Abstract
The excellent impermeability makes graphene film an ideal candidate for thin film encapsulation technology. However, current chemical vapor deposition (CVD) graphene-based barrier films can not provide sufficient moisture barrier performance, suggesting a lack of understanding in mechanism that dominates water diffusion in/through graphene stacks. Herein, we fabricate large-area graphene barrier films with a record-low water vapor transmission rate (WVTR) of 5 × 10−5 g/(m2·day), two orders of magnitude lower than previous works, in which two stacked Janus graphene films are intercalated by toluidine blue O (TBO) sub-monolayer: one side of graphene is decorated with fluorine- and oxygen-containing groups to allow crack-free transfer, while the other side is functionalized with hydroxyl groups to trap water. The intercalated TBO further blocks water transport due to a strong water-TBO interaction. Our work opens a route for surface/interface engineering of CVD graphene and promises its exciting future in the applications for advanced packaging.
Similar content being viewed by others
Introduction
Large-area flexible thin barrier films that can suppress the permeation of moisture and active gasses are highly needed in daily life, from foods, medicines to industries, including flexible electronic and photonic applications1,2,3,4. Ultrathin graphene films that, theoretically, exhibit impermeability to all gasses and liquids, excellent flexibility, optical transparency, and scalability are promising for flexible and inert encapsulation films5,6,7,8. However, traditionally-made graphene barrier films are generally a stack of nano-sized graphene flakes, which are relatively thick, thus inevitably undermining their optical transparency and flexibility8. Therefore, large-area monolayer graphene films produced by CVD with high optical transparency and flexibility are regarded as ideal candidate materials for flexible barrier films9,10,11,12.
Despite their compatibility with batch production, large-scale graphene films produced by CVD suffer from presence of defects, such as point defects, wrinkles, and grain boundaries13,14, which function as the diffusion channels for moisture and gasses7,10,15,16,17; therefore, usually one layer graphene can not efficiently block the water diffusion. One way to address such an issue is to stack graphene layers over large area by forming intimate contacts between the layers to block possible diffusion channels3,10,12. Unfortunately, in practice, the barrier performance (WVTR values) of such stacked thin films is limited to the level of 10−3 g/(m2·day)10,12,18,19, and the diffusion mechanism of moisture between the graphene layers and the determining factors remain elusive7,20,21,22,23,24. In this regard, the deposition of inorganic materials such as Al2O3 and SiO2 on graphene surfaces has been previously reported to ensure sustainable barrier properties under bending conditions25; however, the formation of cracks during the graphene transfer, and the poor adhesion and nucleation difficulty of Al2O3 deposited on graphene surface hinder the further improvement of barrier performance. Meanwhile, conducting another round of CVD growth onto Al2O3/graphene/Cu structure to form a nanolaminating structure would enable a WVTR value below 7 × 10−3 g/(m2·day)19. Furthermore, the self-assembly of hexatriacontane molecules that can block the defects and spaces between stacked graphene layers also delivers a WVTR value of 1.2 × 10−3 g/(m2·day)12. Fabrication of the stacked CVD graphene films involves the growth and transfer steps, during which, the structural defects13,26,27, such as nano-sized defects, cracks, interlayer contamination28, wrinkles29, and surface groups on the graphene surface, are the leaking points which dictate the moisture permeation10. Therefore, clarifying the basic structure-performance relationships would provide vital guidance for designing the growth and stacking methods to improve the barrier performance for potential encapsulation applications.
Herein, instead of integrating other barrier films with graphene, such as oxide or self-assembling molecules, through double-sided surface modification, Janus graphene was fabricated to efficiently block the water diffusion: one side of the graphene surface was decorated with fluorine- and oxygen-containing groups to ensure conformal contact between graphene and the supporting polymer, polyvinylidene difluoride (PVDF), and to retain the hydrophobic surface to prevent water adsorption; the other surface was exposed to oxygen plasma to generate a hydrophilic surface that hindered on-surface water diffusion with reduced slip length (Fig. 1). Intercalating ultrathin small molecules into the stack of as-fabricated two Janus graphene layers further suppresses the water diffusion between the two graphene layers, thereby delivering a record-low WVTR of 5 × 10−5 g/(m2·day), similar to that of commercially available inorganic barrier films, with a thickness at least one order of magnitude thinner than the commercial ones19,30,31,32. The detailed comparison in terms of thickness and WVTR values of graphene-based barrier films in this work with previously reported barrier films and application-specific requirements is presented in Supplementary Fig. 1. Our work not only clarifies the contributing principles for fabricating high-performance graphene barrier films through rational surface modification and intercalation of functional molecular layers, but also provides a route to tackle the current challenge that CVD graphene films are theoretically impermeable but practically poor barriers, and demonstrates their applications in advanced encapsulation techniques with large-area scalability, optical transparency, flexibility, and superior barrier performance.
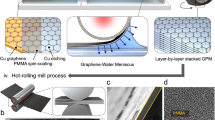
Schematics illustration of steps involved in the preparation of the double-layer graphene barrier films. After the growth, reactive ion etching (RIE) would introduce the fluorine- and oxygen-containing groups, followed by PVDF/ polyethylene naphthalate (PEN)-assisted etching of Cu foils. Oxygen plasma treatment introduces oxygen-containing groups on the other side of graphene. The coating of toluidine blue O (TBO) molecules is conducted on the inner graphene surface decorated by oxygen-containing groups, and the subsequent lamination of another Janus graphene layer would enable the sealing of intercalated TBO molecules possibly through π–π interaction between benzene rings of TBO and graphene planes.
Results
The detailed fabrication process is presented in Supplementary Fig. 2. Briefly, the reactive ion etching (RIE) and oxygen plasma treatment were conducted on different sides of the graphene film to form a Janus structure. Specifically, after CVD growth of graphene on Cu foil, RIE was conducted to decorate the graphene surface with fluorine- and oxygen-containing groups, then a thin PVDF layer was spin-coated on graphene to assist the Cu etching, which would expose the other untreated graphene surface. The other surface would be modified by oxygen plasma with low power and controlled treatment time to obtain a hydrophilic surface. The film was subsequently spin-coated by a TBO layer. Finally, the other Janus graphene film was laminated onto the TBO layer, forming the final barrier film (see Methods section for fabrication details).
First, the surface RIE treatment using CF4 and oxygen gasses allows the crack-free transfer of graphene films from Cu foil. The conventional graphene transfer usually entails the coating of the supporting polymer, etching of Cu, lamination of graphene onto the destination substrates, and the removal of the supporting polymer26,27. In conventional transfer, cracks remain inevitable, although a high intactness of over 99% after transfer has been reported26,33; the 1% cracks that would function as a diffusion (leaking) channel that deteriorates barrier performance. To avoid additional crack formation during the removal of the polymer, the supporting film, PVDF, also functions as the destination substrate, one part of the barrier stack, in this work; therefore, the transfer of graphene only requires the coating of PVDF and the etching of Cu, circumventing polymer removal.
It was observed that crack formation was caused by poor contact between the polymer and graphene; thus, graphene would become suspended and prone to crack owing to the loss of the polymer support after the Cu removal29. In this regard, during the coating of PVDF dissolved in N,N-dimethylformamide (DMF) on a rough graphene/Cu surface, the wettability of the polymer solution on graphene directly determines the contact between graphene and the cured polymer, and therefore, the final intactness after the transfer (Fig. 2a–c).
a, b Typical OM images of graphene transferred onto PEN/PVDF supporting films with (a) and without (b) the RIE-based surface modification. The graphene wrinkles and cracks were highlighted by white arrows. Scale bar, 10 μm. Inset (a): Photograph of A3-sized as-fabricated double-layer graphene barrier film showing the Weiming Lake and Boya Tower at Peking University seen through the transparent graphene barrier films. Yellow dashed lines highlight the graphene region. c The intactness of the graphene transferred onto PVDF/PEN films (red) and the measured contact angle (blue) of PVDF solution on the graphene surface as a function of the RIE treatment time. The data are presented as the means ± standard deviation (SD), n = 50. Inset: Photographs of PVDF solution on graphene surface without (left) and with 10 s RIE treatment time (right), showing the contact angle of 47.8o and 12.0o, respectively. d Summaries of measured average roughness (Ra) of graphene grown on Cu (blue) and graphene transferred onto PVDF/PEN supporting films (red). The data are presented as the means ± SD, n = 20. Inset: Typical AFM images of graphene grown on Cu (right) and graphene transferred onto PVDF/PEN supporting films (left). Scale bar, 1 μm. e Measured normalized conductivity of Ca films covered by transferred multilayer graphene (blue) and as-fabricated double-layer graphene (red) as a function of the measurement time, showing WVTR values of 2 × 10−2 and 5 × 10−5 g/(m2·day) at 25 oC and 45% RH, respectively. The blue dash line is the fitting curve that was used to obtain the WVTR value. Inset: OM image of as-transferred multilayer graphene onto SiO2/Si substrate. Scale bar, 20 μm. Note that the WVTR value of our barrier films were measured after the storing in ambient environment of 3 months after the fabrication. f Summary of previously reported WVTR values of graphene-based barrier films as a function of graphene layers (blue)3,9,10,11,12,19,25,39,41,42,43, Al2O3/graphene composite barrier films (green)3,9,10,11,12,19,25,39,41,42,43, Al2O3-only barrier films (purple)19,30,31,32,44,45,46,47, and WVTR value in our work (red).
RIE treatment of graphene using CF4 and oxygen alters the wettability of the polymer solution, as evidenced by the reduction in contact angles from ~50o to below 20o (Supplementary Fig. 3c), ensuring high intactness of graphene after Cu removal (Fig. 2a). In contrast, without RIE treatment, graphene cracks are visible on the polymer surface in the optical microscopy (OM) image (Fig. 2b). Similarly, the RIE treatment using pure CF4 cannot enable the improved wettability of PVDF on graphene surface, resulting in the crack formation of graphene after the etching of Cu (Supplementary Fig. 4). This improvement in intactness was also confirmed by the statistical results (Supplementary Figs. 5–10). The entirely conformal contact between graphene and the polymer was also supported by the fact that the polymer replicated the surface texture of graphene/Cu even after the removal of Cu, as evidenced by the stepped structures in atomic force microscopy (AFM) images (Inset, Fig. 2d and Supplementary Fig. 11). Furthermore, the prevention of water adsorption on the polymer and graphene surfaces would contribute to improved barrier performance, which is supported by the higher water contact angle on graphene after RIE treatment (Supplementary Figs. 3b, 12). Based on the achieved batch production of large-area graphene films (Supplementary Fig. 13)34, we successfully fabricated A3-sized graphene barrier films with high optical transparency (Supplementary Fig. 14) using the stack of fabricated Janus graphene films and molecular intercalation (Inset, Fig. 2a).
We conducted an electrical Ca test to probe the barrier performance (WVTR values) of the as-fabricated double-layer graphene films by measuring the change in the degree of oxidation of the Ca films over time (Supplementary Fig. 15, and see method for discussion)9,11,35,36,37,38,39. The WVTR was measured to be as low as 5 × 10−5 g/(m2·day) (Fig. 2e), and we also measured the WVTR of multilayer graphene films directly grown via CVD using a high hydrogen-to-methane ratio (Inset, Fig. 2e)40. Interestingly, the multilayer graphene transferred onto the PVDF films exhibited high WVTR values, possibly owing to the presence of defects that penetrated the entire multilayer graphene, such as wrinkles or point defects (Fig. 2e). Our graphene barrier films with only two layers exhibited a record high barrier performance in comparison with previously reported graphene-based barrier films3,9,10,11,12,19,25,39,41,42,43, and such performance was also comparable to or even better than the best WVTR values obtained for inorganic oxides that required a thickness of tens of nanometers and a complex fabrication process (Fig. 2f)19,30,31,32,44,45,46,47. Note that water vapor permeability (WVP) values considering both the thickness and barrier performance of barrier films were calculated and summarized in Supplementary Table 1.
Furthermore, we further investigate the influence of various environmental conditions such as thermal cycle, relative humidity, and ultraviolet (UV) exposure on the barrier performance of as-fabricated barrier films (Supplementary Fig. 16). Meanwhile, regarding the bending resistance of the as-fabricated barrier films, we found that with the dL value larger than 18 mm (initial length L = 35 mm), graphene cracks or wrinkles would be formed presumably owing to the delamination of graphene from the polymer surface, as revealed in the in situ OM observation (Supplementary Fig. 17), which would also result in a higher WVTR value (Supplementary Fig. 18).
To further understand the underlying mechanism, we investigated the contribution of surface modifications and intercalated molecules to the observed barrier performance (Fig. 3a). As evidenced by Raman spectroscopy results, RIE treatment with either CF4 and oxygen (Fig. 3b), or pure CF4 (Supplementary Fig. 19a, b), generated a defect-related D peak caused by the introduction of new groups into the graphene lattice. The intensity of the observed D band reflects the density of defects, i.e., the density of newly introduced groups48,49. The RIE-generated functional groups were further confirmed by X-ray photoelectron spectroscopy (XPS) (Fig. 3d and Supplementary Fig. 19c)48,49,50, in which the intensity of the C-CF and -OH group peaks increases with the RIE treatment time, suggesting such functionalization could be tuned by RIE parameters. However, excessive RIE treatment, e.g., 30 s, directly damaged the graphene lattice by generating nanometer-sized pores, with no 2D bands observed in the corresponding Raman spectrum; therefore, we observed an abrupt increase in WVTR when RIE treatment is longer than 30 s (Supplementary Fig. 20).
a Schematic illustration of the structure of double-layer graphene barrier films in the side view (top) and top view (bottom). TBO molecules would be adsorbed on a graphene surface coated by hydroxyl groups. b, c Raman spectra of pristine graphene, and graphene treated with 5, 10, 15, 20, 30 s RIE (CF4 and oxygen) (b), and with 10 s RIE (CF4 and oxygen) and 1, 3, 5 s oxygen plasma (40 W power) (c). d, e C1s XPS spectra of pristine graphene, and graphene treated with 5, 10, 15, 20, 30 s RIE (CF4 and oxygen) treatment (d), and with 10 s RIE (CF4 and oxygen) and 1, 3, 5 s oxygen plasma (40 W power) (e). f Typical ADF-STEM images showing the graphene lattices in the absence of defects. Scale bar, 1 nm. g, h Photographs showing the contact angle of TBO solution (g) and pure water (h) on pristine graphene (top) and graphene treated with the oxygen plasma (40 W power, 3 s treatment time) (bottom). Note that the graphene was on PVDF films. i, j AFM images of monolayer graphene on SiO2/Si without (i) and with (j) oxygen plasma treatment after the spin-coating of TBO molecules (40 W power, 3 s treatment time). The yellow dashed lines highlight the edge of monolayer graphene. Scale bar, 500 nm. k Schematic illustration of the movement of trapped water layers driven by the pressure gradient between graphene layers with hydrophilic (top) and hydrophobic (bottom) surfaces during the drying process. The blue part is water. l, m AFM images of TBO-coated h-BN surface (l) and graphene/TBO/h-BN surface (m) fabricated by laminating hydrophilic graphene onto TBO-coated h-BN. The yellow dashed line highlights the edge of the graphene-covered region. Inset: Height profile along the white dash line in (m), showing the total ~0.6 nm thickness of the graphene edge and sub-monolayer TBO. Scale bar, 1 μm. n, o Measured normalized conductivity of Ca films at 25 oC and 45% RH covered by double-layer graphene (intercalated with TBO molecules) treated with 5, 10, 15, 20, 30 s RIE (CF4 and oxygen), and with 10 s RIE (CF4 and oxygen) and 1, 3, 5 s oxygen plasma treatment (n) and the summary of the obtained WVTR values (o). The data were presented as the means ± SD, n = 3.
After the RIE treatment and transfer of graphene onto the supporting PVDF films, oxygen plasma treatment was conducted on the other side of the graphene that was previously in contact with Cu. The oxygen plasma treatment alters the water wettability of the graphene surface to a highly hydrophilic surface (Fig. 3g and Supplementary Fig. 12b–d), but also inevitably produces artificial defects in the graphene lattice by introducing the hydroxyl groups, confirmed by an increased intensity of D peak in Raman analysis (Fig. 3c) and enhanced C-OH peak in XPS results (Fig. 3e)48. We found a 3 s oxygen plasma with a power of 40 W is optimal, as either a longer treatment time or a higher power yields a reduction in the 2D band intensity and enhancement of the ratio of D band to G bond (Supplementary Fig. 19d), and more importantly, a higher WVTR value (Supplementary Fig. 20).
To exclude the possibility that our RIE parameters create nanometer-sized holes in graphene lattice allowing water to escape, we observed atomic structure of graphene lattice using the angular dark field scanning transmission electron microscopy (ADF-STEM), and found no lattice defects produced (Fig. 3f and Supplementary Fig. 21a, b). We believe that the introduced fluorine- and oxygen-containing groups can be removed to restore the intrinsic graphene lattice by 12 h vacuum treatment and annealing at around 160 °C, which was conducted before the transmission electron microscope (TEM) observation. To prove the reversible introduction of fluorine- and oxygen-containing groups, we measured the contact angle of graphene directly after the oxygen plasma treatment and after the annealing at 70 °C for one hour. It was revealed that the hydrophilic surface of graphene, enabled by oxygen plasma treatment, switched back to being hydrophobic after annealing, confirming the reversible modification, consistent with ADF-STEM observation (Supplementary Fig. 21c).
The introduction of oxygen-containing groups also enabled the uniform distribution of ultrathin TBO molecules on the graphene surface, followed by the lamination of another Janus graphene film to ensure full sealing of the TBO molecules between layers. After the oxygen plasma treatment, the TBO/ethanol solution fully covered the graphene surface with a near-zero contact angle (Fig. 3h and Supplementary Fig. 22). The uniform distribution of TBO on oxygen plasma-treated graphene was confirmed by AFM. First, monolayer graphene flakes were mechanically exfoliated onto the SiO2/Si surface, followed by oxygen plasma treatment and spin-coating by TBO solution. As illustrated in the AFM images, without oxygen plasma treatment, TBO particles were visible (Fig. 3i), which is in clear contrast with the uniform distribution of TBO on oxygen plasma-treated-graphene surface without the formation of large particles (Fig. 3j).
The hydrophilic nature of graphene prevents the trapping of water molecules and bubble formation during the layer stacking, which was also confirmed by the in situ OM observation (Supplementary Fig. 23 and Supplementary Movie 1): after stacking the two graphene layers, water molecules evaporated from the inner surface of graphene owing to the lower boiling temperature compared to TBO. During water evaporation, both the curvature and movement of the droplets are determined by the wettability of the capillary surface (Fig. 3k and Supplementary Note 1). In this work, the hydrophilic graphene surface ensures that the surface of water droplets between layers becomes concave, and the pressure beneath the droplets is lower than atmospheric pressure by Laplace pressure; such negative pressure would drive the water into the narrower region between layers to reduce the diameter of the droplets. This would enable improved wetting, and complete removal of water from the surfaces and the uniform distribution of dissolved TBO on the graphene surface after ethanol solution evaporation.
The uniform distribution of TBO, free of solution bubbles, was confirmed by AFM characterization of the graphene/TBO/hexagonal boron nitride (h-BN) heterostructure. Owing to the hydrophobic nature of h-BN, the direct TBO coating resulted in the formation of TBO particles, consistent with the results on pristine graphene (Fig. 3l). However, lamination of a hydrophilic graphene layer functioned by oxygen plasma onto the surface of the TBO solution-coated h-BN ensured a very clean and flat surface of the stacks after the evaporation of water, in clear contrast to the TBO particles on the nearby exposed h-BN surface (Fig. 3m). In addition, the height of the sub-monolayer TBO was measured to be around 0.3 nm, considering the 0.33 nm thickness of graphene (Inset, Fig. 3m).
Figure 3n, o summarize the WVTR values of stacked Janus graphene films with different RIE treatment time: RIE treatment of 10 or 15 s delivers a better barrier performance, and the improvement in the barrier performance was caused by suppressed crack formation and the prevention of water adsorption on PVDF and graphene. Furthermore, an additional oxygen plasma treatment further lowered the WVTR to 5 × 10−5 g/(m2·day). Note that, a higher oxygen plasma power caused the creation of holes that allowed for water leakage (Fig. 3o and Supplementary Fig. 20). In this work, the highly improved barrier performance was attributed to Janus surface modification and the intercalation of small molecules. Despite the presence of nanometer-sized point defects or defective wrinkles, the continuous double-layer graphene films formed atomically thin, long, and wide diffusion channels with a large width and height ratio (w/h)22. Therefore, the water flow rate (Q) can be described as
where ρ is the density of water, δ is the slip length of water on the graphene surface, η is the viscosity of diffusing water, P is the driving pressure of water permeation, h is the height of the diffusion channel, and L is the distance of the diffusion length.
Recalling the tortuous pathway theory of graphene oxide (GO) laminates, L was determined by the density of holes that allow for water diffusion7; therefore, high-intactness transfer of graphene without producing cracks would greatly enhance L from nanometer- to micrometer-size and therefore, strongly suppress water leakage.
The interlayer distance between the graphene layers determines h, and a clean interface without air and water bubbles reduces h for high barrier performance. This highlights the importance of suppressed bubble formation and uniformly distributing ultrathin TBO molecules on the graphene surface, which was proven to be enabled by the hydrophilic nature of Janus graphene. We also varied the concentration of the TBO molecules; a higher TBO concentration resulted in the formation of multilayer TBO molecular stacks between the graphene layers51. As indicated in Fig. 4a, b, higher TBO concentration caused the deterioration of barrier performance and also resulted in a larger error bar (Supplementary Fig. 24), indicating the importance of reducing the interlayer distance by controlling the distribution and concentration of TBO. In this regard, sub-monolayer TBO would be preferred, instead of multilayer stack. η is also influenced by the interlayer distance; a smaller distance increases η by forming structured water molecules52.
a, b Measured normalized conductivity of Ca films at 25 °C and 45% RH, covered by double-layer graphene intercalated by TBO with different concentrations, as a function of measurement time (a) and the summary of the obtained WVTR values as a function of TBO concentrations (b). The data were presented as the means ± SD, n = 3. c Calculated adsorption energies and structures of water molecules adsorbed on pristine graphene, hydroxyl-decorated graphene (Gr.-OH) and hydroxyl-decorated graphene surface with backside fluorination (F-Gr.-OH). d Calculated friction coefficients in x (zigzag), y (armchair) direction, and the average friction coefficient value of graphene with 0.12 % hydroxylation within 1 ns simulation calculated by the Green-Kubo equation. Inset: schematic illustration of the structure containing water molecules and double-layer graphene for MD simulation. e The obtained slip length of water molecules on graphene with 0%, 0.12 %, 0.28%, 0.42%, and 0.56% hydroxylation. f The adsorption energies of H2O with TBO, pyrenyl-1-boronic acid pinacol ester (PBA), 1-phenyl-1H-benzo[d]imidazol-2(3H)-one (PBI), 4,5-diazafluoren-9-one (DAFO) and sulfinpyrazone. White, pink, black, blue, red, pale blue, orange, and cyan balls are the H, B, C, N, O, F, S, and Cl atoms, respectively. g–j Measured conductivity of Ca films as a function of measurement time. Note that the Ca films are covered by double-layer graphene films that were intercalated by PBA (g), PBI (h), DAFO (i), and sulfinpyrazone molecules (j).
The slip length of water molecules (δ) on the graphene surface influences the water diffusion22,53,54. As previously reported, a hydrophobic graphene film is predicted to be a frictionless surface with a high δ that enables the fast flow of water molecules22. Furthermore, the water-surface interaction influences δ: for instance, the electrostatic interaction of polar water with hydroxyl groups adsorbed onto the h-BN surface would increase the friction of water on h-BN surfaces with a reduced δ24,55. Therefore, following the strategy of tuning δ through water-surface interactions, the hydroxylated hydrophilic surface of graphene was created by oxygen plasma treatment, which enhances the water-surface interaction. The improved interaction was confirmed by the density functional theory (DFT) calculations (Fig. 4c), in which adsorption energies of water molecules on hydroxyl-functionalized graphene (−0.36 eV) was higher than that of pristine graphene (−0.14 eV). Furthermore, fluorination of the graphene backside can further enhance the adsorption energies (−0.40 eV). Molecular dynamic (MD) simulations56,57 indicate that the friction coefficient (λ) of pristine graphene is about 7 × 103 N·s/m3 with slip length of 108.27 nm (Fig. 4e and Supplementary Fig. 25). In contrast, the 0.12% hydroxylation of graphene increases the λ to 2 × 105 N·s/m3, significantly reducing δ to 3.68 nm (Fig. 4d). Increasing the concentration of hydroxyl groups on graphene surface can further decrease the slip length to around 0.83–3.56 nm (Fig. 4e). Similarly, plasma treatment using air or nitrogen gas would cause the reduction of WVTR values (Supplementary Fig. 26), which, however, is still not comparable with those treated with oxygen plasma presumably owing to the formation of some pyridinic nitrogen and pyrrolic nitrogen in graphene lattice58.
Furthermore, TBO exhibits stronger interactions with water molecules through the interaction between hydrated ions and water molecules, as confirmed by calculated adsorption energies of water molecules on specific positions of the intercalating molecules (Fig. 4f and Supplementary Fig. 27). When the coated TBO molecules do not fully cover the hydrophilic graphene surface in a sub-monolayer manner, TBO molecules form on-surface nanometer-sized channels for water diffusion (Fig. 3a). Such molecular channels with atomic thickness were constructed with TBO walls that can strongly interact with moving water molecules through interaction between ions in TBO and surrounding water molecules. Therefore, similar to the reduction of δ through improved water-graphene surface interactions, the presence of such walls also contributes to the reduced δ and observed excellent barrier performance. To further correlate the water–molecules interaction and the barrier performance, we have used other molecules, and used the DFT to calculate the water–molecules interaction (Fig. 4f and Supplementary Fig. 27), and measured the WVTR values. Clearly, intercalation of other molecules that exhibit reduced interaction with water molecules, cannot achieve a comparable barrier performance with those barrier films with TBO intercalated (Fig. 4g–j). Therefore, our observation confirms the choice of molecules would influence the final barrier performance.
In summary, the main principle for fabricating graphene films with high barrier performances lies in the surface modification and molecular intercalation: (1) the graphene surface that contacts the supporting polymer should be modified for the fine wetting of the polymer solution on the graphene surface to avoid crack formation; (2) the outer surface of the entire film should be hydrophobic to prevent water adsorption; (3) the inner surface of graphene films should be hydrophilic to enhance water-surface interactions that can efficiently suppress water diffusion; (4) intercalated aromatic molecules should be uniformly distributed on the graphene surface and have stronger interactions with diffusing water; (5) the thickness of intercalated molecules should be as thin as possible, such as monolayer, to reduce the interlayer distance. Based on the above principles, we fabricated A3-sized graphene barrier films with record-low WVTR values. Our findings not only enrich the understanding of water diffusion in 2D material systems but also bring graphene films close to real-world applications, such as thin film encapsulation and advanced packaging. Furthermore, to achieve the commercial applications of the developed barrier films, the batch transfer system should be developed based on a roll-to-roll transfer route, possibly equipped with plasma treatment systems, as well as the sensor and controlling system of film tension. In addition, spray coating of TBO and PVDF, and bubbling-based delamination should be introduced, which is relatively compatible with the batch transfer.
Methods
Materials
PVDF (Mw = 1,100,000), DMF, sodium persulfate (Na2S2O8), toluidine blue O (TBO, C15H16N3S+Cl−), pyrenyl-1-boronic acid pinacol ester (PBA, C22H21BO2), 1-phenyl-1H-benzo[d]imidazol-2(3H)-one (PBI, C13H10N2O), 4,5-diazafluoren-9-one (DAFO, C11H6N2O), sulfinpyrazone (C23H20N2O3S), and ethanol (C2H5OH) were purchased from Sigma-Aldrich and used as received. DI water was provided by a Thermo Scientific 7128 RO system. PEN films (50 μm, ELP BT-HR200) were obtained from Nitto. and used as received.
Graphene synthesis
The graphene films were synthesized on commercially available Cu foils (>99.96% purity, 25-μm thick, Dongguan Lidong Metal Foil Co., Ltd.) in a low-pressure CVD system. Electrochemical polishing of Cu foil (A3-sized or A4-sized) was conducted before graphene growth. After the electrochemical polishing, the Cu foil was washed with deionized water, and dried by nitrogen gas. The polished Cu foil in a roll shape was placed into a hot center of the furnace with a quartz tube (diameter: 6 in.) and was heated to 1000 °C under H2 atmosphere (20 sccm, ∼100 Pa) in 45 min and then annealed for 30 min under H2 with same flow rate to remove the carbon contamination and oxide on the Cu surface. Subsequently, 20 sccm of CH4 were introduced into the chamber to initiate the growth of polycrystalline continuous graphene films for 20 min. After the CVD growth, the system was rapidly cooled down to room temperature by moving the furnace. For growing multilayer graphene, additional annealing of Cu at Ar atmosphere (1000 sccm) at 1000 °C was conducted for 30 min before the graphene growth. After the annealing, 200 sccm H2 and 0.1 sccm CH4 was introduced into the chamber to initiate the multilayer growth59. In addition, to further increase the layer number, CO2 with a flow rate of 1 sccm can be introduced into the growth system, according to the previous report60.
Fabrication of double-layer graphene barrier films
Fabrication of Janus graphene films
The graphene film on Cu foil was first treated by reactive RIE with different durations (5, 10, 15, 20, and 30 s). The RIE process was conducted under a pressure of 200 mTorr, an RF power of 30 W, an O2 flow rate of 3 sccm, and a CF4 flow rate of 10 sccm. Subsequently, graphene films on Cu foil were spin-coated with PVDF 4% wt in DMF at 2000 rpm/min and then heated at 60 °C for 30 min. To support the graphene, PEN was applied onto the PVDF/graphene/Cu foil using a commercial laminator (GMP A3 Laminator Machine 320, LSI) at 60 °C with a laminating rate of 2 cm per second. Subsequently, the Cu/graphene/PVDF/PEN was heated at 60 °C over 2 h. Then, the Cu foil was etched using 0.5 mol/L of Na2S2O8 over 20 min. Rinsing the graphene films by deionized water was conducted to remove residual etchant. After the drying, the graphene film on PEN/PVDF was treated under 3 s oxygen plasma (Diener PICO Plasma, 100 W) at a pressure of 0.4 mbar and a radio frequency (RF) power of 40 W, and the O2 flow rate was set to be 10 sccm.
Intercalation of TBO molecules
The Janus graphene film was spin-coated with TBO (1 mg/L, CH3CH2OH) at 2000 rpm/min, followed by lamination of another Janus graphene film using the same lamination condition before. Subsequently, the barrier film was heated at 60 °C overnight to enable the complete evaporation of water and the conformal contact between the graphene layers.
Characterizations
OM images were taken with a Nikon LV100ND instrument. Raman spectra were obtained with a LabRAM HR-800 instrument with a 532 nm laser. Optical transmittance was collected by a PerkinElmer Lambda 950 UV-VIS-NIR Spectrometer. The ADF-STEM images with an atomic resolution of graphene were carried out on a Nion U-HERMES200, 60 kV. Before the STEM imaging, 12 h vacuum treatment and annealing of the sample at around 160 °C were conducted to remove airborne contamination. AFM characterization of graphene was carried out using a Bruker dimension icon microscope based on the Scanasyst mode. C1s XPS spectra were acquired by using a Kratos Analytical Axis-Ultra spectrometer with a monochromatic Al Kα X-ray source. The measurement of contact angle was conducted by using a Biolin optical tensiometer.
Measurement of WVTR
WVTR was measured by using an electrical Ca test, in which the reaction of Ca with the permeated water can result in a conductance change in Ca films. The device for measuring WVTR consists of 200 nm ITO electrodes and 200-nm-thick Ca film that were deposited by thermal evaporation (Supplementary Fig. 12). The barrier film was attached to the Ca test device, and the gap between the testing film and ITO electrodes was sealed by using Nagase Chemtex XNR5570 UV-cured epoxy resin. The above device was fabricated in a glovebox filled with Ar gas. The electrical resistance of the Ca film device was recorded in real time under ambient conditions using a Keithley 4200A-SCS semiconductor parameter analyzer. The relative humidity (RH) and temperature were set to be 45% and 25 °C, respectively.
DFT calculations
The DFT calculations were conducted using the Vienna ab initio Simulation Package (VASP)61, employing the Perdew–Burke–Ernzerh (PBE) functional62 in combination with projector-augmented-wave (PAW) pseudopotentials63. To ensure accuracy, a cutoff energy of 500 eV was chosen. A vacuum layer with a thickness of 15 Å was implemented to prevent interactions between neighboring supercells. Ionic and electronic relaxation criteria were set as 10−2 eV Å−1 and 10−6 eV, respectively. Van der Waals interactions were treated using Grimme’s vdW-D3 approach64. Geometry optimizations utilized a Monkhorst-Pack special k-point mesh to sample the Brillouin zone efficiently65. The adsorption energies can be defined as
Where Etarget is the energy of the target substrate or molecules for water adsorption, including graphene, hydroxyl-functionalized graphene, hydroxyl-functionalized graphene with backside fluorination, TBO, and other molecules. Etotal is the total energy of the target substrate or molecules after the adsorption of water molecules. EH2O is the energy of a water molecule.
MD simulations
The MD simulations were carried out using the large-scale atomic/molecular massively parallel simulation (LAMMPS) software66. The all-atom optimized potentials for liquid simulations (OPLS-AA) force field were employed to develop atomic models of graphene and hydroxyl-functionalized graphene67. The different concentrations of functional groups on one side were designed to represent hydroxyl-functionalized graphene. For the water molecules, the SPC/E model68,69 was used in conjunction with the SHAKE algorithm. The dimension of simulation system is 5 × 5 × 5 nm in x, y, z directions, respectively, where period boundary conditions were used on all dimensions. The van der Waals interactions between water and pristine graphene and hydroxyl-group-coated graphene are represented using the Lennard-Jones 12-6 potential, given by \(V=4\varepsilon \left[{\left(\frac{\sigma }{r}\right)}^{12}-{\left(\frac{\sigma }{r}\right)}^{6}\right]\), where r is the interatomic distance. The parameters of carbon in graphene are ε = 0.105 kcal/mol and σ = 3.851 Å. Regarding the interaction of carbon and atoms in -OH, the parameters εC(C-OH) = 0.070 kcal/mol, σC(C-OH) = 3.550 Å, εO(C-OH) = 0.170 kcal/mol, σO(C-OH) = 3.070 Å, εH(C-OH) = 0.0 kcal/mol, and σH(C-OH) = 0.0 Å70. The characteristic length (σ) and energy parameter (ε) between water molecules and carbon atoms were obtained by the common Lorentz-Berthelot combination rule. The van der Waals interactions were truncated at 1.2 nm, and the long-range Coulomb interactions were computed by utilizing the particle-particle particle-mesh (PPPM) algorithm71. The time step was set to be 1 fs. The total time of simulation is 3 ns and results of the final 1 ns are used for data collection. Water molecules are equilibrated at 300 K using the Berendsen thermostat.
Equilibrium molecular dynamics (EMD) simulations were used to find δ = η/λ, which is given by the ratio of the viscosity (η) to the liquid-solid friction coefficient (λ). λ was calculated from the autocorrelation function of interfacial forces in EMD runs, which can be expressed in terms of the Green-Kubo relation56,57,
where kB is the Boltzmann constant, T is the temperature, and F is the time-dependent interfacial force acting on the surface with an area of S. The density of bulk water of 0.9913 kg/L was used in our system, and the viscosity was predicted to be 0.729 mPa·s from the SPC/E water model at 300 K72.
Data availability
Data supporting the results of this study can be found in this article and its Supplementary Information and are available from the corresponding authors upon request. The source data are provided with this article. Source data are provided with this paper.
References
Sangroniz, A. et al. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 10, 3559 (2019).
Yu, J. et al. High gas barrier coating using non-toxic nanosheet dispersions for flexible food packaging film. Nat. Commun. 10, 2398 (2019).
Choi, K. et al. Reduced water vapor transmission rate of graphene gas barrier films for flexible organic field-effect transistors. ACS Nano 9, 5818–5824 (2015).
Jeong, S. Y. et al. Foldable and washable textile-based OLEDs with a multi-functional near-room-temperature encapsulation layer for smart e-textiles. npj Flex. Electron. 5, 15 (2021).
Bunch, J. S. et al. Impermeable atomic membranes from graphene sheets. Nano Lett. 8, 2458–2462 (2008).
Celebi, K. et al. Ultimate permeation across atomically thin porous graphene. Science 344, 289–292 (2014).
Nair, R., Wu, H., Jayaram, P. N., Grigorieva, I. V. & Geim, A. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 335, 442–444 (2012).
Su, Y. et al. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 5, 4843 (2014).
Seo, Y.-M. et al. Defect-free mechanical graphene transfer using n-doping adhesive gel buffer. ACS Nano 15, 11276–11284 (2021).
Lu, Q. et al. High moisture-barrier performance of double-layer graphene enabled by conformal and clean transfer. Nano Lett. 23, 7716–7724 (2023).
Seo, H.-K. et al. Laminated graphene films for flexible transparent thin film encapsulation. ACS Appl. Mater. Interfaces 8, 14725–14731 (2016).
Kim, Y.-J. et al. Two-dimensional stacked composites of self-assembled alkane layers and graphene for transparent gas barrier films with low permeability. Nano Lett. 22, 286–293 (2022).
Wang, M. et al. Single-crystal, large-area, fold-free monolayer graphene. Nature 596, 519–524 (2021).
Deng, B. et al. Wrinkle-free single-crystal graphene wafer grown on strain-engineered substrates. ACS Nano 11, 12337–12345 (2017).
Vallejos-Burgos, F., Coudert, F.-X. & Kaneko, K. Air separation with graphene mediated by nanowindow-rim concerted motion. Nat. Commun. 9, 1812 (2018).
Shen, J., Liu, G., Han, Y. & Jin, W. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
Xiao, S. et al. Edge-enhanced ultrafast water evaporation from graphene nanopores. Cell Rep. Phys. Sci. 3, 100900 (2022).
Kim, D. J. et al. Degradation protection of color dyes encapsulated by graphene barrier films. Chem. Mater. 31, 7173–7177 (2019).
Sagade, A. A. et al. Graphene-based nanolaminates as ultra-high permeation barriers. npj 2D Mater. Appl. 1, 35 (2017).
Yang, Q. et al. Capillary condensation under atomic-scale confinement. Nature 588, 250–253 (2020).
Xie, Q. et al. Fast water transport in graphene nanofluidic channels. Nat. Nanotechnol. 13, 238–245 (2018).
Radha, B. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016).
Muscatello, J., Jaeger, F., Matar, O. K. & Müller, E. A. Optimizing water transport through graphene-based membranes: insights from nonequilibrium molecular dynamics. ACS Appl. Mater. Interfaces 8, 12330–12336 (2016).
Keerthi, A. et al. Water friction in nanofluidic channels made from two-dimensional crystals. Nat. Commun. 12, 3092 (2021).
Nam, T. et al. A composite layer of atomic-layer-deposited Al2O3 and graphene for flexible moisture barrier. Carbon 116, 553–561 (2017).
Zhao, Y. et al. Large-area transfer of two-dimensional materials free of cracks, contamination and wrinkles via controllable conformal contact. Nat. Commun. 13, 4409 (2022).
Nakatani, M. et al. Ready-to-transfer two-dimensional materials using tunable adhesive force tapes. Nat. Electron. 7, 119–130 (2024).
Haigh, S. J. et al. Cross-sectional imaging of individual layers and buried interfaces of graphene-based heterostructures and superlattices. Nat. Mater. 11, 764–767 (2012).
Song, Y. et al. Transfer‐enabled fabrication of graphene wrinkle arrays for epitaxial growth of AlN films. Adv. Mater. 34, 2105851 (2022).
Carcia, P. F., McLean, R., Reilly, M., Groner, M. & George, S. Ca test of Al2O3 gas diffusion barriers grown by atomic layer deposition on polymers. Appl. Phys. Lett. 89, 031915 (2006).
Dameron, A. A. et al. Gas diffusion barriers on polymers using multilayers fabricated by Al2O3 and rapid SiO2 atomic layer deposition. J. Phys. Chem. C. 112, 4573–4580 (2008).
Weng, Y. et al. Design and fabrication of PDMS/Al2O3 hybrid flexible thin films for OLED encapsulation applications. ACS Appl. Polym. Mater. 5, 10148–10157 (2023).
Ma, L. P., Ren, W. & Cheng, H. M. Transfer methods of graphene from metal substrates: a review. Small Methods 3, 1900049 (2019).
Sun, L. et al. Toward epitaxial growth of misorientation-free graphene on Cu (111) foils. ACS Nano 16, 285–294 (2021).
Graff, G. L., Williford, R. E. & Burrows, P. E. Mechanisms of vapor permeation through multilayer barrier films: lag time versus equilibrium permeation. J. Appl. Phys. 96, 1840–1849 (2004).
Choi, J. H. et al. Evaluation of gas permeation barrier properties using electrical measurements of calcium degradation. Rev. Sci. Instrum. 78, 064701 (2007).
Seo, S.-W., Jung, E., Lim, C., Chae, H. & Cho, S. M. Water permeation through organic–inorganic multilayer thin films. Thin Solid Films 520, 6690–6694 (2012).
Park, M.-H. et al. Flexible lamination encapsulation. Adv. Mater. 27, 4308–4314 (2015).
Sarno, M., Rossi, G., Cirillo, C. & Incarnato, L. Cold wall chemical vapor deposition graphene-based conductive tunable film barrier. Ind. Eng. Chem. Res. 57, 4895–4906 (2018).
Yan, Z. et al. Large hexagonal bi‐ and trilayer graphene single crystals with varied interlayer rotations. Angew. Chem. Int. Ed. 126, 1591–1595 (2014).
Seo, T. H. et al. The role of graphene formed on silver nanowire transparent conductive electrode in ultra-violet light emitting diodes. Sci. Rep. 6, 29464 (2016).
Ko, H., Lee, J. S. & Kim, S. M. 2D heterostructure for enhanced gas barrier performance via synergetic effect. Appl. Sci. Converg. Technol. 27, 144–148 (2018).
Won, S. et al. Graphene-based stretchable and transparent moisture barrier. Nanotechnology 29, 125705 (2018).
Langereis, E., Creatore, M., Heil, S., Van de Sanden, M. & Kessels, W. Plasma-assisted atomic layer deposition of Al2O3 moisture permeation barriers on polymers. Appl. Phys. Lett. 89, 081915 (2006).
Xiao, W. et al. The improvement of thin film barrier performances of organic-inorganic hybrid nanolaminates employing a low-temperature MLD/ALD method. RSC Adv. 4, 43850–43856 (2014).
Choi, D.-w, Park, H., Lim, J. H., Han, T. H. & Park, J.-S. Three-dimensionally stacked Al2O3/graphene oxide for gas barrier applications. Carbon 125, 464–471 (2017).
Kwon, J. H. et al. Functional design of dielectric–metal–dielectric-based thin-film encapsulation with heat transfer and flexibility for flexible displays. ACS Appl. Mater. Interfaces 9, 27062–27072 (2017).
Felten, A., Eckmann, A., Pireaux, J., Krupke, R. & Casiraghi, C. Controlled modification of mono-and bilayer graphene in O2, H2 and CF4 plasmas. Nanotechnology 24, 355705 (2013).
Lim, T. & Ju, S. Control of graphene surface wettability by using CF4 plasma. Surf. Coat. Technol. 328, 89–93 (2017).
Struzzi, C. et al. Probing plasma fluorinated graphene via spectromicroscopy. Phys. Chem. Chem. Phys. 19, 31418–31428 (2017).
Wang, Z. et al. Graphene oxide nanofiltration membranes for desalination under realistic conditions. Nat. Sustain. 4, 402–408 (2021).
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001).
Thomas, J. A. & McGaughey, A. J. Reassessing fast water transport through carbon nanotubes. Nano Lett. 8, 2788–2793 (2008).
Kannam, S. K., Todd, B., Hansen, J. S. & Daivis, P. J. How fast does water flow in carbon nanotubes? J. Chem. Phys. 138, 094701 (2013).
Wu, D. et al. Probing structural superlubricity of two-dimensional water transport with atomic resolution. Science 384, 1254–1259 (2024).
Bocquet, L. & Barrat, J.-L. Hydrodynamic boundary conditions, correlation functions, and Kubo relations for confined fluids. Phys. Rev. E 49, 3079 (1994).
Bocquet, L. & Barrat, J.-L. On the Green-Kubo relationship for the liquid-solid friction coefficient. J. Chem. Phys. 139, 044704 (2013).
Santhosh, N. M. et al. N-graphene nanowalls via plasma nitrogen incorporation and substitution: The experimental evidence. Nano-Micro Lett. 12, 1–17 (2020).
Zhou, H. et al. Chemical vapour deposition growth of large single crystals of monolayer and bilayer graphene. Nat. Commun. 4, 2096 (2013).
Zhang, J. et al. Fast synthesis of large-area bilayer graphene film on Cu. Nat. Commun. 14, 3199 (2023).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Shih, C.-J., Lin, S., Sharma, R., Strano, M. S. & Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: a comparative experimental and molecular dynamics simulation study. Langmuir 28, 235–241 (2012).
Wei, N., Lv, C. & Xu, Z. Wetting of graphene oxide: a molecular dynamics study. Langmuir 30, 3572–3578 (2014).
Falk, K., Sedlmeier, F., Joly, L., Netz, R. R. & Bocquet, L. Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett. 10, 4067–4073 (2010).
Chen, B., Jiang, H., Liu, X. & Hu, X. Observation and analysis of water transport through graphene oxide interlamination. J. Phys. Chem. C. 121, 1321–1328 (2017).
Hockney, R. W. & Eastwood, J. W. Computer Simulation Using Particles (CRC Press, 2021).
González, M. A. & Abascal, J. L. The shear viscosity of rigid water models. J. Chem. Phys. 132, 096101 (2010).
Acknowledgements
This work was supported by National Key Research and Development Program of China (2024YFE0202200). This work were financially supported by the National Natural Science Foundation of China (No. 52372038 and T2188101) and the National Key Research and Development Program of China (2022YFA1204900 and 2023YFB3609900). The authors acknowledge the Molecular Materials and Nanofabrication Laboratory (MMNL) in the College of Chemistry, and Materials Processing and Analysis Center and Peking nanofab at Peking University for the use of instruments. The computations were made with the help of Prof. Wang (Fengchao Wang) from the University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
L.L. supervised the project and conceived the experiment. C.Z., H.G., H.W., and L.Y. conducted the transfer of graphene and fabricated the barrier films. C.Z., Z.L., B.G., and H.W. conducted the transfer of A3-sized graphene films. C.Z., H.G., H.W., Z.H., Y.S., and Q.X. took and analysed the OM, XPS, AFM, and Raman. C.Z., H.G., Y.S., and H.W. conducted measurements of WVTR using the Ca oxidization method. F.L., Y.Z., X.H., and F.F.L. conducted the CVD growth of graphene and TEM characterization. S.B. conducted the DFT calculation and MD simulation. All authors discussed the results. L.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yew Mun Hung,Geng Di Sia, Dongyeop Oh and Ruo Yuan, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, C., Gao, H., Bu, S. et al. Principles for fabricating moisture barrier films via stacked Janus graphene layers. Nat Commun 16, 3512 (2025). https://doi.org/10.1038/s41467-025-58799-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58799-y