Abstract
Schizophrenia (SZ), schizoaffective disorder (SZA), bipolar disorder (BD), and psychotic depression (PD) are associated with premature death due to preventable general medical comorbidities (GMCs). The interaction between psychosis, risk factors, and GMCs is complex and should be elucidated. More research particularly among those with SZA or PD is warranted. We evaluated the association between registry-based psychotic disorders and GMC diagnoses in a large national sample of participants with different psychotic disorders. In addition, we examined whether body mass index (BMI) and smoking as risk factors for GMCs explain differences between diagnostic groups. This was a cross-sectional study of a clinical population of participants (n = 10,417) in the Finnish SUPER study. Registry-based diagnoses of psychotic disorders and hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cancers, ischemic heart disease, and liver disorders were obtained. Participants’ BMI and self-reported smoking were recorded. Total effect of diagnostic category adjusted for age and sex as well as direct effect including known risk factors was calculated using logistic regression. Regardless of diagnostic category, participants had high BMI (average 30.3 kg/m2), and current smoking was common (42.4%). Diabetes and COPD were more common in SZ than in other diagnostic categories. The differences between psychotic disorders were not explained by obesity or smoking status only. Obesity and smoking were prevalent in all diagnostic categories of psychotic disorders, and continued efforts at prevention are warranted. Additional differences in GMC prevalence exist between psychotic disorders that are not explained by obesity and smoking.
Similar content being viewed by others
Introduction
Individuals with psychotic and mood disorders face a higher prevalence of general medical comorbidities (GMCs). Understanding possible differences among these disorders has significant implications for patient care and health policy. Relatively few studies have compared patients with different categories of psychotic disorders directly, especially the less investigated psychotic illnesses such as schizoaffective disorder (SZA) or psychotic depression (PD)1,2.
Schizophrenia (SZ), schizoaffective disorder, and bipolar disorder (BD) are associated with premature death – 7 to 20 years earlier than in the general population – to a large extent due to GMCs3,4,5,6,7. Cardiovascular disorders (CVDs) (including ischemic heart disease (IHD) and stroke), diabetes mellitus (hereafter, diabetes), chronic obstructive pulmonary disease (COPD), and cancer are associated with premature mortality in SZ and mood disorders2,8,9,10. In addition, gastrointestinal illness, especially liver disorders (LDs), are associated with mortality in psychiatric disorders10.
The association between GMCs and psychotic illness is partially explained by the higher prevalence of risk factors among those with psychotic illness. Smoking has been reported to be 2-3 times more common in SZ than in the general population11 BD is similarly associated with a higher prevalence of smoking12. SZ is associated with obesity, diabetes, and metabolic syndrome, and patients with SZ should be considered a high-risk group for metabolic disorders and targeted for preventive intervention13. While mental illnesses are broadly associated with a wide range of physical conditions10,14, recent research also suggests particular risk for specific comorbidities in some categories of mental illness. Specifically, a meta-analysis15 suggested an association between SZA and metabolic syndrome over SZ, and BD has been associated with CVD over depression16. The association between BD and CVD is complex, including the various effects of mental illness and medications but also possibly genetic susceptibility17, while SZA is less studied and the factors underlying the association require elucidation15. PD is relatively less investigated, but a recent national register-based study in Finland found greater all-cause mortality than in nonpsychotic depression, particularly linked to CVDs18.
The SUPER-Finland study19, part of the Stanley Global Neuropsychiatric Genetics initiative, recruited a large sample of participants with different psychotic disorders from Finnish outpatient and inpatient healthcare, with registry-based diagnoses as well as information on BMI and smoking. To elucidate the association between risk factors and comorbidities, we studied the association of different diagnostic categories of psychotic disorders with hypertension, diabetes, IHD, stroke, COPD, cancer, and liver disease (LD) while adjusting for sex and age, and whether this association is explained by differences in obesity and smoking. We hypothesized a direct association of diagnostic category with GMC. Different diagnostic groups were assumed to be associated with different prevalences of the risk factors. Diagnostic group was hypothesized to associate with the comorbidities even after adjustment for current BMI and smoking, but BMI and smoking could explain part of the difference. Therefore, we analyzed total effect models for diagnostic category where the different rates of obesity and smoking are assumed part of the effect of diagnostic category, as well as direct effect models which show the association after adjustment for sex, BMI and smoking. The cross-sectional nature of the data did not permit an analysis of the causal relationships between the variables.
Materials and methods
Study population
The SUPER-Finland research project19 recruited participants with a diagnosis of psychotic illness from psychiatric in- and outpatient care and primary care, and supported housing units in Finland. Participants were also recruited with newspaper advertising. After removal of participants who withdrew consent, as well as removal of duplicates, the sample size was 10417. To be included in the study, a participant had to have a diagnosis of SZ (ICD-10 code F20), SZA (F25), BD (F30, F31), PD (F32.3 or F33.3), or other nonaffective psychotic disorder (ONAP), the ICD-10 codes of which are provided in the supplement. All participants gave written informed consent. Psychiatric diagnosis was the most severe lifetime psychiatric diagnosis received, in the following order of severity: 1. schizophrenia, 2. SAD, 3. BD, 4. psychotic MDD, 5. other psychoses, per the SUPER study protocol. The mean duration of illness in the population is 16.2 years among those aged 18-6520.
Persons under 18 years of age or unable to give informed consent could not participate. The SUPER study was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa (Reference number 202/13/03/00/15).
Participants were interviewed in person at a participating clinic, hospital, or place of residence by research nurses using a pre-set interview form. Height and weight were measured as part of the assessment. Additionally, participants filled in a questionnaire, with items, among others, on smoking history. Phenotype data were linked to data from the Finnish national healthcare registry, the Care Register for Health Care (hyperlink: https://thl.fi/en/web/thlfi-en/statistics-and-data/data-and-services/register-descriptions/care-register-for-health-care). We have previously reported findings on the demographic variables, employment, and living arrangements in the sample20.
Height, weight, BMI, sex, and smoking status
We conceptualized BMI, sex, and smoking status as independent risk factors for GMC diagnoses, but BMI and smoking as also part of the causal pathway from psychiatric illness to GMCs. Participant weight and height were either measured during the interview (8790 and 8669 cases, respectively) or self-reported (897 and 1147 cases, respectively, both measures self-reported in 848 cases). The mean BMI was 30.3 in the group where weight was measured and 30.0 in the self-reporting group, and the mean BMI was 30.4 in the group where height was measured and 29.8 in the self-reporting group. Obesity and overweight were defined by WHO guidelines to be BMI ≥ 30 kg/m2 and BMI ≥ 25 kg/m2, respectively.
Sex was defined in accordance with information in the Finnish population information system and was not queried in the interview or included in the questionnaire.
Information on cigarette smoking was collected using a questionnaire. Lifetime cigarette smoker status was assessed with the question “Have you during your entire life smoked more than 100 cigarettes (5 packages) (or an equivalent amount of cigars/pipe tobacco)” with the response option of no/yes. Current smoking was assessed with the question “How long has it been since you last smoked?” with eight response options ranging from “yesterday or today” to “never”. Current smoker status was assigned to those who reported smoking “yesterday or today”.
GMC diagnoses
All ICD-10, ICD-9, and ICD-8 diagnoses with dates from the healthcare register were read from the Care Register for Health Care data using a previously published algorithm21. Diagnoses were processed using R version 4.2.1 and IBM SPSS 25. Participants were classified as having a particular GMC diagnosis if they had had a healthcare contact where that diagnosis code had been used after the onset of psychotic disorder. Cases where a particular GMC diagnosis was used both before and after onset of psychotic illness were classified in the GMC diagnosis group. The onset of psychotic disorder was the time point of the first of any of the included psychotic disorder diagnoses in the healthcare registers; however, for SZA, BD, and psychotic depression, this could also be the first mood disorder diagnosis if this came before the psychosis diagnosis. All diagnoses available since the year 1969 were used. The registry diagnoses are physician-assigned according to the ICD system.
GMCs were grouped into the studied categories using equivalent ICD-8, ICD-9, and ICD-10 diagnoses. The diagnostic categories and corresponding ICD-10 codes were hypertension (I10), diabetes (E10–E14), COPD (J41–J44), IHD (I20–I25), stroke (I60–I66), cancer (C00–C97), and LD (K70–K77)8,9. We additionally computed the percentage of the study population with BMI over 30 that had been diagnosed with obesity or overweight (E66) as a possible indicator of underdiagnosis.
Missing values
Data were available for 10417 participants. Of the participants, 626 (6.0%) had missing BMI and 152 (1.5%) had missing psychiatric diagnosis. Information on whether the participant had ever smoked 100 cigarettes was missing for 277 cases (2.6%), 127 of which were also missing BMI. Out of the 152 (1.5%) cases missing a psychiatric diagnosis, 8 (5.3%) were missing smoking status and 14 (9.2%) were missing BMI, while 33 (21.7%) had diagnoses of hypertension, 23 (15.1%) diabetes, and 11 (7.2%) stroke.
Statistical analysis
Demographic and illness-related variables were tabulated by diagnostic group. To identify potential correlations between variables, Chi-square tests of independence, one-way analysis of variance, and Pearson or phi correlations between the variables were computed. Covariates for logistic regression were selected based on the literature22 and a hypothetical causal model.
Logistic regression models were fitted using the R package glm (generalized linear model) in R. Model fit was evaluated using Hosmer and Lemeshow’s test and visually. Outliers were excluded due to statistical concerns of model fit and outlier influence. Participants with age under 20 years or over 75 years, or a BMI of less than 18 or more than 50, for a total of 404 participants, were excluded from all analyses to avoid bias. For the final analysis, missing values were imputed with multiple imputation (m = 5) using the R package mice (multivariate imputation using chained equations) for R version 3.15.023 for the following predictor variables: pmm (predictive mean matching) for BMI using active imputation24, polytomous regression imputation for psychiatric diagnostic category, and logistic regression imputation for smoking. GMC diagnoses were not imputed. Multiple imputation was assessed using convergence and strip plots and visual inspection of the imputed data.
Multiply imputed data were analyzed using glm with mice 3.15.0. For sensitivity analysis, models were run for comparison using glm with the original data with observations with missing values excluded. All analyses were performed using R version 4.2.1 and RStudio build 554. Statements about differences between diagnostic groups other than SZ in the multivariate logistic regression results were based on overlap of 95% confidence intervals.
Separate logistic regression models were run 1) for total effects, which did not include BMI and smoking, hypothesized to be part of the causal pathway from mental illness to GMC, and 2) for direct effects adjusted for BMI and smoking.
Results
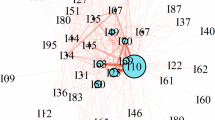
The demographic and diagnosis prevalence results are shown in Table 1, and the overlap of comorbidities can be seen in Fig. 1. Altogether 7414 participants (71.1%) had no comorbidities, and 3003 (28.8%) had at least one of the assessed comorbidities. Furthermore, for stroke, hypertension, diabetes, and IHD, most cases had at least one additional comorbidity. Approximately 42% of participants were current smokers, while ca. 28% were ex-smokers. Risk factors were very prevalent, with the mean BMI of all participants over the threshold of obesity, and even the lowest BMI group (ONAP) over the threshold of overweight. Of those with a BMI ≥ 30, 15.5% had a diagnosis of obesity or overweight. This proportion was 14.6% for SZ, 17.4% for SZA, 19.6% for BD, 15.3% for PD, and 11.1% for ONAP. Using a threshold of BMI ≥ 25, 10.2% of participants with overweight had a diagnosis of obesity or overweight. Current smoking was associated with a slightly lower BMI (mean 29.6 vs 30.9, p < 0.01). This effect was much smaller for lifetime smoking (mean 30.2 vs 30.7, p < 0.01).
Total effect of diagnostic category
There were differences in the rates of all comorbidities between the diagnostic groups. When adjusted for age and sex but not for BMI or smoking, differences emerged between the diagnostic categories relative to SZ in rates of hypertension, diabetes, COPD and stroke. Hypertension was more common in BD, and there was a trend towards increased risk of IHD in the BD group. SZ and SZA were more associated with diabetes and COPD than the other diagnostic categories. Stroke was more associated with SZA and the other psychoses group.
Risk factor variance and direct effect of diagnostic category
There were differences in the prevalence of obesity and overweight. By diagnostic group, the proportion with BMI greater than 30 was 47.1% in SZ, 55.4% in SZA, 47.6% in BD, 43.1% in PD and 34.7% in other psychoses. Mean age and BMI were significantly different between diagnostic groups and likewise between sexes (see Table 1). Females had a history of smoking slightly less often than males.
The differences between diagnostic groups remained when adjusted for smoking and BMI, apart from the reduced association of COPD with other psychoses compared to schizophrenia, indicating that the variance in risk factors between diagnostic groups did not account for the associations. Multivariate logistic regression results from the main analysis are reported in Fig. 2. Multiple imputation affected confidence intervals but did not alter the direction of any results. In general, SZ was associated with a higher risk of comorbidities, but BD was associated with a higher risk of specifically hypertension. However, we did not find significant differences between the diagnostic groups apart from SZ. Results were broadly similar between the total and direct effects models.
Exact logistic regression ORs, Chi-square and ANOVA statistics, Pearson (phi) correlations, and plots of the bivariate logistic regression models for hypertension, diabetes and COPD, as well as the logistic regression models without multiple imputation and with goodness-of-fit and EPV statistics, are reported in the supplement.
Discussion
The SUPER-Finland study included participants with different psychotic disorders, including a large number of participants with SZA and PD, therefore presenting an important opportunity to directly compare participants with these disorders in a large study population. Overall, we found that a significant proportion of the study population had at least one GMC, and risk factors were common. Participants had risk factors more commonly than has been reported for the Finnish general population in the literature. However, there were differences between diagnostic groups in associated comorbidity. The etiology of comorbidity in psychotic disorders is highly complex, and includes the effects of psychiatric medication25, but also metabolic changes in drug-naïve patients26, genetics and behavioral and environmental vulnerabilities27. Below, we describe how our results relate to the general population.
Obesity and smoking
Body mass index has well known limitations as a measure of individual health28, but on the population level, it presents an actionable risk factor for comorbidity and mortality. We found generally high levels of obesity and smoking among SUPER study participants. For comparison, the average BMI in the national FinHealth 2017 health examination survey was 27.329, while the average BMI of our study population was 30.2.
Most participants who were obese did not have a clinical recorded diagnosis of obesity or overweight. By comparison, a register-based study in Denmark found a prevalence of overweight/obesity diagnoses of 10.9% among patients with BMI ≥ 25 kg/m2,30, while in Finland a recent study noted a prevalence of 2.7% for register-based obesity diagnoses versus a 26.1% survey prevalence (BMI ≥ 30 kg/m2)31.
Participants with SZ were more likely to have smoked and to currently smoke. It is known that individuals with SZ smoke more often and more intensely than the general population, a finding that has been suggested to result from a common genetic factor between SZ and tobacco smoking32, self-medication, or a shared vulnerability factor33. Equally high rates of smoking have been reported for BD and lower rates for MDD in a comparative study34. Similarly, we found a lower rate of smoking among individuals with PD than among those with SZ, SZA, and BD.
In 2017, the prevalence of smoking in Finland was 23% among men and 16.1% among women aged 30-59 years (12% total), while 49.6% of men were never-smokers35. By comparison, 70% of our population had smoked in the past and 42% had smoked today or yesterday.
Comorbidities
In general, compared to the other diagnostic groups, SZ was associated with an elevated risk of diabetes and COPD, which persisted after adjusting for the risk factors, while BD was associated with an elevated risk of hypertension. Stroke was more common in the SZA and ONAP groups.
Diabetes
We found a higher prevalence of diabetes (12.8%) than has been reported in the hospital discharge registry for the general population. By comparison, a previous study of Finnish healthcare registry data36 found a prevalence of 8.5% for diagnoses of diabetes. A study based on a general population health examination survey reported an even higher prevalence of type II diabetes T2DM in people with SZ (22.0%) but a lower prevalence of T2DM in people with affective psychoses (3.4%)37 We found that SZ was associated with a higher prevalence of diabetes than diagnostic groups other than SZA, even in the direct effects model adjusted for BMI. An important risk factor not addressed is antipsychotic medication25,38.
Hypertension, IHD, and stroke
Earlier studies have reported rates of 12-16.8% of hypertension diagnoses31,39, compared with the 12.4% in our study. Therefore, unlike diabetes, we did not note a higher level of hypertension diagnoses than in the literature. This might reflect an underdiagnosis of hypertension, considering that risk factors for hypertension were more prevalent in our study population than in the general population.
BD was associated with a higher risk of hypertension than SZ. BD has specifically been associated with cardiovascular disease, an association that has historically predated the use of antipsychotics and lithium40 A recent study of healthcare encounter data found higher 10-year cardiovascular risk scores for BD and 30-year cardiovascular risk scores for SZA based on metabolic data after demographic adjustment41.
We also found a trend towards risk of IHD and a trend towards a higher risk of stroke in BD, although the latter association did not reach statistical significance. Furthermore, SZA and ONAP were associated with increased prevalence of stroke. It should be noted that reverse causation is also possible regarding stroke since post-stroke mania is a rare complication of stroke42, likewise post-stroke psychosis43.
The recent PERFECT Stroke database, a comprehensive survey of national registries, revealed a 1.5% prevalence of living with stroke in Finland44. Thus, stroke was more common in our study population than in the Finnish general population, consistent with a finding previously reported by a large UK-based study45.
COPD
The rate of COPD stages II-IV, as measured by spirometry, has been reported as 4.3% in men and 3.1% in women in the general population in a recent study46. Thus, we report a higher number of COPD diagnoses for participants with SZ (6.7%), despite relying on register diagnoses. A population-based study of people with psychotic disorders using spirometry found a COPD prevalence of up to 12.2% for SZ and 12.4% for affective psychoses47.
We noted a higher prevalence or a trend towards a higher prevalence of COPD in SZ than in the other diagnostic groups. Recent studies have found that individuals with SZ may smoke more cigarettes48,49 and extract more nicotine from a cigarette than healthy smokers49. A previous study has also reported higher cotinine levels in patients with SZ than in those with SZA50. Therefore, intensity of smoking, which was not accounted for in the multivariate logistic regression analysis, may explain part of the connection.
Cancer
The Finnish Cancer Registry reported an overall prevalence of cancer of 5240.4 per 100 000 in 2019, slightly less than in our study population. There were no significant differences between diagnostic categories after age adjustment.
In summary, we found higher overall prevalences of diabetes, COPD, cancers, and stroke than have been reported for the general population using the Finnish Hospital Discharge Register, but not necessarily higher rates of LD or hypertension. One explanation may be that these conditions are underdiagnosed among those living with psychotic disorders.
Strengths and limitations of the study
The main strength of this study was the large number of participants with registry-based diagnoses of serious mental illness and GMCs as well as information on risk factors and measured BMI. We were able to study a large population of participants with clinician-diagnosed psychotic disorders and were able to include known risk factors, which differed by diagnostic group, on GMCs, and study the associations between comorbidities and psychiatric diagnoses when adjusted for these risk factors. Our study opens follow-up possibilities such as associating medication data with data on GMC diagnoses to investigate whether, for example, antipsychotic use could be responsible for part of the association or examining the association between cognition and GMCs.
A limitation of our study is that the sampling strategy may result in over-representation of the chronically ill, as participants are recruited from healthcare settings. This limits the generalizability of the results to the general population, although participants may be representative of the clinical population currently being treated for serious mental illness. We have previously reported that the study participants represented, on average, individuals with long-term illness20. Additionally, the Care Register for Health Care does not include primary care diagnoses that have not been recorded in specialist healthcare.
There are inherent limitations to the use of healthcare registry data compared with survey data, as healthcare registry data represents diagnoses rather than conditions. However, Finnish health care registry data has previously shown good reliability for schizophrenia51 and bipolar disorder52 A particular issue is the reliability of diagnoses of schizoaffective disorder and psychotic MDD. Diagnostic shifts that occur may reflect disease progression or later reassessment53; the highest rate of diagnostic conversion from unipolar depression to psychotic disorders is in the first years after diagnosis54. Together with the hierarchical most severe lifetime rather than latest diagnosis, most diagnostic shifts may have already occurred in the population. Polygenic risk scores in the SUPER study have shown clustering by diagnostic category55.
A significant limitation is not including the role of medication in the models. Psychiatric medication is well-known to affect weight and metabolism, an effect which additionally is mitigated by certain medications25. Antipsychotic polypharmacy56 and dose57 are additional factors that must be considered. Unfortunately, our data did not permit a nuanced study of past medication use. Therefore, the extent to which differences in pharmacotherapy contribute to the similarities and differences in comorbidity between disorders is unknown. However, pharmacotherapies prescribed for different psychotic disorders are overlapping, and we therefore find strong confounding unlikely.
Finally, our sample was a cross-sectional study of participants years to decades after illness onset and does not include follow-up data or information about the presence of the risk factors over the participants’ lifetime. Therefore, we cannot offer an analysis of the causal pathway from psychotic illness to the comorbidities and are limited to offering an analysis of associations in the cross-sectional sample. Present BMI or past smoking does not capture the individual’s lifetime exposure to overweight and smoking, or whether these risk factors were present prior to the onset of psychotic illness or the studied comorbidity. Our results represent associations found in a clinical population, but more nuanced data is needed to elucidate the mechanisms of the association. A lack of a control group without psychotic illness also means the associations with diagnostic group found represent increased or decreased risk relative to the schizophrenia group only.
Conclusions
GMCs and risk factors were prevalent among this large national sample of persons with psychotic illness, recruited mostly from current care settings. Our results showed that there are differences in the prevalence of GMCs between psychotic disorders, and that prevention of general medical comorbidities is a priority in all categories of psychotic illness, not only schizophrenia. The effect of psychotic illness on physical comorbidities was not mediated only by obesity and smoking in our study. Our study highlights the need for continued efforts to combat risk factors for GMCs among those with psychotic disorders regardless of diagnostic category.
Data availability
The SUPER-Finland website can be accessed for further information (https://www.superfinland.fi/english). The data from SUPER-Finland participants who gave biobank consent can be acquired from the THL Biobank when released from the original study (https://thl.fi/en/web/thl-biobank).
Abbreviations
- SZ:
-
schizophrenia
- SZA:
-
schizoaffective disorder
- BD:
-
bipolar disorder
- PD:
-
psychotic depression
- GMCs:
-
general medical comorbidities
- BMI:
-
body mass index
- COPD:
-
chronic obstructive pulmonary disease
- CVD:
-
cardiovascular disorders
- IHD:
-
ischemic heart disease
- LD:
-
liver disease
- ONAP:
-
other nonaffective psychotic disorders
- OR:
-
odds ratio
- ANOVA:
-
analysis of variance
- EPV:
-
events per variable.
References
Vancampfort, D. et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: A systematic review and meta-analysis. World Psych. https://doi.org/10.1002/wps.20252 (2015).
Correll, C. U. et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 16, 163–180 (2017).
Tanskanen, A., Tiihonen, J. & Taipale, H. Mortality in schizophrenia: 30-year nationwide follow-up study. Acta Psychiatr. Scand. 138, 492–499 (2018).
Laursen, T. M. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr. Res 131, 101–104 (2011).
Laursen, T. M. et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic Countries. PLoS One 8, e67133 (2013).
Chang, C. K. et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One 6, 19590 (2011).
Correll, C. U. et al. Mortality in people with schizophrenia: a systematic review and meta‐analysis of relative risk and aggravating or attenuating factors. World Psychiatry 21, 248 (2022).
Crump, C., Winkleby, M. A., Sundquist, K. & Sundquist, J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am. J. Psychiatry 170, 324–333 (2013).
Crump, C., Sundquist, K., Winkleby, M. A. & Sundquist, J. Comorbidities and mortality in bipolar disorder: a swedish national cohort study. JAMA Psychiatry 70, 931–939 (2013).
Momen, N. C. et al. Mortality associated with mental disorders and comorbid general medical conditions. JAMA Psychiatry 79, 444–453 (2022).
De Leon, J. & Diaz, F. J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res 76, 135–157 (2005).
Heffner, J. L., Strawn, J. R., Delbello, M. P., Strakowski, S. M. & Anthenelli, R. M. The co-occurrence of cigarette smoking and bipolar disorder: Phenomenology and treatment considerations. Bipolar Disorders. https://doi.org/10.1111/j.1399-5618.2011.00943.x (2011).
Mitchell, A. J. et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr. Bull. 39, 306 (2013).
Scott, K. M. et al. Association of mental disorders with subsequent chronic physical conditions: world mental health surveys from 17 countries. JAMA Psychiatry 73, 150–158 (2016).
Bartoli, F., Crocamo, C., Caslini, M., Clerici, M. & Carrà, G. Schizoaffective disorder and metabolic syndrome: A meta-analytic comparison with schizophrenia and other non-affective psychoses. J. Psychiatr. Res 66–67, 127–134 (2015).
Goldstein, B. I., Schaffer, A., Wang, S. & Blanco, C. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J. Clin. Psychiatry 76, 163–169 (2015).
Goldstein, B. I. et al. Call to action regarding the vascular-bipolar link: A report from the Vascular Task Force of the International Society for Bipolar Disorders. Bipolar Disord. 22, 440–460 (2020).
Paljärvi, T. et al. Mortality in psychotic depression: 18-year follow-up study. Brit. J. Psych. 222, 37–43 (2023).
Lähteenvuo, M. et al. Cohort profile: SUPER-Finland - The Finnish study for hereditary mechanisms of psychotic disorders. BMJ Open 13, e070710 (2023).
Ahti, J. et al. Differences in psychosocial functioning between psychotic disorders in the Finnish SUPER study. Schizophr. Res 244, 10–17 (2022).
Suokas, K. Suokas, K. hilmo_identify_episodes (1.0.2) [source code]. vol. 2020 https://github.com/kmmsks/hilmo_identify_episodes (2020).
MacMahon, S. et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373, 1083–1096 (2009).
van Buuren, S. & Groothuis-Oudshoorn, K. Mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Wagstaff, D. A., Kranz, S. & Harel, O. A preliminary study of active compared with passive imputation of missing body mass index values among non-Hispanic white youths. Am. J. Clin. Nutr. 89, 1025 (2009).
Akinola, P. S., Tardif, I. & Leclerc, J. Antipsychotic-induced metabolic syndrome: a review. Metab. Syndr. Relat. Disord. 21, 294–305 (2023).
Harris, L. W. et al. Schizophrenia: Metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology 38, 752–766 (2013).
Vessels, T. et al. Integrating electronic health records and polygenic risk to identify genetically unrelated comorbidities of schizophrenia that may be modifiable. Biol. Psychiatry Glob. Open Sci. 4, 100297 (2024).
Rubino, F. et al. Lancet diabetes & endocrinology commission on the definition and diagnosis of clinical obesity. Lancet Diab. Endocrinol. 11, 226–228 (2023).
Vesikansa, A. et al. Obesity and metabolic state are associated with increased healthcare resource and medication use and costs: a Finnish population-based study. Eur. J. Health Econ. https://doi.org/10.1007/S10198-022-01507-0 (2022).
Gribsholt, S. B., Pedersen, L., Richelsen, B. & Thomsen, R. W. Validity of ICD-10 diagnoses of overweight and obesity in Danish hospitals. Clin. Epidemiol. 11, 845–854 (2019).
Tolonen, H. et al. Cross-national comparisons of health indicators require standardized definitions and common data sources. Arch. Public Health 79, 1–14 (2021).
Hartz, S. M. et al. Genetic correlation between smoking behaviors and schizophrenia. Schizophr. Res 194, 86 (2018).
Peterson, R. E. et al. Genome-wide analyses of smoking behaviors in schizophrenia: Findings from the Psychiatric Genomics Consortium. J. Psychiatr. Res 137, 215–224 (2021).
Li, X. H. et al. Prevalence of smoking in patients with bipolar disorder, major depressive disorder and schizophrenia and their relationships with quality of life. Sci. Rep. 7, 1–7 (2017).
Vartiainen, V. A., jousilahti, P., Laatikainen, T. & Vartiainen, E. Contribution of smoking change to 45-year trend in prevalence of chronic bronchitis in Finland. Scand. J. Public Health https://doi.org/10.1177/14034948221104351/ASSET/IMAGES/LARGE/10.1177_14034948221104351-FIG1.JPEG (2022).
Tolonen, H. et al. Cross-national comparisons of health indicators require standardized definitions and common data sources. Arch. Public Health 79, 208 (2021).
Suvisaari, J. et al. Type 2 diabetes among persons with schizophrenia and other psychotic disorders in a general population survey. Eur. Arch. Psychiatry Clin. Neurosci. 258, 129–136 (2008).
Kumar, R. B. & Aronne, L. J. Iatrogenic obesity. Endocrinol. Metab. Clin. North Am. 49, 265–273 (2020).
Koponen, P. et al. Prevalence of hypertension and diabetes in Finland by different data sources. Eur J Public Health. 29, 167 (2019).
Goldstein, B. I., Fagiolini, A., Houck, P. & Kupfer, D. J. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 11, 657 (2009).
Rossom, R. C., Hooker, S. A., O’Connor, P. J., Crain, A. L. & Sperl-Hillen, J. M. Cardiovascular Risk for Patients With and Without Schizophrenia, Schizoaffective Disorder, or Bipolar Disorder. J. Am. Heart Assoc. 11, 21444 (2022).
Santos, C. O., Caeiro, L., Ferro, J. M. & Figueira, M. L. Mania and stroke: a systematic review. Cerebrovasc. Dis. 32, 11–21 (2011).
Stangeland, H., Orgeta, V. & Bell, V. Poststroke psychosis: a systematic review. J. Neurol. Neurosurg. Psychiatry 89, 879–885 (2018).
Meretoja, A. PERFECT Stroke: PERFormance, Effectiveness, and Costs of treatment episodes in Stroke. (2011).
Osborn, D. P. J. et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database. Arch. Gen. Psychiatry 64, 242–249 (2007).
Vasankari, T. M. et al. No increase in the prevalence of COPD in two decades. Eur. Respiratory J. 36, 766–773 (2010).
Partti, K. et al. Lung function and respiratory diseases in people with psychosis: Population-based study. Br. J. Psychiatry 207, 37–45 (2015).
Šagud, M. et al. Nicotine dependence in Croatian male inpatients with schizophrenia. BMC Psychiatry 18, 18 (2018).
Williams, J. M. et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob. Res. 12, 855 (2010).
Tidey, J. W. & Rohsenow, D. J. Smoking expectancies and intention to quit in smokers with schizophrenia, schizoaffective disorder and non-psychiatric controls. Schizophr. Res 115, 310 (2009).
Pihlajamaa, J. et al. The validity of schizophrenia diagnosis in the Finnish Hospital Discharge Register: Findings from a 10-year birth cohort sample. Nord J Psychiatry. 62, 198–203 (2009).
Kieseppa, T., Partonen, T., Kaprio, J. & Lonnqvist, J. Accuracy of register- and record-based bipolar I disorder diagnoses in Finland; a study of twins. Acta Neuropsychiatr. 12, 106–109 (2000).
Fusar-Poli, P. et al. Diagnostic Stability of ICD/DSM First Episode Psychosis Diagnoses: Meta-analysis. Schizophr. Bull. 42, 1395–1406 (2016).
Baryshnikov, I. et al. Diagnostic conversion from unipolar depression to bipolar disorder, schizophrenia, or schizoaffective disorder: A nationwide prospective 15-year register study on 43 495 inpatients. Bipolar Disord. 22, 582–592 (2020).
Kämpe, A. et al. Genetic contribution to disease-course severity and progression in the SUPER-Finland study, a cohort of 10,403 individuals with psychotic disorders. Mol. Psychiatry 7, 1–9 (2024).
Ijaz, S. et al. Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry 18, 275 (2018).
Wu, H. et al. Antipsychotic-induced weight gain: dose-response meta-analysis of randomized controlled trials. Schizophr. Bull. 48, 643 (2022).
Acknowledgements
We would like to thank the collaborating SUPER study group: Aarno Palotie, Erik Cederlöf, Mark Daly, Amanda Elliott, Willehard Haaki, Katriina Hakakari, Elina Hietala. Jarmo Hietala, Steve Hyman, Tuomas Jukuri, Risto Kajanne, Jaakko Keinänen, Martta Kerkelä, Tuomo Kiiskinen, Aija Kyttälä, Kaisla Lahdensuo, Sari Lång-Tonteri, Nina Lindberg, Jonne Lintunen, Jouko Lönnqvist, Ville Mäkipelto, Teemu Männynsalo, Atiqul Mazumder, Zuzanna Misiewicz, Julia Moghadampour, Arto Mustonen, Benjamin Neale, Solja Niemelä, Jussi Niemi-Pynttäri, Olli Pietiläinen, Susanna Rask, Noora Ristiluoma, Elmo Saarentaus, Anssi Solismaa, Andre Sourander, Heidi Taipale, Marjo Taivalantti, Antti Tanskanen, Auli Toivola, Lea Urpa, Imre Västrik, and Juha Veijola for the SUPER study data and support. The SUPER-Finland study has been funded by the Stanley Global Neuropsychiatric Genetics initiative.
Author information
Authors and Affiliations
Contributions
Author JA conducted literature searches and all the statistical analyses, plus drafted the manuscript as the first author. Authors TK and EI supervised statistical analyses and writing of the manuscript, and study design with authors JA, JS and SN. Authors TK, WH, JS, SN, KS, MH, AW, OK, ML, TP, JT, JH and EI have contributed to the national collection of the SUPER-Finland data. All authors have critically reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahti, J., Kieseppä, T., Haaki, W. et al. General medical comorbidities in psychotic disorders in the Finnish SUPER study. Schizophr 10, 124 (2024). https://doi.org/10.1038/s41537-024-00546-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-024-00546-1