Abstract
Body composition is an important predictor in cancer patients, with skeletal muscle loss and high adiposity associated with poorer prognosis. This study evaluated how body composition affects treatment efficacy in 48 women with metastatic breast cancer receiving trastuzumab deruxtecan. Using computed tomography, skeletal muscle, visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were assessed within 60 days before initiating treatment. High SAT and VAT areas were significantly associated with a higher likelihood of dose reductions (Odds Ratio [OR] = 5.34, p = .032 and OR = 5.52, p = 0.032, respectively). Higher SAT areas correlated with a lower objective response rate (OR = 0.22, p = 0.047). Medium SAT and low/medium VAT densities increased the risk of dose reductions. A body mass index over 25 kg/m2 was linked to higher dose reductions (OR = 4.97, p = 0.016). These findings emphasize the need for personalized treatment strategies based on body composition.
Similar content being viewed by others
Introduction
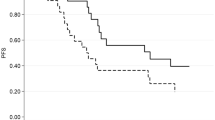
Trastuzumab deruxtecan (T-DXd) is an innovative antibody-drug conjugate (ADC) comprising the anti-HER2 antibody trastuzumab, a cleavable linker, and the chemotherapy agent deruxtecan, an anti-topoisomerase I payload1,2,3. In the pivotal Phase 3 Destiny-Breast03 (DB-03) trial, T-DXd was investigated versus trastuzumab emtansine (TDM1) in patients with HER2 positive metastatic breast cancer (MBC) in the 2nd line treatment setting, improving progression-free survival (PFS) and overall survival (OS)4. Notably, T-DXd is administered intravenously every 3 weeks at a dose of 5.4 mg per kilogram of body weight, regardless of variables such as muscle mass and adiposity. Regarding toxicity profile, severe adverse events (AEs) of grade 3−4 occurred in 56% of patients in the T-DXd group versus 52% in the TDM1 group. The most common drug-related AEs of any grade in the T-DXd group were nausea (72.8% of the patients), fatigue (44.7%), and vomiting (44.0%); As for severe AEs, the most common side effects were neutropenia (19.1%), thrombocytopenia (7.0%), leukopenia (6.6%), and nausea (6.6%). Secondary interstitial lung disease (ILD) appeared in 15% of cases under T-DXd.
Among patients with HER2-negative tumors, approximately 60% will express HER2 at a low level, defined as an immunohistochemistry (IHC) score of +1, or +2 with negative fluorescence in situ hybridization5. In the phase 3 trial Destiny-Breast04, T-DXd was tested in patients with advanced HER2-low MBC, following prior chemotherapy treatment. The study showed significant improvement in PFS and OS. Grade 3−4 AEs occurred in 52.6% of T-DXd patients, with dose reductions in 22.6% and treatment discontinuation due to toxicity in 16.2%6.
Body composition metrics (BCM) refer to the distribution and proportion of various body tissues, including fat and muscle mass. Both Magnetic resonance imaging (MRI) and computed tomography (CT) scans offer high-resolution advanced imaging, allowing for precise measurements of fat and lean tissue distribution, particularly in abdominal and visceral regions7. Understanding body composition is crucial in predicting the pharmacokinetics and pharmacodynamics of pharmacological agents. Specifically, lipophilic (fat-soluble) drugs tend to be primarily distributed in the adipose tissue. Deruxtecan, the cytotoxic payload of the drug, is a small molecule with lipophilic properties. The lipophilic nature of T-DXd allows for a significant bystander effect2. A higher percentage of body fat can increase the volume of distribution for lipophilic drugs, which may result in lower plasma concentrations and, potentially, reduced therapeutic efficacy. Conversely, the increased fat reserve can also act as a drug reservoir, leading to prolonged release of the drug, which could result in extended drug exposure and increased toxicity.
The exploration of body composition’s impact on drug toxicity and efficacy is gaining attention, particularly in oncology, where treatment regimens often lead to severe side effects;
Suboptimal BCM have been associated with poorer outcomes in patients with breast cancer, including shorter survival and higher toxicity8,9,10,11. In a study investigating the correlation between BCM and the toxicity of anthracyclines and taxanes in early-stage breast cancer (N = 151), it was shown that patients with lower skeletal muscle index (SMI) developed higher rates of grade 3−4 toxicity (RR, 1.29; P = 0.002)12 Moreover, a meta-analysis from 38 studies involving a total of 7843 subjects showed that patients with SMI below the specified threshold had a significantly worse OS (HR 1.44, p < 0.001). The impact of low SMI on survival was consistent across different types of tumors and stages of disease13.
While numerous studies have evaluated BCM in cancer patients receiving chemotherapy, none have focused specifically on T-DXd. Our study investigated the correlation between BCM and treatment outcomes, including response rate and toxicity, in patients with MBC treated with T-DXd.
Results and discussion
Patient characteristics and body composition
A total of forty-eight patients diagnosed with HER2-positive or low metastatic breast cancer, treated with T-DXd at Tel Aviv Medical Center (TAMC) between January 2020 and March 2024, met eligibility criteria and were included in the analysis. Patient clinical characteristics, body composition measures, and toxicity outcomes are detailed in Table 1. The median age was 62 years (interquartile range [IQR], 52-74). Sixty percent of the women (n = 29) were treated with T-DXd in ≤ third line setting. Patients’ mean weight was 65.3 kg (standard deviation [SD] ±13.1). Mean body mass index (BMI) was 29.4 ± 7.5 kg/m2, and 62% of patients were considered overweight or obese (BMI ≥ 25 kg/m2). CT-based body composition indices were available and calculated for all included patients. Approximately three-quarters of the patients were sarcopenic (SMI < 41 cm2/m2, n = 37). HER2 status (positive/low) of the study population is presented in Table 1.
Among the study population, 24 patients (50%) experienced a dose reduction of T-DXd and an equal number of patients (n = 24, 50.0%) experienced a dose delay. Out of these 24 patients, 16 patients experienced a single treatment adjustment due to toxicity, while the remaining 8 required a second intervention in dosage or treatment timing. A smaller subset of patients (10 patients, 20.8%) required hospitalization due to treatment-related toxicity.
Anemia was the most prevalent side effect, graded 2 and above, affecting a quarter of the patients (12 patients), followed by neutropenia and nausea, which were both experienced by 23% of the cohort. Three cases of grade 4 neutropenia were observed, all of which led to febrile neutropenia and hospitalization. Treatment discontinuation due to drug toxicity was documented in 7 of the 48 patients assessed (14.6%). Supplementary Table 1 presents differences in patient characteristics, body composition measures, and toxicity outcomes based on sarcopenia status.
Body composition and Toxicity
As presented in Table 2, high subcutaneous adipose tissue (SAT) area (cm2) significantly increased the likelihood of dose reduction compared to Low SAT area (Odds Ratio [OR] = 5.34, p = 0.032). Moreover, medium SAT density (Hounsfield units [HU]), meaning hypodense fat, was also associated with increased probability of a dose reduction compared with high SAT density (OR = 7.75, p = 0.014). Additionally, high visceral adipose tissue (VAT) area (cm2) showed significant impacts on the likelihood of dose reduction compared to Low VAT area (OR = 5.52, p = 0.032). Both low and medium VAT density were significantly associated with increased dose reduction rates (OR = 10.02, p = 0.007 and OR = 7.84, p = 0.015, respectively). In addition, higher BMI, defined as 25 kg/m2 or above, significantly increased the likelihood of dose reduction (OR = 4.97, p = 0.016). Muscle measurements were not associated with toxicity. All analyses were conducted using a multivariate logistic regression model, adjusting for patient age, therapy line, and comorbidities as statistical control variables.
Body composition and treatment response
The analysis revealed a significant association between SAT area and response rate, as detailed in Table 3; High SAT area significantly decreases the likelihood of a favorable response (OR = 0.22, p = 0.047) compared to low SAT. As in the dose reduction analysis, all analyses were conducted using a multivariate logistic regression model, adjusting for patient age, therapy line, and comorbidities as statistical control variables.
Additional analyses assessing the correlation between BCM and AEs, as well as other indirect indicators of T-Dxd toxicity, have been performed and are included as Supplementary Table 2. A higher VAT area (cm2) was significantly associated with an increased risk of severe gastrointestinal diarrhea (OR = 1.02, p = 0.037), while most other associations were not statistically significant. All analyses were adjusted for patient age, therapy line and comorbidities.
Discussion
In this cohort of 48 patients with HER-2-positive/low MBC treated with TDX-d, higher SAT and VAT areas, along with hypodense fat in both SAT and VAT, were associated with higher rates of dose reduction. High BMI showed a similar effect. Most previous studies on body composition in MBC patients did not specifically focus on TDX-d, a crucial component of the current guideline-recommended breast cancer treatment algorithm. To our knowledge, this is the first study to assess the impact of BCM on clinical outcomes in patients with MBC treated with T-DXd.
Previous studies have shown that suboptimal body composition, also characterized by adipose tissue with high density, is associated with poorer outcomes in cancer patients. A systematic review by Kapoor et al. highlighted that increased adipose tissue density is linked to adverse outcomes such as decreased survival and increased tumor recurrence14. Similarly, Cheng et al. found that higher SAT radiodensity was significantly associated with increased risk of overall mortality in non-metastatic breast cancer patients15.
In an exploratory analysis of the ALTTO BIG 2-06 trial, which assessed trastuzumab and/or lapatinib as adjuvant treatments for early-stage HER2-positive breast cancer, patients with obesity exhibited a significantly higher incidence of grade 3/4 AEs (p < 0.001), resulting in a statistically significant increase in treatment discontinuation (p < 0.001)16. These findings suggest that obese patients with HER2-positive breast cancer are at an increased risk of toxicity from HER2-targeted therapies and may require more rigorous monitoring for adverse effects. A large study addressed the value of obesity in trastuzumab-related cardiotoxicity in an elderly cohort of breast cancer. The association between BMNI ≥ 30 kg/m2 and high risk of heart failure has been well established among 5881 individuals17. The mechanisms by which obesity could negatively influence cardiotoxicity are affected by numerous confounding factors. One is increased expression of pro-inflammatory adipokines and reduced anti-inflammatory adipokines, and resultant chronic inflammation18. Another factor can be the activation of neurohormones, increased oxidative stress and increased hemodynamic load19.
TDX-d represents a third-generation antibody-drug conjugate (ADC), enhanced by using site-specific conjugation technology. This technology produces homogenous ADCs with well characterized drug-antibody ratios (DARs) and optimized cytotoxicity20. ADCs with consistent DARs are associated with reduced off-target toxicity and improved pharmacokinetic efficiency21. Hematological toxicity and gastrointestinal reactions observed with ADCs are likely due to the premature release of cytotoxic payloads into the bloodstream22. This effect is consistent with the action of conventional chemotherapy, which primarily targets rapidly proliferating cells. Clinical observations have raised concerns about potential lung toxicity, such as ILD during ADC treatment, with several ILD-related deaths reported23,24.
TDX-d dosing is calculated based on body weight. This means that a patient who weighs twice as much will receive twice the amount of the drug. High fat area, indicative of higher overall adiposity, and low fat density, suggestive of larger adipocytes with greater lipid content, can both impact the efficacy and toxicity of oncological treatments. Compared to the middle range of adipose tissue radiodensity, lower radiodensity is indicative of adipocyte hypertrophy and decreased vascularity, which was reported to be associated with adverse cardiometabolic risk and biomarkers25,26,27,28. In contrast, higher radiodensity may suggest lipid depletion, higher vascularity, extracellular matrix deposition, and stronger inflammatory response26,29. Thus, alterations in adipose tissue radiodensity may reflect the changes of microenvironment and macroenvironment that may contribute to tumor progression and treatment toxicity.
In the case of T-DXd, which has the potential for severe side effects such as interstitial lung disease (ILD), hematologic and gastrointestinal toxicity, identifying frail patients early can help optimize treatment safety. Frail patients may be at higher risk for these toxicities, so clinicians might consider starting with a lower dose to reduce side effects while maintaining therapeutic efficacy. Close monitoring for adverse events, such as fatigue, lung toxicity or hematologic issues, would also be essential. Overall, integrating frailty markers into the management of T-DXd can help balance the therapeutic benefits with the risk of toxicities, offering a more personalized approach that improves patient outcomes and quality of life.
Our study is not without limitations. Firstly, the retrospective design inherently limits the ability to establish causality and may introduce biases related to the selection and availability of data. However, it provides important insights that reflect everyday clinical practice, offering valuable guidance for future prospective studies. The reliance on a single center setting also constrains the generalizability of our findings, as the patient population and treatment protocols at our center may not fully represent broader more diverse populations. At the same time, this ensures consistent treatment protocols and follow-up care, reducing variability due to institutional differences. Additionally, due to the retrospective nature of the study, we could not retrieve data regarding diet, physical activity, sedentary habits, and comorbidities, which could influence both body composition and treatment outcomes. Despite the unavailability of some data, our emphasis on objective body composition measures, such as subcutaneous and visceral adipose tissue, provides reliable indicators of treatment response. Finally, the sample size of 48 patients is relatively small, which may limit the statistical power of our analyses. A small sample size can reduce the ability to detect subtle but clinically important associations and increases the risk of type II errors. This limitation also restricts the extent to which subgroup analyses can be performed, potentially masking differences between various patient groups. Despite the small sample size, it represents a carefully selected cohort of HER-2 positive/low MBC patients treated with TDX-d. This focused sample allows for detailed analysis of body composition’s role in treatment, and significant trends were identified even with the limited sample.
In conclusion, our study showed that patients with larger fat tissue area, lower fat density, and higher BMI, experienced increased rates of dose reduction and treatment-related toxicity. Since some common side effects, fatigue for instance, are challenging to quantify, we used dose reduction rate as an indicator of their occurrence. Our study highlights the importance of these metrics in clinical decision-making. These findings substantiate body composition as an encouraging prognostic tool in the era of modern breast cancer treatment. By integrating body composition assessments into clinical practice, healthcare providers can better tailor treatment plans to individual patients, potentially enhancing treatment efficacy and minimizing adverse effects.
Future prospective studies with larger, more diverse cohorts are needed to validate these findings and further explore the mechanisms underlying the observed associations.
Methods
Patients
This retrospective single-institution analysis included patients with HER2-positive or HER2-low MBC treated with T-DXd at TAMC between January 2020 and March 2024. Eligible participants were females, aged 21 years and older, with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-3. Participants required a baseline abdominal CT scan performed within 60 days prior to initiating therapy with T-DXd, accessible digital images for muscle mass assessment, and complete electronic medical records. Patient data was extracted from the institutional electronic database. The study received approval from the TAMC Institutional Review Board (Helsinki ethics approval number 0611-21-TLV). In this trial, informed consent was not required as the study used pre-existing data from routine clinical care. This approach complies with ethical guidelines for observational research. Clinical parameters included age at the time of metastatic disease diagnosis, HER-2 status, treatment duration, number of prior therapy lines and treatment response by the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1)30.
Toxicity grading measures
Patient demographics and AEs were extracted from the electronic medical records. The severity of AEs was graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE, Version 5.0)31. Our analysis focused on commonly reported AEs in the literature, including hematologic toxicity, gastrointestinal toxicity (diarrhea and nausea), and ILD/pneumonitis. Furthermore, data were collected on dose reductions, treatment delays, and hospitalizations deemed to be caused by drug toxicity, based on physician documentation in the medical records. A dose reduction was defined as a decrease of more than 20% from the initial dose, in line with the manufacturer’s recommendations (FDA label), while a treatment delay was defined as a postponement of more than one week from the scheduled treatment date.
Body composition analysis
Abdominal CT images were obtained from the TAMC Picture Archiving and Communication System (Philips Algotec, Ra’anana, Israel) and were analyzed with the assistance of a radiologist. Axial plane CT images at the level of the third lumbar vertebra (L3) were selected for evaluation. These images were processed using an automated segmentation software, the Data Analysis Facilitation Suite (DAFS) platform (Voronoi Health Analytics Inc., Vancouver Canada). The development and validation of DAFS have been previously described31,32,33,34,35,36.
As demonstrated in Fig. 1, the software detects muscle tissue based on a density range of −29 to +150 HU, utilizing a priori knowledge of the L3 muscle shape to avoid incorrectly labeling adjacent organs with similar HU values. The cross-sectional areas (cm2) of all L3 regional muscles (psoas, paraspinal, and abdominal wall muscles) were calculated for each image, and an average value from two images was computed for each patient. This approach yields a highly accurate estimation of cross-sectional lean tissue area and skeletal muscle area37,38,39. SMI was calculated using the formula: SMI = (L3 muscle area in cm2/(patient height in m2), an SMI of <41 cm2/m2 was considered sarcopenic. Baracos et al. established the sarcopenia threshold of 41 using optimal stratification to identify cutoff values associated with reduced survival, defining skeletal muscle depletion in women across BMI categories as a significant prognostic factor in cancer patients40.
Two patients with metastatic breast cancer. Left (a, b), high SAT and VAT area (cm2), medium SAT and VAT density (HU), high BMI (>25 kg/m2), high treatment toxicity leading to dose reduction; Right (c, d), low SAT and VAT area (cm2), low SAT and high VAT density (HU), low BMI (<25 kg/m2), no treatment toxicity.
The mean skeletal muscle density (SMD) was determined by averaging the HU of skeletal muscle at the level of the L3 vertebra. This attenuation measurement serves as a non-invasive radiological technique to indirectly assess muscle fat content. Higher skeletal muscle density indicates lower muscle fat content, as there is an inverse relationship between muscle density and fat content41.
SAT and VAT density were defined by ranges of −190 to −30 and -150 to −50 Hounsfield Unit (HU), respectively. Muscle and fat areas (cm2) were normalized for the square of height (m2) as indexes (cm2/m2). All body composition values were classified based on tertile distribution, as presented in Supplementary Table 3.
Statistical analysis
For data that conformed to the assumptions of normal distribution, as validated by the Kolmogorov–Smirnov test, the results are presented as mean ± SD. For data that did not follow a normal distribution, the results are presented as median (IQR). A multivariate binary logistic regression model was implemented to estimate the OR for each variable. All analyses were adjusted for patient age, therapy line and comorbidities as control variables to account for potential confounding effects. A p-value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using IBM SPSS version 28.
Data availability
Data are available upon reasonable request. Access to datasets from the Oncology Department, Tel Aviv Sourasky Medical Center (used with permission for this study) should be requested directly from the institution via their data access request forms. Subject to ethical approval by the institutional review board, de-identified data will be made available as a test subset. All implementation details are thoroughly described in the Methods section, enabling independent replication using non-proprietary libraries.
Code availability
Code availability is not applicable, as no custom software or algorithms were used in this study.
References
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Ogitani, Y., Hagihara, K., Oitate, M., Naito, H. & Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 107, 1039–1046 (2016).
Nakada, T., Sugihara, K., Jikoh, T., Abe, Y. & Agatsuma, T. The latest research and development into the antibody-drug conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 67, 173–185 (2019).
Cortés, J. et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
Tarantino, P. et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 38, 1951–1962 (2020).
Modi, S. et al. Trastuzumab Deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Popuri, K., Cobzas, D., Esfandiari, N., Baracos, V. & Jägersand, M. Body composition assessment in axial CT images using FEM-based automatic segmentation of skeletal muscle. IEEE Trans. Med. Imaging 35, 512–520 (2016).
Aleixo, G. F. P., Valente, S. A., Wei, W. & Moore, H. C. F. Association of sarcopenia with endocrine therapy toxicity in patients with early breast cancer. Breast Cancer Res. Treat. 196, 323–328 (2022).
Shachar, S. S. et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin. Cancer Res. 23, 658–665 (2017).
Weinberg, M. S. et al. Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 24, 278–284 (2018).
Prado, C. M. M. et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 15, 2920–2926 (2009).
Shachar, S. S. et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin. Cancer Res. 23, 3537–3543 (2017).
Shachar, S. S., Williams, G. R., Muss, H. B. & Nishijima, T. F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 57, 58–67 (2016).
Kapoor, N. D. et al. Adipose tissue density on CT as a prognostic factor in patients with cancer: a systematic review. Acta Oncol. 59, 1488–1495 (2020).
Cheng, E. et al. Adipose tissue radiodensity and mortality among patients with nonmetastatic breast cancer. Clin. Nutr. 41, 2607–2613 (2022).
Martel, S. et al. Body mass index and weight change in patients with HER2-positive early breast cancer: Exploratory analysis of the ALTTOBIG 2-06 trial. JNCCN. J. Natl Compr. Cancer Netw. 19, 181–189 (2021).
Atish, S. et al. Obesity and the risk of heart failure. N. Engl. J. Med. 347. www.nejm.org (2002).
Nakamura, K., Fuster, J. J. & Walsh, K. Adipokines: a link between obesity and cardiovascular disease. J. Cardiol. 63, 250–259 (2014).
Engeli, S. & Sharma, A. M. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J> Mol. Med. 79, 21–29 (2001).
Strop, P. et al. Site-specific conjugation improves therapeutic index of antibody drug conjugates with high drug loading. Nat. Biotechnol. 33, 694–696 (2015).
Hoffmann, R. M. et al. Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs). OncoImmunology 7. Taylor and Francis Inc. https://doi.org/10.1080/2162402X.2017.1395127 (2018).
Mahalingaiah, P. K. et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol. Ther. 200, 110–125 (2019).
Hackshaw, M. D. et al. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res. Treatment 183, 23–39 (Springer, 2020).
Miles, D. W. et al. 288 P Final results from PERUSE, a global study of pertuzumab (P), trastuzumab (H) and investigator’s chosen taxane as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer (LR/mBC). Ann. Oncol. 31, S356–S357 (2020).
Rosenquist, K. J. et al. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC: Cardiovascular Imaging 6, 762−771 (2013).
Feliciano, E. M. C. et al. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am. J. Clin. Nutr. 114, 1917–1924 (2021).
Torriani, M., Oliveira, A. L., Azevedo, D. C., Bredella, M. A. & Yu, E. W. Effects of Roux-en-Y gastric bypass surgery on visceral and subcutaneous fat density by computed tomography. Obes. Surg. 25, 381–385 (2015).
Côté, J. A. et al. Computed tomography-measured adipose tissue attenuation and area both predict adipocyte size and cardiometabolic risk in women. Adipocyte 5, 35–42 (2016).
Divoux, A. et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Cancer Institute, N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://www.meddra.org/ (2017).
Dabiri, S. et al. Muscle segmentation in axial computed tomography (CT) images at the lumbar (L3) and thoracic (T4) levels for body composition analysis. Computerized Med. Imaging Graph. 75, 47–55 (2019).
Dabiri, S. et al. Deep learning method for localization and segmentation of abdominal CT. Computerized Med. Imaging Graph. 85, 101776 (2020).
Ma, D., Chow, V., Popuri, K. & Beg, M. F. Comprehensive validation of automated whole body skeletal muscle, adipose tissue, and bone segmentation from 3d ct images for body composition analysis: towards extended body composition. https://arxiv.org/abs/2106.00652 (2021).
Anyene, I. et al. Body composition from single versus multi-slice abdominal computed tomography: concordance and associations with colorectal cancer survival. J. Cachexia Sarcopenia Muscle 13, 2974–2984 (2022).
Dietz, M. V. et al. Evaluation of a fully automated computed tomography image segmentation method for fast and accurate body composition measurements. Nutrition 129, 112592 (2025).
Prado, C. M. M. & Heymsfield, S. B. Lean tissue imaging: A new era for nutritional assessment and intervention. J. Parenter. Enter. Nutr. 38, 940–953 (2014).
Looijaard, W. G. P. M., Molinger, J. & Weijs, P. J. M. Measuring and monitoring lean body mass in critical illness. Curr. Opin. Critic. Care 24, 241–247 (2018).
Rollins, K. E., Awwad, A., Macdonald, I. A. & Lobo, D. N. A comparison of two different software packages for the analysis of body composition using computed tomography images. Nutrition 57, 92–96 (2019).
Martin, L. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547 (2013).
Aubrey, J. et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. ActaPhysiol.210, 489–497 (2014).
Acknowledgements
This work was not supported by any specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
S.Y.P., R.K., A.D.L., and Y.B. contributed to the data collection and analysis.Body composition analysis, data was collected by A.D.L., with review and validation carried out by R.K. S.L. was responsible for the statistical analysis and interpretation of the results. A.S. and S.S.S. conceptualized and designed the study. S.Y.P. prepared the manuscript text, with contributions from Y.B. and S.S.S. K.P. and M.F.B. provided technical support and expertise in body composition assessment methodology and prepared the figure. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yaacobi Peretz, S., Kessner, R., Bar, Y. et al. Body composition metrics as a determinant of trastuzumab deruxtecan related toxicity and response. npj Breast Cancer 11, 38 (2025). https://doi.org/10.1038/s41523-025-00754-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-025-00754-7