Abstract
Engineering broadly neutralizing monoclonal antibodies (mAbs) targeting the hemagglutinin (HA) of Influenza A virus (IAV) is a promising approach for intervention of seasonal flu. However, HA plasticity often leads to resistant strains that compromise mAb potency as bivalent IgGs. Here we hypothesize that multimerization of anti-IAV antibodies as IgMs can enhance coverage and neutralization potency. Here, we construct 18 IgM antibodies from known broadly neutralizing IgGs targeting different IAV HA epitopes and evaluate their breadth and potency of neutralization against distinct H1N1 and H3N2 IAVs. The IgM version of receptor binding site-specific IgG F045-092 shows increased breadth and antiviral potency compared to its parental IgG. Engineered IgM molecules overcome IAV strain resistance by expanded avidity, providing potent neutralization in vitro at sub-nanomolar ranges while retaining parental IgG specificity. Intranasal delivery of engineered IgM-F045-092 in female mice demonstrates efficient bio-retention in nasal cavities and lungs, offering protection against lethal doses of H1N1 and H3N2 IAV when administered prophylactically. Optimal epitope selection, trans-crosslinking, decavalent avidity, and intranasal administration contribute to the broader protection and potency of engineered IgM antibodies against diverse IAV subtypes.
Similar content being viewed by others
Introduction
Influenza A viruses (IAVs) are important human pathogens associated with seasonal respiratory infection with significant morbidity and mortality, especially in the elderly and those with underlying diseases1,2. IAVs are classified based on the antigenic composition of their two major glycoproteins: hemagglutinin (HA) and neuraminidase (NA). A total of 18 HA (H1-H18) and 11 NA (N1-N11) subtypes have been identified3,4. HA is critical for initiation of viral infection by binding to sialic acids on host cell surface, and NA is important for the release of mature virions from infected cells5,6,7,8. Both HA and NA are susceptible to mutations9,10, and mutations arising in key residues of HA and NA signify the viral evolution capable of escaping host immune surveillance known as antigenic drift11.
Seasonal influenza vaccination is about 30–60% effective, and the variable efficacy is mainly due to the mismatch of HAs between vaccine strains versus those in circulation. The uncertainty of the current vaccine strategy has prompted research on universal flu vaccines, which can cover dynamic viral evolution especially those of pandemic threats such as avian influenza. However, these efforts, of targeting NA or matrix 2 (M2) protein to T-cell based vaccines, have had limited success so far. In the past 20 years, an increasing number of potent human neutralizing mAbs have been reported, leading to interest in passive prophylaxis especially for those vulnerable to influenza infection.
Numerous human antibodies have been isolated and studied for their neutralizing activity against diverse IAV strains12,13,14,15,16,17. Neutralizing antibodies to HA with breadth against multiple isolates mainly function by blocking the receptor binding site (RBS) located in the head region of HA (HA1), or by preventing host cell membrane fusion mechanism by targeting the stem region (HA2). Administrations of these anti-HA IgG antibodies have been known to confer protection against infection and/or death in preclinical models18,19, and disease symptoms in humans20, although the in vivo protection likely involves Fc-FcγR functions. Due to the highly mutagenic nature of HA, the usage of these antibodies as drugs is still limited by potential viral resistance, leading to reduced strain coverage. Moreover, systemic administration of IgG has not yielded significant efficacy.
We previously demonstrated that increased valency offered by engineered pentameric IgM confer enhanced efficacy and cross-protection against infections caused by SARS-CoV-2 and its variants compared to their IgG counterparts21. Thus, we hypothesize that the potency and breadth of neutralizing IgG antibodies to influenza HA can be enhanced by multimerization to IgM. In this study, a panel of HA-specific IgG antibodies was selected for IgM engineering efforts and paired IgG and IgM versions were evaluated in vitro against a range of H1N1 and H3N2 IAVs from 1934 to 2021 chronologically representative of viral evolution. Here we showed the enhanced potency and breadth coverage conferred by engineered IgMs, especially IgM-F045-092 which targets the RBS. Structural analysis revealed some intriguing features of this antibody with enhanced potency in multimerized IgM format. Evaluating its biodistribution and ability to confer protection against lethal infection in mice demonstrated favorable therapeutic profiles of IgM when administered intranasally. These results provide preclinical proof-of-concept for an experimental candidate that can be further tested in the clinic as a prophylactic or treatment intervention against current and emerging IAVs.
Results
A broad selection of virus strains and recombinant HA proteins
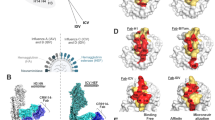
In humans, IAVs use the HA glycoproteins on their surface to form a complex with carbohydrates encompassing host cell surface receptors containing α2-6-linked sialic acids to attach and initiate infection on epithelial cells in the upper respiratory tract22,23. The HA is initially synthesized as a HA0 precursor molecule, and its proteolytic cleavage generates HA1 (head) and HA2 (stem regions) (Supplementary Fig. 1a). The HA1 globular head domain is more prone to mutations that reduce host immune recognition and neutralization. The stem-like HA2 region helps HA anchoring to the virus membrane. Also, this encompasses the fusion peptide, which is released during the proteolytic cleavage of HA0. This fusion process releases viral ribonucleoprotein complexes into the host cell. The H1 and H3 HAs contain immunodominant antigenic regions or domains that differ by viruses and can be recognized by different classes of antibodies. Major antigenic sites on H1 HA head region are termed Sa, Sb, Ca1, Ca2, and Cb based on the names of corresponding antibody classes that were used to characterize the antigenic topology of the A/Puerto Rico/8/1934 virus and its related mutants24,25. The known antigenic sites of H1 are mapped onto A/Puerto Rico/8/1934 HA trimer (PDB ID code: 1RU7)26 (Supplementary Fig. 1b). Similarly, the antigenic regions in the H3 HA viruses were designated Site A–Site E based on the structural and antigenic analysis using A/Aichi/2/1968 virus and its associated mutants27,28. Although the amino acid residues and numbers differ slightly for each strain, the nomenclature used to identify the key antigenic sites have been retained. The antigenic sites of the HA2 are highlighted as I–IV and the antigenic sites of H3 are mapped onto A/Aichi/2/1968 HA trimer (PDB ID code: 3VUN)29,30 (Supplementary Fig. 1c).
As the immunogenically dominant globular head region (HA1) has high variability, human anti-HA1 antibodies display a narrow range of neutralization. However, the RBS, being relatively conserved among HA1 of different subtypes are known to elicit cross-reactivity with potent antibody neutralization capabilities by preventing RBS-mediated receptor binding to the host cell31. In general, the HA2-specific monoclonal antibodies, although some epitopes are less exposed, provide better cross-protection as they are conserved amid various subtypes with reduced antigenic drift32.
The H1N1 subtype has been endemic within the human population since 1918. Since then, this subtype has been replaced, re-emerged, and has undergone significant genetic restructuring which eventually led to the 2009 pandemic. Currently circulating H1N1 viruses are all variants of the 2009 pandemic virus with the necessary adaptations to bind to human host cell receptors and enhanced viral fitness33. To provide a comprehensive validation, we included 7 historical/pre-pandemic (strains or HA proteins) and 8 post-pandemic/contemporary H1N1 IAVs (strains or HA proteins) in our study. The number of amino acid mutations acquired on HA1 and HA2 sites since the pandemic strain (A/California/04/2009) and recently circulating strain (A/Sydney/5/2021) were compared and depicted on a trimeric H1-HA structure (Fig. 1a). The H3N2 subtype originated as a pandemic virus in 1968 and has remained in circulation since then. For viruses belonging to the H3N2 subtype, our selection started from the earlier pandemic strain A/Aichi/2/1968 to the recently circulating A/Delaware/6/2021, comprising 18 H3N2 IAVs (strains or HA proteins) that overall depict the antigenic evolution of HA for more than 5 decades. The HA1 and HA2 sites on H3N2 strains were compared between the pandemic strain (A/Aichi/2/1968), intermediate strain (A/Victoria/361/2011), and recently circulating strain (A/Delaware/6/2021). The number of amino acid mutations acquired on HA1 and HA2 was depicted on a trimeric H3-HA structure (Fig. 1b). The indicative entropy scores for each amino acid position in H1N1 (Fig. 1c) and H3N2 (Fig. 1d) strains used in this study demonstrated higher sequence diversity in HA1 compared to HA2 region34. The phylogenetic tree of the strains used in this study was generated by the Maximum-Likelihood method and Jones–Taylor–Thornton (JTT) matrix-based modeling with full-length HA sequences. A bootstrap consensus tree was generated using 1000 replicates (Supplementary Fig. 1d, e). Furthermore, using a Poisson correction model, the HA antigenic covariance was measured by differences in the number of HA amino acid substitutions per site between input sequences and reference sequences. The estimates of evolutionary divergence analysis between sequences were conducted using MEGA1135 (Supplementary Fig. 1f, g). The oldest sequence A/Puerto Rico/8/1934 for H1N1 or A/Aichi/2/1968 for H3N2 were used as reference strains to construct the phylogenetic tree and evolutionary divergence matrix map29,36.
A schematic representation of HA depicting the amino acid substitution in HA1 (light green) and HA2 regions (dark green) of H1N1 from 1934 to 2021 (a) and H3N2 from 1968 to 2021 (b). HA1 substitutions are highlighted in red and HA2 substitutions are highlighted in yellow. The indicative entropy score for each amino acid position in H1N1 (c) and H3N2 (d) strains used in this study demonstrated higher sequence diversity in HA1 compared to HA2 region. Crystal structures of the broadly neutralizing antibodies targeting HA superimposed on the structure of the H1N1 HA trimer (PDB 3LZG) (e) and H3N2 HA trimer (PDB 3VUN) (f). Each antibody binding region on the HA crystal structure were depicted in different colors, and the antibody overlapping regions were highlighted in red. g Kinetic binding affinity (KD) of broadly neutralizing antibodies to the monomeric HA proteins of H1N1 and H3N2 are represented as heat map with the range of binding affinity from 1 nM (blue) to 10 nM (yellow). Empty boxes depict no binding observed at the tested concentrations. Source data are provided as a Source Data file.
Selection and engineering of broadly neutralizing IgG antibodies and their IgM constructs
To test the hypothesis that multimerization by IgM conversion can drastically change antibodies’ antiviral profiles against IAVs, we identified a panel of 18 broadly neutralizing antibodies from literature, and grouped them into H1-specific, H3-specific, cross-subtypic, and stem-reactive antibodies. The binding regions were superimposed and visualized on the structure of the H1 HA trimer onto A/California/04/2009 pdm09 (PDB 3LZG) for H1-specific antibodies and H3 HA trimer onto A/Aichi/2/1968 (PDB 3VUN) for H3-specific and cross-subtypic antibodies using UCSF ChimeraX37,38,39(Fig. 1e, f). Stem-targeting antibodies were included in both structures, allowing for their visualization with the least overlap. Crystal structures showing the binding sites of each IgG antibody used in this study are depicted in Supplementary Fig. 2. The DNA sequences encoding the heavy and light variable regions were retrieved and the specific genes were synthesized and converted to a fully human immunoglobulin (IgG1) molecule and expressed in Expi293F cells. As part of our initial validation, the binding affinity of each of the constructed IgGs were measured using a bio-layer interferometry (BLI)-based assay against a panel of 19 different recombinant HA proteins that represent the antigenic evolution of human H1N1 and H3N2 subtypes. Among all the classes of antibodies tested, stem-specific antibodies MEDI8852 and FI6v3 displayed strong binding in nM potency and breadth covering nearly the complete panel of 10 H1N1 and 9 H3N2 recombinant HA proteins. This data validates how stem-specific antibodies provide cross-subtypic activity by binding to the conserved region of the HA. It is important to note that 5J8, CH65, CH67, 641 I-9, and H2526 all bind to the RBS domain of HA1. However, as previously reported, only 5J8 and 641 I-9 bind to the 2009 pandemic and post-pandemic H1N1 HAs at a KD of 3 nM and 1 nM, respectively. For the H3-specific antibodies, C585 shows better binding followed by F005-126 and both antibodies exhibit specific overlapping binding residues. Heterosubtypic antibodies FluA-20, F045-092, C05, and K03.12 are RBS-binding antibodies and elicit binding in nanomolar potency. The complete panel of kinetic binding data were depicted as a heatmap generated based on its KD value in nM range (Fig. 1g). The calculated affinity constant values and fitting results are shown in Supplementary Data 1.
We have reported that engineered IgM antibodies exhibited higher binding and neutralizing potentials compared than IgG counterparts against SARS-CoV-2 viruses due to avidity by multimerization, but such enhancement is epitope dependent21. To evaluate anti-HA antibodies in IgM formats, we performed antibody engineering based on the parental IgG1 mAb sequences and expressed in the human IgM scaffolds with the J-chain. For biophysical analysis, we prepared the fragment variable regions as Fab (monovalent), to compare with the corresponding IgG1 form (bivalent) vs. IgM form (decavalent) (Fig. 2a). The characterization of Fab, IgG and IgM by SDS-PAGE and size-exclusion HPLC are shown for F045-092 which represents the data trend observed for all the other antibodies (Fig. 2b, c). The Fab, IgG, and IgM antibody fragments were evaluated using HPLC and showed a purity greater than 90%. Similarly, the comparative binding activity of Fab, IgG, and IgM fragments of F045-092 were evaluated using recombinant HA protein of BEI/95 using BLI-based assay to demonstrate avidity-based affinity enhancement (Fig. 2d). The Fab used in the binding assays revealed no observable binding at the highest concentration tested (400 nM).
a Illustration of antibody engineering from IgG1 into IgM and Fab (Created in BioRender; https://BioRender.com/fvt7dkf). SDS–PAGE (b) and size-exclusion chromatography (c) of fab, IgG, and IgM antibodies analysis showing monoclonal antibody assembly and purity. The gel images were from one experiment. HC heavy chain, LC light chain, and J-chain joining chain. d BLI binding curve depicting the binding affinity and response rate of representative Fab, IgG, and IgM formats against A/Beijing/262/1995 H1N1 recombinant HA protein. e 50% inhibitory concentrations [log10 HI50 (nM)] of 18 neutralizing IgG1 antibodies and their IgM versions were determined by hemagglutination inhibition assay (HAI) against 25 representative H1N1 and H3N2 IAV strains. Blocking potency displayed as a heatmap, with stronger to weaker receptor blocking activity represented from darker to lighter shade. Empty boxes indicate no blocking activity observed at the concentration tested. Dot plot depicting blocking potency and breadth covered by the best performing IgG and IgM antibody specific for H1N1 (f), H3N2 (g), and heterosubtypic (h) from the data displayed in (e). Data represents the average values from two independent experiments performed in duplicates. Source data are provided as a Source Data file.
Enhanced breadth coverage and neutralizing potency by IgM antibodies
To investigate the role of epitope-mediated neutralization potency enhancement upon increased valency, we tested 18 engineered IgM molecules along with their parental IgGs against a panel of 25 different IAV strains (12 H1N1 and 13 H3N2) by hemagglutination inhibition (HAI) assay and microneutralization (MN) assay. Results from the HAI assay demonstrate the ability of head domain binding antibodies to block the virus-host cell receptor attachment, which indicates early infection inhibitory activity40,41. The 50% inhibitory concentration (HI50) measured by HAI assay showed an enhanced HA blocking potency conferred by IgM antibodies over IgGs against the full range of IAV strains tested. Inhibitory potency and IAV strain coverage were displayed as a heatmap for better comparison (Fig. 2e). Interestingly, we observed a significant increase in inhibiting potency, and gain of breadth coverage in IgM versions of head targeting antibodies (5J8, 641 I-9, CH65, CH67, H2526 and F045-092) against the IAV strains that demonstrated no reactivity against their respective parental IgG antibodies at the concentration tested (Supplementary Data 2).
We then segregated the best-performing IgM antibodies under each group to make our initial selection. Both IgG-5J8 and IgM-5J8 neutralized the pandemic (A/California/04/2009) and post-pandemic H1N1 strains, however IgM-5J8 extended its potent blocking activity also by neutralizing historical H1N1 strains including PR/34, BEI/95 and NC/99 which was not observed in its IgG form (Fig. 2f). Considering the H3N2 specific antibodies, C585 shows only increase in potency with no observable gain in breadth coverage in both IgG and IgM versions (Fig. 2g). However, the antibody IgG-F045-092 blocks both historic and recently circulating H3N2 IAVs. Significantly, IgM-F045-092 broadens its inhibitory coverage and potency to pre-pandemic H1N1 strains including PR/34, BEI/95, NC/99, and BR59/07 IAV strains. It is important to note that the IgM-F045-092 does not block the pandemic and post-pandemic H1N1 IAVs (Fig. 2h). Dot plots showing the individual blocking potency and breadth covered by all IgG-IgM pairs are shown in Supplementary Fig. 3. This further validates how the IgM format retains its epitope specificity but enhances its antiviral activity42. Canonically, the inhibitory activity of stem reactive antibodies cannot be assessed by HAI assay as their mechanism is centered on the disruption of the fusion machinery. However, we included stem-targeting antibodies in our HAI assay to understand whether they would gain steric-based inhibitory activity along with FluA-20 antibody43 which is known not to show functional activity in HAI or MN assay. IgM conversion provided no distinctive blocking activity44,45.
The half-maximal neutralization titer (IC50) values evaluated by MN assay confirmed that IgM conversion showed enhanced neutralizing potency compared to their respective parental IgG (Fig. 3a and Supplementary Fig. 4). This result demonstrates that the increased valency of IgM antibodies (decavalent) compared to paratope-matched, parental IgG (bivalent) improved the breadth coverage and antiviral potency. It is noted that IgM versions of antibodies 5J8, 641 I-9, CH65, F045-092, and MEDI8852 gained anti-viral activity by demonstrating efficient neutralization against IAVs. This gain of breadth activity observed in IgG antibodies upon conversion to IgM is in line with other reported works21,46. Not all the IgM molecules conferred expansion on coverage against the panel of IAV strains, as some only demonstrated the enhancement on neutralizing potency (Supplementary Data 3). Antibodies such as HC45 and F005-126 have binding sites that are distant from the RBS and thus, demonstrated a narrow range of activity against IAV strains of the H3 subtype38,40.
a In vitro endpoint microneutralization titer [IC50 (nM)] values of all 18 IgG antibodies and their IgM versions determined by a panel of H1N1 and H3N2 IAVs. Neutralization potency is displayed as a heatmap with stronger to weaker neutralizing activity represented from darker to lighter shade. Empty boxes indicate no blocking activity observed at the highest concentration tested. Cumulative frequency plot depicting neutralization potency and breadth covered by the best performing IgG and IgM antibody specific for H1N1 (b), H3N2 (c), Cross-subtypic (d), and stem (e) from the data displayed in (a). Geometric mean IC50 titer (GMT) of IgGs and IgMs against IAVs shown as a vertical dashed line on each graph with their associated values. The number of strains neutralized and the coverage percentage observed for each antibody is denoted together within its associated plot area. Data represents the average values from two independent experiments performed in duplicates. Source data are provided as a Source Data file.
Antibodies 5J8 (H1-specific), C585 (H3-specific), F045-092 (cross-subtypic), and MEDI8852 (stem-specific) showed better neutralizing efficacy among their respective groups. The IC50 evaluated for H1-head specific antibody IgM-5J8 displayed coverage against 11/12 H1N1 isolates (92% viruses neutralized) as compared to 7/12 H1N1 viruses (58%) for IgG-5J8; in addition, the geometric means for all IC50 demonstrated a shift from 10.87 nM for its IgG form to 0.56 nM in its IgM form, a 19.6-fold increase in neutralization potency (Fig. 3b). For the H3-head specific antibody pairs, IgG-C585 could neutralize all the IAVs tested (13/13 H3N2 viruses) with its IgM form demonstrated around 10-fold gain of potency (4.32 nM for IgG vs. 0.43 nM for IgM) (Fig. 3c). A notable improvement in potency and breadth of F045-092 antibody was observed. IgM-F045-092 conferred protection against 18 of 25 H1N1 (72%), a clear expansion on breadth compared to its IgG form (14 of 25 isolates, 56%). IgM-F045-092 conferred 100% coverage with nanomolar to sub-nanomolar potency for H3N2 and pre-pandemic H1N1 IAVs. Moreover, in contrast to other cross-subtypic antibodies typically with mediocre potency, IgM-F045-092 demonstrated high potency with its geometric mean IC50 titer (GMT) identified at 0.09 nM (Fig. 3d). Among stem-targeting antibodies, IgM-MEDI8852 demonstrated a higher breadth with a moderate neutralization potency (GMT 1.3 nM) (Fig. 3e). Cumulative frequency graphs showing the individual breadth-potency comparison for other IgG-IgM pairs were shown in Supplementary Fig. 5. Overall, the inhibitory activity evaluated by HAI and neutralization activity evaluated by MN for the head binding IgM antibodies 5J8, C585 and F045-092 correlated and displayed similar trend of broader breath coverage and enhanced potency. The avidity-based gain of breadth has been demonstrated previously for RBS-targeting antibodies such as 5J8, where the conversion of Fab to IgG resulted in enhanced binding47,48,49. The antibody Heavy Chain Complementarity-determining region (HCDR3) loops of RBS-targeting antibodies S139/1, C05, K03.12, and F045-092 mostly function by binding to the hydrophobic pockets of the H1 and H3 RBS at residues 130–13817. Unfortunately, an amino acid insertion (lysine or asparagine) at position 133a among currently circulating H1 viruses creates a steric clash that ultimately leads to the loss of binding to these antibodies48,50. Our data of reduced coverage against post-pandemic H1 stains corroborated this structural change.
Enhanced affinity, breadth, and potency of IgM-F045-092
After IgM conversion, F045-092 showed a unique expansion in coverage besides the expected antiviral potency enhancement. To quantify such improvement, we evaluated the binding dynamics of F045-092 in Fab, IgG, and IgM versions measured by ELISA and BLI-based assays against representative H1 (BEI/95 and NC/99) and H3 (AI/68 and HK/19) recombinant HA proteins. The respective half-maximal effective concentration (EC50) values denote the stronger binding ability of IgM-F045-092 (19–65 pM) than its IgG format (0.5–1.5 nM). We observed binding of Fab-F045-092 only for AI/68 which showed EC50 of 5 nM. (Fig. 4a–d). The fold change between Fab, IgG, and IgM for the representative recombinant HA proteins is shown in Supplementary Data 4. The EC50 values suggest at a valency increment directly proportional to higher binding activity. The Fab, which exhibited no binding, was assigned an arbitrary EC50 value of 1000 nM for graphing purposes (Fig. 4e). The kinetic binding assay determines that IgM-F045-092 displayed a faster and stronger association rate with no determined dissociation rate with a recorded KD value of <1 pM against all H1 and H3 HAs tested while IgG-F045-092 showed binding with a certain level of dissociation. However, Fab versions showed no binding at the concentrations tested (data not shown), so the KD values were not determined. (Fig. 4f–i). The individual KD values (nM) and their respective fold differences between IgM and IgG of F045-092 against tested H1 and H3 HA were displayed as a bar graph (Fig. 4j). Individual KD (nM), KD Error (nM), kon (1/Ms), kdis(1/s), and the respective R2 values are shown in Supplementary Data. 5. The end-point neutralization titer (IC50) of IgM-F045-092 antibody exhibited a higher inhibitory effect, enhanced neutralization potency and broader breath coverage compared to its IgG version against five-representative H1N1 and H3N2 IAV strains (Fig. 4k, l).
ELISA data showing the binding of IgG and IgM versions of F045-092 tested against recombinant HA proteins belonging to H1N1, BEI/95 (a), NC/99 (b), and H3N2, AI/68 (c), and HK/19 (d). Data are presented as mean values ± SD and the EC50 values were calculated by nonlinear regression curve fitting. Each point represents the mean of triplicate wells. OD450 nm, optical density at 450 nm. e Line graph showing the comparative EC50 (nM) values observed with respect to change in valency. The dashed line indicates the maximally tested concentration as limit of detection. Binding affinity measurements for H1N1 and H3N2 recombinant HA proteins belonging to H1N1, BEI/95 (f), NC/99 (g), and H3N2, AI/68 (h), and HK/19 (i) against IgG1 and IgM, as determined with BLI-Octet. The kinetic constants of Fab-F045-092 were not determinable. j independent KD values of IgG and IgM-F045-092 were depicted as a bar graph. The respective fold differences between IgG and IgM were indicated on top of each bar associated with HA protein. Comparative neutralization efficiency [IC50 (nM)] of F045-092 in IgG and IgM formats against 5-representative H1N1 (k) and H3N2 (l) IAV strains. Results are represented as an average of at least two technical replicates from one independent experiment. Source data are provided as a Source Data file.
IgM-F045-092 crosslinks with multiple IAV HAs
The structural basis for F045-092 in recognizing H1 HA and H3 HA has been previously reported49. To understand the expansion on strain coverage that we have observed upon IgM conversion, we first conducted a structural analysis using negative stain electron microscopy (ns-EM) single particle analysis using recombinant HA trimers of AI/68 (H3N2) and SI/06 (H1N1) mixed with an excessive molar ratio of F045-092 Fabs. The complex formed between AI/68 HA trimer and F045-092 Fab exhibited a 1:3 HA to antibody binding stoichiometry in 3D reconstruction, with minimal free HA or Fab observed in raw images and 2-dimensional (2D) class averages (Fig. 5a). In contrast, the complex of SI/06 HA and F045-092 displayed a lower antibody occupancy, with either empty HA or HA trimers binding to one Fab (Fig. 5b). This is consistent with our HI and MN data which demonstrated neutralization of AI/68 at lower doses compared to SI/06 indicative of a natural affinity towards H3 over H1 IAV.
Ns-EM images of Fab-F045-092 bound to HK/1968 (a) and SI/06 (b) HA trimers demonstrating varied binding stoichiometry. c Ns-EM images of IgM-F045-092 molecule bound to HA of VI/11 (H3N2) demonstrating different modes of binding. Blue arrow denotes IgM-F045-092 and yellow arrows denote VI/11 HA trimers. HA trimers crosslinked with IgM are circled and shown as enlarged images on the right panels. For the Ns-EM study, n = 3 independent experiments were performed with similar results. d Cartoon representing hypothetical mechanism of valency-based epitope-paratope interaction and cross-linking (Created in BioRender; https://BioRender.com/fvt7dkf).
We next attempted to work with IgM-F045-092 directly to obtain visual insight on how multi-valency interaction can be associated with gain of breadth and potency. The ns-EM micrograph demonstrated well-prepared IgM molecules without aggregates (Supplementary Fig. 5a). The 2D class average revealed a distinct IgM core region and blurred Fabs (Supplementary Fig. 5b), confirming that the linker between the Fab and core is appropriately designed to allow sufficient flexibility for IgM molecule recognition of various IAV virions. We then mixed IgM-F045-092 with VI/11 HA trimer and studied their interaction. Not surprisingly, due to the flexibility of the Fab-IgM linker region, we could not obtain class averages on the interaction of IgM with HA trimers. However, in most ns-EM micrographs, we observed two interesting interaction modes (Fig. 5c). One involved an IgM-F045-092 molecule bound by multiple copies of HA proteins, which might replicate the conditions where IgM cross-links HA on single or even multiple virions to enhance avidity. The other mode depicted two or more IgM-F045-092 molecules connected by binding to different protomers of a single HA trimer. These binding modes suggest that IgM-F045-092, even with lower binding affinity as Fab, can interact with two or three protomer with SI/06 HA trimer, and interact with multiple trimers on the same or even adjacent virions through its decavalent Fab presentation. Expectedly, these binding modes are rather different from bivalent IgG-F045-092, where only one Fab is engaged most of the time (data not shown). Based on these structural findings, we hypothesize a schematic model representing the mechanism of valency-based epitope-paratope interaction and cross-linking of Fab, IgG, and IgM molecules (Fig. 5d).
Retention of F045-092 IgM in the airways
We envision that IgM-F045-092, via inhalation delivery, can be evaluated as a potent antiviral agent against both H1N1 and H3N2 seasonal IAVs. To study its biodistribution and pharmacokinetics upon intranasal delivery in mice, we labeled IgM-F045-092 with Alexa Fluor 750 dye (IgM-750; Supplementary Fig. 7a) and intranasally administered to both nostrils of the mice (Fig. 6a). Whole-body fluorescence imaging demonstrated a high concentration of IgM-750 present and persistent in the nasal cavity and the lungs (Fig. 6b,d and Supplementary Fig. 7b). Using ex vivo organ imaging, IgM-750 was found to be enriched in the lungs that lasted for at least 168 h (Fig. 6c,e and Supplementary Fig. 7c, e), and minimal signals were observed in the other organs collected including blood, liver, spleen, kidney and the heart, suggesting localization towards the major sites associated with intranasal administration and influenza infection22. The presence of nasally administered IgM-F045-092 in the lungs of mice after 24 h was also confirmed by immunofluorescence staining using Alexa Fluor 488-conjugated goat anti-human IgA/IgG/IgM antibody (Supplementary Fig. 7d). This confirms the presence of intact IgM-F045-092 conjugated with Alexa Fluor 750 which justifies the bioavailability of the IgM antibodies. We did not include IgG F045-092 as a comparison in this study, considering no significant differences were observed between the biodistribution pattern of the two antibody modalities when given intranasally in mice in our previous work21.
a Female CD-1 mice (n = 4–5 mice per group) were intranasally administered with IgM-F045-092 antibody conjugated with Alexa Fluor 750 (1.2 mg/kg) and monitored for biodistribution (Created in BioRender; https://BioRender.com/fvt7dkf). b Real-time whole-body images of mice set in dorsal position. c Representative ex vivo organ images of mice taken at different time points demonstrating the spread and retention of antibody in different organs over time. Bl blood (20 µL), Br brain, H heart, K kidney, Lu lung, Lv liver, N nasal cavity, S spleen. d Representative 3D fluorescence/CT images of mice demonstrating the distribution of IgM-750 at 1 h and 48 h after intranasal administration. e Quantification of fluorescent signals detected in the nasal cavity and lungs determined by the average radiant efficiency. Dashed line shows the average autofluorescence of organs. Data are mean ± SD of four independent mice. Source data are provided as a Source Data file.
Protection against lethal challenge in mice by IgM-F045-092 administered intranasally
Next, we evaluated the in vivo protective efficacy of prophylactically administered IgM-F045-092 antibody and its parental IgG-F045-092 in a BALB/c mouse model of lethal influenza infection. Mice were first treated with either IgG-F045-092 or IgM-F045-092 antibody at 1 mg kg−1 dose 6 h before challenge with a lethal dose of mouse-adapted PR/34 (H1N1) or A/Hong Kong/1/1968 (HK/68; H3N2). For comparison, we included mice that were administered the same dose of control IgM (vehicle), and mice that were administered with IgM-F045-092 but not challenged (unchallenged). Body weights, and clinical signs of illness were monitored daily for the duration of the study (Fig. 7a). Intranasal administration of F045-092 conferred partial protection from weight loss and infection in mice challenged with PR/34 (Fig. 7b, c, d), with all surviving mice demonstrating significant weight gain from day 8 indicating recovery from infection. Two mice from both the IgG- and IgM-F045-092 administered group succumbed to infection indicating similar effectiveness of both antibody formats. However, mice administered with IgM-F045-092 demonstrated reduced morbidity and delayed mortality with overall improved clinical symptoms. Complete sterilizing protection from HK/68 was observed in all treated mice with no observed clinical complications (Fig. 7e, f, g). No significant increase in protective efficacy was observed by using the IgM format over IgG in vivo against either challenge strain, but it is important to note that the superiority of a testing molecule cannot be determined in a mouse model of infection based on survival alone.
a Experimental design of IgM-F045-092 or IgG-F045-092 against mouse adapted H1N1 and H3N2 IAV infection (Created in BioRender; https://BioRender.com/fvt7dkf). b, e Percentage of body weight change observed in mice (n = 8 mice per group) after intranasal administrations of 1 mg kg−1 per dose of IgM-F045-092, IgG-F045-092, or IgM-Vehicle control followed by intranasal challenge of PR/34 (b; Set 1) or HK/68 (e, Set 2). Kaplan–Meier plot representing the mean percentage of surviving mice for Set 1 (c) or Set 2 (f). Clinical scoring of mice treated with IgM-F045-092, IgG-F045-092, or IgM-Vehicle control after challenge with PR/34 (d) or HK/68 (g). # denotes the number of mice that were euthanized due to an observed body weight loss of >20%. Data are presented as mean values ± SEM. Two-sided Mann–Whitney test was used to compare IgM-F045-092 and IgM-Vehicle control groups for (d) with letters indicating statistical significance. A, p = 0.002331 and B, p = 0.000155. Source data are provided as a Source Data file.
The in vivo evaluation used in this study serves as a tool for the confirmation of IgM-F045-092 as a therapeutic regimen, and it has no statistical power to differentiate between the different modalities. Further dose de-escalation studies with virus titration from sites of infection might be required to identify any potential differences between the two antibody modalities in an in vivo setting. This data is similar to other published work which demonstrate in vivo efficacy for IgG to other multimeric formats (IgA, IgM)51,52. Validating the IgM modality over IgG is subjective considering the inaccuracies associated with mouse-adaptation of IAVs. We strongly believe that our two different in vitro functional studies demonstrate significant avidity-based protective enhancement upon IgM conversion that will be translatable to clinical settings53,54,55.
Discussion
Seasonal vaccination is still widely implemented as the most effective means of mitigating the severity and spread of influenza infections. However, the constantly changing antigenic landscape hampers the development of effective vaccine-induced immunity beyond the effective range of antigenic distance. Waning immunity and/or poor mucosal immunity in infants and older people makes them highly susceptible to severe infection as these individuals cannot mount sufficient immune responses. Recent findings have demonstrated that anti-influenza antibodies generated by the elderly often target conserved but less potent epitopes on the HA protein, further demonstrating the inability to elicit an effective protective response, especially against novel influenza strains56. One of the central approaches to universal vaccine is focused on developing broadly neutralizing antibodies against conserved epitopes of the IAV HA and several candidates are at different stages of clinical trials57,58,59,60,61. Broadly neutralizing antibodies against IAVs primarily target the RBS, vestigial esterase domain, the HA stem, or the NA head domain17. Virus entry into cells is directly inhibited by antibodies targeting the RBS domain, which is often considered the most potent mode for inhibiting entry. Stem-targeting antibodies are the most broadly neutralizing with the breadth of activity observed across subtypes at the cost of neutralizing potency. With the identification of different anti-influenza antibody types, therapeutic administration of monoclonal antibodies has been considered as a suitable means to supplement current vaccination strategies to reduce the burden associated with influenza infections.
This study explored whether enhancing antibody valency would boost neutralizing profiles of known anti-IAV antibodies that bind to different epitopes on the HA. We identified, constructed, and evaluated 18 broadly neutralizing IgG1 antibodies with known reactivity profiles from published sources. Using our multimerization platform, we constructed pentameric IgM antibodies from their parental IgG1 sequences. We tested their binding and neutralizing activity against a panel of H1N1 and H3N2 IAVs representing a broad HA antigenic landscape spanning over 5 to 8 decades. Our results showed that an increased valency offered by IgM significantly increased the binding affinity, overall receptor blocking capability, and enhanced neutralization potency providing a suitable platform to develop broad coverage against seasonal IAV infections. These characteristics are particularly evident among RBS-targeting antibodies such as 5J8, 641 I-9, and F045-092 compared to stem-specific antibodies where the breadth is retained with limited enhancement to potency.
It is important to note that IAV-specific antibodies have been known to inhibit viral entry or release by means of HA crosslinking62. The epitope location determines the antibody binding geometry in two patterns termed cis- or trans-crosslinking. Crosslinking between antigens of the same membrane leads to cis binding pattern that disrupts HA protein diffusion. This mode of activity is typical for stem-specific antibodies as the membrane-proximal stem restricts its access to adjacent surface proteins. It has been demonstrated by other groups that the bivalent antibody IgG-FI6v3 could effectively cis-crosslink the trimeric viral surface proteins and inhibit the viral release significantly compared to its monovalent form (Fab-FI6v3). On the other hand, RBS-specific antibodies S139/1 and C05 favor trans-crosslinking by binding antigens across membrane to induce viral aggregation as the membrane-distal HA head domain could expose epitopes efficiently63,64.
In this study, RBS specific antibody, F045-092 displays trans-crosslinking pattern. By converting IgG to IgM further improved its trans-crosslinking potency that induced efficient neutralization. These results suggest the potential for F045-092 IgM to engage multiple HA molecules on a single virion or to crosslink HA molecules on different virions, owing to its multivalent binding capability. However, this study does not provide direct evidence for such crosslinking and would require further investigation. Previous work done by Saito and colleagues demonstrates that the recombinant tetrameric antibody SIgA constructs (secretory IgAs) of F045-092 enhanced the breadth of activity in vitro where the IgG antibody is known to bind at a lower affinity but will only have limited effect in terms of enhancing the peak neutralization potency51. However, our study showed that decavalent IgM-F045-092 demonstrated an improved target breadth and higher binding altogether, suggesting the advantage of using engineered multivalent IgM antibodies to prevent and block infection of antigenically drifted IAVs.
The mucosal layer of the lung epithelia contains numerous innate and adaptive immune functions to prevent viral infection52. These barriers are often sufficient to protect against severe infection but among the elderly and the immunocompromised, the sheer quantity of infecting virus particles might overwhelm, leading to an exacerbated infection. Furthermore, newly budding virions are released from the apical side of the infected lung cells, leading to further release and persistence of infection. Airway delivery of therapeutics has been proposed as a viable option for conferring protection against respiratory infections21,65. This simplified intranasal administration makes these engineered IgM antibodies appealing for preventing and treating IAV infections. We selected IgM-F045-092, which displayed significant neutralization potency and broader breadth coverage among all the IgM antibodies tested in vitro.
Our in vivo biodistribution study demonstrated that a single intranasally administered dose of IgM-F045-092 antibodies was well tolerated and retained in the nasal cavities and lungs for up to 168 h. Prophylactic administration conferred protection against lethal challenges, allowing host immune cells to clear infection effectively without further clinical complications. In our analysis, our model was not able to provide any differentiation between IgG and IgM antibody modality of F045-092, on the point of estimate on efficacy based on the change of mortality rate, or weight loss with statistical significance. As such, this study serves as a binary measure for assessing IgM modality and does not have the quantitative power or precision to differentiate or rank candidates, therapeutic regimens, or formulations. We can speculate that the desirable pharmacokinetic and safety profiles offered by IgM antibodies are comparable to IgG. Our in vitro functional data suggest that blocking virus attachment and entry into target cells would effectively prevent IAV infection and allow for immune cells to clear the infection before reaching significant severity.
These results, coupled with our structural findings highlight not only the avidity-based affinity enhancement, but also the importance of epitope binding region and trans-crosslinking pattern that play a critical role in conferring efficacy. Based on our structural results which show F045-092 crosslinking the multiple trimeric HAs, we can hypothesize that these IgMs may similarly function by crosslinking virus particles that may provide an added mechanistic advantage to ultrapotent antibodies effective in suppressing the initiation of infection. This could also provide sufficient time for further antigen processing by the immune system to generate potent antibodies and CD8 T cell response before the severity of infection increases. Current advancements in therapeutic antibody engineering have allowed the production of different antibody-based constructs with high efficiency. As such, with subsequent process optimization using stable cell line, we can achieve higher productivity (>1 g L−1) of engineered therapeutic IgM antibodies with high purity and stability. By engineering IgM antibody constructs of known broadly neutralizing antibodies, we provide an alternative approach for designing and administering these molecules via nasal delivery. The inherently “adhesive” nature of IgM antibodies provided by the J chain would act as a “molecular mask” in the mucosal lining that blocks or reduces the infectivity of circulating IAVs with favorable pharmacokinetics and safety profiles.
Methods
Cells, viruses, and proteins
Madin-Darby Canine Kidney (MDCK; ATCC: CCL-34) cells were cultured in DMEM media (Gibco) containing 10% FBS and 100 U mL−1 penicillin G, 100 μg mL−1 streptomycin (Gibco), and L-glutamine (2 mM) (Thermo) at 37 °C in a 5% CO2 incubator. EXPI293F cells (Gibco) were maintained in Expi293 expression medium without FBS. ExpiCHO-S cells (Thermo Fisher Scientific) were maintained in ExpiCHO expression medium without FBS. CHO cells (Horizon Discovery) were maintained in ex-cell advanced CHO fed-batch medium (Millipore-Sigma) supplemented with 4 mM L-glutamine.
All influenza viruses used in this study were obtained from BEI Resources and IRR. List of viruses H1N1 viruses are A/Puerto Rico/8/1934, A/Beijing/262/1995, A/New Caledonia/20/1999, A/Solomon Islands/3/2006, A/Brisbane/59/2007, A/California/04/2009, A/Georgia/T51700/2012 pdm09, A/Michigan/45/2015 A/Wisconsin/505/2018, A/Hawaii/70/2019, A/Indiana/02/2020, A/Sydney/5/2021 and H3N2 strains are A/Aichi/2/1968, A/Nanchang/933/1995, A/Sydney/5/1997, A/Netherlands/22/2003, A/Brisbane/10/2007, A/Victoria/361/2011, A/Texas/50/2012, A/Singapore/INFIMH-16-0019/2016, A/Kansas/08/2017, A/Hong Kong/45/2019, A/Tasmania/503/2020, A/Delaware/01/2021, A/Darwin/06/2021 strains. Viruses were propagated in MDCK cells as previously described66 in DMEM media with 1% BSA, 1% HEPES, 100 U;mL−1 penicillin G and 100 μg mL−1 streptomycin, and 2 µg mL−1 of TPCK-treated trypsin (Sigma). HA titers were determined immediately upon harvesting the viruses from the cell culture supernatant and were subsequently passaged if the titers were lower than required for the assays performed. Viruses were stored in −80 °C immediately.
The recombinant HA proteins with His-tag used in this study are purchased from Sino Biological. For H1N1, A/Puerto Rico/8/1934, A/Denver/JY2/1957, A/Taiwan/01/1986, A/Beijing/262/1995, A/New Caledonia/20/1999, A/Solomon Islands/3/2006, A/California/04/2009 pdm09, A/Texas/05/2009, A/Michigan/45/2015 were used. For H3N2 specific HA proteins, A/Aichi/2/1968, A/Sydney/5/1997, A/Fujian/411/2002, A/California/07/2004, A/Wisconsin/67/2005, A/Ohio/14/2008, A/Victoria/208/2009, A/Singapore/INFIMH-16-0019/2016A/Hong Kong/45/2019 and A/Cambodia/e0826360/2020 were used. In addition, recombinant HA protein of A/Darwin/6/2021 with His-tag was purchased from ACRObiosystems. Complete names of abbreviations used for IAV strains or HA proteins are shown in Supplementary Data 6.
The trimeric HA proteins of A/Solomon Islands/3/2006 (H1N1), A/Hong Kong/1/1968 (H3N2), and A/Victoria/361/2011 (H3N2), used for structural analyses were kindly donated from Vaccine Research Center (NIH).
Sequence and structural visualization
The sequences of all above-mentioned H1N1 and H3N2 were retrieved from GSIAID database and the HA amino acid sequences were aligned using MUSCLE67. Antigenic covariance were measured with amino acid sequences as we wanted to focus on the expressed protein mutations rather than the nucleotide sequences which are prone to more mutations with limited change in the amino acid landscape. The phylogenetic tree was created using Maximum Likelihood method with JTT matrix-based model of 1000 bootstrap replicates in MEGA 11.0.13. The antigenic regions and binding domains of the IAV HA trimer proteins were identified, superimposed onto A/Puerto Rico/8/1934 (PDB 1RU7)26 for H1 and A/Aichi/2/1968 (PDB 3VUN) for H3 and visualized using UCSF ChimeraX.
Selection of broadly neutralizing IgG antibodies
Anti-influenza monoclonal antibodies with known H1N1, H3N2, and heterosubtypic broadly neutralizing activity were selected and its respective variable regions were retrieved from the published sources. The antibodies used in this study are categorized into HA-head-specific antibodies namely 5J8 (4M5Z)47,68, 641 I-9 (4YK4)69, CH65 (5UGY)70, CH67 (4HKX)71,72, CL6649 (5W6G)73, H2526 (4YJZ)69, HC45 (1QFU)44,74, F005-126 (3WHE)45, C585 (6PDX)75, S139/1 (4GMS)76, C05 (4FP8)48, K03.12 (5W08)77, FLUA-20 (6OCB)43, F045-092 (4O5I)78,79 and HA-stem specific antibodies CR6261 (3GBN)80,81, CR8020 (3SDY)82, FI6V3 (3ZTJ)83 and MEDI8852 (5JW3)84. The respective PDB codes are mentioned in parenthesis. Crystal structures of the antibodies are retrieved from the PDB sources and the epitope-paratope interaction regions were analyzed using ePISA database85. The binding regions were superimposed and visualized on the structure of the H1 HA trimer onto A/California/04/2009 (PDB 3LZG) for H1 specific antibodies and H3 HA trimer onto A/Aichi/2/1968 (PDB 3VUN) for H3 specific and heterosubtypic antibodies using UCSF ChimeraX.
Engineering and production of IgG and IgM antibodies
The DNA sequences of the VH and Vκ/λ were retrieved from published sources and the corresponding genes of heavy and light chains were synthesized and inserted into their corresponding IgG1 heavy and light chain backbones by In-Fusion cloning (Takara Bio). The plasmids were co-transfected into Expi293F cells and cultured for 1 week. The antibodies secreted into the supernatants were purified using Protein A resin (Genscript) and reconstituted in PBS. Further, the expression yield was quantified using nanodrop and the purity measured by analytical HPLC and SDS-PAGE. The respective yields of each IgG antibody were mentioned in Supplementary Data 7. We synthesized IgM antibodies containing the Fab2 arm of the parental IgG1 antibody into the IgM scaffold. IgM heavy chain constant regions contain one major sequence (Cµ1, Cµ2, Cµ3, and Cµ4 with tailpiece; Accession #: S37768). The constant regions of all IgMs used in this study were wildtype (WT) sequences with WT J chain. The J chain has one sequence (Accession #: ABI63362) and the light chains used were the same kappa or lambda chains as the IgG1 antibodies. Engineering and production of IgM antibodies were carried out as previously described21,86. IgM antibody constructs were purified by mixed-mode chromatography and anion-exchange chromatography. The titer for recombinant IgM transient transfection is in the range of 100–200 mg L−1. For SDS–PAGE, all antibodies were run on 10% Mini-PROTEAN TGX gels (Bio-Rad). The gels were stained with Coomassie blue R-250 (Bio-Rad)87. To evaluate the purity, integrity and size of the IgG and IgM molecules, 10 μl of each antibody sample at the concentration of 1 mg ml−1 were injected into the MAbPac™ SEC-1 Size Exclusion Chromatography HPLC Column and were analyzed using Chromelon 7.
Biolayer Interferometry
Binding affinities of constructed IgGs to recombinant HA proteins were confirmed using the ForteBio Octet RED96 system. The constructed IgG antibodies were captured on Protein A biosensor for 60 s at the concentration of 5 μg ml−1 diluted in 1× KB buffer and we then tested against a panel of IAV H1N1 and H3N2 recombinant HA proteins in a dose dependent manner ranging 25 μg ml−1 or 12.5 μg ml−1 respectively. Both the association and dissociation kinetics were recorded for 300 s. ForteBio Octet Data Analysis software was used to fit the KD data using the global fitting method.
The measurement of antibody avidity to recombinant HA proteins was performed on the ForteBio Octet RED96 system. His-tagged HA proteins (15 μg ml−1) were captured on the Ni-NTA biosensor (Sartorius). Following 30 s of baseline run in kinetics buffer, the sensors were dipped in threefold serially diluted Fab, IgG1, and IgM antibodies (0.18–44 nM) for 200 s to record association kinetics. Then, the sensors were dipped into kinetics buffer for 200 s to record dissociation kinetics. ForteBio Octet Data Analysis software was used to fit the KD data using the global fitting method.
Hemagglutination inhibition (HAI) assay
HAI was performed based on the protocol available through the World Health Organization Manual for the laboratory diagnosis and virological surveillance of influenza88. Briefly, influenza viruses were diluted to 8 HA units in 50 µL of PBS and combined with an equal volume of two-fold serially diluted antibodies (starting concentration, 10 µg mL−1) and incubated for 1 h at room temperature. An equal volume of 0.5% Turkey red blood cells (RBCs) (Innovative Research) or 1% Guinea Pig RBCs (Innovative Research) were added to the wells and incubated for further 30–45 min at RT. Tear-drop formation upon tilting the plates at a 45° angle was scored as evidence of hemagglutination. Button formation without any tear-drop formation was also considered as positive for hemagglutination. All experiments were carried out in duplicates and performed twice for statistical significance. Turkey RBCs were used to titer all H1N1 viruses and H3N2 viruses until 2007. Guinea pig RBCs were used to titrate against more contemporary H3N2 IAVs due to a reduced HA activity against avian-origin RBCs. The inhibition titers were calculated determined as the lowest antibody concentration negative for hemagglutination89.
Microneutralization assays
Influenza virus neutralization was determined using cell-based MN assay as described previously90. MDCK cells seeded in 96-well plates at a concentration of 2.5 × 104 cells/well overnight. Two-fold dilutions of antibodies in DMEM containing TPCK-treated trypsin (starting concentration, 20 µg mL−1) were mixed with 100 TCID50 of viruses and incubated for 1 h at room temperature. Cells were then infected with the antibody-virus mixture and incubated at 37 °C for 1 h. After inoculum removal, antibody dilutions were added to the plates and further incubated for 72 h at 37 °C in DMEM media containing TPCK-treated trypsin.
For the HA test, an equal volume of 0.5% Turkey RBCs or 1% Guinea Pig RBCs were added to the wells and incubated for further 30–45 min at RT. Tear-drop formation upon tilting the plates at a 45° angle was scored as evidence of hemagglutination. Button formation without any tear-drop formation was also considered as positive for hemagglutination. All experiments were carried out in duplicates and performed twice for statistical significance.
ELISA binding assay
Antibody binding to recombinant HA proteins was evaluated using ELISA as follows. High-binding 96-well clear polystyrene ELISA plates (Pierce 15042) were coated with 100 μl per well of 1 μg ml−1 recombinant His-tagged HA protein overnight at 4 °C followed by blocking with 5% non-fat milk at the volume of 300 μl per well and kept at room temperature for 1 h. Fab-, IgG-, and IgM-F045-092 were serially diluted in PBS from (10–0.00016 μg ml−1) and incubated at 37 °C for 2 h. The plates were then washed thrice with PBST and PBS and incubated with HRP-conjugated F(ab′)2 fragment goat anti-human IgA + IgG + IgM (H + L) antibody (Jackson ImmunoResearch) for 1 h. Plates were then washed thrice with PBST and PBS. TMB (3,3′,5,5′-tetramethylbenzidine) substrate was added at 100 μl per well for color development. The reaction was stopped by adding 50 μl per well 2 M H2SO4. The OD450 nm was read by a SpectraMax microplate reader and analyzed with GraphPad Prism 10.
Negative-staining electron microscopy analysis for the structure of Fab- and IgM-HA complex
Fab and IgM of F045-092 antibody and IAV HA trimers were mixed together with either a 2:1 molar ratio of Fab to HA, or an equal molar ratio of HA to IgM, and diluted in EM dilution buffer (10 mM HEPES, pH 7.4, 150 mM NaCl) and allowed to adsorb on a fresh glow-discharged, carbon-coated copper grid, and stained with 0.75% uranyl formate. Images were collected at a magnification of 57,000 using Serial EM on a Tecnai TF20 microscope equipped with a 2 × 2k CMOS camera and operated at 200 kV. The pixel size was 3.69 Å for the camera. Particle picking, reference-free 2D classification, 3D reconstruction, and refinement were performed using cryoSPARC. Structural analysis and illustration were performed by using pyMOL and Chimera.
Tracking intra-nasal administered IgM antibody bio-distribution in mice
IgM-F045-092 antibodies were conjugated with Alexa Fluor 750 dye for in vivo imaging studies and monitored for their biodistribution and retention in mice upon intranasal (IN) administration. Two milligrams per milliliter solution of IgM-F045-092 in PBS (pH 7.4) was reacted with Alexa Fluor 750 succinimidyl ester (AF750-NHS, Thermo Fisher Scientific) in the presence of 3% DMSO and 10% sodium bicarbonate buffer (v/v, pH 8.3) using the molar ratio of 1:10 antibody to fluorescent probe at room temperature for 1 h. The labeled F045-092-AF750 antibody was purified by dialysis, Zeba spin desalting column (MWKO 7 kDa), and concentrated with an Amicon ultra centrifugal unit (MWKO 3 kDa).
Mice studies were carried out following approval of animal procedures by University of Houston Institutional Animal Care and Use Committee (IACUC; Protocol #16-041). Female CD-1 mice (6–8 weeks old, n = 4–5 mice per group) were anesthetized with 2% isoflurane inhalation and positioned in a supine position. Alexa Fluor 750-labeled IgM was intranasally administered to both nostrils of the mice using a fine pipette tip to achieve a final antibody dose of 1.2 mg kg−1. All mice were housed under ABSL-1 conditions. The in vivo near-infrared fluorescence was monitored at predetermined time points (5 min, 30 min, 1, 2, 4, 6, 9, 24, 48, 72, 96, 120, 144, and 168 h) after a single dose of administration using an IVIS Spectrum CT imager preprogrammed with Ex = 745 nm, Em = 800 nm, and auto-exposure settings. 3D fluorescence imaging with computed tomography (CT) was acquired at 1 h and 48 h and co-registered. Upon euthanasia, 20 μl of blood, the heart, lung, liver, spleen, kidney, brain, and nasal cavity samples were excised and imaged. Regions of interest (ROIs) were drawn and average radiant efficiency [(p s−1 cm−2 sr−1)/(μW cm−2)] was measured. This parameter represents the sum of the radiance from each pixel inside the ROI divided by the number of pixels. All images were processed using Living Image software (Perkin Elmer) and the same fluorescence threshold was applied for group comparison. Frozen lung tissue sections (20 µm) from 24 h post treatment with IgM-750 were fixed with 4% paraformaldehyde, blocked with 5% BSA, and incubated with Alexa Fluor® 488-conjugated AffiniPure™ F(ab′)2 fragment goat anti-Human IgA + IgG + IgM (H + L) (Jackson ImmunoResearch, 1:250 dilution) for 1 h at room temperature. Lung tissue sections from untreated mice were used as control. After counterstaining with DAPI, the slides were imaged using Nikon confocal microscopy.
In vivo efficacy studies
The evaluation of prophylactic efficacy of IgG-and IgM-F045-092 in a BALB/c mouse model of lethal IAV infection was evaluated at Bioqual Inc. (Maryland) following approval of animal procedures by the Testing Facility’s IACUC (Protocol # 23-101). Eight- to ten-week-old female mice were purchased from Envigo (Indiana) and maintained in ABSL-2 facilities throughout. The A/Puerto Rico/8/1934 (H1N1) challenge was generated from seed stock (Lot #: NR348; BEI Resources) and expanded at BIOQUAL (Lot #: 071515-1415). The stock titer was reported to be 1010.45 TCID50 mL−1. The A/Hong Kong/1/68 ×31 (H3N2) challenge was generated from seed stock (Lot #: MD-4/2/22/02, NVX) expanded at BIOQUAL (BIOQUAL Lot #: 062614-1147). The stock titer was reported to be 1010.54 TCID50 mL−1. Viruses were stored at −80 °C until used for challenging mice. Six hours prior to challenge on day 0, mice (n = 8 mice per group) were anesthetized via IP injection with 80 mg kg−1 of ketamine and 5 mg kg−1 xylazine and intranasally administered (25 µL per nostril) with IgM-F045-092, IgG-F045-092, or IgM-Vehicle control (IgM containing the same backbone with unrelated Fab region) at 1 mg kg−1 per mouse. At the time of challenge, mice were anesthetized as above, and inoculated with lethal doses of PR/34 (challenge dose: 4.27 × 103 TCID50), HK/68 (5.74 × 103 TCID50), or PBS control. During administration of antibodies and challenge inoculum, animals were held upright such that the nostrils of the animals were pointed toward the ceiling; the animals’ heads were kept tilted back in this position for ~20 s following administration. The animals were then returned to their housing units and observed until fully recovered from anesthesia. Body weights were measured daily, and clinical signs of infection were recorded twice per day during the study duration. All mice were euthanized on day 14. A body weight loss of ≥20% and survival for the duration of the study (0–14 days) were used as major end points.
Statistical analysis
All statistical analysis was performed using GraphPad Prism 10 and statistical tests are described in the indicated figure legends. Nonlinear regression curve fitting was performed to calculate the EC50 values.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data associated with this study are provided in the Manuscript and Supplementary Materials. Source data are provided with this paper.
References
Caini, S. et al. Distribution of influenza virus types by age using case-based global surveillance data from twenty-nine countries, 1999-2014. BMC Infect. Dis. 18, 269 (2018).
Cromer, D. et al. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J. Infect. 68, 363–371 (2014).
Wu, Y., Wu, Y., Tefsen, B., Shi, Y. & Gao, G. F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 22, 183–191 (2014).
Tong, S. et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 9, e1003657 (2013).
McAuley, J. L., Gilbertson, B. P., Trifkovic, S., Brown, L. E. & McKimm-Breschkin, J. L. Influenza virus neuraminidase structure and functions. Front. Microbiol. 10, 39 (2019).
Sriwilaijaroen, N. & Suzuki, Y. Molecular basis of the structure and function of H1 hemagglutinin of influenza virus. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 88, 226–249 (2012).
Rogers, G. N. & D’Souza, B. L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology 173, 317–322 (1989).
Rogers, G. N. & Paulson, J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127, 361–373 (1983).
Pauly, M. D., Procario, M. C. & Lauring, A. S. A novel twelve class fluctuation test reveals higher than expected mutation rates for influenza A viruses. Elife 6, https://doi.org/10.7554/eLife.26437 (2017).
Nobusawa, E. & Sato, K. Comparison of the mutation rates of human influenza A and B viruses. J. Virol. 80, 3675–3678 (2006).
McDonald, N. J., Smith, C. B. & Cox, N. J. Antigenic drift in the evolution of H1N1 influenza A viruses resulting from deletion of a single amino acid in the haemagglutinin gene. J. Gen. Virol. 88, 3209–3213 (2007).
Laursen, N. S. & Wilson, I. A. Broadly neutralizing antibodies against influenza viruses. Antivir. Res. 98, 476–483 (2013).
Guthmiller, J. J. et al. Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 602, 314–320 (2022).
Ekiert, D. C. & Wilson, I. A. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr. Opin. Virol. 2, 134–141 (2012).
Sun, X. et al. Broadly neutralizing antibodies to combat influenza virus infection. Antivir. Res. 221, 105785 (2024).
Biswas, M., Yamazaki, T., Chiba, J. & Akashi-Takamura, S. Broadly neutralizing antibodies for influenza: passive immunotherapy and intranasal vaccination. Vaccines 8, https://doi.org/10.3390/vaccines8030424 (2020).
Jiao, C., Wang, B., Chen, P., Jiang, Y. & Liu, J. Analysis of the conserved protective epitopes of hemagglutinin on influenza A viruses. Front Immunol. 14, 1086297 (2023).
Nachbagauer, R. et al. Defining the antibody cross-reactome directed against the influenza virus surface glycoproteins. Nat. Immunol. 18, 464–473 (2017).
Friesen, R. H. et al. New class of monoclonal antibodies against severe influenza: prophylactic and therapeutic efficacy in ferrets. PLoS ONE 5, e9106 (2010).
Tharakaraman, K., Subramanian, V., Cain, D., Sasisekharan, V. & Sasisekharan, R. Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure. Cell Host Microbe 15, 644–651 (2014).
Ku, Z. et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature 595, 718–723 (2021).
Shinya, K. et al. Influenza virus receptors in the human airway. Nature 440, 435–436 (2006).
Couceiro, J. N. S. S., Paulson, J. C. & Baum, L. G. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 29, 155–165 (1993).
Gerhard, W., Yewdell, J., Frankel, M. E. & Webster, R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 290, 713–717 (1981).
Caton, A. J., Brownlee, G. G., Yewdell, J. W. & Gerhard, W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31, 417–427 (1982).
Gamblin, S. J. et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303, 1838–1842 (2004).
Wiley, D. C., Wilson, I. A. & Skehel, J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 289, 373–378 (1981).
Wilson, I. A., Skehel, J. J. & Wiley, D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 289 366–373 (1981).
Ye, J., Shao, H. & Perez, D. R. Passive immune neutralization strategies for prevention and control of influenza A infections. Immunotherapy 4, 175–186 (2012).
Varecková, E., Mucha, V., Wharton, S. A. & Kostolanský, F. Inhibition of fusion activity of influenza A haemagglutinin mediated by HA2-specific monoclonal antibodies. Arch Virol. 148, 469–486 (2003).
Sun, X., Ling, Z., Yang, Z. & Sun, B. Broad neutralizing antibody-based strategies to tackle influenza. Curr. Opin. Virol. 53, 101207 (2022).
Ghafoori, S. M. et al. Structural characterisation of hemagglutinin from seven Influenza A H1N1 strains reveal diversity in the C05 antibody recognition site. Sci. Rep. 13, 6940 (2023).
Swanson, N. J. et al. 2019-2020 H1N1 clade A5a.1 viruses have better in vitro fitness compared with the co-circulating A5a.2 clade. Sci. Rep. 13, 10223 (2023).
Visher, E., Whitefield, S. E., McCrone, J. T., Fitzsimmons, W. & Lauring, A. S. The mutational robustness of influenza A Virus. PLOS Pathog. 12, e1005856 (2016).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Creanga, A. et al. A comprehensive influenza reporter virus panel for high-throughput deep profiling of neutralizing antibodies. Nat. Commun. 12, 1722 (2021).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Zacour, M. et al. Standardization of hemagglutination inhibition assay for influenza serology allows for high reproducibility between laboratories. Clin. Vaccin. Immunol. 23, 236–242 (2016).
Reber, A. & Katz, J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Rev. Vaccines 12, 519–536 (2013).
Chen, Y. Q., Lan, L. Y., Huang, M., Henry, C. & Wilson, P. C. Hemagglutinin stalk-reactive antibodies interfere with influenza virus neuraminidase activity by steric hindrance. J. Virol. 93, https://doi.org/10.1128/JVI.01526-18 (2019).
Bangaru, S. et al. A site of vulnerability on the influenza virus hemagglutinin head domain trimer interface. Cell 177, 1136–1152.e1118 (2019).
Fleury, D. et al. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site. Nat. Struct. Biol. 6, 530–534 (1999).
Iba, Y. et al. Conserved neutralizing epitope at globular head of hemagglutinin in H3N2 influenza viruses. J. Virol. 88, 7130–7144 (2014).
Hale, M. et al. IgM antibodies derived from memory B cells are potent cross-variant neutralizers of SARS-CoV-2. J. Exp. Med. 219, https://doi.org/10.1084/jem.20220849 (2022).
Hong, M. et al. Antibody recognition of the pandemic H1N1 Influenza virus hemagglutinin receptor binding site. J. Virol. 87, 12471–12480 (2013).
Ekiert, D. C. et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489, 526–532 (2012).
Lee, P. S. et al. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc. Natl. Acad. Sci. USA 109, 17040–17045 (2012).
Simmons, H. C. et al. A new class of antibodies that overcomes a steric barrier to cross-group neutralization of influenza viruses. PLoS Biol. 21, e3002415 (2023).
Saito, S. et al. IgA tetramerization improves target breadth but not peak potency of functionality of anti-influenza virus broadly neutralizing antibody. PLoS Pathog. 15, e1007427 (2019).
Shen, C. et al. An IgM antibody targeting the receptor binding site of influenza B blocks viral infection with great breadth and potency. Theranostics 9, 210–231 (2019).
Verschoor, C. P. et al. Microneutralization assay titres correlate with protection against seasonal influenza H1N1 and H3N2 in children. PLoS ONE 10, e0131531 (2015).
Ilyushina, N. A. et al. Adaptation of pandemic H1N1 influenza viruses in mice. J. Virol. 84, 8607–8616 (2010).
Choi, E.-J. et al. The effect of mutations derived from mouse-adapted H3N2 seasonal influenza A virus to pathogenicity and host adaptation. PLoS ONE 15, e0227516 (2020).
Henry, C. et al. Influenza virus vaccination elicits poorly adapted B cell responses in elderly individuals. Cell Host Microbe 25, 357–366.e356 (2019).
Koszalka, P., Subbarao, K. & Baz, M. Preclinical and clinical developments for combination treatment of influenza. PLoS Pathog. 18, e1010481 (2022).
Lim, J. J. et al. A phase 2 randomized,https://doi.org/10.1093/ofid/ofab630 double-blind, placebo-controlled trial of MHAA4549A, a monoclonal antibody, plus oseltamivir in patients hospitalized with severe influenza A virus infection. Antimicrob. Agents Chemother. 64, https://doi.org/10.1128/aac.00352-20 (2020).
Vanderven, H. A. et al. Understanding the treatment benefit of hyperimmune anti-influenza intravenous immunoglobulin (Flu-IVIG) for severe human influenza. JCI Insight 8, https://doi.org/10.1172/jci.insight.167464 (2023).
Lim, J. J. et al. A phase 2 randomized, double-blind, placebo-controlled trial of the monoclonal antibody MHAA4549A in patients with acute uncomplicated influenza A infection. Open Forum Infect. Dis. 9, https://doi.org/10.1093/ofid/ofab630 (2021).
Ali, S. O. et al. Evaluation of MEDI8852, an anti-influenza a monoclonal antibody, in treating acute uncomplicated influenza. Antimicrob. Agents Chemother. 62, https://doi.org/10.1128/AAC.00694-18 (2018).
Wu, Y. et al. A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat. Commun. 6, 7708 (2015).
He, Y., Guo, Z., Subiaur, S., Benegal, A. & Vahey, M. D. Antibody inhibition of influenza A virus assembly and release. J. Virol. e0139823 https://doi.org/10.1128/jvi.01398-23 (2024).
Williams, J. A., Gui, L., Hom, N., Mileant, A. & Lee, K. K. Dissection of epitope-specific mechanisms of neutralization of influenza virus by intact IgG and Fab fragments. J. Virol. 92, https://doi.org/10.1128/JVI.02006-17 (2018).
Vigil, A., Frias-Staheli, N., Carabeo, T. & Wittekind, M. Airway delivery of anti-influenza monoclonal antibodies results in enhanced antiviral activities and enables broad-coverage combination therapies. J. Virol. 94, https://doi.org/10.1128/JVI.00052-20 (2020).
Karakus, U., Crameri, M., Lanz, C. & Yanguez, E. Propagation and titration of influenza viruses. Methods Mol. Biol. 1836, 59–88 (2018).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Krause, J. C. et al. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J. Virol. 85, 10905–10908 (2011).
Schmidt, AaronG. et al. Viral receptor-binding site antibodies with diverse germline origins. Cell 161, 1026–1034 (2015).
Whittle, J. R. R. et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 108, 14216–14221 (2011).
Xu, H. et al. Key mutations stabilize antigen-binding conformation during affinity maturation of a broadly neutralizing influenza antibody lineage. Proteins 83, 771–780 (2015).
Schmidt, A. G. et al. Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc. Natl. Acad. Sci. USA 110, 264–269 (2013).
Raymond, D. D. et al. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl. Acad. Sci. USA 115, 168–173 (2018).
Gigant, B., Fleury, D., Bizebard, T., Skehel, J. J. & Knossow, M. Crystallization and preliminary X-ray diffraction studies of complexes between an influenza hemagglutinin and Fab fragments of two different monoclonal antibodies. Proteins 23, 115–117 (1995).
Qiu, Y. et al. Mapping of a novel H3-specific broadly neutralizing monoclonal antibody targeting the hemagglutinin globular head isolated from an elite influenza virus-immunized donor exhibiting serological breadth. J. Virol. 94, https://doi.org/10.1128/jvi.01035-19 (2020).
Yoshida, R. et al. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLOS Pathog. 5, e1000350 (2009).
McCarthy, K. R. et al. Memory B cells that cross-react with group 1 and Group 2 influenza A viruses are abundant in adult human repertoires. Immunity 48, 174–184.e179 (2018).
Ohshima, N. et al. Naturally occurring antibodies in humans can neutralize a variety of influenza virus strains, including H3, H1, H2, and H5. J. Virol. 85, 11048–11057 (2011).
Lee, P. S. et al. Receptor mimicry by antibody F045-092 facilitates universal binding to the H3 subtype of influenza virus. Nat. Commun. 5, 3614 (2014).
Throsby, M. et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B Cells. PLoS ONE 3, e3942 (2008).
Ekiert, D. C. et al. Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009).
Ekiert, D. C. et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333, 843–850 (2011).
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A Hemagglutinins. Science 333, 850–856 (2011).
Kallewaard, N. L. et al. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166, 596–608 (2016).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Keyt, B. A., Baliga, R., Sinclair, A. M., Carroll, S. F. & Peterson, M. S. Structure, function, and therapeutic use of IgM antibodies. Antibodies 9, https://doi.org/10.3390/antib9040053 (2020).
Vorauer-Uhl, K., Wallner, J., Lhota, G., Katinger, H. & Kunert, R. IgM characterization directly performed in crude culture supernatants by a new simple electrophoretic method. J. Immunol. Methods 359, 21–27 (2010).
World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza (World Health Organization, 2011).
Broszeit, F. et al. Glycan remodeled erythrocytes facilitate antigenic characterization of recent A/H3N2 influenza viruses. Nat. Commun. 12, 5449 (2021).
Cuevas, F., Kawabata, H., Krammer, F. & Carreno, J. M. Correction: an in vitro microneutralization assay for influenza virus serology. Curr. Protoc. 2, e567 (2022).
Acknowledgements
This work was supported in part by grants from IGM Biosciences and the Welch Foundation (AU-0042-2003061 to Z.A.), as well as from the NIH (U01-AI173348 and UH2-AI171611 to K.X.). The authors would like to thank Hui Deng for her services in maintaining and preparing the cells for antibody expression, Dr. Xiong Wei for his input in designing and expressing recombinant IgG antibodies used in this study, and Dr. Giovanna Grandinetti at The Ohio State University for her assistance in EM data collection. Electron microscopy was performed at the Center for Electron Microscopy and Analysis (CEMAS) at The Ohio State University. Molecular graphics and analyses performed with UCSF ChimeraX, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from National Institutes of Health R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Contributions
A.K.R., J.P.S., X.Y., and H.G. selected the influenza virus strains, recombinant proteins, and IgG1 antibodies. A.K.R., J.P.S., and X.Y. performed bioinformatics analysis of virus sequences. A.K.R. and J.P.S. carried out HA mapping and antibody structural visualization. X.Y. constructed the IgG1 clones. X.Y., J.P.S., transfected cell, purified and characterized IgG1 mAb binding avidities. J.P.S. performed SEC, ELISA EC50 assay. A.K.R. performed SDS-PAGE, comparative IgG, IgM, and Fab binding avidity. A.K.R. and J.P.S. performed cell and virus culture, blocking and neutralization assays. H.G., S.H. engineered the IgM mAbs. Y.X., H.D., T.Z., and K.X. performed structural studies. X.L. and L.Y. performed antibody biodistribution. H.G. and C.C. organized and facilitated in vivo challenge studies and compiled the report. N.Z., J.W.S., W.R.S., T.M.F., and Z.A. supervised the study. A.K.R. and J.P.S. wrote the original draft with input from the team. T.M.F. and Z.A. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.G., C.C., S.H., J.W.S., W.R.S., and T.M. are/were employees of IGM Biosciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ramesh, A.K., Sivaccumar, J.P., Ye, X. et al. An intranasally administered IgM protects against antigenically distinct subtypes of influenza A viruses. Nat Commun 16, 4025 (2025). https://doi.org/10.1038/s41467-025-59294-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59294-0