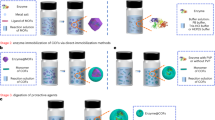
Abstract
Precisely regulating protein conformation (folding) for biomanufacturing and biomedicine is of great significance but remains challenging. In this work, we innovate a covalent organic framework (COF)-directed protein refolding strategy to modulate protein conformation by rationally designed covalent organic frameworks with adapted pore structures and customizable microenvironments. The conformation of denatured protein can be efficiently recovered through a simple one-step approach using covalent organic framework treatment in aqueous or buffer solutions. This strategy demonstrates high generality that can be applied to various proteins (for example, lysozyme, glucose oxidase, trypsin, nattokinase, and papain) and diverse covalent organic frameworks. An in-depth investigation of the refolding mechanism reveals that pore size and microenvironments such as hydrophobicity, π-π conjugation, and hydrogen bonding are critical to regulating protein conformation. Furthermore, we use this covalent organic framework platform to build up solid-phase columns for continuous protein recovery and achieved a ~ 100% refolding yield and excellent recycling performance (30 cycles), enabling an integrated process for the extracting and refolding denatured proteins (such as the harvest of protein in inclusion bodies). This study creates a highly efficient and customizable covalent organic framework platform for precisely regulating proteins refolding and enhancing their performance, opening up a new avenue for advanced protein manufacturing.
Similar content being viewed by others
Introduction
Proteins, the essential components of life, play pivotal roles in biological manufacturing and biomedicine1,2,3. Their structure and function are determined by multi-level structures4, and correct folding and conformation are crucial for maintaining function5. However, due to the structural complexity and fragile nature of proteins, misfolding often occurs, leading to protein denaturation and loss of activity6,7,8. For example, the production of recombinant proteins in inclusion bodies typically involves overexpression of the proteins9,10,11, often leading to their misfolding or denaturation. Therefore, properly refolding proteins is essential to harvest them and ensure productivity and activity12,13. Currently, strategies to address protein misfolding are very limited, mainly relying on refolding agents (e.g., surfactants, amino acids) or protein engineering14,15. These approaches often suffer from low yield, limited scalability, operational complexity, and time-consuming processes16,17,18. Traditional refolding techniques, including dilution, adsorption-and-release, and chromatography19,20,21, aim to minimize aggregation and promote denatured protein refolding by controlling denaturant removal. However, these methods have limitations, such as high buffer use, time-intensive optimization, and limited compatibility or capacity. Thus, developing high-performance platforms that facilitate proper protein folding is urgently demanded. In natural systems, the Endoplasmic Reticulum and the Golgi apparatus provide microenvironments that prevent incorrect molecular interactions, ensuring correct folding and conformation of proteins. Inspired by nature, the dramatic advancement of material science that can mimic natural systems will open up new opportunities to resolve the abovementioned issues.
A feasible nature-mimicking approach is constructing porous materials with confined spaces to accommodate proteins and facilitate protein refolding, which is still underexplored. Mesoporous organosilica and supramolecular nanotubes have been applied as porous solid carriers to demonstrate their ability to assist protein refolding by the controlled release of unfolded proteins22,23. However, these amorphous porous materials lack well-defined structures, hindering the precise tuning of the refolding efficiency and understanding of the underlying mechanisms. Covalent organic frameworks (COFs), an emerging class of crystalline porous materials24,25,26, offer distinct advantages in addressing these challenges. The high crystallinity and well-defined porous structures of COFs enable in-depth mechanistic studies of the refolding process. Additionally, the rational design and tunability of COFs allow for precise modulation of interface interactions between confined spaces and proteins, such as electrostatic interactions, π-π conjugation, hydrophobic forces, and hydrogen bonding, facilitating the provision of suitable microenvironments to accommodate proteins. Therefore, these characteristics make COFs promising candidates for regulating protein folding, but remain unexplored.
In this work, to demonstrate the proof-of-concept, we innovated a COF-directed protein refolding strategy by designing and constructing a versatile platform based on COFs to correct protein misfolding and facilitate their recovery (Fig. 1a). The ordered and customizable pore structures of COFs can accommodate proteins and precisely control their microenvironment, which enables proper interactions and the recovery of protein activity. Characterizations have confirmed that these well-defined structural and microenvironmental features are crucial for achieving high renaturation efficiency, with results showing high refolding for various proteins. Furthermore, we developed a continuous solid-phase column refolding system using COFs for denatured proteins, even the inclusion body proteins, by simulating real-world scenarios (Fig. 1b).
Results and discussion
Platform preparation and refolding evaluation
Lysozyme, a glycosidic bond-breaking enzyme with dimensions of ~3.0 nm × 3.0 nm × 4.5 nm, is widely present in human saliva, tears, human milk, and egg white27,28. The extensively characterized structure of lysozyme, coupled with its low molecular weight, makes it an ideal model protein for investigating the processes and mechanisms of protein folding. To ensure compatibility between the confined space of the COFs and the structure and conformation of lysozyme, as well as to optimize the interfacial interactions between the COF and lysozyme, we rationally designed and synthesized a mesoporous COF, NKCOF-122, as a representative example by reacting 3,8-diamino-6-phenylphenanthridine (DP) with 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (Fig. 2a). Following the Optimization of solvothermal reaction conditions, such as temperature and solvents, NKCOF-122 with high crystallinity was prepared in a mixed solvent system of 1,4-dioxane, mesitylene, and acetic acid at 120 °C for 72 h. The crystal structure of NKCOF-122 was characterized using powder X-ray diffraction (PXRD) patterns (Fig. 2b). Pawley refinements were performed using data from the PXRD patterns, yielding unit cell parameters of a = 3.50 Å, b = 48.23 Å, c = 48.34 Å, α = 60.24°, β = 90°, γ = 90°, with a weighted profile R-factor Rwp of 5.77% and an unweighted profile R-factor Rp of 3.22%. These refinements revealed the presence of one-dimensional (1D) channels ~4.1 nm wide, with a distance of 3.50 Å between stacked two-dimensional sheets. The chemical structure of NKCOF-122 was confirmed through Fourier transform infrared spectrum (FT-IR) and solid-state 13C nuclear magnetic resonance (NMR) spectroscopy. In the FT-IR spectrum (Fig. 2c), NKCOF-122 exhibited a characteristic vibration band for the −CH=N− in 1600–1640 cm−1. The vibration bands corresponding to –CHO (1701 cm–1) and –NH2 (3300–3500 cm–1) were significantly decreased. Additionally, solid-state 13C NMR spectroscopy revealed aromatic carbon signals between 50 and 160 ppm in NKCOF-122 (Fig. 2d), with carbon signals at C=N at 158 ppm, confirming the formation of imine-linked COFs. Subsequently, the morphology and microstructure of COF materials were examined using scanning electron microscopy and transmission electron microscopy, which revealed that NKCOF-122 exhibited a characteristic layered morphology (Supplementary Figs. 1 and 2). The permanent porosity of NKCOF-122 was evaluated through N2 sorption isotherms at 77 K (Fig. 2e), with a specific surface area of 1699 m2/g (calculated by the BET model). Density functional theory fitting of the adsorption isotherm curve revealed desorption hysteresis (0.4 < P/P0 < 1), characteristic of a type IV adsorption isotherm, indicating its mesoporous property. The pore size distribution of NKCOF-122 was calculated, exhibiting an average pore diameter of ~4.1 nm, consistent with the pore size calculated from the AA layer stacking model. Notably, NKCOF-122 demonstrated excellent chemical stability, maintaining its original PXRD characteristics after exposure to various organic solvents and buffer solutions for 24 h (Fig. 2f).
a Synthesis scheme of NKCOF-122. b PXRD patterns of NKCOF-122: experimental patterns (red), refinement profile (black), simulated pattern for the eclipsed AA stacking model (pink), and refinement differences (blue). c FT-IR spectra of DP (3,8-diamino-6-phenylphenanthridine), TFPT (2,4,6-Tris(4-formylphenyl)-1,3,5-triazine), NKCOF-122. d 13C solid-state NMR spectrum of NKCOF-122. e N2 sorption isotherms and pore size distribution of NKCOF-122. f PXRD patterns of NKCOF-122 under different treatment conditions for 24 h. Source data are provided as a Source Data file.
To evaluate the capability of COFs to regulate protein folding, NKCOF-122 was incubated with denatured lysozyme (LD) in denaturants and native lysozyme (LN) in PBS buffer (20 mM), respectively. As shown in Fig. 3a, the Bradford method to measure the lysozyme content (Supplementary Fig. 65) revealed that NKCOF-122 could adsorb LD (0.30 g/g, product named LD@NKCOF-122), but not adsorb LN (product named LN + NKCOF-122). FT-IR spectra29 showed that LD@NKCOF-122 exhibited the characteristic peak at 1674 cm−1 of LD (Supplementary Fig. 3), corresponding to a β-sheet structure, which is commonly associated with the C=O stretching vibration in β-sheets. Furthermore, the Bradford method to measure the lysozyme content in the supernatant revealed that LD@NKCOF-122 could completely release lysozyme (0.29 g/g) in PBS buffer (Fig. 3b). It is purposed that the release is attributed to the reduced hydrophobic interactions (Supplementary Fig. 6), induced by the recovery of denatured proteins within the pores (Supplementary Figs. 4 and 5). These findings were further supported by N₂ sorption measurements and confocal laser scanning microscopy (CLSM) images. NKCOF-122* (labeled * to distinct with pristine NKCOF-122) after releasing lysozyme exhibited a similar pore size (4.1 nm) and specific surface area (1659 m²/g) as pristine NKCOF-122, further confirming the entire unloading of lysozyme (Fig. 3c and Supplementary Fig. 7). In the CLSM images (using FITC-labeled lysozyme30), green fluorescence was observed, indicating the presence of adsorbed LD (Fig. 3d), while no fluorescence signal was detected for LN + NKCOF-122 or NKCOF-122*. This adsorption selectivity could result from the pore size and microenvironment of NKCOF-122. Similarly, Taketomi et al.‘s study on MIL-101 showed higher adsorption capacity for LD over LN through pore and cage size tuning31.
a Time evolution of the LN (native lysozyme) and LD (denatured lysozyme) adsorption amount in NKCOF-122. b Time evolution of the LD amount in NKCOF-122*. c N2 sorption isotherms, d CLSM images of pristine NKCOF-122 (no green fluorescence), LD@NKCOF-122 (NKCOF-122 was incubated with LD, green fluorescence), LN + NKCOF-122 (NKCOF-122 was incubated with LN, no green fluorescence), and NKCOF-122* (NKCOF-122 after releasing lysozyme, no green fluorescence). All error bars mean ± s.d. received from three independent experiments (n = 3). Source data are provided as a Source Data file.
Subsequently, the spectral characterization of the released lysozyme (LR) from NKCOF-122 was conducted. In the fluorescence spectra32, a blue shift from 359 nm (LD) to 344 nm (LR) was observed, attributed to the burying of aromatic amino acid side chains, indicating a restoration trend in the conformation (Supplementary Fig. 8). The appearance of characteristic absorption peaks at 281 nm (LR) in the UV spectra33, as supported by Supplementary Fig. 9, also suggests partial structural recovery. Changes in the secondary structure were observed in the circular dichroism (CD) spectra33. LR displayed the same negative peaks of LN at 208 nm, indicative of α-helix (Fig. 4a). Raman spectra (Fig. 4b) also revealed LR showed an amide I band near 1659 cm−1 as LN34. The 1H NMR spectra of both native and refolded lysozyme were measured. The results show that the spectra are consistent, with chemical shifts (6.5–10.0 ppm) indicating proper protein folding and identical line widths ruling out the possibility of oligomerization (Supplementary Fig. 10). Moreover, the activity of LR was monitored at 25 °C using Micrococcus lysodeikticus as the substrate, showing ~93% activity compared to LN and confirming recovery of lysozyme activity (Supplementary Fig. 11 and Supplementary Table 1).
a CD spectra, b Raman spectra of lysozyme in different states: native, denatured, and recovered. c Refolding yield of lysozyme (1 mg/mL) with different contents of NKCOF-122. d Reusability of NKCOF-122 for refolding. All error bars mean ± s.d. received from three independent experiments (n = 3). Source data are provided as a Source Data file.
The above charming results inspired us to try whether COFs can directly renature proteins in one step, i.e., put NKCOF-122 into a solution with LD to directly achieve refolding of lysozyme. Thereupon, a comprehensive assessment of NKCOF-122’s ability to regulate lysozyme folding and conformation was conducted. The refolding yield was assessed by measuring the enzymatic activity of lysozyme after the refolding process. We found that NKCOF-122 could effectively renature LD in water (>94%) and various buffer solutions such as PB (>93%), PBS (>94%) and Tris (>93%) (Supplementary Fig. 12). This result indicated that the refolding ability of NKCOF-122 was tolerant to different salt ions in the buffers. Subsequently, the refolding capacity of NKCOF-122 was investigated by varying the COF content (0 to 5 mg/mL) while maintaining a fixed concentration of 1 mg/mL LD. As the content of NKCOF-122 increased, the refolding yield also rose, peaking at ~95% at 3 mg/mL (Fig. 4c and Supplementary Table 2). The reusability of NKCOF-122 was investigated through in-depth research, revealing that it could still maintain >80% refolding yield after 20 cycles (Fig. 4d). Moreover, PXRD and N2 sorption isotherms of NKCOF-122 after recycle retained as the pristine NKCOF-122 (Supplementary Fig. 13), indicating the high robustness of NKCOF-122 with preserved crystallinity and regular pore structure.
Mechanism study
The results presented above have successfully verified the effective recovery of LD achieved by NKCOF-122. To further investigate the mechanism and guide the regulation of different protein activities, the role of COF pore size in regulating protein refolding and activity was investigated. Considering the size of lysozyme (~3.0 × 3.0 × 4.5 nm), a series of NKCOFs with varied pore sizes were designed and synthesized (Fig. 5a and Supplementary Figs. 14–19 and 55–58). As depicted in Fig. 5b, a correlation between COF pore size and the refolding yield of lysozyme was observed. NKCOF-122, with a pore size close to lysozyme, exhibited the highest refolding yield at ~95%. In contrast, NKCOF-123, with a larger pore size of ~5.4 nm, achieved a lower refolding yield of ~53%. It was possibly due to weaker interactions between the pore microenvironment and lysozyme, resulting in less effective assistance in lysozyme recovery. Furthermore, NKCOF-121-3O, with a pore size of ~1.8 nm smaller than the size of lysozyme, demonstrated a very low refolding yield (~18%), because lysozyme could only interact with the COF particle surface rather than COF pores. Additionally, the refolding yield of lysozyme for traditional materials was also measured. The results showed that ZIF-8 (~11.4%), D152 (~5.3%), and D311 (~8.6%) also exhibited lower yields, attributed to their pore sizes being mismatched with lysozyme, emphasizing the importance of pore size in refolding (Supplementary Figs. 20–22). To further clarify the pore-directed effects, glucose oxidase (GOx, ~6.0 × 5.2 × 7.7 nm) was selected as another model protein35,36 (Fig. 5c). The results revealed that NKCOF-123 with a pore size close to GOx exhibited the highest refolding yield at ~81%, while NKCOF-121-3O and NKCOF-122 with smaller pore sizes showed a much lower refolding yield (<15%). Subsequently, the impact of COFs’ crystallinity was also explored. Amorphous NKCOF-122, which was prepared by treating NKCOF-122 with concentrated sulfuric acid (Supplementary Fig. 23), showed a diminished refolding yield (12%), highlighting the importance of regular pore structures of COFs for regulating protein folding.
a COFs with different pore sizes. b Refolding yield of lysozyme with COFs of different pore sizes. c Refolding yield of glucose oxidase with COFs of different pore sizes. d Electrostatic interactions (refolding yield of lysozyme with COFs of positive and negative charges). e π-π conjugation interaction (adsorption of aromatic amino acids by NKCOF-122). f Hydrophobicity (refolding yield of lysozyme with COFs of different hydrophobicity). All error bars mean ± s.d. received from three independent experiments (n = 3). Source data are provided as a Source Data file.
Furthermore, we also studied whether electrostatic interactions, π-π conjugation, hydrophobic forces, and hydrogen bonding could contribute to protein refolding and maintaining activity37,38,39,40,41. It was found that NKCOF-122 (-) and NKCOF-125 (+), with a similar pore size of ~3.8 nm but opposite zeta potentials, both exhibited a refolding yield of over 90% for lysozyme (Fig. 5d). This implies that electrostatic interaction may not be the primary force for regulating protein folding, especially with similar pore sizes (Supplementary Figs. 24–30 and 59–62). Given the prevalence of π-conjugated systems in COFs, the potential π-π conjugated interaction between COFs and lysozyme was investigated using the dye 6-carboxytetramethylrhodamine (TAMRA)41, which possessed a conjugated structure. NKCOF-122 showed significant adsorption of TAMRA (~85%), indicating the presence of π-conjugated systems. Control experiments with 6-carboxyfluorescein (FAM, a non-conjugated negatively charged dye, ~7%) excluded the interference from electrostatic interactions (Supplementary Fig. 31 and 63). Furthermore, since lysozyme contains aromatic amino acids like tryptophan, tyrosine, and phenylalanine, the adsorption of NKCOF-122 to these amino acids was measured, yielding values of 0.05 g/g, 0.11 g/g, and 0.04 g/g, respectively (Fig. 5e and Supplementary Fig. 64). These results further supported the hypothesis that π-π interactions promoted protein refolding. Moreover, to explore the impact of hydrophobicity further, a series of COFs with similar pore sizes, including NKCOF-121 (~108°), NKCOF-121-1O (~103°), NKCOF-121-2O (~83°), and NKCOF-121-3O (~61°) (Supplementary Fig. 32–40 and 49–54), were synthesized by varying the number of hydroxyl groups to reduce hydrophobicity (Supplementary Fig. 41). It was found that as hydrophobicity decreased, as the refolding efficiency decreased (NKCOF-121: 40%, NKCOF-121-1O: 33%, NKCOF-121-2O: 25%, NKCOF-121-3O: 16%) (Fig. 5f). This suggests that hydrophobic interactions play a key role in promoting protein refolding, possibly by allowing hydrophobic/hydrophilic groups in COFs to segregate partially folded proteins and reduce misfolding. To explore the contribution of hydrogen bonding, a heating test at 95 °C was conducted41, as hydrogen bonds can be broken at this temperature. Compared to LD@NKCOF-122 (0.30g/g), a higher release of denatured proteins (0.08g/g) was observed in the heating test (Supplementary Table 3), indicating the presence of hydrogen bonds. Thus, hydrogen bonding interaction also plays an important role in promoting protein refolding. Additionally, the adsorption capacity of COFs for lysozyme may influence refolding. By measuring the adsorption capacity of different COFs for denatured lysozyme and their corresponding refolding yields (Supplementary Table 4), it was found that the adsorption capacity correlates with the refolding yield, as seen in the comparison between NKCOF-122 (0.29g/g, ~95%) and NKCOF-123 (0.16g/g, ~53%). However, the comparison between NKCOF-121 (0.08g/g, ~40%, ~108°) and NKCOF-121-2O (0.07g/g, ~25%, ~83°) suggests that the differences in refolding yield are more likely due to hydrophobic interactions rather than adsorption capacity. In conclusion, while a higher adsorption capacity promotes refolding, it is not the only factor determining a high refolding yield.
Overall, all the above results thoroughly verify the critical role of COFs’ internal environment (hydrogen bonding, π-π conjugation, and hydrophobicity) in modulating protein folding (Supplementary Table 5), which may stabilize key intermediates and potentially lower the activation energy for correct folding.
Universality and practical study
To investigate the universality of COFs in regulating protein folding, various proteins, including trypsin (spherical diameter: ~3.8 nm), nattokinase (spherical diameter: ~4.0 nm), and papain (spherical diameter: ~3.6 nm), were investigated. Trypsin is commonly employed in treating pancreatic insufficiency and as a digestive enzyme replacement therapy42. Nattokinase is utilized as a thrombolytic agent, primarily to prevent cardiovascular diseases43. Papain is frequently used for digestive support, aiding in the digestion of proteins, and is also incorporated into skincare products44. Similar to the lysozyme refolding tests mentioned above, we measured the CD spectra (Fig. 6b, Supplementary Figs. 42 and 43), fluorescence spectra (Fig. 6c, Supplementary Figs. 44 and 45), and UV spectra (Supplementary Fig. 46) of the native, denatured, and renatured proteins. These characterizations all indicated that the denatured proteins could be renatured by NKCOF-122 treatment. Enzyme activity assays revealed that NKCOF-122 demonstrated high refolding efficiencies for these proteins (>70%) (Fig. 6a). Additionally, the zeta potential of these proteins was assessed (positive charges in nattokinase and papain, and negative charges in trypsin), and it was found that they did not affect the high refolding yield of these proteins, further demonstrating that electrostatic interaction was not the primary driving force behind the regulation of protein folding in COFs (Supplementary Fig. 47). Moreover, the refolding yields of different hydrophobic COFs were measured. It was found that as hydrophobicity decreased, the refolding efficiency also decreased: NKCOF-121 (~108°, trypsin: ~29%, papain: ~30%, and nattokinase: ~31%) >NKCOF-121-1O (~103°, trypsin: ~25%, papain: ~26%, and nattokinase: ~27%) > NKCOF-121-2O (~83°, trypsin: ~20%, papain: ~22%, and nattokinase: ~25%) >NKCOF-121–3O (~61°, trypsin: ~14%, papain: ~17%, and nattokinase: ~19%). This further highlights the importance of hydrophobic interactions in protein folding (Supplementary Table 6). We conducted refolding experiments with other NKCOFs for the three types of proteins (Supplementary Table 7). The refolding efficiency of NKCOF-121-3O (~1.8 nm) was worst for all test proteins (trypsin: ~14%, nattokinase: ~19%, and papain: ~17%) due to its relatively small pore size, whereas NKCOF-123 (~5.4 nm) showed moderate performance (trypsin: ~31%, nattokinase: ~37%, and papain: ~33%) due to its relatively large pore size. These results further confirmed the significance of pore size in modulating protein folding and activity.
a Regulation of the recovery of various proteins (trypsin, nattokinase, papain) by NKCOF-122. b CD spectra of papain in different states: native, denatured, and recovered. c Fluorescence spectra of papain in different states: native, denatured, and recovered. d Regulating lysozyme recovery through solid-phase column of NKCOF-122. e PXRD of NKCOF-122 as a solid-phase column after recycling. All error bars mean ± s.d. received from three independent experiments (n = 3). Source data are provided as a Source Data file.
To enhance the operability of COF-directed protein refolding to fit industrialization requirements, a solid-phase column using NKCOF-122 as the medium was prepared (Fig. 6d and Supplementary Table 8). The refolding yield of lysozyme on the column was initially evaluated. The results demonstrated that ~100% activity recovery could be achieved by gradually pumping an LD solution through the column. Following thorough refolding tests, rapid and effective elution was carried out. The column refolded ~5 mL (1mg/mL) of lysozyme solution, requiring only 50 mL of eluent for the elution process, with the lysozyme refolding yield reaching 97% and the recovery rate reaching 98%. Remarkably, the column maintained its high refolding yield and recovery rate even after 30 cycles of refolding, elution, and regeneration. Moreover, PXRD patterns indicated that NKCOF-122 retained good crystallinity after 30 cycles (Fig. 6e), suggesting the high durability and robustness of NKCOF-122 platform.
Furthermore, we investigated a real biomanufacturing scenario using the inclusion body proteins, which are a key issue for biochemistry and biotechnology, with current methods being complex and time-consuming. Therefore, we used the solid-phase column to directly refold solubilized inclusion body proteins, achieving an integrated process for refolding and separation (Supplementary Fig. 48). Specifically, the HEWL-6His gene was introduced into E. coli BL21 (DE3). After fermentation and high-pressure homogenization, the inclusion body proteins were obtained. Treating the inclusion bodies with denaturants, passing them through a solid-phase column, and eluting with PBS buffer yielded lysozyme (4.15 mg/L, ~92% activity compared to commercial lysozyme), in contrast to Ni-purification followed by dialysis (3.12 mg/L, ~52%), demonstrating a more efficient purification and refolding process (Supplementary Table 9). These findings highlight the industrial potential of COFs in regulating protein folding, offering promising prospects for applications in biotechnology and biomedicine.
In this study, a COF-directed protein refolding strategy was innovated, which allowed for precise modulation over COF microenvironments and interface interaction, enabling highly efficient recovery of denatured proteins. A robust imine-linked mesoporous COF, NKCOF-122, has been rationally designed to accommodate lysozyme, resulting in highly efficient recovery of denatured lysozyme. The regulation of protein refolding by factors (pore size, pore orderliness, and interactions) has been clarified. It has been proved that appropriate pore size and ordered pores play a critical role. Moreover, it has been explored that hydrophobic interactions, π-π conjugated interactions, and hydrogen bonding could serve as assisted regulators of protein activity. Additionally, we successfully demonstrated the high generality and practical application of this protein-refolding strategy, which is applicable to different proteins and COFs. A solid-phase column using NKCOF-122 achieved high refolding efficiency (~100%) and excellent recycling performance (30 cycles). Furthermore, an integrated process for the purification and refolding of inclusion bodies was also achieved, showing much higher efficiency for recombinant proteins (4.15 mg/L, 92%) than traditional Ni-purification followed by dialysis. This work introduces a versatile strategy for highly efficient protein refolding and points out that the customizable features of COFs can enhance the utility and versatility in biomanufacturing and biopharmaceutical applications.
Methods
Synthesis of NKCOFs
DP (42.8 mg, 0.15 mmol) and 2,4,6-Tris(4-formylphenyl)-1,3,5-triazine (39.3 mg, 0.10 mmol) were dispersed in a solvent containing 0.5 mL of 1,4-dioxane, 0.5 mL of mesitylene, and 0.1 mL of 6 M HAc in a tube. The tube was then flash-frozen at 77 K (liquid N2 bath) and evacuated, then flame-sealed. Next, the tube was heated at 120 °C for 3 days. After the reaction, the yellow product was filtered out and washed with anhydrous tetrahydrofuran using Soxhlet extraction for 24 hours. It was dried under vacuum at 100 °C to afford NKCOF-122. The synthesis method of other NKCOFs is similar to that of NKCOF-122. Detailed methods can be found in the Supplementary Information.
Preparation of denatured proteins
Aggregated lysozyme (10 mg/mL), Trypsin (20 mg/mL) were added to the denaturant solution that included 8 M Urea, 20 mM DTT, and PBS buffer (pH = 8.0, 20 mM). Aggregated Glucose Oxidase (1 mg/mL), Nattokinase (20 mg/mL), and Papain (20 mg/mL) were added to the solution that included 8 M Urea and PBS buffer (pH = 8.0, 20 mM). The resulting solution was left for enough time at 40 °C to ensure denaturation.
Refolding of denatured proteins
Protein refolding experiments were conducted by introducing denatured protein solutions into a refolding buffer, either with or without the addition of NKCOFs. In a typical procedure, NKCOFs were first suspended in PBS buffer at a concentration of 3 mg/mL to prepare the refolding solutions. Subsequently, 0.1 mL of the denatured protein solution was gently mixed with 0.9 mL of this refolding solution. The mixture was then incubated at 25 °C for 4 hours to facilitate protein refolding. The efficiency of refolding was assessed by evaluating the recovered enzymatic activity of the treated proteins.
Data availability
All data supporting the findings of this study are available within the article, as well as the Supplementary Information file, or available from the authors on request. Source data are provided with this paper.
References
Goodman, M. Sales of biologics to show robust growth through to 2013. Nat. Rev. Drug Discov. 8, 837–837 (2009).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Walsh, G. Post-translational modifications of protein biopharmaceuticals. Drug Discov. Today 15, 773–780 (2010).
Vendruscolo, M. et al. Protein folding and misfolding: a paradigm of self-assembly and regulation in complex biological systems. Philos. Trans. R. Soc. Lond. A 361, 1205–1222 (2003).
Dishman, A. F., Tyler, R. C. & Fox, J. C. Evolution of fold switching in a metamorphic protein. Science 371, 86–90 (2021).
Balchin, D., Hayer-Hartl, M. & Hartl, F. U. In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016).
Baneyx, F. & Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22, 1399–1408 (2004).
Louros, N., Schymkowitz, J. & Rousseau, F. Mechanisms and pathology of protein misfolding and aggregation. Nat. Rev. Mol. Cell Biol. 24, 912–933 (2023).
Beygmoradi, A., Homaei, A., Hemmati, R. & Fernandes, P. Recombinant protein expression: challenges in production and folding related matters. Int. J. Biol. Macromol. 233, 123407 (2023).
De Brabander, P., Uitterhaegen, E., Delmulle, T., De Winter, K. & Soetaert, W. Challenges and progress towards industrial recombinant protein production in yeasts: a review. Biotechnol. Adv. 64, 108121 (2023).
Rani, A. K., Katiyar, R. & Rathore, A. S. Bioprocessing of inclusion bodies from E. coli. to produce bioactive recombinant proteins. Biochem. Eng. J. 203, 109188 (2024).
Vendruscolo, M. Enzymatic activity in disordered states of proteins. Curr. Opin. Chem. Biol. 14, 671–675 (2010).
Cellini, B. et al. Role of misfolding in rare enzymatic deficits and use of pharmacological chaperones as therapeutic approach. Front. Biosci. 26, 1627–1642 (2021).
Rozema, D. & Gellman, S. Artificial chaperones: protein refolding via sequential use of detergent and cyclodextrin. J. Am. Chem. Soc. 117, 2373–2374 (1995).
Katina, N. et al. Influence of amino acid substitutions in ApoMb on different stages of unfolding of amyloids. Molecules 28, 7736 (2023).
Mittal, J. & Best, R. B. Dependence of protein folding stability and dynamics on the density and composition of macromolecular crowders. Biophys. J. 98, 315–320 (2010).
Hartl, F. U. & Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16, 574–581 (2009).
Koppl, C. et al. Fusion tag design influences soluble recombinant protein production in Escherichia coli. Int. J. Mol. Sci. 23, 7678 (2022).
Yang, Y. et al. Characterization of structural and functional properties of soybean 11S globulin during the renaturation after the guanidine hydrochloride denaturation. Food Hydrocoll. 130, 107715 (2022).
Jungbauer, A., Kaar, W. & Schlegl, R. Folding and refolding of proteins in chromatographic beds. Curr. Opin. Biotech. 15, 487–494 (2004).
Geng, X. & Wang, C. Protein folding liquid chromatography and its recent developments. J. Chromatogr. B 849, 69–80 (2007).
Wang, X. Q. et al. Protein refolding assisted by periodic mesoporous organosilica. Langmuir 23, 5735–5739 (2007).
Kameta, N. & Ding, W. Stacking of nanorings to generate nanotubes for acceleration of protein refolding. Nanoscale 13, 1629–1638 (2021).
Chen, H. et al. Covalent organic frameworks as crystalline sponges for enzyme extraction and production from natural biosystems. Chem. Eng. J. 444, 136624 (2022).
Jin, C. et al. Enzyme immobilization in porphyrinic covalent organic frameworks for photoenzymatic asymmetric catalysis. ACS Catal. 12, 8259–8268 (2022).
Li, M. et al. Fabricating covalent organic framework capsules with commodious microenvironment for enzymes. J. Am. Chem. Soc. 142, 6675–6681 (2020).
Luo, R. et al. Lysozyme aptamer-functionalized magnetic nanoparticles for the purification of lysozyme from chicken egg white. Foods 8, 67 (2019).
Zhang, Y., Feng, W. & Zhang, W. Tetrahedral DNA enhanced antibacterial effect of lysozyme. ACS Mater. Lett. 6, 2409–2415 (2024).
Islam, Z. et al. Probing the fibrillation of lysozyme by nanoscale-infrared spectroscopy. J. Biomol. Struct. Dyn. 39, 1481–1490 (2021).
Zhong, C., Wang, Y., Ma, G. & Li, R. Measurement of the onset temperature of irreversible inactivation of proteins using FITC as a fluorescent reporter. Anal. Methods 8, 3809–3815 (2016).
Taketomi, H., Hosono, N. & Uemura, T. Selective removal of denatured proteins using MOF nanopores. J. Am. Chem. Soc. 146, 16369–16374 (2024).
Chen, B. et al. Unfolding mechanism of lysozyme in various urea solutions: Insights from fluorescence spectroscopy. J. Mol. Struct. 1076, 524–528 (2014).
Nakamoto, M., Nonaka, T., Shea, K. J., Miura, Y. & Hoshino, Y. Design of synthetic polymer nanoparticles that facilitate resolubilization and refolding of aggregated positively charged lysozyme. J. Am. Chem. Soc. 138, 4282–4285 (2016).
Xing, L., Lin, K., Zhou, X., Liu, S. & Luo, Y. Multistate mechanism of lysozyme denaturation through synchronous analysis of raman spectra. J. Phys. Chem. B 120, 10660–10667 (2016).
Fu, L. H., Qi, C., Hu, Y. R., Lin, J. & Huang, P. Glucose oxidase-instructed multimodal synergistic cancer therapy. Adv. Mater. 31, 1808325 (2019).
Wang, X., Lan, P. C. & Ma, S. Metal-organic frameworks for enzyme immobilization: beyond host matrix materials. ACS Cent. Sci. 6, 1497–1506 (2020).
Das, S., Lin, Y. H., Vernon, R. M., Forman-Kay, J. D. & Chan, H. S. Comparative roles of charge, pi, and hydrophobic interactions in sequence-dependent phase separation of intrinsically disordered proteins. Proc. Natl. Acad. Sci. USA 117, 28795–28805 (2020).
Jena, S. et al. Noncovalent interactions in proteins and nucleic acids: beyond hydrogen bonding and pi-stacking. Chem. Soc. Rev. 51, 4261–4286 (2022).
Lee, M. et al. Surface hydrophobicity modulates the key characteristics of cancer spheroids through the interaction with the adsorbed proteins. Adv. Funct. Mater. 31, 2100775 (2021).
Shao, J. et al. The role of tryptophan in π interactions in proteins: an experimental approach. J. Am. Chem. Soc. 144, 13815–13822 (2022).
Peng, S. et al. Metal-organic frameworks for precise inclusion of single-stranded DNA and transfection in immune cells. Nat. Commun. 9, 1293 (2018).
Momeni, L., Mahmodian, S., Shareghi, B., Saboury, A. A. & Farhadian, S. The functional and structural stabilization of trypsin by sucrose. Int. J. Biol. Macromol. 99, 343–349 (2017).
Yuan, L., Liangqi, C., Xiyu, T. & Jinyao, L. Biotechnology, bioengineering and applications of bacillus nattokinase. Biomolecules 12, 980 (2022).
Tacias-Pascacio, V. G. et al. Immobilization of papain: a review. Int. J. Biol. Macromol. 188, 94–113 (2021).
Acknowledgements
Y.C. gratefully acknowledges financial support from the National Key Research and Development Program of China (2021YFC2102100), Tianjin Natural Science Foundation (23JCJQJC00140), National Natural Science Foundation of China (22371136), Haihe Laboratory of Synthetic Biology (22HHSWSS00008), and the CAS Youth Interdisciplinary Team program. W.C. gratefully acknowledges financial support from the Recruitment Program of Global Experts (A4220040). Z.Z. gratefully acknowledge financial support from Nankai University & Cangzhou Bohai New Area Institute of Green Chemical Engineering (NCC2022-PY-04).
Author information
Authors and Affiliations
Contributions
Y.C. designed the project. J.G. performed the major experiments, X.S. and W.C. helped to analyze protein conformation, J.W. and Z.Z. performed part of industrialized experiments, Y.H. conducted cyclic experiments for NKCOF-122 refolding lysozyme, J.T. and M.Y. measured CD spectra and the hydrophobic angles of COFs. Y.C. and J.G. analyzed the results and wrote the manuscript with contributions from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications Guosheng Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, J., Sun, X., Wang, J. et al. Precise modulation of protein refolding by rationally designed covalent organic frameworks. Nat Commun 16, 4122 (2025). https://doi.org/10.1038/s41467-025-59368-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59368-z