Abstract
Charge overpotential for oxygen evolution reaction is a crucial parameter for the energy conversion efficiency of lithium-oxygen (Li-O2) batteries. So far, the realization of low charge overpotential via catalyst design is a grand challenge in this field, which usually exceeds 0.25 V. Herein, we report an orbital reconstruction strategy to significantly decrease the charge overpotential to the low 0.11 V by employing PdCo nanosheet catalyst under a low-loading mass (0.3 mg/cm2) and capacity (0.3 mAh/cm2). Experimental and theoretical calculations demonstrate that the precise d-d orbital coupling (dxz-dxz, dyz-dyz and dz2-dz2) between the low-electronegativity Co and Pd leads to the reconstruction of Pd 4 d orbitals in PdCo nanosheets, thereby resulting in a downward shift of all the three active Pd 4 d orbitals (dz2, dxz and dyz) relative to that of Pd nanosheets. Furthermore, the highest energy level of the Pd 4dz2 orbital in PdCo is lower than the lowest energy levels of the Pd 4dxz and 4dyz orbitals in pure Pd, significantly decreasing the charge activation energy and achieving a highest energy conversion efficiency of 91%. This finding provides the orbital-level tuning into rational design of highly efficient electrocatalysts for Li-O2 batteries.
Similar content being viewed by others
Introduction
Lithium-oxygen (Li-O2) battery has garnered wide attention due to its highest theoretical energy density of 3500 Wh kg-1 among all the battery systems1,2, making it a highly promising candidate for the market competition in long-range electric vehicles and massive energy storages3,4,5,6,7. However, the sluggish oxygen evolution reaction (OER) leads to high charge overpotential and thus, low energy conversion efficiency, which is the bottleneck problem in the development of Li-O2 batteries8,9,10. In addition, such high charge overpotential can lead to the degradation of electrolytes and the deposition of insulating parasitic products, causing inferior cycle stability11,12,13,14. To date, significant efforts have been devoted to reducing the charge overpotential by designing cathode electrocatalysts, such as noble metal-based alloys15,16,17, single-atom catalysts18,19,20,21, sulfides22,23,24, transition metal oxides25,26,27, and metal-organic frameworks28,29,30,31, etc. Recently, Pd-based nanocatalysts are highly considered as the promising candidates for Li-O2 batteries due to their tunable orbital electronic configuration and energy levels32,33,34. The main strategy for decreasing the charge overpotential of Pd-based catalysts is to reduce the overall d-band energy level (d-band center) of Pd, thereby weakening the adsorption interaction towards intermediate5,35. However, only the regulation of the d-band center does not ensure a simultaneous reduction of the three active orbitals (dxz, dyz, and dz2) out of the total five orbitals (the other two inactive orbitals are dx2−y2 and dxy). Consequently, the charge overpotentials have not been reduced to the desired value of below 0.25 V, which is the threshold of a reliable battery system with an energy conversion efficiency above 85%12,34,36,37.
Herein, we propose an orbital reconstruction strategy to remarkably reduce the charge overpotential to 0.11 V by using PdCo at a low loading mass (0.3 mg/cm2) with a low capacity (0.3 mAh/cm2). Due to the lower electronegativity of Co over other transition metals, and the perfect alignment of d orbitals between Pd and Co (energy level differences are less than 0.5 eV), the desired d-d orbital coupling of Pd and Co (dxz-dxz, dyz-dyz, and dz2-dz2) in PdCo nanosheets induced Pd 4 d orbital reconstruction and effectively lowered the energy levels of the active dxz, dyz, and dz2 orbitals of Pd. Theoretical calculations further indicated that the top energy level of the reconstructed dz2 orbital of Pd in PdCo nanosheets was even lower than the bottom energy level of Pd dxz/yz orbitals in Pd nanosheets, which significantly lowered the activation energy of the OER rate-determining step (LiO2 = Li+ + O2) in Li-O2 batteries and achieved the high energy conversion efficiency of 91%.
Results
Synthesis and characterization of Pd72Co28 nanosheets
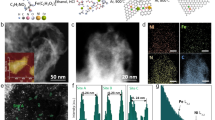
The PdCo nanosheet was synthesized through a conventional colloidal chemistry method. The obtained PdCo nanosheets exhibited a highly curved morphology (Fig. 1a) with a thickness of approximately 3-4 nm in atomic force microscopy (AFM, Fig. 1b). Similarly, Pd nanosheets synthesized by the same method demonstrated a comparable curved morphology and uniform distribution of Pd elements (Supplementary Fig. 1). The X-ray diffraction (XRD) patterns of PdCo nanosheets showed a slight shift towards lower angles compared to that of Pd nanosheets due to the alloying and tensile strain effect (Fig. 1c). On one hand, due to the slightly smaller radius of Co compared to Pd, the XRD peak of Pd72Co28 shifts slightly towards higher angles caused by the alloying effect. On the other hand, the thickness of Pd72Co28 nanosheets is about 0.9 nm less than that of Pd nanosheets (Supplementary Fig. 1d), resulting in a stronger tensile strain effect. Consequently, the XRD peak of PdCo notably shifts towards lower angles compared to the Pd nanosheets. Therefore, the weak alloying effect and the strong tensile strain effect resulted in the XRD peak of Pd72Co28 shifting towards lower angles compared to the Pd nanosheet. The fast Fourier transform (FFT) analysis of PdCo nanosheets from the selected white dashed region exhibited distinct crystal facets oriented along the [011] zone axis (Fig. 1d), which aligned with the results from the XRD patterns. The lattice spacing of 0.248 nm observed in the high-angle annular dark-field scanning TEM (HAADF-STEM) corresponded to the (111) facets of PdCo (Fig. 1d, e). The line scan analysis and energy-dispersive X-ray spectroscopy (EDS) mapping images of PdCo nanosheets demonstrated a uniform distribution of Pd and Co elements with a molar ratio of Pd/Co near 72:28 (Pd72Co28, Fig. 1f-h and Supplementary Fig. 2).
a The HAADF-STEM images of PdCo nanosheets. b AFM images and a corresponding height of PdCo nanosheets. c XRD patterns of Pd and PdCo nanosheets. d Atomic-resolution HAADF-STEM image and FFT patterns of PdCo nanosheets. The inset is an FFT pattern of PdCo nanosheets from the white dash box. e Pixel intensity of PdCo from blue dash boxes in (d). f EDX spectra of PdCo nanosheets. g, h Linear elemental distribution of Pd and Co in PdCo nanosheets.
Electrocatalytic performances of Pd72Co28 nanosheets
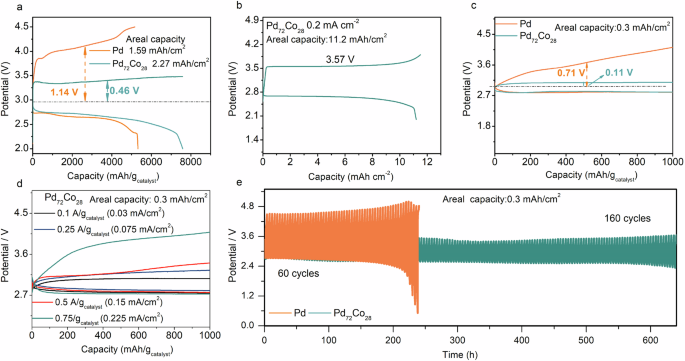
The Pd72Co28 and Pd nanosheets were used as OER electrocatalysts in Li-O2 batteries, respectively. Due to the larger specific surface area of Pd72Co28/C compared to Pd/C (Supplementary Fig. 3), more Li2O2 will deposit on the surface of Pd72Co28/C. Therefore, under full charge and discharge conditions, PdCo exhibited a discharge capacity of 7586 mAh/gcatalyst (2.27 mAh/cm2) at a current density of 0.1 A/gcatalyst (0.03 mA/cm2), surpassing that of the Pd (5325 mAh/gcatalyst or 1.59 mAh/cm2, Fig. 2a). It is noteworthy that as the charging voltage steadily increased to 4.5 V, the increased charging capacity was a result of electrolyte decomposition (Supplementary Figs. 4–6). Meanwhile, the Pd72Co28 electrocatalysts show a lower charge overpotentials of 0.46 V than that of Pd (1.14 V). When the loading mass of PdCo is increased to 3 mg/cm2, the Pd72Co28 catalyst exhibits an areal capacity of 11.2 mAh/cm2 (Fig. 2b), good rate performance (Supplementary Fig. 7), and an energy density of 1177 Wh/kg (including the mass of the catalysts, electrolytes, and Li2O2, as shown in Supplementary Table 1) according to the calculation method in the literature38, far better than that of Li-ion batteries39. Meanwhile, the Pd72Co28 electrocatalyst can efficiently promote the decomposition of Li2O2 without any obvious parasitic products (Supplementary Figs. 8–12).
a The full discharge-charge profiles of Pd and Pd72Co28 nanosheets at a low loading mass (0.3 mg/cm2). b The full discharge-charge profiles of Pd72Co28 based at high loading mass (3 mg/cm2). c The charge-discharge curves of Pd and Pd72Co28 at a current density of 0.1 A/gcatalyst (0.03 mA/cm2) with a limited capacity of 1000 mAh/gcatalyst (0.3 mAh/cm2). d Rate performance of Pd72Co28 nanosheets at a low loading mass of 0.3 mg/cm2 and a low capacity of 0.3 mAh/cm2. e Cycles performance of Pd and Pd72Co28 nanosheets with a limited capacity of 1000 mAh/gcatalyst (0.3 mAh/cm2) at 0.5 A/gcatalyst (0.15 mA/cm2).
When the discharge capacity is restricted to 1000 mAh/gcatalyst (0.3 mAh/cm2), Pd72Co28 demonstrated a remarkably low charge overpotential of 0.11 V with an energy conversion efficiency of 91% at a low loading mass (0.3 mg/cm2), outperforming Pd with an overpotential of 0.71 V (energy conversion efficiency: 76%, Fig. 2c). The cyclic voltammetry (CV) results demonstrated that the exchange current density of the Pd72Co28 surpassed that of pure Pd, confirming the good OER kinetics of Pd72Co28 (Supplementary Fig. 13). The rate performance further indicated that Pd72Co28 exhibited a low charge overpotential even at high current density of 0.75 A/gcatalyst (0.225 mA/cm2 in Fig. 2d), which was significantly advantageous compared to Pd (Supplementary Fig. 14). Such low charge overpotential of Pd72Co28 also demonstrated significant superiority over the reported OER electrocatalysts in Li-O2 batteries (Supplementary Fig. 15 and Supplementary Table 2)12,15,17,19,30,31,35,40,41,42,43,44,45,46,47. Moreover, when the discharge capacity is limited to 1000 mAh/gcatalyst (0.3 mAh/cm2), Pd72Co28 showed no noticeable degradation in the charge-discharge profiles after 160 cycles (640 h) at a current density is 0.5 A/gcatalyst (0.15 mA/cm2 in Fig. 2e). In contrast, Pd can only sustain cycling for 60 cycles under the same conditions due to severe charge polarizations (Fig. 2e). It is worth noting that that although Pd72Co28 demonstrates low charge overpotential and good cycle stability at a low loading mass (0.3 mg cm-2) and a low depth of discharge (0.3 mAh cm−2), the performance of Pd72Co28 at high loading mass (3 mg cm-2 or higher) and depths of discharge (11.2 mAh cm−2 or higher) should be enhanced to meet the requirements of practical Li-O2 batteries.
The reversibility analysis of Pd72Co28 nanosheet
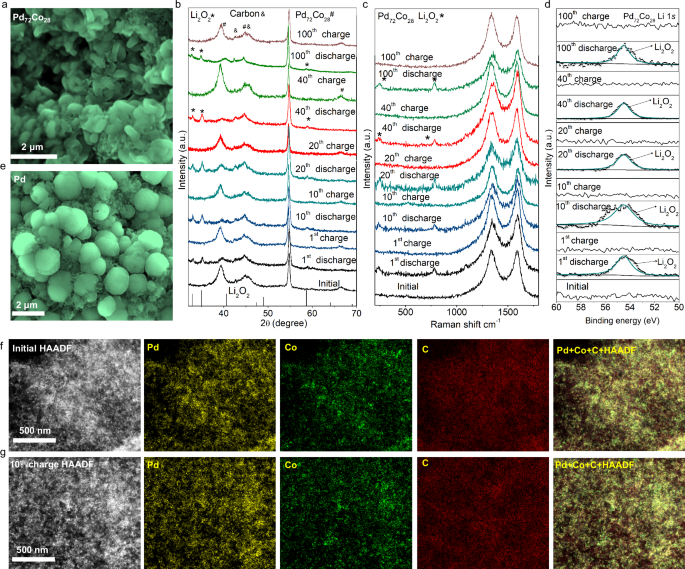
To reveal the microstructure change of discharged Pd72Co28 nanosheets, the morphologies of Pd72Co28 and Pd were conducted by scanning electron microscopy (SEM). At a limited discharge capacity of 1000 mAh/gcatalyst (0.3 mAh/cm2), the disk-shaped discharge products were observed on Pd72Co28 (Fig. 3a). These disk-shaped products (~0.7 μm) were identified as Li2O2 based on XRD patterns, Raman spectra, X-ray photoelectron spectroscopy (XPS) results, Fourier-transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectra (Fig. 3b–d and Supplementary Figs. 16 and 17), which were different from the sphere-shape Li2O2 particles (~1.1 μm) with a slightly larger diameter on Pd (Fig. 3e and Supplementary Figs. 18–20). In comparison to Pd/C, the higher specific surface area of the Pd72Co28/C catalyst and the smaller size of the discharge products on the PdCo/C positive electrode facilitate good interface contact between Li2O2 and Pd72Co28/C according to the previous report48,49,50,51, resulting in low charge transfer resistance (193 Ω in Supplementary Fig. 21 and Supplementary Table 3) and good OER kinetics for the Pd72Co28/C electrocatalyst.
a SEM images of 1st discharge Pd72Co28 nanosheets. b–d XRD patterns (b), Raman (c), and XPS (d) spectra of Pd72Co28 electrocatalysts at different cycles. e SEM images of 1st discharge Pd nanosheets. f, g Comparative TEM imaging and EDX elemental mapping in C, Pd, and Co distribution for Pd72Co28 electrocatalysts during the initial state (f) and the 10th cycle (g).
The reversibility of Pd72Co28 and Pd catalysts in Li-O2 batteries were analyzed through XRD patterns, Raman spectra, and XPS spectra under various discharge and charge cycling conditions. Throughout the 1 st, 10th, 20th, 40th, and 100th cycles, the reversible formation and decomposition of Li2O2 are confirmed through XRD patterns and Raman spectra on Pd72Co28 (Fig. 3b, c). The good reversibility of Pd72Co28 an also be confirmed by XPS results. Only Li 1 s and C 1 s spectra were utilized to identify the discharge products, considering the overlapping of O 1s signals from Li2CO3, Pd, and Pd72Co28 (Supplementary Figs. 22 and 23). Obviously, the reversible formation and decomposition of Li2O2 can be obtained on Pd72Co28 during 100 discharge-charge cycles (Fig. 3d and Supplementary Fig. 24). Moreover, no noticeable changes are observed in the oxidation states of Pd and Co in Pd72Co28 after 100th cycles, highlighting the good stability of the Pd72Co28 (Fig. 3f, g and Supplementary Figs. 25 and 26). In contrast, the high overpotential during the charging process of the Pd led to the formation of byproducts on Pd, including Li2CO3 and unreacted Li2O2, even in the 1st cycle (Supplementary Figs. 27 and 28).
The d-d orbital coupling between Pd and Co in Pd72Co28 nanosheets
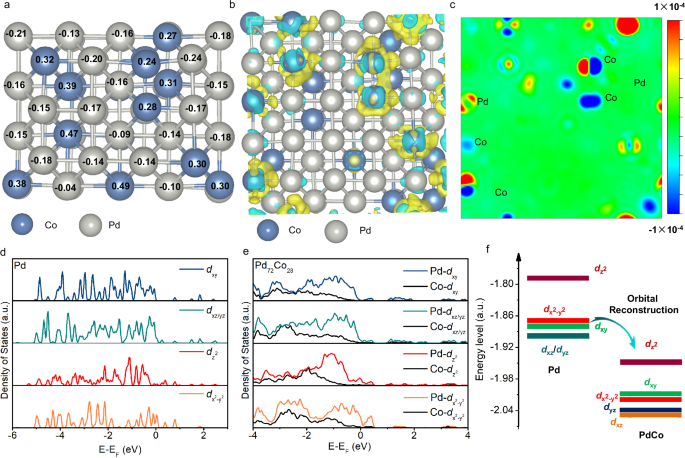
Bader charge analysis was used to analyze the valence state changes of Pd in Pd72Co28 to elucidate the underlying mechanism for the outstanding OER catalytic activity of Pd72Co28 nanosheets. Due to the higher electron affinity of Pd than that of Co, electrons transferred from Co to Pd, resulting in a lower valence state of Pd in Pd72Co28 (Fig. 4a), which was in accordance with the XPS results (Supplementary Fig. 29). The charge transfer between Pd and Co was further confirmed by the analysis of differential charge density in two-dimension maps, revealing a higher electron density near pure Pd atoms than that of Pd atoms in Pd72Co28 nanosheets (Fig. 4b, c).
a Bader charge results for Pd72Co28 nanosheets. b The differential charge density of Pd72Co28. The yellow and cyan areas indicate the gain and loss of electrons, respectively. c Two-dimensional charge density differences of Pd72Co28 nanosheets. The red and blue areas represent the gain and loss of electrons. d DOSs of Pd 4 d orbital for dxy, dyz/xz, dz2, and dx2−y2 in Pd nanosheets. e DOSs of Pd and Co for dxy, dyz/xz, dz2, and dx2−y2 in Pd72Co28 nanosheets. f Schematic diagram demonstrating the orbital reconstruction of Pd in Pd72Co28 compared to pure Pd nanosheets.
The electron transfer between Pd and Co ultimately resulted in a change of the density of state (DOS) for Pd in Pd72Co28 nanosheets compared to that of Pd nanosheets. For the pure Pd nanosheets in Fig. 4d, its Pd 4d-band was consisted of five distinct orbitals with varying energy levels, 4dz2 (− 1.79 eV), 4dx2−y2 (− 1.87 eV), 4dxy (− 1.88 eV), 4dxz (− 1.90 eV) and 4dyz (− 1.90 eV). On the other hand, the Pd 4d-band in PdCo nanosheets exhibited increased electron localization, particularly noticeable in the 4dz2 orbitals (Fig. 4e). Furthermore, the Pd 4dz2 orbital in Pd72Co28 crossed the Fermi level, while the dxy, dxz, dyz, dz2, and dx2−y2 orbitals of both Pd and Co exhibited resonance within the energy ranges of approximately − 4 to − 2.7 eV, − 4 to − 2.4 eV, − 4 to −2.4 eV, − 4 to − 1.8 eV, and − 4 to − 1.6 eV, respectively, indicating a strong d-d orbital coupling between Pd and Co in Pd72Co28 (Fig. 4e). The d-d orbital interactions between Pd and Co ultimately led to orbital reconstruction and a decrease in the energy levels of Pd 4d-band in Pd72Co28. In comparison to the Pd 4 d orbitals in pure Pd, the reconstructed Pd 4 d orbitals in Pd72Co28 underwent an energy splitting of dxz and dyz. Finally, the five independent Pd 4 d orbitals in PdCo were rearranged as dz2 (− 1.95 eV), dxy (− 2.01 eV), dx2−y2 (− 2.02 eV), dyz (− 2.04 eV), and dxz (− 2.05 eV) (Fig. 4f). Most interestingly, the top energy level of the reconstructed Pd 4 dz2 in Pd72Co28 nanosheets was even lower the bottom energy level of Pd 4dxz/yz in Pd nanosheets. The decreased energy level Pd 4d in Pd72Co28 is also confirmed by the ultraviolet photoelectron spectroscopy (Supplementary Fig. 30), where Pd72Co28 exhibits a lower d-band center (− 12.28 eV) than that of Pd (− 11.84 eV).
The orbital interaction between adsorbed LiO2 and Pd72Co28
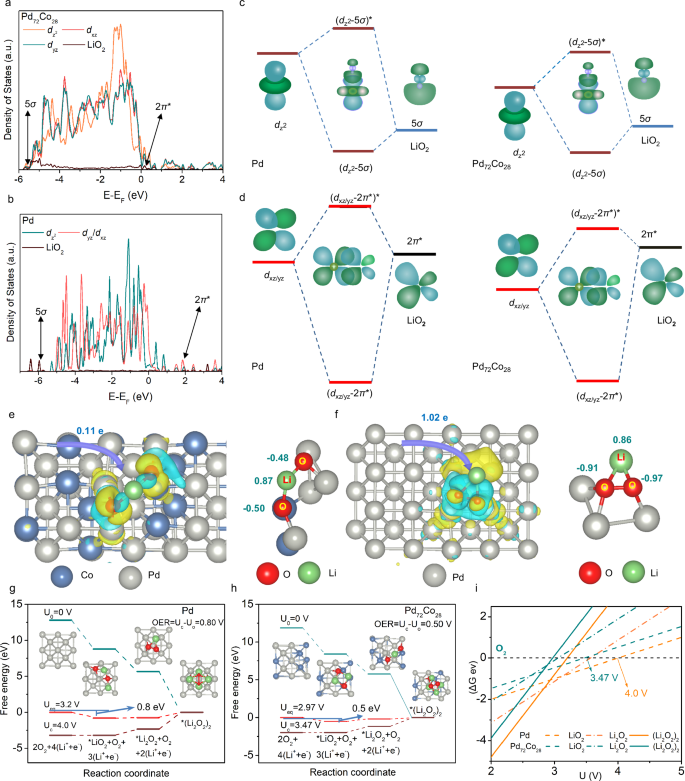
Since the interaction between catalysts and intermediates is a key factor in determining OER catalytic performance, the orbital interactions between electrocatalysts (Pd72Co28 and Pd) and intermediates have been investigated. The DOSs of Pd dz2 orbital and dxz/yz orbitals overlaps with the 5σ and 2π* orbitals of LiO2, respectively (Fig. 5a, b), resulting in the formation of bonding orbitals (dz2-5σ and dxz/yz-2π*) and antibonding orbitals ((dz2-5σ)* and (dxz/yz-2π*)*). Considering the demonstrated orbital reconstruction and the energy level decrease, the antibonding orbitals exhibited a decrease in energy level when the 4 dxy, 4dyz, and 4dz2 orbitals of Pd in Pd72Co28 interacted with the 2π* and 5σ orbitals of LiO2, respectively (Fig. 5c, d). As a result, the electrons from Pd 4d orbitals intended to occupy the antibonding orbitals, leading to a weaker orbital interaction (− 2.30 eV) between Pd72Co28 and LiO2 compared to that of Pd (− 2.67 eV). The weak adsorption interaction between Pd72Co28 and LiO2 is further supported by ultraviolet-visible (UV-Vis) spectra (Supplementary Fig. 31), with Pd72Co28 showing higher adsorption compared to Pd electrocatalysts. Additionally, the weak orbital interaction can also be confirmed through the analysis of the differential charge density and Bader charge results. The O atoms in LiO2 connecting to Pd72Co28 exhibited higher oxidation states compared to Pd, indicating a less amount of electron transfer from Pd72Co28 to LiO2 (0.11 e- in Fig. 5e) relative to that of Pd (1.02 e- in Fig. 5f).
a, b The DOSs for the orbital hybridization between Pd 4 d orbitals and adsorbed LiO2 for Pd (a) and Pd72Co28 (b). c, d A schematic representation of the hybridization between LiO2 and Pd (or Pd72Co28) 4 d orbitals. e, f The difference charge density of LiO2 adsorption on Pd72Co28 (e) and Pd (f), visualized using an isosurface of 0.005 e/Bohr3. The yellow and cyan isosurfaces indicate the gain and loss of electrons, respectively. g, h The Gibbs free energy profiles of Pd72Co28 and Pd at various potentials. i Phase diagram of the positive electrode reaction on the Pd and Pd72Co28.
The weak orbital interaction between Pd72Co28 and LiO2 enhanced the OER electrocatalytic activity, as confirmed by the Gibbs free energy results. The OER overpotential is defined as \({\eta }_{{{\rm{OER}}}}={U}_{{{\rm{c}}}}-{U}_{{{\rm{eq}}}}\). Here, Ueq represents the equilibrium potential of the overall reaction, while Uc indicates the minimum potential at which the Gibbs free energy gradually increases during the charge process. Furthermore, U0 represents the Gibbs free energy of the relevant species when the potential is 0. During the charging process, superoxide species are identified as intermediates for Pd and Pd72Co28 electrocatalysts based on UV-Vis results, confirming that the decomposition of Li2O2 proceeds in two steps (Supplementary Figs. 32 and 33). For both Pd and Pd72Co28, the rate-determining steps of the OER process involve the oxidation of LiO2 to O2 and Li+. Therefore, the weak orbital interaction between Pd72Co28 and LiO2 led to lower activation energy (0.5 eV in Fig. 5h) than that of Pd nanosheets (0.8 eV in Fig. 5g), which is consistent with the electrochemical impedance spectra (EIS) (Supplementary Fig. 34). The lower activation energy of Pd72Co28 indicates a low charge overpotentials of Pd72Co28 (0.5 V) relative to that of Pd nanosheets (0.8 V). This result was also apparent in the phase diagram, where the intersection of LiO2 and O2 occurred at 3.47 V for Pd72Co28 and 4.00 V for Pd, respectively (Fig. 5i).
Three different contents of Co in PdCo catalysts (Pd81Co19, Pd72Co28, and Pd56Co44) are prepared to further investigate the effect of Co content on the charge overpotential in Li-O2 batteries (Supplementary Figs. 35 and 36). The charge overpotential of the PdCo catalyst is mainly determined by two factors: the OER activity of the Pd atom and the number of Pd sites. With a slight increase in the Co content from Pd81Co19 to Pd72Co28, the d-band center of Pd in Pd72Co28 (− 12.28 eV) shifts downward compared to Pd81Co19 (− 11.92 eV, Supplementary Fig. 37), suggesting weaker adsorption towards LiO2 and enhanced OER catalytic activities of Pd in Pd72Co28. Hence, despite a slight reduction in the number of active Pd sites, the moderate increase of Co content results in a lower overpotential for Pd72Co28 (0.11 V) compared to Pd81Co19 (0.53 V, Supplementary Fig. 38) at a low loading mass (0.3 mg/cm2) with a low capacity (0.3 mAh/cm2). With a further increase in the Co content from Pd72Co28 to Pd56Co44, although the decreased d-band center of Pd56Co44 (− 13.12 eV, Supplementary Fig. 37) enhances the OER activity of Pd, the significant decrease in the number of Pd sites results in a higher charge overpotential for Pd56Co44 (0.38 V, Supplementary Figs. 38) compared to Pd72Co28 (0.11 V) at a low loading mass (0.3 mg/cm2) with a low capacity (0.3 mAh/cm2). Therefore, the Pd72Co28 catalyst with a moderate Co content can guarantee relatively high OER catalytic activity and a significant number of Pd sites, resulting in low charge overpotential.
Discussion
In summary, an orbital reconstruction strategy has been reported in Pd72Co28 nanosheets as a highly efficient electrocatalyst in Li-O2 batteries with a low charge overpotential of 0.11 V at a low loading mass (0.3 mg/cm2) and a low capacity (0.3 mAh/cm2). Experimental results and theoretical calculations revealed that the d-d orbital coupling between Pd and Co in Pd72Co28 nanosheets leads to Pd 4 d orbital reconstruction and significantly reduced the energy levels of the electrochemical active 4dxy, 4dyz, and 4dz2 orbitals of Pd rather than the only band center shift in the conventional approaches. As a result, the interactions between the energetically lowered 4dxy/4dyz/4dz2 orbitals of Pd in Pd72Co28 and 2π*/5σ orbitals of LiO2 optimized the activation energy of the rate-determining step and enhanced the OER kinetics. This study developed a highly efficient Li-O2 batteries with a reported highest energy conversion efficiency of 91%, and offered a guideline for the precise manipulation of d orbitals in designing efficient noble metal-based electrocatalysts.
Methods
Chemicals and materials
Palladium(II) acetylacetonate (Pd(acac)2, ≥ 99.95%), cobalt(II) acetylacetonate (Co(acac)2, ≥ 97%), molybdenum carbony (Mo(CO)6, ≥ 98%), oleylamine (C18H37N, 80–90%), cyclohexane (C₆H12, ≥ 99.9%), ethanol (C2H5OH, ≥ 99.7%), acetic acid (CH3COOH, ≥ 99.5%), dimethyl sulfoxide (C2H6SO, ≥ 99.9%), 1-methyl-2-pyrrolidone (NMP, C6H13NO, ≥ 99.5%), tetraethylene glycol dimethyl ether (C10H22O5, 99%) were purchased from Macklin. Poly(vinylidene fluoride) (PVDF, average molecular weight ~275000), lithium trifluoromethanesulfonate (LiCF3SO3, ≥ 99.5%), and lithium foil (thickness of 0.4 mm, diameter of 16 mm, ≥ 99.9%) were obtained from Aladdin. Carbon paper (thickness of 0.21 mm, resistivity of 8 mΩ cm²) was purchased from Shanghai Hesen. A separator (thickness of 0.26 mm, diameter of 18 mm) was purchased from Whatman. The pure O2 (99.5%) was obtained from Linde HKO Ltd. XC72R carbon(≥ 98%) was purchased from Energy. Parafilm (thickness of 127 μm) was purchased from Bemis. To ensure the reliability and reproducibility of the experimental results, all experiments were independently repeated at least three times.
Synthesis of Pd and PdCo (Pd81Co19, Pd72Co28, and Pd56Co44) nanosheets
10 mg of Pd(acac)2, 10 mg of Co(acac)2, and 30 mg of Mo(CO)6 were dissolved in 5 mL of oleylamine, followed by sonicating for 2 h to ensure uniform dispersion. Next, the solution underwent a 5 h reaction in a 180 °C oil bath. After the completion of the reaction and cooling to room temperature, the product was collected by centrifugation. Subsequently, the product underwent three rounds of centrifugal washing using a mixture of cyclohexane and ethanol (in a 9:1 volume ratio) to obtain the Pd72Co28. The synthesis of Pd81Co19 and Pd56Co44 is similar to that of Pd72Co28, differing only in the amount of metal salts added. To synthesize Pd81Co19, 10 mg of Pd(acac)2 and 5 mg of Co(acac)2 are needed, whereas for Pd56Co44, 8 mg of Pd(acac)2 and 12 mg of Co(acac)2 are required. In addition, the synthesis process for Pd nanosheets closely resembles that of Pd72Co28, with the only difference being the exclusion of Co(acac)2 as a required component.
Preparation of Pd/C and PdCo/C (Pd81Co19/C, Pd72Co28/C, and Pd56Co44/C)
The synthesized PdCo nanosheets (80 mg) was dispersed in cyclohexane (60 mL), followed by homogeneous mixing with XC72R carbon (20 mg), and subjected to continuous sonication for 60 min. The products were obtained by centrifugation and subsequent washing with a mixed solvent of cyclohexane/ethanol (9/1, v/v). Subsequently, the products was continuously stirred overnight with the addition of acetic acid to remove any residual surfactants. Finally, the resulting product PdCo/C was obtained through centrifugation. The loading mass of PdCo in PdCo/C was 80.0 wt%. The synthesis process for Pd/C was the same as that for PdCo/C.
Characterizations
The crystal structures of both Pd and PdCo were investigated using a powder X-ray diffraction (XRD, a Bruker D2 Phaser XE-T X-ray diffractometer) utilizing Cu Kα radiation (λ = 1.5406 Å). The microstructural features of Pd and PdCo were examined through high-resolution transmission electron microscopy (HR-TEM) using the Tecani-G2 T20 instrument and field-emission scanning electron microscopy (SEM) employing the Thermo Fisher Quattro S system. The discharge products of Pd and PdCo were identified by FT-IR using a PerkinElmer Spectrum Two Spectrometer with the wavenumber range of 400–4000 cm−1 and NMR (Bruker). The valence bands of Pd and PdCo were analyzed by ultraviolet photoelectron spectroscopy (UPS, Thermo escalab 250Xi). Raman spectroscopy was employed to analyze the discharge products of Pd and PdCo, which was conducted using a WITec alpha 300 Raman System equipped with a 532 nm wavelength laser. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo Fisher ESCALAB XI + instrument to investigate the valence states of Pd in Pd and PdCo, and discharge products during the cycling process. Prior to ex situ measurements, the discharged or charged PdCo or Pd positive electrodes were transferred to an Ar-filled glovebox and disassembled at a temperature of 20 °C, with both O2 and H2O concentrations in the glovebox maintained below 0.1 ppm. The PdCo (or Pd) positive electrodes were washed by dimethyl sulfoxide to remove residual electrolytes and salts. Subsequently, the PdCo or Pd positive electrode was tightly wrapped with Parafilm to prevent contamination. To ensure the accuracy of the characterization results, the positive electrode sample was taken out of the glovebox 5 min before performing ex situ characterizations.
Battery assembly and performances
In order to fabricate the positive electrode (PdCo/C) for Li-O2 batteries, the 10 wt% poly(vinylidene fluoride) binder was dispersed in NMP under continuous magnetic stirring. Subsequently, the PdCo/C (90 wt%) was transferred into the PVDF solution and further stirred for 8 h. Then, the obtained slurry was drop-casted onto a carbon paper current collector (thickness of 0.21 mm, a diameter of 10 mm) using a pipette, followed by vacuum drying at 60 °C for 24 h to obtain the PdCo/C electrode. The carbon paper current collector discs were obtained by cutting the carbon paper using a slicing machine (Shenzhen Kejing, Model: MSK-T10). In addition, Pd/C electrocatalysts were utilized as a positive electrode for comparative analysis. The loading mass for both PdCo/C and Pd/C ranges from 0.3 to 3 mg/cm2. A 2032 coin cell with 13 holes was assembled in a glove box under an Ar atmosphere, where the content of both H₂O and O₂ were below 0.1 ppm. The cell configuration included a positive electrode, a lithium foil (Aladdin, thickness of 0.4 mm, a diameter of 16 mm), a separator (Whatman, thickness of 0.26 mm, dimension of 18 mm), and solvent (10 μL, 1 M lithium trifluoromethanesulfonate in tetraethylene glycol dimethyl ether). The cell assembly was conducted using a hydraulic crimping machine (Shenzhen kejing, Model: MSK-110). The lithium foil was prepared prior to cell assembly. Prior to battery measurements, the assembled Li-O2 batteries were placed into a container filled with ultra-pure dry O2 (99.99%, 1.0 atm.) and left to stand in a constant-temperature chamber with convection heating (25 °C, Neware, Model: MIHW-200-160CH) for 24 h. Following that, galvanostatic experiments were conducted on the battery using a LAND cell system. The cyclic voltammogram was carried out using an electrochemical workstation (CHI760e) from 2.0 to 4.5 V at 0.3 mV/s. EIS was analyzed by a CHI760E workstation, with an amplitude voltage of 10 mV and a frequency range from 105 Hz to 0.1 Hz (number of data points: 31). The system was held at open-circuit voltage for 10 min prior to electrochemical impedance spectroscopy (EIS) measurements. Each electrochemical data was analyzed based on the measurements of three cell samples.
DFT calculations
The DFT calculations were performed using the Vienna Ab initio simulation package (VASP). We adopted the generalized gradient approximation of Perdew-Burke-Ernzerhof (GGA-PBE) to characterize the exchange-correlation potential in our calculations. To account for the interactions between ion cores and valence electrons, the projector augmented-wave (PAW) method was utilized. A fixed plane-wave cutoff energy of 450 eV was applied in the calculations. The structural models were relaxed until achieving Hellmann-Feynman forces below − 0.02 eV/Å and an energy change below 10−5 eV. To account for dispersion interactions between all atoms in the adsorption models, Grimme’s DFT-D3 methodology was employed. The optimized configuration of Pd and Pd72Co28 are shown in Supplementary Data 1, 2.
Data availability
The data that support the conclusions of this study are available within the paper and Supplementary information. The DOI for the dataset on Figshare is https://doi.org/10.6084/m9.figshare.27683289. Source data are provided in this paper.
References
Ye, H. & Li, Y. A perspective on sulfur-equivalent cathode materials for lithium-sulfur batteries. Energy Lab 1, 220003-220001–220003-220009 (2023).
Peng, J., Yi, X., Fan, L., Zhou, J. & Lu, B. Molecular extension engineering constructing long-chain organic elastomeric interphase towards stable potassium storage. Energy Lab 1, 220014-220011–220014-220017 (2023).
Zaidi, S. S. H. & Li, X. Li–O2/air batteries using ionic liquids–a comprehensive review. Adv. Energy Mater. 13, 2300985 (2023).
Askins, E. J. et al. Triarylmethyl cation redox mediators enhance Li–O2 battery discharge capacities. Nat. Chem. 15, 1247–1254 (2023).
Tian, J. et al. Sabatier relations in electrocatalysts based on high-entropy alloys with wide-distributed d-band centers for Li-O2 batteries. Angew. Chem. Int. Ed. 62, e202310894 (2023).
Han, X., Zhao, L., Wang, J., Liang, Y. & Zhang, J. Delocalized electronic engineering of Ni5P4 nanoroses for durable Li–O2 batteries. Adv. Mater. 35, 2301897 (2023).
Bai, T. et al. Atomic Ni-catalyzed cathode and stabilized Li metal anode for high-performance Li–O2 batteries. eScience 5, 100310 (2025).
Jia, C. et al. Bifunctional photoassisted Li–O2 battery with ultrahigh rate-cycling performance based on siloxene size regulation. ACS Nano 17, 1713–1722 (2023).
He, Y. et al. A “Trinity” design of Li-O2 battery engaging the slow-release capsule of redox mediators. Adv. Mater. 35, 2308134 (2023).
Liu, Y. et al. Tailoring the adsorption behavior of superoxide intermediates on nickel carbide enables high-rate Li–O2 batteries. eScience 2, 389–398 (2022).
Cai, Y. et al. Ionic liquid electrolyte with weak solvating molecule regulation for stable Li deposition in high-performance Li-O2 batteries. Angew. Chem. Int. Ed. 135, e202218014 (2023).
Zhou, Y. et al. Cascaded orbital–oriented hybridization of intermetallic Pd3Pb boosts electrocatalysis of Li-O2 battery. Proc. Natl. Acad. Sci. USA 120, e2301439120 (2023).
Liang, Y. L. et al. A rotating cathode with periodical changes in electrolyte layer thickness for high-rate Li-O2 batteries. Adv. Mater. 36, 2403230 (2024).
Xiao, F. et al. D-band center regulation for durable catalysts and constructing a robust hybrid layer on Li anode enable long-life Li-O2 batteries. Adv. Energy Mater. 14, 2303766 (2024).
Zhou, Y. et al. Engineering eg orbital occupancy of Pt with Au alloying enables reversible Li−O2 batteries. Angew. Chem. Int. Ed. 61, e202201416 (2022).
Hong, J., Hyun, S., Tsipoaka, M., Samdani, J. S. & Shanmugam, S. RuFe alloy nanoparticle-supported mesoporous carbon: efficient bifunctional catalyst for Li-O2 and Zn–air batteries. ACS Catal. 12, 1718–1731 (2022).
Zhang, E. et al. Electron localization in rationally designed Pt1Pd single-atom alloy catalyst enables high-performance Li–O2 batteries. J. Am. Chem. Soc. 146, 2339–2344 (2024).
Zheng, L.-J. et al. Regulating electrochemistry kinetics and discharge product selectivity with near-free cobalt single-atom catalyst in Li−O2 batteries. Energy Storage Mater. 56, 331–341 (2023).
Hu, X. et al. Ru single atoms on N-doped carbon by spatial confinement and ionic substitution strategies for high-performance Li–O2 batteries. J. Am. Chem. Soc. 142, 16776–16786 (2020).
Zhang, Y. et al. Single-atom-mediated spinel octahedral structures for elevated performances of Li-oxygen batteries. Angew. Chem. Int. Ed. 135, e202218926 (2023).
Bai, T. et al. Recent progress on single-atom catalysts for lithium–air battery applications. Energy Environ. Sci. 16, 1431–1465 (2023).
Li, D. et al. Activating MoS2 nanoflakes via sulfur defect engineering wrapped on CNTs for stable and efficient Li-O2 batteries. Adv. Funct. Mater. 32, 2108153 (2022).
He, B. et al. MoSe2@CNT core-shell nanostructures as grain promoters featuring a direct Li2O2 formation/decomposition catalytic capability in lithium-oxygen batteries. Adv. Energy Mater. 11, 2003263 (2021).
Lin, X. et al. An open-structured matrix as oxygen cathode with high catalytic activity and large Li2O2 accommodations for lithium-oxygen batteries. Adv. Energy Mater. 8, 1800089 (2018).
Cao, D. et al. Crystal phase-controlled modulation of binary transition metal oxides for highly reversible Li–O2 batteries. Nano Lett. 21, 5225–5232 (2021).
Agyeman, D. A. et al. Synergistic catalysis of the lattice oxygen and transition metal facilitating ORR and OER in perovskite catalysts for Li–O2 batteries. ACS Catal. 11, 424–434 (2020).
Sun, Z. et al. Synergized multimetal oxides with amorphous/crystalline heterostructure as efficient electrocatalysts for lithium–oxygen batteries. Adv. Energy Mater. 11, 2100110 (2021).
Yuan, M. et al. Ultrathin two-dimensional metal–organic framework nanosheets with the inherent open active sites as electrocatalysts in aprotic Li–O2 batteries. ACS Appl. Mater. Interfaces 11, 11403–11413 (2019).
Zhou, Y. et al. Orbital coupling of PbO7 node in single-crystal metal–organic framework enhances Li-O2 battery electrocatalysis. Nano Lett. 23, 10600–10607 (2023).
Lv, Q. et al. Atomic ruthenium-riveted metal–organic framework with tunable d-band modulates oxygen redox for Lithium–oxygen batteries. J. Am. Chem. Soc. 144, 23239–23246 (2022).
Lv, Q., Zhu, Z., Ni, Y., Geng, J. & Li, F. Spin-state manipulation of two dimensional metal-organic framework with enhanced metal-oxygen covalency for lithium-oxygen batteries. Angew. Chem. Int. Ed. 61, e202114293 (2022).
Tian, S. L. et al. A force-assisted Li−O2 battery based on piezoelectric catalysis and band bending of MoS2/Pd cathode. Adv. Energy Mater. 14, 2303215 (2024).
Zheng, J. et al. Single-atom Pd-N4 catalysis for stable low-overpotential lithium-oxygen battery. Small 19, 2204559 (2023).
Guo, L. et al. Pd cluster decorated free standing flexible cathode for high performance Li-oxygen batteries. Nano Res. 17, 2678–2686 (2024).
Zhou, Y. et al. Lewis-acidic PtIr multipods enable high-performance Li–O2 batteries. Angew. Chem. Int. Ed. 60, 26592–26598 (2021).
Cao, C. et al. Mechanistic insight into the synergetic catalytic effect of Pd and MnO2 for high-performance Li–O2 cells. Energy Storage Mater. 12, 8–16 (2018).
Li, K., Dong, H., Wang, Y., Yin, Y. & Yang, S. Preparation of low-load Au-Pd alloy decorated carbon fibers binder-free cathode for Li-O2 battery. J. Colloid Interface Sci. 579, 448–454 (2020).
Liang, Z., Wang, W. & Lu, Y.-C. The path toward practical Li-air batteries. Joule 6, 2458–2473 (2022).
Jenkins, M. et al. A high capacity gas diffusion electrode for Li–O2 batteries. Adv. Mater. 36, 2405715 (2024).
Wang, P. et al. Atomically dispersed cobalt catalyst anchored on nitrogen-doped carbon nanosheets for lithium-oxygen batteries. Nat. Commun. 11, 1576 (2020).
Lu, J. et al. A lithium–oxygen battery based on lithium superoxide. Nature 529, 377–382 (2016).
Song, L.-N. et al. Tuning lithium-peroxide formation and decomposition routes with single-atom catalysts for lithium–oxygen batteries. Nat. Commun. 11, 2191 (2020).
Ke, S.-W. et al. Covalent organic frameworks with Ni-Bis (dithiolene) and Co-porphyrin units as bifunctional catalysts for Li-O2 batteries. Sci. Adv. 9, eadf2398 (2023).
Zhang, J. et al. A long-life lithium-oxygen battery via a molecular quenching/mediating mechanism. Sci. Adv. 8, eabm1899 (2022).
Zhang, L. et al. Metal telluride nanosheets by scalable solid lithiation and exfoliation. Nature 628, 313–319 (2024).
Asadi, M. et al. A lithium–oxygen battery with a long cycle life in an air-like atmosphere. Nature 555, 502–506 (2018).
Xiong, Q. et al. Hydrogen-bond-assisted solution discharge in aprotic Li–O2 batteries. Adv. Mater. 34, 2110416 (2022).
Chen, Y. et al. Metal-air batteries: progress and perspective. Sci. Bull. 67, 2449–2486 (2022).
Shi, W. et al. High-entropy alloy stabilized and activated Pt clusters for highly efficient electrocatalysis. SusMat 2, 186–196 (2022).
Liu, L., Zhou, C., Fang, W., Hou, Y. & Wu, Y. Rational design of Ru/TiO2/CNTs as cathode: promotion of cycling performance for aprotic lithium-oxygen battery. Energy Mater. 3, 300011 (2023).
Zhou, Y. & Guo, S. Recent advances in cathode catalyst architecture for lithium–oxygen batteries. eScience 3, 100123 (2023).
Acknowledgements
This work is supported from the Green Tech Fund (GTF202220105), Guangdong Basic and Applied Basic Research Foundation (2024A1515011008), National Natural Science Foundation of China (52372229 and 52172241), General Research Fund of Hong Kong (CityU11308321, CityU11315622, and CityU11310123), the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. PDFS2425−1S03), and the Shenzhen Research Institute, City University of Hong Kong.
Author information
Authors and Affiliations
Contributions
Y.Z. and K.Y. contributed equally to this work. S.G., and G.H. conceived the project. G.H. and W.Z. secured funding and supervised the project. Y.Z., K.Y., Y.H., J.L., A.Z., D.L., and G.G. finished the catalysts preparation, characterizations, performance testing, and DFT calculations. J.Z., Kai L., T.Z., Kunlun L., C.L., H.Y., and H.C. contributed to the analysis of the experimental data and participated in the result discussion. Y.Z. wrote the manuscript. G.H., S.G., and W.Z. revised the manuscript. All the authors commented on the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Graham Leverick, Jiefang Zhu, and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Y., Yin, K., Huang, Y. et al. D-orbital Reconstruction Achieves Low Charge Overpotential in Li-oxygen Batteries. Nat Commun 16, 3353 (2025). https://doi.org/10.1038/s41467-025-58640-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58640-6