Abstract
Transitions to sustainable food systems require shifts in food production and availability, particularly the replacement of animal-based protein with plant-based protein. To explore how this transition may relate to demographic patterns, we undertake an ecological analysis of global associations between age-specific mortality, total national macronutrient distributions, and protein substitution. Our dataset includes per capita daily food supply and demographic data for 101 countries from 1961–2018. After adjusting for time, population size, and economic factors, we find associations between low total protein supplies and higher mortality rates across all age groups. Early-life survivorship improves with higher animal-based protein and fat supplies, while later-life survival improves with increased plant-based protein and lower fat supplies. Here, we show that the optimal balance of protein and fat in national food supplies, which correlates with minimal mortality, varies with age, suggesting that reductions in dietary protein, especially from animal sources, may need to be managed with age-specific redistributions to balance health and environmental benefits.
Similar content being viewed by others
Introduction
In an era marked by the pressing need for decarbonization and environmental sustainability, global food systems, which contribute a third of all greenhouse gas emissions, are under increasing scrutiny1. Food systems are complex social-ecological networks that encompass all processes and infrastructure involved in feeding a population2. Many have argued that a reduction in the production of animal-based foods is key to achieving more sustainable food systems3, however, reduced production of animal-based foods is likely to result in a substitution of animal-based protein (ABP) for plant-based protein (PBP) and/or a reduction in total protein supplies within food systems4. For many populations, this transition therefore represents a substantial change in the nutritional environment. The capacity of post-transition PBP-rich nutritional environments to support the health of different demographics and age-classes has become a focus of debate5.
In general, the nutritional environment, encompassing factors such as the quality, quantity, and source of nutrients available for consumption, has been identified as a critical determinant of health and survivorship at different ages6. Malnutrition, including ‘undernutrition’, ‘overnutrition’ and imbalanced nutrition, are major risk factors for numerous age-related chronic diseases7, which remain the leading cause of death and age-related disability globally8. Furthermore, the health of older adults may be especially sensitive to the nutritional environment, as this demographic displays elevated risk of both under- and over-nutrition in a food-insecure environment9. At the other end of life, stunting or chronic malnutrition primarily occurs during the first 1000 days from conception due to insufficient maternal or child nutrition10. This often results from a food system that provides limited access to adequate and high-quality foods and is linked to an increased risk of death.
The availability of macronutrients, and particularly protein, at the national level is of interest when considering how nutritional environments affect health and survivorship as a function of age. There is abundant evidence that at the dietary level (i.e. below that of the nutritional environment) protein intake affects health. At the level of diet composition, recent reviews11,12 suggest that protein content, and, particularly, its amounts relative to the quantity of carbohydrate, plays a vital role in shaping age-related health outcomes. At the level of individual intakes, protein appears to exhibit a non-linear relationship with all-cause mortality, relative to total caloric intake in the diet, with both low and high protein intakes associated with increased mortality risk13,14. Furthermore, researchers have advocated for increases in protein intake recommendations for adults of all ages, but especially among older individuals15,16.
At the dietary level, the associations between protein and mortality also appear to vary depending on the source, with ABP potentially having a greater impact on mortality than PBP sources17,18. In particular, high intake of processed meats and—with less certainty—unprocessed red meats, has been linked to an increased risk of certain chronic diseases, including cardiovascular disease, type 2 diabetes, and some types of cancer19,20. On the other hand, plant protein sources, such as legumes, nuts, and whole grains, have been associated with a lower risk of chronic diseases and overall mortality18,21. Researchers have proposed that a predominantly plant-based diet is one of the key common denominators contributing to the extended vitality and longevity observed in long-lived communities, also known as “blue zones“22,23. These “blue zones,” including Okinawa, Japan; Ikaria, Greece; and Loma Linda, California; among others, have been found to have diets high in predominantly plant-based foods and low in animal-based protein. The differential associations of protein sources have been attributed to various dietary factors, such as nutrient composition and bioactive compounds24. While the exact causes remain the focus of ongoing work, there is sufficient evidence to suspect that transitioning from ABP to PBP sources can, at the very least, lead to a shift in the overall nutrient profile of the diet. The nutritional variation reflected in this shift may contribute to differences in health outcomes among humans.
A tool that can be used to parse the differential effects of nutrients and their interactions on outcomes of interest is the geometric framework for nutrition (GFN)25. The GFN is a comprehensive multi-dimensional framework that allows simultaneous examination of undernutrition, overnutrition, nutrient amounts, and compositions within a single framework. It enables insights into the combined macronutrient landscape associated with food environments, and identifies interactive relationships between nutritional components and response variables. The aim of the current study is to assess how the nutritional environment, with special reference to national per capita supplies of ABP and PBP, associates with patterns of age-specific mortality (ASM). Within this context, ASM emerges as an indicator of population-wide, and age-specific, health26. ASM serves as a standardised measure to assess the broader impact of nutritional environments on the morbidity of different age groups.
In this study, we used the GFN to investigate the associations between PBP versus ABP supplies on ASM using global demographic data. Our dataset comprises complete country- and year-specific entries from 101 different countries, spanning 1961–2018. We used data on macronutrient supply quantities available for human consumption from food balance sheets (FBS; see Methods for full explanation) compiled by the Food and Agriculture Organization of the United Nations (FAO). Specifically, these data reflect the average availability of food and nutrients at the national level, which are crucial for understanding the nutritional environment’s ability to support population health. This FBS supply data was combined with sex-specific mortality data (specifically, the proportion of individuals within a cohort that survive to each age) from life tables provided at the Human Mortality Database, economic statistics from the Maddison Project Database, and age, sex specific population estimates from the population division of the United Nations. We explored interactions between macronutrient supplies, time, country, and wealth, with statistical corrections to control for possible confounding by such factors. Using the best approximating model, we predicted the effects of ABP, PBP, fat and carbohydrate supplies on human life table parameters. Additionally, we considered age-specific macronutrient and energy availabilities for optimising survival over the human life course.
Results
Animal-based vs plant-based protein
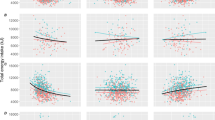
The collated data comprised complete entries for macronutrient supplies from national food balance sheets (FBSs), survivorship at ages 5 and 60 (\({l}_{5}\) and \({l}_{60}\), i.e., the proportion of a cohort still alive at ages 5 and 60) from national life tables, and gross domestic product per capita (GDP) across both sexes, for 101 countries. Briefly, per capita food supply data provided by FBSs refers to the average amount of food available for consumption per person within a nation for a given year, a foundational element of the nutritional environments contributing to health outcomes across different populations. Quantities of food items available for consumption are calculated based on the total quantities produced and imported, adjusting for any changes in stocks including exports. FBSs also give daily per capita supplies in terms of total energy, and specific energy sources, based on composition factors27. Here we have analysed daily per capita protein supplies from animal and vegetal sources (i.e., animal-based protein and plant-based protein; ABP and PBP), as well as daily per capita supplies of total fat supplies and carbohydrate (note that carbohydrate was estimated as total available energy less energy from alcohol, ABP, PBP and fat). Our final dataset spanned from 1961 to 2018 with data from 4000 country-, year-, and sex-specific life tables. We begin by summarising the trends and global coverage of daily per capita protein supplies in our dataset. Figure 1A and B show the ratio of ABP to PBP supplies across different countries in historic (1961–1990) and more recent (1991–2018) data, respectively. The geographic distribution of supplies shows a temporal trend towards convergence, where areas with higher ratios of ABP to PBP supplies in earlier years, such as northern America, north and west Europe, Argentina, Hong Kong, and New Zealand, tend to show a reduction in later years. In contrast, areas where supply is predominantly PBP in early years, including most parts of central and south America, Japan, South Korea, and Taiwan, show an increase in the ratio of ABP to PBP supplies during more recent years. Figure 1C illustrates an average convergence of total ABP and PBP supply quantities, toward parity around the mid 1990’s and remaining relatively similar thereafter. Notably, the total protein supplies available within countries has tended to increase over time, largely attributable to increased ABP (Fig. 1C). Within the data there is a positive association between total protein supplies and economic wealth, with a greater proportion of PBP to ABP where GDP is lower and the ratio of ABP to PBP increases up to GDP values of ~2011 $ 12,000 per capita (Fig. 1D). Additionally, Fig. 1E and 1F show a greater proportion of PBP supplies relative to ABP where fat supplies are lower and carbohydrate supplies are higher, and vice versa.
A Average log ratio of animal-based to plant-based protein supplies for each country, from 1961–1990, and B 1991 –2018, using all available data; grey shows an absence of data. (C) Supplies of plant- and animal-based protein as a function of year, D GDP per capita, (E) fat supplies (kcal/capita/day), and (F) carbohydrate supplies (kcal/capita/day). C–F The individual data points show the raw data values. The red, green, and blue smoothed lines represent the predicted values for ABP, PBP, and total protein, respectively, based on year (C), GDP (D), fat supplies (E), and carbohydrate supplies (F). Predictions were obtained using generalized additive mixed models, with random effects for countries. The shaded areas around the lines represent the 95% confidence intervals centred on these predictions. The smooth term significance is detailed in Supplementary Table 1 to Supplementary Table 12. GDP gross domestic product per capita (expressed in international-$ at 2011 prices). A, B Map data from Natural Earth, facilitated by rnaturalearth R package94.
Age-specific mortality
Using the model life table approach proposed by the World Health Organisation (WHO)28, a comprehensive picture of ASM in human populations can be built by taking survivorship to ages 5 (\(l\)5) and 60 (\(l\)60) as markers of early and late life survival, respectively. Hence, we assessed the \(l\)5 and \(l\)60 data using separate analyses. We further stratified the data into male and female survivorship, yielding four subsets for statistical modelling (i.e., \(l\)5 male; \(l\)60 male; \(l\)5 female; \(l\)60 female). Our primary tool was the generalised additive mixed model (GAMM). To each subset we fitted several models exploring a range of different potential predictors of survivorship including daily per capita nutrient supplies, time, and wealth. All models accounted for differences in relative precision of estimates of survivorship coming from countries with different total population sizes. The best fit model for all four stratifications of the dataset was selected based on lowest AIC score. In all four stratifications, the favoured model captured the 3D interactive effects of ABP, PBP and fat supply varying across time, with the additive effects of GDP, and carbohydrate supply (Supplementary Table 13 and Supplementary Table 14). This indicates that within our dataset, ASM is best predicted by nutritional supplies in a time-dependent manner. Furthermore, there are interactive effects of ABP, PBP, and fat supplies on survival in both early and late life and in both sexes.
Figure 2 uses the surfaces-based approach from the GFN to visualise the associations between ABP, PBP, fat supplies and \(l\)₅, as estimated by the AIC-favoured GAMM. Note here and below, model predictions were made for 2017, this being the most recent year for which comprehensive data were available. These figures show the associations of survivorship with ABP and PBP supply, at low, medium, and high fat supplies (while assuming a median carbohydrate supply, and GDP for 2017). For both sexes our model predicted a strong positive association between \(l\)₅ and absolute amount of ABP, while any beneficial effects of PBP were found to be much weaker (Fig. 2). The grey dashed isolines on Fig. 2 at 400 kcal/capita/day total protein energy (PE) supplies, show the effects of isocalcorically substituting ABP for PBP (and vice versa) while holding total energy supplies constant at 3023 (non-protein energy (NPE) + PE = 2623 + 400), 3120 (NPE + PE = 2720 + 400) and 3228 (NPE + PE = 2828 + 400) kcal/capita/day (Fig. 2A and D, Fig. 2B and E, and Fig. 2 C and 2 F, respectively). Moving in the direction of the isolines illustrates improvements in \(l\)₅ associated with the substitution of ABP from PBP without changing the amounts of total protein and total energy supplies. \(l\)₅ also demonstrated positive association with total fat supplies. At low fat supplies, \(l\)₅ showed similar improvements as protein supplies were increased in the direction of either ABP or PBP, however \(l\)₅ was ultimately maximised where both fat and ABP supplies were high (Fig. 2A vs C and Fig. 2D vs F). There was a non-linear association between \(l\)₅ and carbohydrate supplies, where \(l\)₅ appeared to be maximised with lower carbohydrate supplies (~1200 kcal/cap/day) in both males and females (Supplementary Fig. 4).
Survivorship outcomes were predicted based on 2017 global nutrient supply, GDP, and population data, using the GAMM selected as the best approximating model for the entire dataset. A–C shows the effects of animal-based protein, plant-based protein, and fat supplies on \({l}_{5}\) in females. D–F shows the effects of animal-based protein, plant-based protein, and fat supplies on \({l}_{5}\) in males. The colour map and contour lines on each surface shows predicted \({l}_{5}\) associated with the aligning proportions of protein supplies on the \(x\) and \(y\) axes. Survivorship values increase from blue to red, as shown by the colour bar scale. Moving from (A–C) and from (D–F) across each row of surfaces, the quantity of fat supplies increases. The surfaces for low (A, D), median (B, E) and high (C, F) fat show predicted effects at the first, second, and third quartiles of global fat supplies in 2017, respectively. A–F assume the median carbohydrate supply and GDP for 2017 (1571 kcal/capita/day; 2011-Intl$30902). Total non-protein energy (NPE) is the sum of energy supplies from fat and carbohydrate for each surface. The grey dashed isoline indicates a constant total protein energy (PE) supply at 400 kcal/capita/day on all surfaces (A–F). The smooth terms for macronutrient supply were statistically significant in both sexes (Supplementary Table 16). GAMM generalized additive mixed model, GDP gross domestic product per capita (in international-$ at 2011 prices).
Figure 3 shows the associations estimated between nutrient-supplies and \(l\)₆₀. Here we see beneficial associations when elevating average daily protein supplies in the animal or plant-based dimension, with low levels of either being associated with poor survival. However, per unit of energy, PBP was predicted to improve \(l\)₆₀ more than ABP, and peak \(l\)₆₀ was predicted at high PBP and lower ABP supplies (Fig. 3A–F). While there were positive associations between fat and \(l\)₅, this appeared to reverse at older age with \(l\)₆₀ peaking at low fat (Fig. 2A–F vs Fig. 3A–F). For both sexes, moderate carbohydrate supplies (~1500 kcal/cap/day) maximised later-life survival (Supplementary Fig. 4), though survivorship in males appeared more sensitive to carbohydrate supplies than in females.
Survivorship outcomes were predicted across the 2017 global nutrient supply space, as described in Fig. 2. A–C and D–F show female and male \({l}_{60}\) outcomes, respectively. The colour map and contour lines show predicted \({l}_{60}\) survivorship, as described in Fig. 2. Survivorship values increase from blue to red, as specified by the colour bar legend (min = 0.849, max = 0.959). Fat supplies are fixed at low, median and high for surfaces (A, D), (B, E) and C, F, respectively, based on the first, second and third quartiles of global fat supplies in 2017. Carbohydrate supplies and GDP are held constant across all surfaces (A–F), as specified in Fig. 2. Total non-protein energy (NPE) held constant as indicated on each surface, as specified in Fig. 2. The grey dashed isoline on each surface indicates total protein energy (PE) supplies of 400 kcal/capita/day. The smooth terms for macronutrient supply were statistically significant in both sexes (Supplementary Table 17). GDP gross domestic product per capita (in international-$ at 2011 prices).
Using the \(l\)₅ and \(l\)₆₀ values predicted in Figs. 2 and 3, we derived entire life tables using the WHO-proposed model life table method (see Methods). We visualised the effects of macronutrient supplies on the rederived life table functions, such as: life-expectancy at birth \(\left({e}_{0}\right)\) and the age-specific risk of mortality (\({q}_{{{\rm{x}}}}\)). Put another way, for every predicted point in the nutrient space shown in Figs. 2 and 3 we generated a complete life table, with mortality patterns of age specific survivorship. Predicted effects of nutrient supply on the derived \({e}_{0}\) are shown in Fig. 4. For both sexes, \({e}_{0}\) appeared to decrease when combined total protein energy from both ABP and PBP supplies are low (Fig. 4). A beneficial association between PBP and \({e}_{0}\) was seen with low to moderate ABP and fat supplies (Fig. 4A, B and Fig. 4D, E), such that \({e}_{0}\) is maximised where PBP is high and ABP and fat supplies are low. Additionally, with low fat supplies, improvements in \({l}_{60}\) were associated with the isocaloric substitution of PBP from ABP (grey-dashed lines in Fig. 4A, B).
e0 values were derived from pairs of corresponding \(l\)₅ and \(l\)₆₀, as shown in Figs. 2, 3, and visualised across the same nutrient supply space. A–C and (D–F) show female and male \({e}_{0}\) outcomes, respectively. The colour map and contour lines in (A–F) show the \({e}_{0}\) in years, corresponding to the aligning proportions of protein supplies given by the \((x,y)\) coordinates. In (A–F), life expectancy values increase from blue to red, as specified by the colour bars legend (min = 70 years, max = 85 years). Moving from (A–C) and from (D–F) across each row of surfaces, the quantity of fat and total non-protein energy (NPE) supplies increase. The surfaces for low (A, D), median (B, E) and high (C, F) fat supplies show predicted effects at the first, second, and third quartiles of global fat supplies in 2017, respectively. (A–F) assume the median carbohydrate supply and GDP for 2017 (1571 kcal/capita/day; $30902). Total non-protein energy (NPE) is the sum of energy supplies from fat and carbohydrate for each surface. Total protein energy (PE) supplies are held constant at 400 kcal/capita/day along the grey dashed isoline shown on each surface. \({l}_{5}\) = survivorship to age 5; \({l}_{60}\)= survivorship to age 60. GDP gross domestic product per capita (in international-$ at 2011 prices).
Finally, Fig. 5 shows the logged hazard ratio (based on the predicted qx) at each age for an ABP-rich vs PBP-rich nutritional environment. A negative value for a given age class indicated better survival under an ABP-rich supply vs a PBP-rich supply and vice versa. A higher proportion of ABP supplies was found to support a lower \({q}_{{{\rm{x}}}}\) during early life. Whereas, from early adulthood, \({q}_{x}\) was lower with a higher proportion of PBP supplies. For both sexes, beyond age 70 there was a clear reduction in \({q}_{x}\) suggesting that differences between survival in the two nutritional environments becomes less prominent.
Optimal macronutrient supply ratios for sex-specific survival at ages 5 and 60 were computed using a general-purpose optimization and selected as animal- and plant- based model environments (detailed in Supplementary Table 18). The \({q}_{x}\) schedules from birth to 95 years in each model environment were then derived. The log hazard ratio for the animal- based nutritional environment was taken as the log of the ratios of \({q}_{x}\) for animal- vs plant-based nutritional environments. The total energy for the primarily animal-based composition is 3150 kcal for males and 3110 kcal for females. Total energy for the primarily plant-based composition is 3710 kcal for males and 3700 kcal for females. Within model environments, the percentage of carbohydrates and fats differed by <0.5% across sexes (Supplementary Table 18). \({q}_{x}\) = probability of mortality at age \(x\).
Discussion
We investigated associations between national daily per capita PBP and ABP supplies and patterns of ASM across countries and time, after accounting for international variation in national prosperity, population size, and the supplies of fat and carbohydrate. Robust disparities existed between the association of ABP and PBP with early and late-life mortality. Higher ABP supplies were associated with enhanced survivorship during infancy and early childhood, independent of total calorie supplies, while PBP-rich environments were associated with improved survivorship in later life and overall life expectancy at birth.
Our results align with previous ecological studies, indicating negative effects of energy-deficient nutritional environments on population survivorship across age groups26,29. In our analysis, low total protein supplies were consistently associated with reduced survivorship and diminished life expectancy. These results mirror epidemiological studies at both the ecological and cohort level, linking protein-energy malnutrition (PEM) to various mortality risk factors30,31,32. After mitigating undernutrition by ensuring an adequate availability of energy and total protein, an age-specific association with different protein sources (specifically, the balance between animal- and plant-based) emerged in our analysis, prompting a consideration of potential nutritional imbalances between PBP and ABP.
We have leveraged nutrient supply data from FBSs as a metric of the nutritional environment. This method offers a complementary, but different, perspective relative to assessments of individual dietary habits and nutrient intake. Firstly, FBS data facilitate extensive temporal and trans-national comparisons of food availability and consumption patterns, which can reveal long-term trends and regional differences; such extensive coverage is not currently available for intra-national data on diet pattern. Secondly, the FBS data capture the nutritional environment, which is likely to be highly responsive to policy interventions (e.g., agricultural, trade, and environmental policies affecting food systems), where individual diets can prove hard to affect.
Food and nutrient supplies, as captured by FBSs, are an upstream and key determinant of individual diet, and population-wide dietary patterns; the food supply influences the accessibility, affordability, and distribution of various food groups and nutrients, as well as setting a hard upper limit on average intake33. Indeed, several studies have shown that at the level of nutrient composition and food patterns, average national diet is correlated with nutrient supply34,35. Despite these correlations, nutrient supply cannot be considered a direct surrogate for intake, the latter being influenced by myriad intervening factors including inefficiencies in the food system such as food waste. Hence, our results should not be interpreted as evidence for the effects of nutrient intake on health. We can, however, look to the literature on dietary intake and health to provide potential nutritional explanations for the differential associations between ABP and PBP supplies and mortality that we have observed. We pay particular attention to both deficiencies of specific nutrients and how the consumption of certain nutritional compounds has been linked to chronic disease. Finally, we consider the limitations of this study including residual confounding.
Early-life survivorship
Our findings suggest that the composition of the nutritional environment may play a role in determining early life survival outcomes. In low-fat environments, early life survivorship was reduced when overall protein supplies were low, with no significant distinction between ABP and PBP sources. This result points to the general importance of protein supplies in energy-deficit environments36. Conversely, in higher fat environments (i.e., with sufficient net calories), increasing ABP supplies showed a distinct advantage for early life survivorship over PBP. This suggests a possible link between essential nutrient availability and survival up to age 5.
A possible explanation for this result is that ABP may offer some advantages over PBP in terms of bioavailability, digestibility, and essential animo acid content. ABPs provide a complete and balanced profile of indispensable amino acids37, which are the building blocks of proteins. They also have higher bioavailability and digestibility than most PBPs10,38, thus are more easily absorbed and utilised by the body. Moreover, ABPs are rich in other bioactive compounds including iron, zinc, and vitamin A, which are essential for growth and development. These nutrients are often lacking in developing regions, where under-5 mortality is high39. For example, growth and development increase the demand for iron in pregnant and lactating women, and children, putting them at higher risk of rapidly developing iron deficiency anaemia and increasing the risk of child mortality40. Noting that iron deficiency has been reported to be less frequent in countries where meat constitutes a significant part of the diet41, it is possible that the increased content and bioavailability of micronutrients, particularly iron, present in ABP may contribute to the stronger association between improved early life survivorship and increased ABP supplies, compared to PBP supplies42. Additionally, complementary consumption of ABPs with PBPs has been found to counteract the negative effects of some anti-nutrients commonly produced in many PBP, such as phytic acids, tannins and lectins, which can impair the absorption of iron and zinc from PBP sources10,43. Furthermore, nutritional environments may indirectly impact infant and early childhood survivorship due to maternal undernutrition and nutritional deficiencies, which have previously been connected to major causes of child mortality44. Therefore, increased ABP supplies may enhance early life survivorship by addressing maternal undernutrition, reducing nutritional deficiencies, improving breast milk quality and quantity, and lowering the risk of infant health problems39.
While our findings reinforce the benefits of ABP for early life survivorship, it is crucial to acknowledge the potential role of PBP, particularly in resource-constrained environments. In these settings – specifically, regions with limited ABP and fat supplies – our results indicated that adding energy from any protein source, plant or otherwise, was beneficial. This supports the idea that our findings do not refute the advantage of incorporating PBP in a malnourished environment8,45. In resource-constrained environments where there is limited access to diverse and protein-rich foods, heightened energy demands, and a reliance on less nutrient-dense diets, vegetarian dietary patterns show promise if they are balanced and complete46. PBPs may be tailored to mimic the AA profile of ABPs37 and can also be combined to achieve a balanced indispensable AA profile, by exploiting the complementarity between different sources, such as cereals and legumes47. Ensuring completeness in these dietary patterns for early life may require addressing potential deficiencies, such as vitamin B deficiency, which can be detrimental during infant development48.
Consistent with previous ecological analyses, our results demonstrated positive associations between fat supplies and early life survivorship26,29, highlighting potential benefits associated with dietary fat in early life. Further research is needed to fully understand this association. However, a plausible nutritional explanation is that fat, particularly from animal based sources, serves as a rich energy source and a carrier of indispensable fatty acids and essential fat-soluble vitamins38,49,50. Essential fatty acids, required for growth, development, and immune function, must be obtained from the diet, supporting the observed benefit of increasing fat-energy supplies during early life51. Specific fat subtypes and ABP are often correlated in certain animal-based foods, such as red meats and full-fat dairy products, which can complicate attributing health effects to specific nutrients52. The GFN can help to disentangle these effects by considering their relative balance and interactions with other nutrients, provided sufficient data are available.
Later-life survivorship
Our investigation revealed distinct associations with nutrient supplies for later-life survivorship compared to early-life survivorship. Maximal survivorship to age 60 and overall life expectancy at birth were observed in nutritional environments with lower fat supplies and a higher proportion of protein from PBP sources. This finding does mirror existing research at the level of individual diets, which show the long-term health benefits and reduced mortality risks associated with PBP- over ABP-based foods. These benefits include a reduced risk of type 2 diabetes, all-cause mortality, and cardiovascular disease and dementia mortality18,53,54. One possible explanation for the positive association between PBP and increased later-life survivorship is the higher dietary fibre and wholegrain content associated with PBP food sources55. High intake of whole grains has been linked to decreased risks of mortality from all causes, cardiovascular disease, and cancer in the general population56. Furthermore, the observed advantage of low ABP combined with low-fat supplies for later life survivorship is consistent with studies that emphasise the potential mid-life harms resulting from overconsumption of saturated fatty acids and cholesterol found in red and processed meat57.
Whilst the reduced benefit to later life survivorship in ABP-rich environments relative to PBP-rich environments may stem from differences in nutritional components, it may also be indicative of poorer overall dietary trends influenced by factors such as income, urbanisation, and increased access to processed foods. Specifically, as income and urbanisation rise, so does the consumption of meat, sugar, fats, oils, and processed foods58,59. This dietary pattern is particularly pronounced in countries with a higher Socio-Demographic Index (SDI), where the abundance of ABP – largely sourced from modern supermarkets – reflects greater access to ultra-processed and ready-made foods60. Numerous studies have consistently linked high intake of processed foods to an increased risk of age-related ill health, and premature death, providing a plausible explanation for the negative impact on later-life survivorship observed in ABP-rich environments, compared to PBP-rich ones19,61,62,63.
Several pre-clinical studies have suggested that reducing the ratio of total protein to carbohydrate in the diet may extend longevity56,64,65. It is important to note that findings from animal models may not always directly translate to human health outcomes, especially at the population/ecological level. However, our analyses, which statistically control for total carbohydrates, correlate with these findings. Further, we see additional independent benefits of replacing ABP with PBP, within human populations, irrespective of the protein-carbohydrate ratio. Some researchers have suggested that the observed benefits of protein restriction during mid and early-late life may be driven, in part, by reduced intake of branched chain amino acids (BCAAs), of which excess consumption is associated with several age-related chronic health problems66. Such a mechanism may explain our result, showing that iso-calorically substituting ABP supplies, generally higher in BCAAs, with PBP, generally lower in BCAAs67, is associated with gains in population life-expectancy without any restriction of total protein supplies or altering the ratio of protein to carbohydrates in the food supply.
Additionally, we observed unfavourable associations between higher fat and survivorship in later life, potentially explained by overnutrition resulting from the higher energy density of dietary fat compared to carbohydrate and protein68. This detrimental relationship was particularly pronounced when combined with ABP consumption, indicating a synergistic negative influence. The involvement of fat-rich processed foods in this association is also conceivable, particularly when considering westernised dietary patterns69,70.
As an ecological study, our findings overlook intra-population variations in both the nutritional environment and dietary patterns and are based on national food supply data rather than individual dietary intake. This distinction is important as it highlights the broader availability of nutrients rather than specific consumption behaviours, and the possibility of unaccounted for confounding variables should be considered. Causal relationships and the exact mechanisms underlying the observed associations between protein sources and mortality cannot be established based on this study alone. National-level data are unable to capture within country heterogeneity, potentially overlooking regional and inter-individual variations in access to nutrients, dietary habits and health outcomes71. Additionally, the ecological fallacy limitation must be acknowledged; based on the relationships illustrated by our findings, it cannot be concluded that every individual who replaces ABP with PBP sources will or has experienced an increased likelihood of survival to age 60. While our results are based on indicative supplies available for human consumption, they are not a direct measure of dietary intake. As detailed elsewhere and above72, discrepancies can arise between foods supplies and habitual diets due to factors such as food wastage, processing losses and variations in individual consumption patterns. Additionally, to maintain the interpretability of our models, our analyses do not account for the heterogeneity within the carbohydrate and fat supplies, such as the differences between refined carbohydrates, fibre, and sugar, as well as between saturated and polyunsaturated fats. The current analyses focus on the impacts of protein sources. Specifically, we examine the potential effects of reduced animal-based food production, resulting in the substitution of ABP for PBP and/or a reduction in total protein supplies on age-specific mortality. It should therefore be noted that our results may not fully capture the true impact of variations in carbohydrate and fat supplies on mortality outcomes. While these issues make assigning the effect of the nutritional environment directly to diet a vexed issue, our findings emphasise the potential side effects of shifts to more sustainable food systems on population survivorship.
With regards to confounding, despite adjusting for time, GDP, other nutritional supplies, and population size, we acknowledge that other potential confounding factors may exist. For example, GDP only provides a partial reflection of the disparities between countries that influence survival. Nutritional factors that are potentially unaccounted for include levels of foodborne illness and food safety, which may be more influential in lower income countries73. Further confounding factors that have not been accounted for may include broader socio-economic factors such as food access, affordability, and cultural dietary preferences74,75.
Finally, while we have undertaken broad corrections, there remains the possibility of residual confounding associated with the type of fat or carbohydrates. Our analyses incorporated statistical adjustments for total fat and carbohydrate supplies. However, limitations in the data prevented us from further separating out the effects of different fat and carbohydrate types, as well as other nutrients such as fibre. It is important to note such nutritional covariances represent potential confounders. Considering fat, for instance, it has been shown that ABP and PBP sources tend to be enriched with saturated and unsaturated fat, respectively76,77,78. At the dietary level, saturated fats are associated with increased rates of mortality, while unsaturated fats appear protective79. These complexities are not unique to our study system, even representing a challenge to the most granular level of clinical trials. One 12 week clinical trial in healthy Finnish adults replacing ABP with PBP sources resulted in improved plasma lipoprotein levels55. However, the dietary transition was also associated with improved overall dietary fat quality and increased intake of dietary fibre and complex carbohydrates55. All of these dietary dimensions have been linked, variably, to decreased risks of chronic diseases and mortality from all causes, cardiovascular diseases, and cancer in the general human population56,80,81, making it hard to pin-point if any one individual dietary change was causal.
Our analysis of historical associations between protein supplies and age-specific mortality patterns predicts the potential impact of transitioning from ABP to PBP. Our results indicate that low overall protein supplies negatively associate with survivorship across all age intervals. In the context of energy sufficiency, higher proportions of ABP supplies associate with greater improvement in early life survivorship, while nutritional environments richer in PBP maximise later life survivorship and overall life expectancy. We also note, similarly, high fat supplies may be beneficial in early life, but detrimental later throughout life. However, it is important to note that while national food supplies can highlight potential trends and associations, they are not definitive in establishing the direct biological effects of specific nutrient levels on survival. Although we cannot establish causality, these patterns align with existing literature linking diet and healthspan in humans, particularly the long-term advantages of consuming a plant-based diet. In the context of advocating for decarbonisation of food systems, especially the shift from animal-based to plant-based products, our findings stress the importance age-specific considerations.
Methods
Data
This study uses only publicly available data and therefore compliance from an ethics committee was not deemed applicable. To build our dataset we combined data from several sources, incorporating ecological design principles to ensure a comprehensive analysis of the environmental impacts of dietary shifts. Macronutrient supply data was obtained from the United Nations’ Food and Agriculture Organisation Statistical Database (FAOSTAT)82. FAOSTAT provides Food Balance Sheet (FBS) data which present global, regional, and national statistics of food availability. This includes the total food supply allocated for potential human consumption as per capita estimates of dietary energy supply, specific to a country and year. For all available (country, year) combinations, average per capita food supplies were extracted in terms of fat and protein content (g/person/day). Given the current goal to distinguish the effects of plant and animal-based protein supplies, we took separate values for protein supply – expressed in terms of protein content from vegetal and animal products – provided by the database. Protein, fat, and alcohol supply quantities were subtracted from the reported total food supplies per capita, given in terms of caloric value (kcal/person/day) for each (country, year) population. The difference was used to represent the average per capita nutritional contribution of energy from carbohydrate, for the specified (country, year) combination.
For each (country, year) combination in our dataset we included a value for GDP, obtained from the 2020 update of the Maddison Project Database83. This was found to be the most historically and globally comprehensive dataset available for worldwide, economic statistics.
Lastly, our dataset included ASM patterns from life tables in the Human Lifetable Database (HLD)84. The HLD has been developed as part of the Human Mortality Database project. It hosts a collection of individual life tables for numerous developed and developing, national and subnational populations around the world. The HLD has been prepared from a range of sources, including official reports, as well as statistical and scientific publications and datasets. The database assembles and recalculates standardized complete and abridged life tables that cover many years, providing a quantitative description of the evolution of human mortality. More specifically, each life table contains a series of inter-related age-specific mortality statistics, including survivorship to age \(x\), denoted \({l}_{x}\); life expectancy at age \(x\), denoted \({e}_{x}\); probability of mortality at age x, denoted qx. For our analysis we required the values for survivorship (i.e., proportion of a cohort surviving at a given age) from each life table. For this reason, we used the complete (unabridged) lifetables provided by the HLD, since they are constructed based on single year age intervals. Furthermore, we calculated the standard error for each estimated survival value by adding population estimates to the HLD life table data. This data was obtained by age and sex from the United Nations Population Division85. The Kaplan-Meier product-limit estimator was used to re-estimate the survival values and confirm their consistency with the HLD life table data. Standard error was then calculated using Greenwood’s formula86, which considers the number of people at risk of death in each age interval. This allowed us to assess stochasticity in, and sampling variance of, the estimated survival values (i.e., estimates from small populations are expected to vary year-to-year, more than those in large populations). Following this, the HLD lifetable values and standard errors for male and female survivorship to ages 5 (\(l\)₅) and 60 (\(l\)₆₀) were added to the already collated nutrient supply and wealth data for all matching (country, year) combinations. Briefly, we focus on these parameters, \(l\)₅ and \(l\)₆₀, because they give critical information about early and late-age mortality and can be used with model life tables to rederive the complete set of life table parameters across all ages, for downstream interpretation. We discuss this in further detail below.
Statistical analysis
The complete dataset was stratified by sex, giving country by year specific values for \(l\)₅ and \(l\)₆₀ in both males and females. Each of the four stratified datasets was then analysed separately. To quantify covariances between demographics and macronutrient supplies, with corrections for potential confounders, a series of generalised additive mixed models (GAMMs) were used to estimate the response variables: \(l\)₅ and \(l\)₆₀. In short, GAMMs combine the flexibility of Generalized Additive Models, in terms of including conventional linear parametric, and non-parametric non-linear smoothed terms, with the ability to account for both within-group correlations and between-group variability using random effects (e.g., as in generalised linear-mixed models)87. Additionally, GAMMs can handle non-normal responses by using a distributional transformation (i.e., link-functions) of the response variable to make it more appropriate for modelling. In the current case, since \(l\)₅ and \(l\)₆₀ are bounded response variables (i.e., between 0 and 1), we chose to specify the Beta distribution family and logit link transformation function. In summary, by using GAMMs as our chosen statistical tool we have been able to model complex, non-linear relationships between the response variables, \(l\)₅ and \(l\)₆₀, and multiple predictor variables, including macronutrient supply, time, and economic wealth, while also accounting for random effects of country. All GAMMs have been fitted in R using the gam function from the mcgv package88.
To explore the effects of macronutrient supply, time, and economic wealth, we constructed a comprehensive series of models containing subsets of these predictor variables and their interactions (Supplementary Table 13). All models accounted for differences in typical survival among countries as a random effect. The macronutrient supplies considered were ABP, PBP, carbohydrate and fat in kcal/capita/day. We restricted our most complex terms for nutrient supply to a 3-dimensional (3D) smooth term. This was because higher-dimensional models, such as 4-dimensional nutritional effects, are overly complex and difficult to interpret. We tested a series of models to determine the effects of PBP and ABP supplies on survival. The models included 3D terms comprising ABP, PBP, and carbohydrate or ABP, PBP, and fat. The other nutrient dimension outside the 3D term was then included as an additional additive smoothed term. Supplementary Table 13 and Supplementary Table 14 list the full set of candidate models that we explored. Inclusion of the GDP value, to reflect economic wealth, aimed to control for the potential confounding and/or interacting effect of wealth.
Akaike Information Criterion (AIC) was used for model scoring and selection. The set of models was tested and scored using AIC values, for each outcome and sex. To rank the relative fit and parsimony of each model to the dataset, differences (∆) from the lowest AIC value (AICmin) were computed over all candidate models in the set (i.e., for model i: Δ = AICi – AICmin). Based on a conventionally accepted threshold, all models with AIC differences >2 (∆ > 2) were discarded. Among models with AIC differences of ∆ ≤ 2 we selected the model with the lowest parameters using the effective degrees of freedom for smooth terms (EDF, i.e., the least complex model).
Finally, to improve the generalizability of our models and mitigate model bias from outliers, survivorship observations were weighted based on their standard errors, considering lower uncertainties to be better representations of the true long-term population survival rates. Weights were calculated by scaling the standard errors of survivorship using the inverse of the squared standard errors and normalisation, relative to the mean. New weights were predicted for each observation as a function of year (to account for the fact that the global population has grown over the time-course covered by our data), while accounting for variance in uncertainty levels between countries. These weights were then applied to each GAMM using Weighted Least Squares to provide more reliable model estimates of survivorship probabilities and accommodate heteroscedasticity in the data.
Model life tables and derived mortality statistics
In several instances, we used the \(l\)₅ and \(l\)₆₀ values predicted by AIC-favoured GAMMs to re-derive entire life tables including life-expectancy (ex) and hazards of mortality (qx), for all age classes between 0 and 110. This was done using the modified version of the Brass-relational model life table system89 proposed by Murray et al. (2003)90. In the context of generating model life tables representative of human mortality, the modified system has been shown to accurately capture the extent to which mortality varies across populations with respect to age and sex. Specifically, survivorship to age x for life table k (\({l}_{x}^{k}\)) was calculated using the inverse logit of the transformation shown in Eq. (1), where (\({l}_{5}^{k}\), \({l}_{60}^{k}\)) are the pairs of \(l\)₅ and \(l\)₆₀ values predicted by the GAMM for life table k and (\({l}_{5}^{s}\), \({l}_{60}^{s}\)) come from a sex-specific global standard life table (s).
In the above functions, Logit is the standard logit transformation, and \({l}_{x}^{s}\) is the survivorship to age x, obtained from s. The γₓ and θₓ parameters make age-specific corrections and are also taken from life table s. Here, s refers to the global standard life table provided in Wilmoth et al. (2012)91, specific to the sex in question (Supplementary Table 15). Having derived the pattern of survivorship from age x = 0 to 110, for lifetable k, we calculated the related mortality statistics (e.g., life-expectancy, ex, and probability of death, qx) using the LifeTable function from the R package, MortalityLaws92.
Sensitivity analysis
To assess the sensitivity of our results to biases in the available data we conduced analyses with imputed values for missing data in certain countries and years. The imputation was performed using the ‘mice’ function from the R packages mice and miceadds53,54. The variables subjected to imputation included: (1) protein supply (1.7% missing), (2) animal-based protein supply (1.7% missing), (3) plant-based protein supply (1.7% missing), (4) carbohydrate supply (1.7% missing), (5) fat supply (1.7% missing), and (6) log of GDP per capita as given by the Maddison project27 (14.95% missing). We employed a two-level imputation approach, treating countries as a clustering factor at the second level. The imputation method used was ‘2 l.pmm’ where ‘pmm’ refers to ‘predictive mean matching’. This semi-parametric method selects the closest observed values based on prediction to impute missing values. The choice of 50 imputed datasets was made to account for uncertainty in the imputation process. The convergence of the imputation process was assessed over 20 iterations. While common in multiple imputation, the standard approach of fitting independent models to each imputed dataset before averaging was not suitable for our analysis due to the presence of GAMM smooth terms. Instead, the average of the imputed datasets was used to refit selected GAMM as described in methods. It’s worth noting that this imputation strategy provides a robust method for handling missing data and enhances the reliability of our analyses.
This study does not constitute human-subject research and meets the criteria for IRB exemption under 45 CFR 46.102. The data used were obtained from sources that ensure the information is de-identified and publicly accessible therefore, no IRB review was required.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data used in this study were obtained from publicly accessible databases. We have complied with all data usage conditions and licensing agreements associated with these databases. Population estimates were sourced from the United Nations Population Division [available at https://population.un.org/wpp/ and downloaded on 02 Feb 2023]. Life table data were sourced from the Human Lifetable Database [available at https://www.lifetable.de/ and downloaded on 03 August 2023]. Food supply data were sourced from the Food and Agriculture Statistical Database [available at https://www.fao.org/faostat/en/ and downloaded on 11 August 2023]. Historical economic data were sourced from the Maddison Project Database [available at https://www.rug.nl/ggdc/historicaldevelopment/maddison/releases/ and downloaded on 21 March 2022]. For any future analyses, it is recommended to obtain current data directly from the referenced sources for the most up-to-date information.
Code availability
All code for the current analyses can be found at GitHub, https://doi.org/10.5281/zenodo.1496353893.
References
Crippa, M. et al. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2, 198–209 (2021).
Béné, C. et al. When food systems meet sustainability – current narratives and implications for actions. World Dev. 113, 116–130 (2019).
Augustin, M. A. & Cole, M. B. Towards a sustainable food system by design using faba bean protein as an example. Trends Food Sci. Technol. 125, 1–11 (2022).
Lonnie, M. & Johnstone, A. M. The public health rationale for promoting plant protein as an important part of a sustainable and healthy diet. Nutr. Bull. 45, 281–293 (2020).
Willett, W. et al. Food in the anthropocene: the EAT-lancet commission on healthy diets from sustainable food systems. Lancet 393, 447–492 (2019).
Tripathi, A. D., Mishra, R., Maurya, K. K., Singh, R. B. & Wilson, D. W. Chapter 1 - estimates for world population and global food avilability for global health. In The Role of Functional Food Security in Global Health (eds. Singh, R. B., Watson, R. R. & Takahashi, T.) 3–24 (Academic Press, 2019).
Fanzo, J., Bellows, A. L., Spiker, M. L., Thorne-Lyman, A. L. & Bloem, M. W. The importance of food systems and the environment for nutrition. Am. J. Clin. Nutr. 113, 7–16 (2021).
Viroli, G., Kalmpourtzidou, A. & Cena, H. Exploring benefits and barriers of plant-based diets: health, environmental impact, food accessibility and acceptability. Nutrients 15, 4723 (2023).
Pereira, M. H. Q., Pereira, M. L. A. S., Campos, G. C. & Molina, M. C. B. Food insecurity and nutritional status among older adults: a systematic review. Nutr. Rev. 80, 631–644 (2022).
FAO. Contribution of Terrestrial Animal Source Food to Healthy Diets for Improved Nutrition and Health Outcomes - An Evidence and Policy Overview on the State of Knowledge and Gaps. https://openknowledge.fao.org/server/api/core/bitstreams/0c1bfa99-18d4-42e4-b94f-27160126f826/content (2023).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 23, 56–73 (2022).
Longo, V. D. & Anderson, R. M. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell 185, 1455–1470 (2022).
Lagiou, P. et al. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J. Intern. Med. 261, 366–374 (2007).
Chen, Z. et al. Dietary protein intake and all-cause and cause-specific mortality: results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 35, 411–429 (2020).
Bauer, J. et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 14, 542–559 (2013).
Deutz, N. E. P. et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin. Nutr. 33, 929–936 (2014).
Huang, J. et al. Association between plant and animal protein intake and overall and cause-specific mortality. JAMA Intern. Med. 180, 1173–1184 (2020).
Sun, Y. et al. Association of major dietary protein sources with all-cause and cause-specific mortality: prospective cohort study. J. Am. Heart Assoc. 10, e015553 (2021).
van den Brandt, P. A. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in the Netherlands cohort study. Eur. J. Epidemiol. 34, 351–369 (2019).
Etemadi, A. et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP diet and health study: population based cohort study. BMJ 357, j1957 (2017).
Aune, D. Plant foods, antioxidant biomarkers, and the risk of cardiovascular disease, cancer, and mortality: a review of the evidence. Adv. Nutr. 10, S404–S421 (2019).
Buettner, D. & Skemp, S. Blue zones: lessons from the world’s longest lived. Am. J. Lifestyle Med. 10, 318–321 (2016).
Chacón, A. M., Jiménez, C. C. & Campos, H. Dietary habits and lifestyle among long-lived residents from the Nicoya Peninsula of Costa Rica. Rev. Hispanoam. de. Cienc. de. la Salud (RHCS) 3, 53–60 (2017).
Virtanen, H. E. K. et al. Dietary proteins and protein sources and risk of death: the Kuopio Ischaemic heart disease risk factor study. Am. J. Clin. Nutr. 109, 1462–1471 (2019).
Simpson, S. J. et al. The geometric framework for nutrition as a tool in precision medicine. Nutr. Healthy Aging 4, 217–226 (2017).
Gage, T. B. & O’Connor, K. Nutrition and the variation in level and age patterns of mortality. 1994. Hum. Biol. 81, 551–574 (2009).
Food And Agriculture Organization The United Nation. Food Balance SHEETS - A Handbook. https://www.fao.org/4/X9892E/X9892e02.htm#P430_31575 (2001).
Murray, C. J. L., Ahmad, O. B., Lopez, A. D. & Salomon, J. A. WHO System of Model Life Tables. https://apps.who.int/iris/bitstream/handle/10665/67747/a78509.pdf (2000).
Senior, A. M., Nakagawa, S., Raubenheimer, D. & Simpson, S. J. Global associations between macronutrient supply and age-specific mortality. Proc. Natl Acad. Sci. Usa. 117, 30824–30835 (2020).
Batool, R., Butt, M. S., Sultan, M. T., Saeed, F. & Naz, R. Protein-energy malnutrition: a risk factor for various ailments. Crit. Rev. Food Sci. Nutr. 55, 242–253 (2015).
Grover, Z. & Ee, L. C. Protein energy malnutrition. Pediatr. Clin. North Am. 56, 1055–1068 (2009).
Zhang, X. et al. Global, regional, and national burden of protein-energy malnutrition: a systematic analysis for the global burden of disease study. Nutrients 14, 2592 (2022).
Smith, M. R., Micha, R., Golden, C. D., Mozaffarian, D. & Myers, S. S. Global expanded nutrient supply (GENuS) model: a new method for estimating the global dietary supply of nutrients. PLoS ONE 11, e0146976 (2016).
Remans, R., Wood, S. A., Saha, N., Anderman, T. L. & DeFries, R. S. Measuring nutritional diversity of national food supplies. Glob. Food Sec. 3, 174–182 (2014).
Del Gobbo, L. C. et al. Assessing global dietary habits: a comparison of national estimates from the FAO and the global dietary database. Am. J. Clin. Nutr. 101, 1038–1046 (2015).
Ghosh, S., Suri, D. & Uauy, R. Assessment of protein adequacy in developing countries: quality matters. Br. J. Nutr. 108, S77–S87 (2012).
Dimina, L., Rémond, D., Huneau, J.-F. & Mariotti, F. Combining plant proteins to achieve amino acid profiles adapted to various nutritional objectives-an exploratory analysis using linear programming. Front Nutr. 8, 809685 (2021).
Prache, S. et al. Review: Quality of animal-source foods. Animal 16, 100376 (2022).
Neumann, C., Harris, D. M. & Rogers, L. M. Contribution of animal source foods in improving diet quality and function in children in the developing world. Nutr. Res. 22, 193–220 (2002).
Scott, S. P., Chen-Edinboro, L. P., Caulfield, L. E. & Murray-Kolb, L. E. The impact of anemia on child mortality: an updated review. Nutrients 6, 5915–5932 (2014).
Uzel, C. & Conrad, M. E. Absorption of heme iron. Semin. Hematol. 35, 27–34 (1998).
Pasricha, S.-R., Tye-Din, J., Muckenthaler, M. U. & Swinkels, D. W. Iron deficiency. Lancet 397, 233–248 (2021).
Samtiya, M., Aluko, R. E. & Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod. Process. Nutr. 2, 1–14 (2020).
Black, R. E. et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013).
McDermott, J. & Wyatt, A. J. The role of pulses in sustainable and healthy food systems. Ann. N. Y. Acad. Sci. 1392, 30–42 (2017).
Sebastiani, G. et al. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 11, 557 (2019).
Nolden, A. A. & Forde, C. G. The nutritional quality of plant-based foods. Sustain. Sci. Pract. Policy 15, 3324 (2023).
Green, R. & Miller, J. W. Chapter Fifteen - Vitamin B12 deficiency. In Vitamins and Hormones (ed. Litwack, G.) 405–439 (Academic Press, 2022).
Wood, J. D. et al. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 78, 343–358 (2008).
Lands, W. E. M. Dietary fat and health: the evidence and the politics of prevention: careful use of dietary fats can improve life and prevent disease. Ann. N. Y. Acad. Sci. 1055, 179–192 (2005).
Millward, D. J. Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev. 30, 50–72 (2017).
Wang, Y., Hill, E. R., Campbell, W. W. & O’Connor, L. E. Plant- and animal-based protein-rich foods and cardiovascular health. Curr. Atheroscler. Rep. 24, 197–213 (2022).
Zheng, J. et al. The isocaloric substitution of plant-based and animal-based protein in relation to aging-related health outcomes: a systematic review. Nutrients 14, 557 (2022).
Qian, F., Liu, G., Hu, F. B., Bhupathiraju, S. N. & Sun, Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern. Med. 179, 1335–1344 (2019).
Ekmekcioglu, C. Nutrition and longevity - from mechanisms to uncertainties. Crit. Rev. Food Sci. Nutr. 60, 3063–3082 (2020).
Capurso, C. Whole-grain intake in the mediterranean diet and a low protein to carbohydrates ratio can help to reduce mortality from cardiovascular disease, slow down the progression of aging, and to improve lifespan: a review. Nutrients 13, 2540 (2021).
Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 281, 106–122 (2017).
International Food Policy Research Institute (IFPRI). Urbanization and the Nutrition Transition. https://gfpr.ifpri.info/2017/03/10/chapter-4-chapter-4/ (2017).
Henchion, M., McCarthy, M., Resconi, V. C. & Troy, D. Meat consumption: trends and quality matters. Meat Sci. 98, 561–568 (2014).
Downs, S. M., Ahmed, S., Warne, T., Fanzo, J. & Loucks, K. The global food environment transition based on the socio-demographic index. Glob. Food Security 33, 100632 (2022).
Levy, R. B. et al. Ultra-processed food consumption and type 2 diabetes incidence: a prospective cohort study. Clin. Nutr. 40, 3608–3614 (2021).
Zhang, Y. & Giovannucci, E. L. Ultra-processed foods and health: a comprehensive review. Crit. Rev. Food Sci. Nutr. 63, 10836–10848 (2023).
Dehghan, M. et al. Ultra-processed foods and mortality: analysis from the prospective urban and rural epidemiology study. Am. J. Clin. Nutr. 117, 55–63 (2023).
Senior, A. M. et al. Dietary macronutrient content, age-specific mortality and lifespan. Proc. Biol. Sci. 286, 20190393 (2019).
Solon-Biet, S. M. et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 11, 1529–1534 (2015).
Fontana, L. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530 (2016).
Young, V. R. & Pellett, P. L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 59, 1203S–1212S (1994).
Rolls, B. J. The relationship between dietary energy density and energy intake. Physiol. Behav. 97, 609–615 (2009).
Monteiro, C. A., Moubarac, J.-C., Cannon, G., Ng, S. W. & Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 14, 21–28 (2013).
da Costa, G. G., da Conceição Nepomuceno, G., da Silva Pereira, A. & Simões, B. F. T. Worldwide dietary patterns and their association with socioeconomic data: an ecological exploratory study. Glob. Health 18, 31 (2022).
Lu, C. Y. Observational studies: a review of study designs, challenges and strategies to reduce confounding. Int. J. Clin. Pr. 63, 691–697 (2009).
Thar, C.-M., Jackson, R., Swinburn, B. & Mhurchu, C. N. A review of the uses and reliability of food balance sheets in health research. Nutr. Rev. 78, 989–1000 (2020).
Liguori, J. et al. How do food safety concerns affect consumer behaviors and diets in low- and middle-income countries? a systematic review. Glob. Food Security 32, 100606 (2022).
Placzek, O. Socio-Economic and Demographic Aspects of Food Security and Nutrition. https://www.oecd-ilibrary.org/content/paper/49d7059f-en (2021).
Karanja, A., Ickowitz, A., Stadlmayr, B. & McMullin, S. Understanding drivers of food choice in low- and middle-income countries: a systematic mapping study. Glob. Food Security 32, 100615 (2022).
Siri-Tarino, P. W., Sun, Q., Hu, F. B. & Krauss, R. M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 91, 502–509 (2010).
Li, D. Chemistry behind vegetarianism. J. Agric. Food Chem. 59, 777–784 (2011).
Li, D. Effect of the vegetarian diet on non-communicable diseases: effect of the vegetarian diet on non-communicable diseases. J. Sci. Food Agric. 94, 169–173 (2014).
Wang, D. D. et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern. Med. 176, 1134–1145 (2016).
Afshin, A. et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 393, 1958–1972 (2019).
Augustin, L. S. A. et al. Dietary fibre consensus from the international carbohydrate quality consortium (ICQC). Nutrients 12, 2553 (2020).
FAOSTAT. Food Balances. https://www.fao.org/faostat/en/#data (2024).
Bolt, J. & Luiten van Zanden, J. Maddison Style Etimates of the Evolution of the World Economy. https://www.rug.nl/ggdc/historicaldevelopment/maddison/publications/wp15.pdf (2020).
HLD. Human Life-Table Database. https://www.lifetable.de/ (2024).
United Nations, Department of Economic and Social Affairs, Population Division (2022). World Population Prospects 2022. https://population.un.org/wpp/Download/Archive/Standard/ (2022).
Tutz, G. & Schmid, M. Modeling Discrete Time-to-Event Data 1st edn, Vol. 247 (Springer, 2016).
Wood, S. N. Generalized Additive Models: An Introduction with R 2nd edn (CRC Press, 2017).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol 73, 3–36 (2011).
Brass, W. Mortality models and their uses in demography. Trans. Fac. Actuaries 33, 123–142 (1971).
Murray, C. J. L. et al. Modified logit life table system: principles, empirical validation, and application. Popul. Stud. 57, 165–182 (2003).
Wilmoth, J., Zureick, S., Canudas-Romo, V., Inoue, M. & Sawyer, C. A flexible two-dimensional mortality model for use in indirect estimation. Popul. Stud. 66, 1–28 (2012).
Pascariu, M. D. Parametric Mortality Models, Life Tables and HMD [R package MortalityLaws version 1.9.4]. https://cran.r-project.org/web/packages/MortalityLaws/readme/README.html (2022).
Andrews, C. J. cand8403/ABP_PBP_FS: (v1.0.0) [computer software]. Zenodo https://doi.org/10.5281/zenodo.14963539 (2025).
Massicotte, P. & South, A. rnaturalearth: World map data from natural Earth. R Package version 1.0.1. GitHub https://github.com/ropensci/rnaturalearth, https://docs.ropensci.org/rnaturalearth/ (2025).
Acknowledgements
C.J.A. discloses funding support for the research of this work from the University of Sydney, Faculty of Science. AMS received support from the University of Sydney Horizon Program and the Australian Research Council Future Fellowship scheme.
Author information
Authors and Affiliations
Contributions
C.J.A., A.M.S., S.J.S., and D.R. designed research and contributed to interpretation of results; C.J.A. performed analysis. C.J.A., and A.M.S. wrote the first draft of the paper. C.J.A., A.M.S., S.J.S., and D.R. contributed to revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Andrews, C.J., Raubenheimer, D., Simpson, S.J. et al. Associations between national plant-based vs animal-based protein supplies and age-specific mortality in human populations. Nat Commun 16, 3431 (2025). https://doi.org/10.1038/s41467-025-58475-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58475-1