9:1 ratio for 7 min on ice in the dark. The excess of fluid from the grids was absorbed on a Whatman filter paper n°1. Grids were air-dried for 1 h before storage. Observations were performed on a Transmission Electron Microscope (TEM) TECNAI 12BT 120 kV (ThermoFisher/FEI) equipped with a CCD Orius 1000 (Gatan) camera. Image acquisition was performed with DigitalMicrograph. EV diameters were measured using ImageJ software.
Immunolabelling for TEM
Five μL of P100-P40 pellets were deposited on copper formvar coated, carbonated grids (Electron Microscopy Sciences - EM-FCF200-CU-SB) and incubated for 20 min at room temperature. Grids were fixed afterward in 2% paraformaldehyde in 100 mM phosphate buffer pH 7.4 for 5 min at room temperature. The grids were washed 3 times in distilled water for 2 min, and next incubated in a solution of 50 mM ammonium chloride in 100 mM phosphate buffer pH 7.4 for 30 min at room temperature. The grids were washed 3 times in 0.1% BSA-c (AURION) in 100 mM phosphate buffer pH 7.4 for 2 min each, and next incubated in the same buffer supplemented with primary antibody (either antibody against TET8 purchased from PhytoAB (PHY2750A), or anti-UGPase(purchased from Agrisera) at a 1:50 ratio for 60 min in a humid chamber at room temperature. The grids were washed 6 times in 0.1 % BSA-c in 100 mM phosphate buffer pH 7.4 for 2 min, then incubated in the same buffer with a 1:20 ratio of goat anti-rabbit (GAR) gold conjugate reagent (6 nm, AURION) for 60 min in a humid chamber at room temperature in the dark. The grids were washed 6 times in 0.1 % BSA-c in 100 mM phosphate buffer pH 7.4 for 2 min, then washed 3 times in 100 mM phosphate buffer pH 7.4 for 2 min. Grids were fixed afterward in 2% glutaraldehyde in 100 mM phosphate buffer pH 7.4 for 5 min at room temperature. The grids were washed 6 times in distilled water for 2 min, and next incubated in a solution of 2% methylcellulose/1% uranyl acetate mixed at a 9:1 ratio for 7 min on ice in the dark for embedding and contrast. The excess of fluid from the grids was absorbed on a Whatman filter paper °1. Grids were air-dried for 3 h before storage. Observations were performed on a Transmission Electron Microscope (TEM) TECNAI 12BT 120 kV (ThermoFisher/FEI) equipped with a CCD Orius 1000 (Gatan) camera. Image acquisition was performed with DigitalMicrograph.
Separation of long and small RNA fractions
From 100 μg of total RNAs extracted with the Tri-reagent (Sigma, St. Louis, MO), long and small RNA fractions were separated using the mirVana miRNA isolation kit (Ambion®) according to the manufacturer’s instructions. The long and small RNA fractions were visualized by agarose gel electrophoresis and further analyzed using a Bioanalyzer 2100 (Agilent Technologies).
In vitro synthesis of inverted repeat RNAs
In vitro synthesized RNAs were generated following the instructions of the MEGAscript® RNAi Kit (Life Technologies, Carlsbad, CA). Templates were amplified by PCR using gene-specific primers containing the T7 promoter. PCR amplification was done in two steps with two different annealing temperatures to increase the specificity of primer annealing. After the amplification step, PCR products were purified by gel extraction using the PCR Clean-up kit (Macherey-Nagel) to eliminate any unspecific PCR products. To produce dsRNAs, the purified PCR products were used as templates for in vitro transcription performed according to the MEGAscript® RNAi Kit instructions. After purification with the filter cartridges, the corresponding dsRNAs were processed in 18–25 nt sRNA duplexes by incubation with ShortCut® RNase III (NEB, Ipswich, MA) for 20 min at 37 °C. Small RNAs were then precipitated overnight at −20 °C in presence of 3 volumes of 100% EtOH and 0.1 volume of 3 M NaCl, and then were washed once with 70% EtOH. Each step of this process was followed by gel electrophoresis (TAE 1X, 1% agarose gel for DNA amplification and 2% agarose gel for RNAs) to verify the quality of RNAs.
RNA gel blot analyses
Accumulation of low molecular weight RNAs was assessed by Northern blot analysis as previously described80. Total RNA was extracted using Tri-reagent (Sigma, St. Louis, MO) according to the manufacturer’s instructions and stabilized in 50% formamide. RNA gel blot analysis of low molecular weight RNAs was performed on 30 μg of total RNAs and as described previously80. Prior to RNA extraction from P40 or P100-P40 pellets, 600 to 700 μL of those fractions were separated in equal volumes, and each fraction was further treated (with mock or the enzyme of interest). The corresponding total RNAs were subsequently extracted as described for total RNA fraction. All the extracted RNAs were loaded on gel for further low molecular weight northern blot analyses as previously described80. Prior to RNA extraction from SN fraction, 12 mL of SN fraction were separated in 3 equal volumes, each fraction (4 mL) was further treated (with mock or the enzyme(s) of interest), and the corresponding RNAs were precipitated overnight at −20 °C using 400 μL NaCl 5 M, 12 mL EtOH 100%. RNAs from the resulting pellets were extracted as described before and loaded on gel for further low molecular weight northern blot analyses as previously described80. Two hundred nanograms of total RNAs from IR-CFA6/HRPL#4 plants were also used as control in the northern blot experiments in parallel of the SN samples (as shown for instance in Fig. 4e). Detection of U6 RNA was used to confirm equal loading of intracellular RNAs. DNA oligonucleotides complementary to miR167-5p and U6 sequences were end-labeled with γ-32P-ATP using T4 Polynucleotide Kinase (PNK) (Promega) following the manufacturer’s instructions. To detect anti-cfa6/hrpL sRNAs, specific 32P-radiolabeled probes were generated by amplifying regions of 150 bp to 300 bp with gene-specific primers (see Supplementary Data 5) and the amplicons were labeled by random priming (Prime-a-Gene® Labeling System, Promega). The indicated probes were hybridized to the same membrane by sequential rounds of probing and stripping.
P19 pull-down assays to purify sRNA duplexes
To pull-down sRNA duplexes, purified MBP-p19 proteins associated with chitin beads were used. For this purpose, 40 μg of total RNAs, or 2 mL of SN fraction, were resuspended in binding buffer at a final concentration of 20 mM Tris-HCl pH 7.0, 100 mM NaCl, 1 mM DTT, 0.1 mg/mL BSA, 1X Protease Inhibitor Cocktail (Sigma-Aldrich), and were then incubated with 100 units of purified MBP-p19-CBD proteins (gift from New England Biolabs) for 1 h at room temperature. Forty μL of chitin magnetic beads (NEB) were first blocked overnight at 4 °C in 200 μL of pretreatment buffer (20 mM Tris-HCl pH 7.0, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, 1 mg/mL BSA) before to be added to the sample and were then incubated with rotation for 1 h at room temperature. To elute sRNA duplexes bound to p19, the beads were first washed 5 times in 500 μL of wash buffer (20 mM Tris-HCl pH 7.0, 100 mM NaCl, 1 mM DTT, 100 µg/mL BSA) and then resuspended in 30 μL of elution buffer (20 mM Tris-HCl pH 7.0, 100 mM NaCl, 0.5% SDS) and incubated for 10 min at 37 °C, followed by 10 min at RT under stirring. The elution step was repeated twice and both elution fractions were pooled. Each pooled elution fraction was divided into two parts for the detection of anti-cfa6/hrpL sRNAs (Northern blot analysis) and to perform activity test (stomatal reopening assay).
In vitro bacterial incubation with ATTO 565-labeled anti-hrpL sRNA duplexes
A Pto DC3000-GFP culture was grown to OD600nm = 0.6. Bacterial cells were harvested by centrifugation (4000 rpm, 5 min, same settings for all centrifugations in this section), washed with Milli-Q water, and diluted to OD600nm = 0.2. A 200 µl aliquot of this bacterial suspension was then incubated with 2 µM of anti-hrpL-ATTO 565 duplexes in a 24-well plate at room temperature on an orbital shaker (Heidolph Polymax 1040). The incubation was carried out for 24 h at 20 rpm. Following incubation, bacteria were collected by centrifugation and washed with Milli-Q water and resuspended in 500 µl of Milli-Q water. To cross-link the bacteria, 1% formaldehyde was added and incubated for 15 min at room temperature. The bacteria cells were again pelleted and washed with Milli-Q water and resuspended in 500 µL of Milli-Q water. A 1/10 dilution of the bacterial suspension was prepared for fluorescence analysis. GFP fluorescence was observed using a Leica SP8 laser scanning confocal microscope (excitation: 488 nm; emission: 500–550 nm), and ATTO 565 fluorescence was detected (excitation: 565 nm; emission: 580–630 nm).
In vitro bacterial incubation with salicylic acid-treated Arabidopsis leaves
Five-week-old IR-CYP51 and IR-CFA6/HRPL#4 transgenic plants were sprayed with either water (mock) or SA (2 mM) solution supplemented with 0.02% Silwet L-77. For each condition, 48 h after the spray, 8 pieces of 1 cm2 were cut from 2–3 leaves and incubated in 6-well plates with 2 mL of Pto DC3000 hrpL-FLAG at 5 × 107 cfu mL−1 in hrpL-derepressing medium (HDM, 10 mM fructose, 50 mM potassium phosphate buffer, 7.6 mM (NH4)2SO4, 1.7 mM NaCl, pH 6.0)81,82 for 2 h. Bacterial cells were then collected and hrpL-FLAG expression was assessed at the RNA level, by RT-qPCR analysis, and at the protein level, by western blot analysis.
Incubation of bacteria with SN fraction and EDTA treatment
One mL of Pto DC3000 bacteria at OD600nm = 0.4 was incubated with 1 mL of SN from IR-CFA6/HRPL#4 plants for 2 h at room temperature. Then, bacteria were pelleted, washed with PBS, and resuspended in 400 µL of TSE buffer (0.2 M Tris-HCl pH 8.0, 0.5 M sucrose, 1 mM EDTA) + 100 µL of 0.5 M EDTA to lyse the outer membrane. After 15 min incubation at room temperature, bacteria were pelleted and washed with PBS again before RNA extraction. To assess the effectiveness of EDTA treatment in disrupting the outer membrane, 20 µL of EDTA-treated bacterial suspension were diluted in a final volume of 200 µL H2O, containing 1 µL of propidium iodide. The fluorescence of the propidium iodide, bound to bacterial DNA, was measured using an excitation wavelength of 535 nm and an emission wavelength of 617 nm. To evaluate the effect of EDTA treatment on bacterial viability, the treated bacteria were resuspended in 500 µL of H2O. A 100 µL aliquot of the EDTA-treated bacteria was inoculated into 500 µL of NYGB medium in a 96-well plate, and optical density (OD600nm) was recorded 24 h after inoculation.
Bacterial RNA extraction and real-time quantitative PCR analysis
Total RNAs from bacterial cells were extracted with Tri-Reagent (Sigma, St. Louis, MO) according to the manufacturer’s instructions, followed by DNAse (RQ1, Promega) digestion at 37 °C to remove the genomic DNA. One μg of total RNA was reverse-transcribed using RevertAid H minus Reverse Transcriptase (Thermo Fisher Scientific) and random hexamers according to the manufacturer’s instructions. Gene expression was quantified by real-time qPCR, using Takyon SYBR Green Supermix and gene-specific primers with the following parameters: a 384-well optical reaction plate was heated at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, and elongation at 72 °C for 40 s. A melting curve was performed at the end of the amplification by steps of 1 °C (from 95 °C to 50 °C). Expression was normalized to that of the bacterial gene gyrA. Primer sequences are listed in Supplementary Data 5.
Protein extraction and western blot analysis
Total protein extraction from bacterial cells or plant vesicles was done according to Hurckman and Tanaka83. Equal amounts of proteins were resolved on SDS-PAGE in Laemmli buffer and were then electroblotted on Immobilon®-P PVDF Membrane (Millipore). Membranes were blocked for 1 h in a 5% milk solution in 1X PBS-Tween 0.1%, then incubated overnight at 4 °C with specific antibodies. Antibodies against PEN3, UGPase, and EF-Tu were purchased, respectively, from Agrisera and Hycult Biotech. Antibody against PEN1 was a gift from Prof. Thordal-Christensen. The next day, membranes were washed 3 times for 10 min in PBS-Tween 0.1% then incubated 2 h at room temperature in PBS-Tween milk 5% with the secondary antibody. Membranes were washed again 3 times for 10 min in PBS-Tween 0.1% and revelation was performed with Chemicapt ImageQuant400 with Immobilon forte HRP substrate (Sigma).
Bioinformatic analysis – thermodynamic energy analysis
Unique reads mapping to cfa6 and hrpL genes of Pto DC3000 from two sRNA libraries were used for all BLAST analyses (Supplementary Fig. 1e). The 500 most abundant anti-cfa6 and anti-hrpL sRNA reads were selected and blasted against annotated Arabidopsis genes (TAIR10) and Pto DC3000 coding sequences (CDS) (-e-value 10, -word_size 4, -ungapped, -reward 1, -penalty -1)84. The free energy of binding for each read paired with its top target was calculated using RNAup85. In Fig. 3c unique reads mapping to the HrpL gene of Pto DC3000 in the two sRNA libraries presented in Fig. 1d were used for BLAST analysis against the sequences in Fig. 1c, and the thermodynamic energy analysis was performed as described earlier. Target prediction was made by aligning the reverse complement of each sRNA against the Pto DC3000 genome using blastn86 (version 2.12.0+, Parameters: -outfmt 6 -evalue 20 - -max_hsps 10000 -max_target_seqs 1000 -word_size 4 -ungapped -reward 1 -penalty -1). Alignments were processed to keep up to 5 mismatches, where wobble pairs (G-U) matches were considered as a partial (0.5) mismatch. Predicted energy of the RNA-RNA pair between the sRNA and the target sequence was calculated using RNAup85.
Small RNA sequencing
Small RNA libraries were constructed and sequenced from 4- to 5-week-old leaves of IR-CFA6/HRPL#4 plants. Raw reads have been deposited at the NCBI SRA under Bioproject (PRJNA587213). Libraries were mapped against the Arabidopsis thaliana genome (v TAIR10.1 GCF_000001735.4), the IR-CFA6/HRPL sequence, and the Pto DC3000 genome using ShortStack (v 3.8.4) and Bowtie2 (v 2.3.4) with parameters --mismatches 0 --dicermin 15 --dicermax 30 --bowtie_m 20 --mmap f87. Coverage of mapped loci was obtained with the Genomic Alignments package in R88. Unique reads mapping to Cfa6 and HrpL in both replicates were extracted and quantified from ShortStack, and results were mapped using samtools89.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw reads have been deposited at the NCBI SRA under Bioproject PRJNA587213. Raw data from other analyses included in this study can be found in the accompanying Source data file. Source data are provided with this paper.
References
Lopez-Gomollon, S. & Baulcombe, D. C. Roles of RNA silencing in viral and non-viral plant immunity and in the crosstalk between disease resistance systems. Nat. Rev. Mol. Cell Biol. 23, 645–662 (2022).
Staiger, D., Korneli, C., Lummer, M. & Navarro, L. Emerging role for RNA-based regulation in plant immunity. N. Phytol. 197, 394–404 (2013).
Pumplin, N. & Voinnet, O. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat. Rev. Microbiol. 11, 745–760 (2013).
Bologna, N. G. & Voinnet, O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu. Rev. Plant Biol. 65, 473–503 (2014).
Liu, L. & Chen, X. Intercellular and systemic trafficking of RNAs in plants. Nat. Plants 4, 869–878 (2018).
Melnyk, C. W., Molnar, A. & Baulcombe, D. C. Intercellular and systemic movement of RNA silencing signals. EMBO J. 30, 3553–3563 (2011).
Cai, Q. et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360, 1126–1129 (2018).
Guo, Z., Li, Y. & Ding, S.-W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 19, 31–44 (2019).
Zhang, T. et al. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2, 1–6 (2016).
Wang, M. et al. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2, 16151 (2016).
Hou, Y. et al. A phytophthora effector suppresses trans-kingdom RNAi to promote disease susceptibility. Cell Host Microbe https://doi.org/10.1016/j.chom.2018.11.007 (2018).
He, B. et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat. Plants 7, 342–352 (2021).
Weiberg, A. et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342, 118–123 (2013).
Dunker, F. et al. Oomycete small RNAs bind to the plant RNA-induced silencing complex for virulence. eLife 9, e56096 (2020).
Ren, B., Wang, X., Duan, J. & Ma, J. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science 365, 919–922 (2019).
Baulcombe, D. C. VIGS, HIGS and FIGS: small RNA silencing in the interactions of viruses or filamentous organisms with their plant hosts. Curr. Opin. Plant Biol. 26, 141–146 (2015).
Koch, A. et al. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase–encoding genes confers strong resistance to Fusarium species. PNAS 110, 19324–19329 (2013).
Xin, X.-F., Kvitko, B. & He, S. Y. Pseudomonas syringae: what it takes to be a pathogen. Nat. Rev. Microbiol. 16, 316–328 (2018).
Melotto, M., Underwood, W., Koczan, J., Nomura, K. & He, S. Y. Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980 (2006).
Melotto, M., Underwood, W. & He, S. Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 46, 101–122 (2008).
Bender, C. L., N-Chaidez, F. A. & Gross, D. C. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 63, 27 (1999).
Brooks, D. M. et al. Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 17, 162–174 (2004).
Sreedharan, A., Penaloza-Vazquez, A., Kunkel, B. N. & Bender, C. L. CorR regulates multiple components of virulence in Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 19, 768–779 (2006).
Fouts, D. E. et al. Genomewide identification of Pseudomonas syringae pv. tomato DC3000 promoters controlled by the HrpL alternative sigma factor. Proc. Natl Acad. Sci. USA 99, 2275–2280 (2002).
Koch, A. et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12, e1005901 (2016).
Plesse, B. et al. Effects of the polyubiquitin gene Ubi. U4 leader intron and first ubiquitin monomer on reporter gene expression in Nicotiana tabacum. Plant Mol. Biol. 45, 655–667 (2001).
Daxinger, L. et al. Unexpected silencing effects from T-DNA tags in Arabidopsis. Trends Plant Sci. 13, 4–6 (2008).
Mlotshwa, S. et al. Transcriptional silencing induced by Arabidopsis T-DNA mutants is associated with 35S promoter siRNAs and requires genes involved in siRNA-mediated chromatin silencing. Plant J. 64, 699–704 (2010).
Vargason, J. M., Szittya, G., Burgyán, J. & Hall, T. M. T. Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115, 799–811 (2003).
Ye, K., Malinina, L. & Patel, D. J. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature 426, 874–878 (2003).
Rutter, B. D. & Innes, R. W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 173, 728–741 (2017).
Théry, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. CP Cell Biol. 30, 3–22 (2006).
Baldrich, P. et al. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “tiny” RNAs. Plant Cell 31, 315–324 (2019).
Zand Karimi, H. et al. Arabidopsis apoplastic fluid contains sRNA- and circular RNA–protein complexes that are located outside extracellular vesicles. Plant Cell 34, 1863–1881 (2022).
Zhang, K., Hodge, J., Chatterjee, A., Moon, T. S. & Parker, K. M. Duplex structure of double-stranded RNA provides stability against hydrolysis relative to single-stranded RNA. Environ. Sci. Technol. 55, 8045–8053 (2021).
Koeppen, K. et al. Let-7b-5p in vesicles secreted by human airway cells reduces biofilm formation and increases antibiotic sensitivity of P. aeruginosa. Proc. Natl Acad. Sci. USA 118, e2105370118 (2021).
Alamillo, J. M., Saénz, P. & García, J. A. Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco. Plant J. 48, 217–227 (2006).
Campos, L. et al. Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Biochem 77, 35–43 (2014).
Diao, P. et al. miR403a and SA are involved in NbAGO2 mediated antiviral defenses against TMV infection in Nicotiana benthamiana. Genes 10, 526 (2019).
Alazem, M., Kim, K.-H. & Lin, N.-S. Effects of abscisic acid and salicylic acid on gene expression in the antiviral RNA silencing pathway in Arabidopsis. Int J. Mol. Sci. 20, 2538 (2019).
Xie, Z., Fan, B., Chen, C. & Chen, Z. An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc. Natl Acad. Sci. USA 98, 6516–6521 (2001).
Carr, J. P., Lewsey, M. G. & Palukaitis, P. Signaling in induced resistance. Adv. Virus Res. 76, 57–121 (2010).
Lee, W.-S. et al. Salicylic acid treatment and expression of an RNA-dependent RNA polymerase 1 transgene inhibit lethal symptoms and meristem invasion during tobacco mosaic virus infection in Nicotiana benthamiana. BMC Plant Biol. 16, 15 (2016).
Lam, H. N. et al. Global analysis of the HrpL regulon in the plant pathogen Pseudomonas syringae pv. tomato DC3000 reveals new regulon members with diverse functions. PLoS ONE 9, e106115 (2014).
Montavon, T. et al. A specific dsRNA-binding protein complex selectively sequesters endogenous inverted-repeat siRNA precursors and inhibits their processing. Nucleic Acids Res. 45, 1330–1344 (2017).
Max, K. E. A. et al. Human plasma and serum extracellular small RNA reference profiles and their clinical utility. Proc. Natl Acad. Sci. USA 115, E5334–E5343 (2018).
Tosar, J. P. & Cayota, A. Extracellular tRNAs and tRNA-derived fragments. RNA Biol. 17, 1149–1167 (2020).
Koeppen, K. et al. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 12, e1005672 (2016).
He, X. et al. Human tRNA-derived small RNAs modulate host–oral microbial interactions. J. Dent. Res. 97, 1236–1243 (2018).
Yang, M. et al. Targeting Fusobacterium nucleatum through chemical modifications of host-derived transfer RNA fragments. ISME J. 17, 880–890 (2023).
Kusch, S., Singh, M., Thieron, H., Spanu, P. D. & Panstruga, R. Site-specific analysis reveals candidate cross-kingdom small RNAs, tRNA and rRNA fragments, and signs of fungal RNA phasing in the barley–powdery mildew interaction. Mol. Plant Pathol. 24, 570–587 (2023).
Panstruga, R. & Spanu, P. Transfer RNA and ribosomal RNA fragments - emerging players in plant-microbe interactions. N. Phytol. 241, 567–577 (2024).
Thompson, A. et al. tRex: a web portal for exploration of tRNA-derived fragments in Arabidopsis thaliana. Plant Cell Physiol. 59, e1 (2018).
Nobori, T. et al. Multidimensional gene regulatory landscape of a bacterial pathogen in plants. Nat. Plants 6, 883–896 (2020).
Wang, M., Thomas, N. & Jin, H. Cross-kingdom RNA trafficking and environmental RNAi for powerful innovative pre- and post-harvest plant protection. Curr. Opin. Plant Biol. 38, 133–141 (2017).
Mi, S. et al. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133, 116–127 (2008).
Montgomery, T. A. et al. Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133, 128–141 (2008).
Yan, Y., Ham, B.-K., Chong, Y. H., Yeh, S.-D. & Lucas, W. J. A plant SMALL RNA-BINDING PROTEIN 1 family mediates cell-to-cell trafficking of RNAi signals. Mol. Plant 13, 321–335 (2020).
Lühmann, K. L., Seemann, S., Martinek, N., Ostendorp, S. & Kehr, J. The glycine-rich domain of GRP7 plays a crucial role in binding long RNAs and facilitating phase separation. Sci. Rep. 14, 16018 (2024).
Buzás, E. I., Tóth, E. Á., Sódar, B. W. & Szabó-Taylor, K. É. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 40, 453–464 (2018).
Sundaram, K. et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. iScience 21, 308–327 (2019).
Teng, Y. et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 24, 637–652.e8 (2018).
Dalakouras, A. et al. Delivery of hairpin RNAs and small RNAs into woody and herbaceous plants by trunk injection and petiole absorption. Front Plant Sci. 9, 1253 (2018).
Koch, B. L., Rutter, B. D. & Innes, R. W. Arabidopsis produces distinct subpopulations of extracellular vesicles that respond differentially to biotic stress. Preprint at https://doi.org/10.1101/2024.04.18.589804 (2024).
Li, S. et al. MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell 153, 562–574 (2013).
Brodersen, P. et al. Widespread translational inhibition by plant miRNAs and siRNAs. Science 320, 7 (2008).
Iwakawa, H. & Tomari, Y. Molecular insights into microRNA-mediated translational repression in plants. Mol. Cell 52, 591–601 (2013).
Holmqvist, E. & Vogel, J. RNA-binding proteins in bacteria. Nat. Rev. Microbiol. 16, 601–615 (2018).
Horman, S. R. et al. Akt-mediated phosphorylation of Argonaute 2 downregulates cleavage and upregulates translational repression of MicroRNA targets. Mol. Cell 50, 356–367 (2013).
Jonas, S. & Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet 16, 421–433 (2015).
Mishima, Y. et al. Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish. Proc. Natl Acad. Sci. USA 109, 1104–1109 (2012).
Larivera, S., Neumeier, J. & Meister, G. Post-transcriptional gene silencing in a dynamic RNP world. Biol. Chem. 404, 1051–1067 (2023).
Dutta, T. & Srivastava, S. Small RNA-mediated regulation in bacteria: a growing palette of diverse mechanisms. Gene 656, 60–72 (2018).
Lalaouna, D., Simoneau-Roy, M., Lafontaine, D. & Massé, E. Regulatory RNAs and target mRNA decay in prokaryotes. Biochim. Biophys. Acta (BBA) - Gene Regul. Mech. 1829, 742–747 (2013).
Shen, X. & Corey, D. R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 46, 1584–1600 (2018).
Lampropoulos, A. et al. GreenGate—a novel, versatile, and efficient cloning system for plant transgenesis. PLoS ONE 8, e83043 (2013).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Castillo-Lizardo, M. G. et al. Contribution of the non-effector members of the HrpL regulon, iaaL and matE, to the virulence of Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Microbiol. 15, 165 (2015).
Halter, T. et al. The Arabidopsis active demethylase ROS1 cis-regulates defense genes by erasing DNA methylation at promoter-regulatory regions. eLife https://doi.org/10.7554/eLife.62994 (2021).
Navarro, L., Jay, F., Nomura, K., He, S. Y. & Voinnet, O. Suppression of the microRNA pathway by bacterial effector proteins. Science 321, 964–967 (2008).
Huynh, T. V., Dahlbeck, D. & Staskawicz, B. J. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 245, 1374–1377 (1989).
Peñaloza-Vázquez, A., Preston, G. M., Collmer, A. & Bender, C. L. Regulatory interactions between the Hrp type III protein secretion system and coronatine biosynthesis in Pseudomonas syringae pv. tomato DC3000. Microbiology 146, 2447–2456 (2000).
Hurkman, W. J. & Tanaka, C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 81, 802–806 (1986).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Lorenz, W. A. & Clote, P. Computing the partition function for kinetically trapped RNA secondary structures. PLoS ONE 6, e16178 (2011).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinforma. 10, 421 (2009).
Axtell, M. J. ShortStack: comprehensive annotation and quantification of small RNA genes. RNA 19, 740–751 (2013).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011).
Acknowledgements
We thank S.-H. He for providing the Pto DC3118-GFP strain, C. Ramos for providing the Pto DC3000 hrpL mutant strain, M. J. Filiatrault and S. Cartinhour for providing the Pto DC3000 hrpL-FLAG strain and Pto pBS181, H. Thordal-Christensen for providing PEN1 antibody, L. Martin-Jaular and F. Cocozza from C. Théry Lab for their help on the use of the NTA, H. Jin for providing an anti-TET8 antibody, J. Alfano for providing the anti-EF-Tu antibody, BioRender.com for some illustrations shown in the figures of this article, and members of the Navarro laboratory for critical reading of the manuscript. This work was supported by the French funding research agency “Agence nationale de la recherche” ANR-18-CE200020-NEPHRON (to L.N.), ANR-21-CE20-0039-02-SYMBISIL (to L.N.), European Research Council (ERC) “Silencing & Immunity”, grant no. 281749 (to L.N.), A.R. received Ph.D. funding support from CNRS, A.R. and M.S.-R. received Ph.D. funding support under the program “Investissements d’Avenir” and implemented by ANR (ANR‐10‐LABX‐54 MEMO LIFE, ANR‐10‐IDEX‐0001‐02PSL). O.T., J.Z., and A.E.F. received support from the French programs “Jeune Entreprise Innovante” (JEI) and “Crédit Impôt Recherche” (CIR). This article also received support from COST Action, RNA communication across kingdoms: new mechanisms and strategies in pathogen control (exRNA-PATH), supported by COST (European Cooperation in Science and Technology).
Author information
Authors and Affiliations
Contributions
A.R., J.Z., M.S.-R., O.T., and L.N. designed research; A.R., J.Z., M.C., M.S.-R., O.T., A.C., and L.L. performed research; A.R., J.Z., M.C., M.S.-R., O.T., A.E.F., and V.M. generated resources; A.P-Q. and L.C. conducted the bioinformatic analyses; A.R., J.Z., M.C., M.S.-R., O.T., A.E.F., L.C., L.L., and L.N. analyzed data; A.R., M.S.-R., and L.N. wrote the manuscript; all the authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.R., M.S.-R., M.C., V.M., A.P.-Q., A.C., L.L., and L.C. declare no competing interests. O.T. is an employee of IRT. J.Z. and A.E.F. were employees of IRT and are now employees of ENgreen Technologies. L.N. is Research Director at CNRS and co-founder of IRT.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ravet, A., Zervudacki, J., Singla-Rastogi, M. et al. Vesicular and non-vesicular extracellular small RNAs direct gene silencing in a plant-interacting bacterium. Nat Commun 16, 3533 (2025). https://doi.org/10.1038/s41467-025-57908-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57908-1