Abstract
The identification of poliovirus in stool samples post-Oral Poliovirus Vaccine (OPV) immunization is essential for implementing post-eradication monitoring of poliomyelitis. This research presents the inaugural assessment of the virus shedding rate and the genetic diversity of OPV shedding strains across different Inactivated Poliovirus Vaccine (IPV)-OPV sequential immunization schedules. Our findings revealed that the shedding rate of different serotypes in each sequential immunization groups peaked within 7 days following the initial administration of OPV, and then gradually decreased. Prior vaccination with OPV reduced the rate and shortened the duration of virus shedding. Additionally, we observed a slight increase in the shedding rate of type3 after the removal of type2 from trivalent oral poliovirus vaccine (tOPV). The comprehensive analysis of the whole-genome high-throughput sequencing results for the shedding strain revealed that the variation sites among samples from different sequential immunization groups were distributed throughout the entire genome. The mutation frequencies within the 5’NCR, 2 C, 3 A, 3 C, and 3D regions were elevated of type1 and type3, while type2 had higher frequencies within the 5’NCR, VP1 and 3D regions. Consequently, it is imperative to expedite the transition from OPV to IPV, and to discontinue OPV as soon as wild poliovirus strains and vaccine-derived poliovirus (VDPVs) are eliminated.
Similar content being viewed by others
Introduction
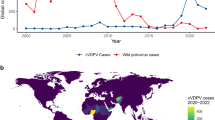
Since the global initiative to eradicate polio was launched in 1988, the worldwide incidence of polio has decreased by over 99%1. Currently, nations use full dose Inactivated Poliovirus Vaccine (IPV) or IPV-Oral Poliovirus Vaccine (OPV) sequential immunization in their routine immunization schedule2. Both IPV and OPV are capable of inducing humoral immunity; however, OPV also promotes mucosal immunity and provides superior primary intestinal immunity compared to IPV, thereby more effectively preventing the transmission of poliovirus3. Nevertheless, the genetic instability associated with OPV presents challenges to eradication efforts4,5. Although the vaccine strain is unable to invade the central nervous system following attenuation, it retains a significant reproductive capacity within the intestine6. Vaccine strains replicate in populations with inadequate immunisation levels for an extended period and reverted to neurovirulence on rare occasions, causing the outbreaks of vaccine-derived poliovirus (VDPV). VDPV exhibits similar pathogenic and transmission characteristics to wild poliovirus (WPV)7,8. Typically, when children receive OPV, they and their close contacts can transmit the virus through feces and secretions within a week, with this transmission potentially continuing for several weeks9,10. Consequently, the replication of vaccine strains may result in the loss of attenuating mutations. In rare cases, this reverted OPV can circulate within the community as a circulating vaccine-derived poliovirus (cVDPV), resulting in poliomyelitis outbreaks in areas with low vaccination coverage11. In recent years, the incidence of WPV has declined, with wild poliovirus type2 (WPV2) and wild poliovirus type3 (WPV3) have been eradicated; however, wild poliovirus type1 (WPV1) continues to cause disease in certain regions. The latest polio data from the World Health Organization (WHO) indicates that between 2018 and 2023, the annual number of WPV cases reported were 33, 176, 140, 6, 30, and 12, while cVDPV cases were 105, 378, 1107, 688, 879, and 527. These statistics suggest that the majority of global paralytic poliomyelitis cases are now attributed to poliovirus shedding from OPV rather than WPV, with circulating vaccine-derived poliovirus type 2 (cVDPV2) accounting for the highest number of cases12.
The eradication of WPV2 and the rise in reported cases of cVDPV2 have led to the removal of poliovirus type2 (PV2) from OPV. To mitigate the risk of compromised population immunity to PV2 and the heightened risk of vaccine-associated paralytic poliomyelitis (VAPP), particularly following the initial OPV dose13. WHO mandated that all countries currently using OPV only should be added at least 1 dose of IPV to the polio vaccine schedule. IPV is recognized as a safe and effective alternative for polio control and eradication, as it eliminates the risks associated with VAPP and VDPV14. However, IPV has been shown to induce only limited primary intestinal immunity and does not effectively halt viral replication upon subsequent exposure to either the vaccine virus or through fecal-oral transmission of poliovirus15,16. Furthermore, mucosal immunity has been observed to decline following the transition of the immunization schedule17. This deficiency in mucosal immunity presents a considerable challenge to the eradication of polio and the prevention of poliovirus transmission. As a result, the immunization schedule in most countries that only use OPV shifted from the full dose trivalent oral poliovirus vaccine (tOPV) to a regimen consisting of one dose of IPV (type1, 2, and 3) followed by three doses of bivalent oral poliovirus vaccine (bOPV, type1 and 3). Considering it is essential for adequate humoral immunity provided by at least two doses of IPV, the WHO recommended to add one dose of IPV in the existing sequential immunization schedule 1IPV + 3bOPV and converted into 2IPV + 2bOPV in 20193,18,19.
During transitional period of IPV-OPV sequential immunization schedule, genetic variations in the attenuated strains present a considerable public health challenge20. The primary concern associated with OPV is the genetic instability of RNA viruses, which can lead to the emergence of mutant strains during in vivo replication, potentially reverting to a neurovirulence level that surpasses of the attenuated poliovirus. Early studies have shown that virulence-related sites in Sabin strains are not limited to the specific regions of 5’NCR, but are instead distributed throughout the entire genome21. The detection of poliovirus in environmental sewage and stool samples after OPV immunization can provide insights into the immunogenicity of mucosal immunity and the surveillance of poliomyelitis post-eradication. This information is vital for halting polio transmission. In this study, we collected stool samples at different stages following OPV immunization, and identified poliovirus in these samples to examine the shedding in different sequential immunization schedules. Furthermore, 20 poliovirus-positive samples from each group were chosen for whole-genome high-throughput sequencing to evaluate the genetic stability of shedding strains. Understanding the dynamics of shedding and the genetic stability of these strains in infants is of paramount importance for the global initiative to eradicate polio.
Results
We enrolled 1200 healthy 2-month-old infants and randomized assigned into 6 sequential immunization schedules: 1sIPV + 2bOPV(immunization with sIPV, bOPV, bOPV at 2, 3, and 4 months old), 1cIPV + 2bOPV(immunization with cIPV, bOPV, bOPV at 2, 3, and 4 months old), 2sIPV + 1bOPV(immunization with sIPV, sIPV, bOPV at 2, 3, and 4 months old), 2cIPV + 1bOPV(immunization with cIPV, cIPV, bOPV at 2, 3, and 4 months old), 2sIPV + 1tOPV(immunization with sIPV, sIPV, tOPV at 2, 3, and 4 months old), and 2cIPV + 1tOPV(immunization with cIPV, cIPV, tOPV at 2, 3, and 4 months old). The schematic diagram of the immunization schedule and sample collection time is shown in Fig. 1. Additionally, we collected stool samples from the first 10% of participants in each group prior to the second dose, as well as on days 7, 14, and 28 following the second and third doses of the polio vaccine, resulting in a total of 7 sampling occasions. The baseline characteristics across the six groups are presented in Fig. 2, indicating no statistically significant differences among these sequential immunization schedules.
a–c The sex, weight, and ethnic distribution of infants in each sequential immunization group. d–f The number of seropositive and negative infants of type1 (d), type2 (e), type3 (f) in each group before immunization. g–i Serum neutralizing antibody of type1 (g), type2 (h), type3 (i) for polio in each group before immunization.
Number of shedding infants following OPV immunization
We gathered a total of 802 stool samples, from which 249 poliovirus-positive samples were successfully isolated utilizing two distinct cell lines: human RD (rhabdomyosarcoma) and L20B (a transgenic mouse cell line that expresses the human poliovirus receptor). The number of positive samples in each immunization group were presented in Fig. 3. Ongoing surveillance of stool shedding in infants post-immunization with OPV indicated that more than 80% of infants in each group, who received the initial dose of bOPV or tOPV, tested positive for poliovirus in their stool. Notably, 102 out of 112 stool samples from participants who received the first OPV dose were poliovirus-positive on day 7. The rate of poliovirus shedding decreased on days 14 and 28 following OPV immunization in these groups; however, over 10% of infants continued to shed poliovirus. This suggested that viral shedding may persist for several weeks. Furthermore, we noted that the shedding rate following the second dose of bOPV was significantly lower compared to the first dose within the same observation period.
a The poliovirus shedding rate of 1sIPV + 2bOPV sequential immunization group. b The poliovirus shedding rate of 1cIPV + 2bOPV sequential immunization group. c The poliovirus shedding rate of 2sIPV+bOPV sequential immunization group. d The poliovirus shedding rate of 2cIPV+bOPV sequential immunization group. e The poliovirus shedding rate of 2sIPV + 1tOPV sequential immunization group. f The poliovirus shedding rate of 2cIPV + 1tOPV sequential immunization group.
Virus shedding of different serotypes following OPV immunization
To further investigate the various serotypes of poliovirus shedding following immunization with bOPV or tOPV, we implemented a gold standard assay utilizing mixed antisera pools to accurately type the positive isolate after evaluating the virus titers of the positive samples. The serotype distribution of poliovirus-positive samples across each group is illustrated in Fig. 4. For type1, with the exception of the 2cIPV + 1tOPV group, the virus shedding rate in other groups were below 60% on day 7 and dropped to below 20% on day 28 after the initial dose of bOPV or tOPV (Fig. 4a, b). Type2 was exclusively observed in 2sIPV + 1tOPV and 2cIPV + 1tOPV groups, with shedding rates surpassing 60% on day 7 after the first tOPV dose, before experiencing a rapid decline by day 14 (Fig. 4c, d). For type3, the shedding rate in 1sIPV/1cIPV + 2bOPV and 2sIPV/2cIPV + 1bOPV groups was higher than that in 2sIPV/2cIPV + 1tOPV groups (Fig. 4e, f). In addition, we observed that the shedding rate of different serotypes in each sequential immunization group reached their peak within 7 days post the initial administration of bOPV or tOPV, followed by a gradual decrease (Fig. 4g–j). While the shedding rate of type1 in 1sIPV + 2bOPV and 1cIPV + 2bOPV groups exhibited a slight increase on day 7 following the second dose of bOPV immunization (Fig. 4g, h). Additionally, we noted a slight increase in the shedding rate of type3 after the removal of type2 from tOPV (Fig. 4k, l).
a The poliovirus shedding rate of type1 in sIPV/OPV sequential immunization group after the first dose of OPV. b The poliovirus shedding rate of type1 in cIPV/OPV sequential immunization group after the first dose of OPV. c The poliovirus shedding rate of type2 in sIPV/OPV sequential immunization group. d The poliovirus shedding rate of type2 in cIPV/OPV sequential immunization group. e The poliovirus shedding rate of type3 in sIPV/OPV sequential immunization group after the first dose of OPV. f The poliovirus shedding rate of type3 in cIPV/OPV sequential immunization group after the first dose of OPV. g The poliovirus shedding rate of type1 in 1sIPV + 2bOPV group after immunization with bOPV. h The poliovirus shedding rate of type1 in 1cIPV + 2bOPV group after immunization with bOPV. i The poliovirus shedding rate of type3 in 1sIPV + 2bOPV group after immunization with bOPV. j The poliovirus shedding rate of type3 in 1cIPV + 2bOPV group after immunization with bOPV. k, l The rates of poliovirus shedding across each sequential immunization group.
Subsequently, we evaluated the poliovirus shedding among various sequential immunization following the administration of the initial dose of bOPV or tOPV. In sIPV/OPV sequential immunization schedules, our findings indicated no significant differences in type1 shedding among the different immunization schedules. In comparison with 1sIPV + 2bOPV group, we noted a marked reduction in the type3 shedding rate following the first dose of OPV in 2sIPV + 1tOPV group (Table 1). In evaluating the type1 and type3 shedding rates across diverse immunization groups within cIPV/OPV sequential immunization schedules, we found no significant differences among these immunization groups (Supplementary Table 1). Furthermore, there were no significant differences between the same immunization schedules of sIPV/OPV sequential immunization groups and cIPV/OPV sequential immunization groups (Table 2).
Sequencing of positive stool samples
The mutant strains were classified as VDPVs when there is ≥1% nucleotide sequence mutation in viral protein 1 (VP1) compared to the parental Sabin reference strain for poliovirus types1 and 3, and a 0.6% nucleotide variation for poliovirus type2. To enhance our understanding of the genetic variation in shedding strains, we selected 20 poliovirus-positive samples from each group, resulting in a total of 120 stool samples from six groups that underwent high-throughput sequencing. We obtained 61 high-quality gene sequences, including 5 samples from 1sIPV + 2bOPV group, 14 samples from 2sIPV + 1bOPV group, 11 samples from 2sIPV + 1tOPV group, 10 samples from 1cIPV + 2bOPV group, 11 samples from 2cIPV + 1bOPV group, and 10 samples from 2cIPV + 1tOPV group.
The analysis of sequence alignment in the genome of poliomyelitis vaccine shedding strains reveals that mutation sites from different sequential immunization groups were distributed throughout the entire genome. Sequencing data indicated that mutation frequency is particularly elevated in the 5’NCR, 2 C, 3 A, 3 C, and 3D regions of type1 and type3, with the highest mutation rate found in the 3D region (Fig. 5a,c). While type2 had higher frequencies within the 5’NCR, VP1 and 3D regions (Fig. 5b). Analyzing the mutation ratios at specific sites in stool samples from the 2sIPV + 1bOPV and 2cIPV + 1bOPV groups indicated that the majority of high base content mutation sites are concentrated in the 5’NCR, 3 A, 3B, and 3D regions (Fig. 5d–g).
a–c Mutation of different regions of type1 (a), type2 (b), type3 (c) in the whole genome of shedding strain of different sequential immunization groups. d, e Comparison of mutation ratio of type1 within 2sIPV+bOPV group (d) and 2cIPV+bOPV group (e). f, g Comparison of mutation ratio of type3 within 2sIPV+bOPV group (f) and 2cIPV+bOPV group (g). Each dot represents a poliovirus-positive stool sample.
Characteristics of mutation hotspots in the shedding strain
To further investigate the genetic variation patterns and frequencies of nucleotide variation sites in shedding strains, we evaluated mutation hotspots of Sabin strain. In type1 of shedding strains, we identified five mutation hotspots: nt480, nt525, nt2747, nt2749, and nt6203, with mutation rates of 74.2%, 67.7%, 41.9%, 35.5%, and 48.4%, respectively. Notably, mutations at nt2747, nt2749, and nt6203 resulted in corresponding changes in coding amino acids. For type2 poliovirus shedding strains, 12 samples exhibited mutations at the nt481 site, while five samples showed an A to G transition at the nt2908 site. Additionally, 8 samples displayed mutations at nt2909, where T mutated to C/A. Significant amino acid changes occurred after mutations at the nt2908 and nt2909 site. In type3 poliovirus shedding strains, we identified three mutation hotspots: nt472, nt2034, and nt2636, with mutations at nt2034 and nt2636 leading to amino acid alterations (Table 3).
Ile Isoleucine, Leu Leucine, Met Methionine, His Histidine, Tyr Tyrosine, Val Valine, Thr Threonine, Asn Asparagine, Phe Phenylalanine, Ser Serine, Ala Alanine.
Analysis of point mutation rates in the entire genome of shedding strains across different sequential immunization groups revealed no significant differences in gene mutation rates for type1 between 2sIPV + 1bOPV and 2cIPV+bOPV groups, as well as between 1cIPV + 2bOPV and 2cIPV + 1bOPV groups (Fig. 6a, b). Additionally, when examining the gene mutation rate for type3 among different groups, we observed no significant differences between 1sIPV + 2bOPV and 2sIPV + 1bOPV groups, 1cIPV + 2bOPV and 2cIPV + 1bOPV groups, and 2sIPV + 1bOPV and 2cIPV + 1bOPV groups (Fig. 6c–e). By analyzing the cumulative rate of mutations at key attenuated sites, including the G480A and U525C sites for type1 and the U472C site for type3, the mean mutation rate of 480-A + 525-C in type1 was 29.4%, while the mean mutation rate of 472-C in type3 reached 89.2% (Fig. 6f, g).
a P-value distribution of mutation ratio of type1 between 2sIPV+bOPV group and 2cIPV+bOPV group. b P-value distribution of mutation ratio of type1 between cIPV+2bOPV group and 2cIPV+bOPV group. c P-value distribution of mutation ratio of type3 between sIPV+2bOPV group and 2sIPV+bOPV group. d P value distribution of mutation ratio of type3 between cIPV+2bOPV group and 2cIPV+bOPV group. e P value distribution of mutation ratio of type1 between2sIPV+bOPV group and 2cIPV+bOPV group. f The mean mutant rate of 480-A + 525-C in type1. g The mean mutant rate of of 472-C in type3.
In addition, we compared the variations in gene mutations at mutation hotspots among shedding strains following immunization with sIPV/OPV. The analysis revealed statistically significant differences in the number of mutations only at the nt525 site in type1 between 1sIPV + 2bOPV group and 2sIPV + 1bOPV group. Furthermore, when contrasting the IPV/bOPV and IPV/tOPV sequential immunization groups, notable differences emerged in the count of mutation samples at nt525 and nt2747 in type1 between 2sIPV + 1bOPV and 2sIPV + 1tOPV groups (Table 4). When comparing 1cIPV + 2bOPV group to 2cIPV + 1bOPV group, significant differences appeared only at the nt2749 site in type1. Additionally, when comparing 2cIPV+bOPV and 2cIPV+tOPV group, significant differences were observed in the number of mutation samples at nt2034 in type3 (Supplementary Table 2). Comparison between sIPV/OPV and cIPV/bOPV, the difference in the number of mutations was only statistically significant at the nt525 in type1 (Table 5).
Effect of humoral immunity and intestinal mucosal immunity on viral shedding
To investigate the effects of humoral immunity on poliovirus shedding following OPV immunization, we assessed serum-neutralizing antibodies in poliovirus positive or negative infants. Our analysis revealed no significant differences in serum neutralizing antibodies relative to poliovirus shedding (Fig. 7a). Additionally, we investigated the influence of serum-neutralizing antibodies on the duration of virus shedding, yielding comparable results (Fig. 7b). Furthermore, we assessed the levels of polio-specific intestinal mucosal IgA in both poliovirus positive and negative infants (Fig. 7c–e). For type1, the levels of polio-specific intestinal mucosal IgA in poliovirus negative infants were significantly higher than those in poliovirus positive infants on days 7 and 28 following the second dose of immunization (Fig. 7c). For type2, poliovirus positive infants exhibited lower levels of polio-specific intestinal mucosal IgA compared to their negative counterparts at various intervals after the third dose of immunization (Fig. 7d). The levels of type3-specific intestinal mucosal IgA were found to be similar in both poliovirus positive and negative infants (Fig. 7e).
a Serum neutralizing antibody for polio of poliovirus positive and negative infants. b Comparison the level of serum neutralizing antibody between poliovirus virus shedding time ≥ 2 weeks and virus shedding time < 2 weeks. c–e Comparison the level of polio-specific intestinal mucosal IgA of poliovirus positive and negative infants. A t-test was used for comparison of the serum neutralizing antibody and polio-specific intestinal mucosal IgA between the poliovirus positive and negative infants. *P < 0.05.
Discussion
Today most paralytic poliomyelitis cases around the world are no longer caused by WPV but are due to poliovirus shedding from OPV. The latest polio data published by WHO show that cVDPV cases have far surpassed the global number of WPV cases. A study conducted in Mexico from 2010 to 2011 also proved that infants who received two doses of IPV with one dose of tOPV and infants in close contact with them showed virus shedding (the virus shedding rate was 42.2% in those who received OPV and 2.3% in those who did not receive OPV22. In some areas that in incomplete vaccine coverage, vaccine strains can be transmitted from person to person and cause community spread23. Therefore, the detection of poliovirus shedding after OPV immunization and environmental surveillance are very important for the eradication of poliomyelitis. In our study, we detected the poliovirus shedding in different sequential immunization schedules, our findings showed that poliovirus shedding was affected by different immune procedures. Because IPV induces a limited mucosal immune response, the poliovirus shedding rate of each immune procedure group reached its peak within 7 days after the first dose of OPV immunization. Then the shedding rate gradually decreased and the shedding period was gradually shortened with the increase in bOPV dose, which was consistent with the results of foreign clinical trials24,25. It might be because after the immunization of OPV, the poliovirus replicates in the intestinal tracts of infants, inducing mucosal immune response and secreting a large amount of polio-specific IgA, it can quickly neutralize the virus and reduce virus shedding after subsequent bOPV immunization26.
In this study, the serotyping outcomes of poliovirus-positive samples showed that poliovirus shedding occurred within a week following the initial administration of bOPV or tOPV, with the shedding rate peaked within 7 days post-vaccination with OPV and subsequently declined gradually, aligning with results from other clinical trials27. This result suggested that OPV can quickly trigger an intestinal mucosal immune response. Notably, we observed that type3 exhibited a longer shedding duration and a higher shedding rate compared to type1 following OPV immunization. This discrepancy may be attributed to the varying replication efficiencies of different poliovirus types within the intestinal environment28,29. Additionally, we assessed the mucosal immune response subsequent to OPV immunization. The results showed that the level of type3 specific IgA was lower than that of type1 specific IgA, this indicated that type1 can induce a stronger intestinal mucosal immune response30. This may be the main factor contributing to the higher shedding rate for type3 compared to type1. Importantly, we found a slightly increase in the shedding rate of type3 following the removal of type2 from tOPV, which may be attributed to the fact that different types of polioviruses have a certain inhibitory effect on the replication of other types upon simultaneous entry into the intestinal tract29. Consequently, the titers of the three poliovirus types in tOPV are ranked as type1> type3> type2 to ensure their replication capabilities maintain a relatively stable equilibrium after intestinal entry. The removal of the type2 virus from tOPV disrupts this equilibrium, leading to a diminished suppression of the type3 virus28. Since May 2016, the routine immunization schedule for polio vaccine in China has been revised to IPV+3bOPV. Monitoring of acute flaccid paralysis (AFP) cases and poliovirus in environmental sewage in China indicated that type2 was the dominant strain of poliovirus before the polio vaccine immunization schedule switch, while the separation rate of type1 and type3 in the environment after the switching was significantly higher than that of type2 before the polio vaccine immunization schedule was changed in May 201631,32. This conclusion is consistent with our research findings. Additionally, the latest polio data published by the GPEI indicates that VDPV1 has supplanted VDPV2 and has emerged as the predominant local epidemic strain in numerous nations33.
The accumulation of genomic mutations associated with vaccine-derived poliovirus, isolated from stool samples of recipients of IPV-OPV, results in a reversal of neurotoxicity. While the mutation sites are distributed throughout the entire genome of OPV strains, the mutation patterns vary across different gene regions. The results of our study showed that poliovirus positive samples from different sequential immunization groups of sIPV-OPV and cIPV-OPV were prone to mutation in the 5’NCR and 3D region, which may be closely related to the fact that the most important attenuation sites are located in 5’NCR, and the 3D region is crucial for RNA replication. The frequency of high-content mutations was observed in the order of type3> type2> type1, suggesting that during poliovirus replication in the human intestine, reverted attenuated mutant determinants accumulate most rapidly in type3 vaccine strains. This phenomenon may be attributed to the relatively higher degree of attenuation in type1, which possesses more attenuation sites, resulting in a reduced likelihood of simultaneous reversion mutations. Conversely, Sabin strains of type2 and type3 exhibit a lower degree of attenuation and fewer sites, allowing for a rapid recovery of some neurotoxicity to enhance their growth adaptability in the intestinal environment34,35. Consequently, a single mutation reversal in type2 or type3 vaccine strains can lead to a significant increase in neurovirulence, thereby elevating the risk of VAPP. The genome of RNA viruses is relatively small, however, it exhibits a high degree of evolution and diversity, leading to the emergence of multiple viral mutation strains. Generally, the mutation probability for each nucleotide within the poliovirus RNA genome is uniform. Nevertheless, the actual mutation sites are not randomly chosen; rather, certain sites can be identified as mutation hotspots due to their significantly elevated mutation rates compared to the average probability14. For instance, in type1, the mutation hotspots are located at the nt480 and nt525 sites in the 5’NCR, nt2747 and nt2749 in the VP1 region, and nt6203 in the 3D region. In type2, hotspots are found at the nt481 site in the 5’NCR and at nt2908 and nt2909 in the VP1 region. Type3 hotspots are identified at the nt472 site in the 5’NCR and at nt2034 and nt2636 in the VP1 region, all exhibiting mutation rates exceeding 50% across nearly all samples. This observation suggests that these sites experience heightened selection pressure, making them more prone to revert to wild-type mutations. Furthermore, the alterations at these sites are predominantly non-synonymous mutations that have direct phenotypic consequences, resulting in changes to the coding amino acids within the structural components of VP136. Although OPV strains isolated from the stool samples of individuals immunized through various sequential immunization protocols may exhibit a certain degree of reversion mutations-aligning with the early mutation characteristics of VDPV—there remains a notable evolutionary gap when compared to VDPV. The transition from tOPV to bOPV has led to a decrease in the accumulation of reverse-mutated bases in type2 strains. However, 2IPV+bOPV sequential immunization schedule cannot entirely prevent the emergence of VDPV, as immunization with bOPV results in the shedding of type1 and type3 viruses, with instances of cVDPV1 and cVDPV3 detected in environmental samples.
In summary, considering that type3 exhibited a longer shedding duration and a higher shedding rate, there have been no cases of WPV3 since November 2012. Nations with low risk of poliovirus importation and currently using OPV should consider transitioning from bOPV to monovalent type1 OPV(mOPV1). Furthermore, the poliovirus shedding rate was higher on days 7 following the first dose of OPV, and then gradually decreased, this result suggests that one dose of OPV can establish mucosal immunity. In addition, IPV has the potential to significantly enhance mucosal immunity in children previously immunized with OPV2. Therefore, we recommend that countries currently using 2IPV + 2bOPV sequential immunization schedule can replace the fourth dose from bOPV to IPV and transform to a “sandwich” immunization strategy-2IPV+bOPV+IPV, which can not only reduce the poliovirus shedding from OPV, but also strengthen humoral immunity to type2. Although there were no significant differences in virus shedding between cIPV/OPV and sIPV/OPV sequential immunization groups, the production of cIPV requires stricter biosafety and biosecurity measures37. Furthermore, clinical trials have indicated that sIPV demonstrates safety, immunogenicity and robust neutralization protection capabilities18,38,39,40. Therefore, WHO encourages to use of the Sabin attenuated strain to produce IPV.
Although we detected the poliovirus shedding in different immunization schedules, the study still had some limitations. First, we only monitored the virus shedding after OPV immunization for three weeks and not monitored it longer-term dynamics. In addition, due to volume limitation of stool samples, the amount of virus in stool samples could not be detected. Therefore, future work will further explore the duration and amount of poliovirus shedding after OPV immunization.
Methods
Study design and participants
The study was expanded from the original completed phase III clinical trial “Randomized, Double Blind, Single Center, Clinic Trial to Evaluate the Safety and Immunogenicity by Different Sequential Schedules of bOPV and IPV”, registered in 2015 (ClinicalTrials. gov ID:NCT03614702). This study represents the first clinical trial globally focused on the virus shedding rate and the genetic diversity of OPV shedding strains across different sIPV-OPV sequential immunization schedules. Approval for the study was granted by the Ethics Committee of the GuangXi Province Center for Disease Prevention and Control, and informed consent was secured from the legal guardians of the infants prior to their enrollment.
We enrolled 1200 eligible Infants who had not yet received essential vaccinations against polio were recruited for the study. Healthy 2-month-old infants, born from full-term pregnancies with birth weights exceeding 2.5 kg and without any medical defects, were selected based on the established inclusion and exclusion criteria. The eligibility criteria encompass the following: the guardian has been notified, consented, and signed the informed consent document; the guardian and family members have adhered to the stipulations of the clinical trial protocol; there is no prior history of postnatal immunoglobulin administration (with the exception of hepatitis B immunoglobulin); no live vaccine has been administered within 28 days prior to vaccination; no inactivated vaccine has been given within 14 days before vaccination; and the axillary temperature is below 37.1 °C.
Randomization and masking
The infants were randomly assigned to 6 sequential immunization schedules as follows: 1sIPV + 2bOPV, 1cIPV + 2bOPV, 2sIPV+bOPV, 2cIPV+bOPV, 2sIPV+tOPV, 2cIPV+tOPV. The vaccine administered to the infants was anonymized during the blinded trial, with the vaccine code affixed to the large packaging, entirely obscuring the original label. Each participant was associated with three distinct vaccine codes. The grouping outcomes (study number and vaccine code) were documented in the original notebook and vaccination card, which the participants retained for their vaccination process.
Immunization
Infants in each immunization schedules were administered vaccines at 2, 3, and 4 months of age in accordance with the immunization schedule. IPV was delivered by injection, and bOPV was given as oral. A volume of 2.5 ml of venous blood was drawn prior to vaccination and again 28 days following complete immunization. The samples were allowed to sit at room temperature for one hour before being transferred to a refrigerator set at 4 °C for overnight storage. Subsequently, the samples were centrifuged at 3000 rpm for 10 min to separate the serum, which was then stored at −20 °C for the analysis of poliovirus neutralizing antibodies.
Sample collecting
The first 10% of participants in each group during the enrollment phase and allocate sampling containers and plastic bags for stool sample collecting. The stool samples were obtained prior to the administration of the second dose and on the 7th, 14th, and 28th days following the second and third doses, resulting in a total of seven collection instances. The stool samples were transferred to plastic collection containers, the participant identification number was attached, and the collection time was documented, then stored at −70 °C until further analysis was conducted. Stool samples were labeled with the study number rather than any other grouping information.
Poliovirus isolation
Add 5 mL PBS(+) buffer, 1 glass, 0.5 mL chloroform, and 1 g fecal samples to each tube, shake for 30 min to make 20% fecal suspension, 4 °C, 8500 rpm centrifuge for 30 min, then collect the supernate and stored at −70 °C until further testing.
Poliovirus amplification
In accordance with the guidelines provided by the WHO, we employed L20B and RD cell lines for the isolation of poliovirus. Each tube received a total of 200 µL of inoculum. The inoculated tubes were subsequently incubated in a roller at 36 °C for a duration of 1 to 7 days, until a complete cytopathic effect (CPE) was evident under an inverted microscope. If the cultures demonstrated positivity for poliovirus in L20B cells, they were then subcultured onto RD cells, and the reverse was done for confirmation of positivity. Uninfected cells from both cell lines served as the negative control.
Poliovirus serotype identification
Upon assessing the virus titers of positive samples, we determined the serotypes of poliovirus through a neutralization assay. A neutralization technique utilizing mixed antisera pools was employed to classify the clinical isolates, adhering to the established protocols. In summary, high-titer polyclonal antisera were combined with approximately 50 TCID50 of the unidentified virus isolate. A back titration isolate was incorporated into each assay, facilitating the calculation of the viral titer present in the sample. Configure the following four combinations of poliovirus standard antisera for the purpose of identifying serotypes in poliovirus-positive samples: anti-type1+type2+type3 mixed standard serum, anti-type1+type2 mixed standard serum, anti-type1+type3 mixed standard serum, anti-type2+type3 mixed standard serum; each serum type contains 20 units of neutralizing antibodies in a volume of 50ul. The serum/virus mixtures were incubated for 3 h at 37 °C. Following this, a cell suspension was introduced to the microtiter plates, which were monitored daily for CPE over a period of up to 5 days. Ultimately, we identified the serotype of poliovirus in positive samples based on the manifestation of CPE.
Sequencing of positive stool samples
The selected poliovirus positive samples were commissioned for the construction of a genomic library and subsequent second-generation sequencing by Shanghai Source Sequence Biotechnology. The library was developed utilizing the KAPA Stranded mRNA-Seq Kit (KAPA Biosystems, USA). Samples from various sequential immunization groups were compared in pairs as required, and a T-test was performed to assess the differences in gene mutation proportions across all sites between the two groups using Plink software. The P-values reflecting the differences in gene mutation proportions for each site between the two groups were calculated. A Manhattan plot illustrating the relationship between each site and -lg (P) across the entire viral genome was generated using the Manhattan package in R, highlighting the sites with significant differences in gene mutation ratio distribution between the two groups.
Statistical analysis
All the statistical analyses were performed by using SPSS Statistical Software 22.0 or GraphPad Prism Version 10, the graphs were performed by using GraphPad Prism Version 10. P < 0.05 was considered statistically significant.
Data availability
The sequencing data of poliovirus shedding strain during the current study is available in the NCBI repository, the Genbank Accession numbers is PRJNA1232836. All other data supporting the findings of this study are available within the article and its Supporting Information files.
References
Yeh, M. T. et al. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 27, 736–751 (2020).
Polio vaccines: WHO position paper–June 2022. https://iris.who.int/bitstream/handle/10665/357168/WER9725-277-300-eng-fre.pdf?sequence=1&isAllowed=y.
Brickley, E. B. et al. Intestinal Immunity to Poliovirus Following Sequential Trivalent Inactivated Polio Vaccine/Bivalent Oral Polio Vaccine and Trivalent Inactivated Polio Vaccine-only Immunization Schedules: Analysis of an Open-label, Randomized, Controlled Trial in Chilean Infants. Clin. Infect. Dis. 67, S42–S50 (2018).
Wilkinson, A. L. et al. Immunogenicity of novel oral poliovirus vaccine type 2 administered concomitantly with bivalent oral poliovirus vaccine: an open-label, non-inferiority, randomised, controlled trial. Lancet Infect. Dis. 23, 1062–1071 (2023).
Burns, C. C., Diop, O. M., Sutter, R. W. & Kew, O. M. Vaccine-derived polioviruses. J. Infect. Dis. 210, S283–S293 (2014).
Guo, J., Bolivar-Wagers, S., Srinivas, N., Holubar, M. & Maldonado, Y. Immunodeficiency-related vaccine-derived poliovirus (iVDPV) cases: a systematic review and implications for polio eradication. Vaccines 33, 1235–1242 (2015).
Jenkins, H. E. et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N. Engl. J. Med 362, 2360–2369 (2010).
Nathanson, N. Eradication of poliovirus: fighting fire with fire. J. Infect. Dis. 203, 889–890 (2011).
Troy, S. B. et al. Use of a novel real-time PCR assay to detect oral polio vaccine shedding and reversion in stool and sewage samples after a Mexican national immunization day. J. Clin. Microbiol. 49, 1777–1783 (2011).
Tan, S. K. et al. Metagenomic sequencing of stool samples in Bangladeshi infants: virome association with poliovirus shedding after oral poliovirus vaccination. Sci. Rep. 10, 15392 (2020).
Ming, L. C., Hussain, Z., Yeoh, S. F., Koh, D. & Lee, K. S. Circulating vaccine-derived poliovirus: a menace to the end game of polio eradication. Glob. Health 16, 63 (2020).
Burki, T. Vaccine-derived poliovirus cases exceed wild types. Lancet Infect. Dis. 19, 140 (2019).
Yao, N. et al. Detection of a Highly Divergent Type 3 Vaccine-Derived Poliovirus in a Child with a Severe Primary Immunodeficiency Disorder - Chongqing, China. MMWR Morb. Mortal. Wkly Rep. 71, 1148–1150 (2022).
Yeh, M. T. et al. Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3. Nature 619, 135–142 (2023).
Hird, T. R. & Grassly, N. C. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 8, e1002599 (2012).
Brickley, E. B. et al. Intestinal antibody responses to a live oral poliovirus vaccine challenge among adults previously immunized with inactivated polio vaccine in Sweden. BMJ Glob. health 4, e001613 (2019).
Zhao, T. et al. Reduced mucosal immunity to poliovirus after cessation of trivalent oral polio vaccine. Hum. Vaccin Immunother. 17, 2560–2567 (2021).
He, H. et al. Immunogenicity of three sequential schedules with Sabin inactivated poliovirus vaccine and bivalent oral poliovirus vaccine in Zhejiang, China: an open-label, randomised, controlled trial. Lancet Infect. Dis. 20, 1071–1079 (2020).
O’Ryan, M. et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect. Dis. 15, 1273–1282 (2015).
Singanayagam, A. et al. Asymptomatic immunodeficiency-associated vaccine-derived poliovirus infections in two UK children. Nat. Commun. 14, 3413 (2023).
Ren, R. B., Moss, E. G. & Racaniello, V. R. Identification of two determinants that attenuate vaccine-related type 2 poliovirus. J. Virol. 65, 1377–1382 (1991).
Ferreyra-Reyes, L. et al. Assessing the individual risk of fecal poliovirus shedding among vaccinated and non-vaccinated subjects following national health weeks in Mexico. Plos One 12, e0185594 (2017).
Troy, S. B. et al. Real-time Polymerase Chain Reaction Analysis of Sewage Samples to Determine Oral Polio Vaccine Circulation Duration and Mutation After Mexican National Immunization Weeks. J. Pediatr. Infect. Dis. Soc. 1, 223–229 (2012).
Gast, C. et al. Fecal Shedding of 2 Novel Live Attenuated Oral Poliovirus Type 2 Vaccine Candidates by Healthy Infants Administered Bivalent Oral Poliovirus Vaccine/Inactivated Poliovirus Vaccine: 2 Randomized Clinical Trials. J. Infect. Dis. 226, 852–861 (2022).
Zaman, K. et al. Evaluation of the safety, immunogenicity, and faecal shedding of novel oral polio vaccine type 2 in healthy newborn infants in Bangladesh: a randomised, controlled, phase 2 clinical trial. Lancet 401, 131–139 (2023).
Zhao, T. et al. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. NPJ Vaccines 5, 47 (2020).
Laassri, M. et al. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J. Infect. Dis. 192, 2092–2098 (2005).
Sanden, S. V. D. et al. Shedding of vaccine viruses with increased antigenic and genetic divergence after vaccination of newborns with monovalent type 1 oral poliovirus vaccine. J. Virol. 83, 8693–8704 (2009).
Lentz, K. N. et al. Structure of poliovirus type 2 Lansing complexed with antiviral agent SCH48973: comparison of the structural and biological properties of three poliovirus serotypes. Structure 5, 961–978 (1997).
Resik, S. et al. Does Simultaneous Administration of Bivalent (Types 1 and 3) Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine Induce Mucosal Cross-immunity to Poliovirus Type 2?. Clin. Infect. Dis. 67, S51–s56 (2018).
Zhao, C. et al. Prevalence and Bayesian Phylogenetics of Enteroviruses Derived From Environmental Surveillance Around Polio Vaccine Switch Period in Shandong Province, China. Food Environ. Virol. 12, 321–332 (2020).
Ma, Y. et al. Poliovirus Detection and Genetic Characteristic from Sewage in Heilongjiang Province from 2013 to 2016. Jpn J. Infect. Dis. 71, 442–447 (2018).
Global Polio Eradication Initiative Global circulating vaccine-derived (cVDPV) AFP cases and environmental samples 2021-2024. https://polioeradication.org/circulating-vaccine-derived-poliovirus-count/. Accessed 29th Nov 2024.
Macadam, A. J. et al. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 192, 18–26 (1993).
Minor, P. D. The molecular biology of poliovaccines. J. Gen. Virol. 73, 3065–3077 (1992).
Gavrilin, G. V., Cherkasova, E. A., Lipskaya, G. Y., Kew, O. M. & Agol, V. I. Evolution of circulating wild poliovirus and of vaccine-derived poliovirus in an immunodeficient patient: a unifying model. J. Virol. 74, 7381–7390 (2000).
Okayasu, H., Sutter, R. W., Jafari, H. S., Takane, M. & Aylward, R. B. Affordable inactivated poliovirus vaccine: strategies and progress. J. Infect. Dis. 210, S459–S464 (2014).
Capeding, M. R. et al. Safety and immunogenicity of a new inactivated polio vaccine made from Sabin strains: a randomized, double-blind, active-controlled, phase 2/3 seamless study. J. Infect. Dis. 226, 308–318 (2022).
Resik, S. et al. Reactogenicity and immunogenicity of inactivated poliovirus vaccine produced from Sabin strains: a phase I Trial in healthy adults in Cuba. Vaccine 32, 5399–5404 (2014).
Hu, Y. et al. Immunogenicity and Safety of a Sabin Strain-Based Inactivated Polio Vaccine: A Phase 3 Clinical Trial. J. Infect. Dis. 220, 1551–1557 (2019).
Acknowledgements
We would like to extend our heartfelt appreciation to Xiaolei Yang and convey our utmost respect and gratitude for her unwavering commitment and significant contributions to this research. Although she is no longer with us, her efforts in this endeavor will continue to support the global initiative for polio eradication. We thank the Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention, Liuzhou City Center for Disease Control and Prevention of Guangxi Zhuang Autonomous Region, and the researchers, project managers and laboratory personnel at all clinical study sites in Liujiang. We thank China National Institute for Food and Drug Control for providing professional third-party services authorized by the state for serum neutralizing antibody testing in this clinical trial project. We are particularly grateful to the experts from the Center for Drug Evaluation of the State Food and Drug Administration of China and the Chinese Center for Disease Control and Prevention for providing valuable opinions and suggestions to the clinical research plan, which enables the clinical trial to be carried out more smoothly and provides effective basis and guidance for the actual and future polio immunization strategy in China. This work was supported by the Yunnan Province Major Science and Technology Special Project (202002AA100009, 202302AA310003, 202402AA310022), CAMS Innovation Fund for Medical Sciences (2021-I2M-1-043), the Special Project in Basic Research of Yunnan Province (202401AT070147).
Author information
Authors and Affiliations
Contributions
Jingsi Yang, Changgui Li, Teng Huang, Zhaojun Mo carried out the clinical study design. Jingsi Yang and Li Shi revised and verified the manuscript.Yuting Fu and Rufei Ma isolated and detect the virus, analyzed the data and drafted the manuscript. Zhimei Zhao, Xiaolei Yang, Ting Zhao verified the experimental results and statistical analysis of the data. Zhifang Ying and Jianfeng Wang detected the neutralizing antibodies of poliovirus. Jing Li, Hui Ye, Guoliang Li, Xiaochang Liu, Jiangli Liang, Ling Ping, Junhui Tao, Qinghai Yang, Dingkai Wei collected the samples. Jingyan Li, Li Yi, Hongbo Chen, Lei Yu, Wei Cai, Wei Yang, Lei Yue, Mingxue Xie, Chao Hong, Lukui Cai, Yan Deng, Jiana Wen, Yan Ma, Na Gao, Xiaoyu Wang, Hongwei Liao, Qiuyan Ji, Guang Ji, Wenzhu Hu, Qin Gu, Jian Zhou, Yu Wen responsible for production and quality control of the vaccine used in the clinical trial. Ruiju Jiang, Qiongzhou Yin, Jing Pu supervised the field investigation in the clinical trial. Xiaoyue He, Han Chu, Yixian Fu provided experimental assistance. All authors participated in the evaluation and refinement of the report, had complete access to all data involved in the study, and held ultimate accountability for the decision to proceed with publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, Y., Ma, R., Zhao, Z. et al. Poliovirus shedding after sequential immunization of Sabin-strain inactivated polio vaccines and oral attenuated polio vaccines. npj Vaccines 10, 81 (2025). https://doi.org/10.1038/s41541-025-01134-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01134-9