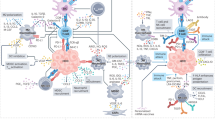
Abstract
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related mortality and has an increasing incidence worldwide. Locoregional therapies, defined as imaging-guided liver tumour-directed procedures, play a leading part in the management of 50–60% of HCCs. Radiofrequency is the mainstay for local ablation at early stages and transarterial chemoembolization (TACE) remains the standard treatment for intermediate-stage HCC. Other local ablative techniques (microwave ablation, cryoablation and irreversible electroporation) or locoregional therapies (for example, radioembolization and sterotactic body radiation therapy) have been explored, but have not yet modified the standard therapies established decades ago. This understanding is currently changing, and several drugs have been approved for the management of advanced HCC. Molecular therapies dominate the adjuvant trials after curative therapies and combination strategies with TACE for intermediate stages. The rationale for these combinations is sound. Local therapies induce antigen and proinflammatory cytokine release, whereas VEGF inhibitors and tyrosine kinase inhibitors boost immunity and prime tumours for checkpoint inhibition. In this Review, we analyse data from randomized and uncontrolled studies reported with ablative and locoregional techniques and examine the expected effects of combinations with systemic treatments. We also discuss trial design and benchmarks to be used as a reference for future investigations in the dawn of a promising new era for HCC treatment.
Key points
-
Locoregional treatments for hepatocellular carcinoma (HCC) are aimed at eliminating or reducing tumoural viability, delaying progression and ultimately extending overall survival; options include local ablative techniques and intra-arterial techniques.
-
Radiofrequency ablation is considered the standard treatment option among local ablative techniques for very early stage tumours (<2 cm) and for tumours at early stages not suitable for surgical therapies.
-
Transarterial chemoembolization is established as the standard of care for intermediate-stage lesions (multinodular liver-only disease in asymptomatic patients with compensated liver function) leading to median survivals of 25–30 months.
-
The role of radioembolization and stereotactic body radiotherapy is still to be defined, and further trials are required to delineate a patient subset deriving benefit from these treatments.
-
No systemic therapy tyrosine kinase inhibitor has been able to improve survival when tested in combination with locoregional treatment but there is rationale for combination therapies with immunotherapy in HCC.
-
Single-agent and combination therapies are being investigated in randomized controlled trials both in an adjuvant setting and combined with intra-arterial therapies, and results are expected to change HCC management within the next 5 years.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2, 16018 (2016).
Kulik, L. & El-Serag, H. B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 156, 477–491.e471 (2019).
Galle, P. R. et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68, 723–750 (2018).
Llovet, J. M., Montal, R., Sia, D. & Finn, R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15, 599–616 (2018).
Greten, T. F., Lai, C. W., Li, G. & Staveley-O’Carroll, K. F. Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology 156, 510–524 (2019).
Nault, J.-C., Cheng, A.-L., Sangro, B. & Llovet, J. M. Milestones in the pathogenesis and management of primary liver cancer. J. Hepatol. 72, 209–214 (2020).
Park, J. W. et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 35, 2155–2166 (2015).
Livraghi, T. et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 214, 761–768 (2000).
Nault, J. C., Sutter, O., Nahon, P., Ganne-Carrie, N. & Seror, O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J. Hepatol. 68, 783–797 (2018).
Llovet, J. M. et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359, 1734–1739 (2002).
Llovet, J. M. & Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37, 429–442 (2003).
Lencioni, R., de Baere, T., Soulen, M. C., Rilling, W. S. & Geschwind, J. F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology 64, 106–116 (2016).
Han, G. et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology 72, 198–212 (2019).
Palmer, D. H., Malagari, K. & Kulik, L. M. Role of locoregional therapies in the wake of systemic therapy. J. Hepatol. 72, 277–287 (2020).
Salem, R., Mazzaferro, V. & Sangro, B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology 58, 2188–2197 (2013).
Llovet, J. M. et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology https://doi.org/10.1002/hep.31327 (2020).
Raoul, J. L. et al. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat. Rev. 72, 28–36 (2019).
Kadalayil, L. et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 24, 2565–2570 (2013).
Attallah, A. M. et al. HCC-ART score, a simple, highly sensitive and specific test for early diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Br. J. Cancer 109, 1657–1665 (2013).
Meyer, T. et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2, 565–575 (2017).
Lencioni, R. et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J. Hepatol. 64, 1090–1098 (2016).
Kudo, M. et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology 60, 1697–1707 (2014).
Vogel, A. et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv238–iv255 (2018).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Finn, R. S. et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 38, 2960–2970 (2020).
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020).
Ohri, N. et al. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J. Natl Cancer Inst. 108, djw133 (2016).
Vibert, E., Schwartz, M. & Olthoff, K. M. Advances in resection and transplantation for hepatocellular carcinoma. J. Hepatol. 72, 262–276 (2020).
Lencioni, R. A. et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228, 235–240 (2003).
Lin, S.-M., Lin, C.-J., Lin, C.-C., Hsu, C.-W. & Chen, Y.-C. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 127, 1714–1723 (2004).
Lin, S. M., Lin, C. J., Lin, C. C., Hsu, C. W. & Chen, Y. C. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 54, 1151–1156 (2005).
Shiina, S. et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 129, 122–130 (2005).
Cho, Y. K., Kim, J. K., Kim, M. Y., Rhim, H. & Han, J. K. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 49, 453–459 (2009).
Germani, G. et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J. Hepatol. 52, 380–388 (2010).
Orlando, A., Leandro, G., Olivo, M., Andriulli, A. & Cottone, M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 104, 514–524 (2009).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370 (2017).
Breen, D. J. & Lencioni, R. Image-guided ablation of primary liver and renal tumours. Nat. Rev. Clin. Oncol. 12, 175–186 (2015).
Livraghi, T., Lazzaroni, S., Meloni, F. & Solbiati, L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br. J. Surg. 92, 856–858 (2005).
Sasaki, A. et al. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer 103, 299–306 (2005).
Liao, M. et al. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: a prospective randomized trial. J. Surg. Oncol. 115, 971–979 (2017).
Minami, Y. & Kudo, M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int. J. Hepatol. 2011, 104685 (2011).
Poggi, G., Tosoratti, N., Montagna, B. & Picchi, C. Microwave ablation of hepatocellular carcinoma. World J. Hepatol. 7, 2578–2589 (2015).
Lam, V. W. et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J. Am. Coll. Surg. 207, 20–29 (2008).
Lee, D. H. et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270, 900–909 (2014).
Lencioni, R. et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 234, 961–967 (2005).
N’Kontchou, G. et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 50, 1475–1483 (2009).
Sala, M. et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 40, 1352–1360 (2004).
Livraghi, T. et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 47, 82–89 (2008).
Bale, R. et al. Stereotactic radiofrequency ablation of hepatocellular carcinoma: a histopathological study in explanted livers. Hepatology 70, 840–850 (2019).
Rossi, S. et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology 53, 136–147 (2011).
Brunello, F. et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand. J. Gastroenterol. 43, 727–735 (2008).
Shiina, S. et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 107, 569–577; quiz 578 (2012).
Casadei Gardini, A. et al. Radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of overall survival and recurrence-free survival. Onco Targets Ther. 11, 6555–6567 (2018).
Doyle, A. et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3cm in potentially transplantable patients. J. Hepatol. 70, 866–873 (2019).
Hermida, M. et al. Multimodal percutaneous thermal ablation of small hepatocellular carcinoma: predictive factors of recurrence and survival in western patients. Cancers 12, 313 (2020).
Chen, M. S. et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 243, 321–328 (2006).
Feng, K. et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 57, 794–802 (2012).
Huang, J. et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 252, 903–912 (2010).
Ng, K. K. C. et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br. J. Surg. 104, 1775–1784 (2017).
Izumi, N. et al. A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). J. Clin. Oncol. 37, 4002–4002 (2019).
Yu, J. et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut 66, 1172 (2017).
Giorgio, A. et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer. Res. 31, 2291–2295 (2011).
Wang, C. et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 61, 1579–1590 (2015).
Peng, Z. W. et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J. Clin. Oncol. 31, 426–432 (2013).
Chen, K. et al. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J. Hepatol. 61, 1304–1311 (2014).
Tak, W. Y. et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin. Cancer Res. 24, 73–83 (2018).
Xia, Y. et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 6, 255–263 (2019).
Majumdar, A. et al. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst. Rev. 3, Cd011650 (2017).
Cho, Y. K., Kim, J. K., Kim, W. T. & Chung, J. W. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology 51, 1284–1290 (2010).
Cucchetti, A. et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 59, 300–307 (2013).
Poon, R. T. et al. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann. Surg. Oncol. 14, 1835–1845 (2007).
Liu, Y. et al. Effects of various interventions on the occurrence of macrovascular invasion of hepatocellular carcinoma after the baseline serum γ-glutamyltransferase stratification. Onco Targets Ther. 12, 1671–1679 (2019).
Tsuchiya, K. et al. Expression of keratin 19 is related to high recurrence of hepatocellular carcinoma after radiofrequency ablation. Oncology 80, 278–288 (2011).
Hoshida, Y. et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Engl. J. Med. 359, 1995–2004 (2008).
Kang, T. W. et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology 280, 300–312 (2016).
Hakime, A. et al. Clinical evaluation of spatial accuracy of a fusion imaging technique combining previously acquired computed tomography and real-time ultrasound for imaging of liver metastases. Cardiovas. Intervent. Radiol. 34, 338–344 (2011).
Harari, C. M. et al. Microwave ablation: comparison of simultaneous and sequential activation of multiple antennas in liver model systems. Radiology 278, 95–103 (2016).
Vietti Violi, N. et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 3, 317–325 (2018).
Shibata, T. et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 223, 331–337 (2002).
Abdelaziz, A. et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg. Endosc. 28, 3429–3434 (2014).
Xu, Y. et al. Microwave ablation is as effective as radiofrequency ablation for very-early-stage hepatocellular carcinoma. Chin. J. Cancer 36, 14 (2017).
Tan, W., Deng, Q., Lin, S., Wang, Y. & Xu, G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int. J. Hyperthermia 36, 264–272 (2019).
Glassberg, M. B. et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 12, 6407–6438 (2019).
Facciorusso, A., Di Maso, M. & Muscatiello, N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int. J. Hyperthermia 32, 339–344 (2016).
Loriaud, A. et al. Hepatocellular carcinoma abutting large vessels: comparison of four percutaneous ablation systems. Int. J. Hyperthermia 34, 1171–1178 (2018).
Lencioni, R., de Baere, T., Martin, R. C., Nutting, C. W. & Narayanan, G. Image-guided ablation of malignant liver tumors: recommendations for clinical validation of novel thermal and non-thermal technologies - a western perspective. Liver Cancer 4, 208–214 (2015).
Xu, J. et al. Radiofrequency ablation vs. cryoablation for localized hepatocellular carcinoma: a propensity-matched population study. Anticancer. Res. 38, 6381–6386 (2018).
Kim, R. et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: therapeutic efficacy and vascular complications. Eur. Radiol. 29, 654–662 (2019).
Cheng, R. G., Bhattacharya, R., Yeh, M. M. & Padia, S. A. Irreversible electroporation can effectively ablate hepatocellular carcinoma to complete pathologic necrosis. J. Vasc. Interv. Radiol. 26, 1184–1188 (2015).
Sutter, O. et al. Safety and efficacy of irreversible electroporation for the treatment of hepatocellular carcinoma not amenable to thermal ablation techniques: a retrospective single-center case series. Radiology 284, 877–886 (2017).
Di Costanzo, G. G. et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J. Gastroenterol. Hepatol. 30, 559–565 (2015).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 16, 1344–1354 (2015).
Kong, G. et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 60, 6950–6957 (2000).
Celik, H. et al. Radiofrequency ablation duration per tumor volume may correlate with overall survival in solitary hepatocellular carcinoma patients treated with radiofrequency ablation plus lyso-thermosensitive liposomal doxorubicin. J. Vasc. Interv. Radiol. 30, 1908–1914 (2019).
Lawrenceville, N. J., Celsion corporation receives recommendation from independent data monitoring committee to consider stopping the phase III optima study. News Release, 13 July 2020, https://bit.ly/2ZomTD5.
Duffy, A. G. et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 66, 545–551 (2017).
Sugimoto, K. et al. Irreversible electroporation versus radiofrequency ablation: comparison of systemic immune responses in patients with hepatocellular carcinoma. J. Vasc. Intervent. Radiol. 30, 845–853.e846 (2019).
Partridge, B. R. et al. High-frequency irreversible electroporation for treatment of primary liver cancer: a proof-of-principle study in canine hepatocellular carcinoma. J. Vasc. Intervent. Radiol. 31, 482–491.e484 (2020).
Greaney, S. K. et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol. Res. 8, 246 (2020).
Bertot, L. C., Sato, M., Tateishi, R., Yoshida, H. & Koike, K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur. Radiol. 21, 2584–2596 (2011).
Kasugai, H., Osaki, Y., Oka, H., Kudo, M. & Seki, T. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology 72 (Suppl. 1), 72–75 (2007).
Giorgio, A. et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma in cirrhosis: analysis of complications in a single centre over 20 years. Br. J. Radiol. 90, 20160804 (2017).
Park, M. J. et al. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J. Vasc. Intervent. Radiol. 22, 771–779 (2011).
Woo, S. et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system — mid-term results. Radiology 268, 589–600 (2013).
Seror, O. et al. Hepatocellular carcinoma within milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology 280, 611–621 (2016).
de Baère, T. et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. Am. J. Roentgenol. 181, 695–700 (2003).
Nakagomi, R. et al. Drastically reduced neoplastic seeding related to radiofrequency ablation for hepatocellular carcinoma. Am. J. Gastroenterol. 109, 774–776 (2014).
Yu, J. et al. Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur. J. Radiol. 81, 2495–2499 (2012).
Hakime, A., Tselikas, L., Otmezguine, Y., Deschamps, F. & de Baere, T. Artificial ascites for pain relief during microwave ablation of subcapsular liver tumors. Cardiovas. Interven. Radiol. 38, 1557–1562 (2015).
Lee, S. et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas: factors related to intraprocedural and postprocedural pain. Am. J. Roentgenol. 192, 1064–1070 (2009).
Lo, C. M. et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35, 1164–1171 (2002).
Llovet, J. M., Bru, C. & Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 19, 329–338 (1999).
de Baere, T. et al. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovas. Interv. Radiol. 39, 334–343 (2016).
Okusaka, T. et al. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: a randomized phase III trial. J. Hepatol. 51, 1030–1036 (2009).
Kudo, M. et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 47, 2117–2127 (2011).
Yu, S. C. et al. Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology 270, 607–620 (2014).
Golfieri, R. et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 111, 255–264 (2014).
Kudo, M. et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol. Hepatol. 3, 37–46 (2018).
Ikeda, M. et al. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a phase III randomized trial. J. Gastroenterol. 53, 281–290 (2018).
Kudo, M. et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 69, 1492–1501 (2019).
Park, J. W. et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J. Hepatol. 70, 684–691 (2019).
Vilgrain, V. et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 18, 1624–1636 (2017).
Chow, P. K. H. et al. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-pacific patients with hepatocellular carcinoma. J. Clin. Oncol. 36, 1913–1921 (2018).
Ricke, J. et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 71, 1164–1174 (2019).
Kudo, M. et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 3, 424–432 (2018).
Salem, R. et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 151, 1155–1163.e1152 (2016).
Lammer, J. et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovas. Interv. Radiol. 33, 41–52 (2010).
Meyer, T. et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br. J. Cancer 108, 1252–1259 (2013).
Lencioni, R. et al. Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiov. Interv. Radiol. 35, 980–985 (2012).
Bolondi, L. et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 32, 348–359 (2012).
Kudo, M. et al. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified Bolondi’s subclassification (Kinki criteria). Dig. Dis. 33, 751–758 (2015).
Forner, A., Gilabert, M., Bruix, J. & Raoul, J. L. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 11, 525–535 (2014).
Yin, L. et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J. Hepatol. 61, 82–88 (2014).
Becker, G. et al. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World J. Gastroenterol. 11, 6104–6109 (2005).
Pitton, M. B. et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovas. Interv. Radiol. 38, 352–360 (2015).
Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J. Clin. Oncol. 33, 550–558 (2015).
Edeline, J. et al. A multicentre comparison between child pugh and albumin-bilirubin scores in patients treated with sorafenib for hepatocellular carcinoma. Liver Int. 36, 1821–1828 (2016).
Waked, I. et al. Transarterial chemo-embolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br. J. Cancer 116, 448–454 (2017).
Wang, Q. et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J. Hepatol. 70, 893–903 (2019).
Mazzaferro, V. et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet. Oncol. 10, 35–43 (2009).
Lencioni, R. & Llovet, J. M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 30, 52–60 (2010).
Llovet, J. M. & Lencioni, R. mRECIST for HCC: performance and novel refinements. J. Hepatol. 72, 288–306 (2020).
Vincenzi, B. et al. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS ONE 10, e0133488 (2015).
Hilgard, P. et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 52, 1741–1749 (2010).
Lewandowski, R. J. et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am. J. Transplant. 9, 1920–1928 (2009).
Mazzaferro, V. et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 57, 1826–1837 (2013).
Salem, R. et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 138, 52–64 (2010).
Sangro, B. et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 54, 868–878 (2011).
Kolligs, F. T. et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 35, 1715–1721 (2015).
Mazzaferro, V. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 334, 693–699 (1996).
OPTN/UNOS Policy Notice Changes to HCC Criteria for Auto Approval. https://optn.transplant.hrsa.gov/media/2027/liver_policynotice_201612.pdf (2016).
Llovet, J. M. & Finn, R. S. Negative phase 3 study of (90)Y microspheres versus sorafenib in HCC. Lancet. Oncol. 19, e69 (2018).
Sposito, C. & Mazzaferro, V. The SIRveNIB and SARAH trials, radioembolization vs. sorafenib in advanced HCC patients: reasons for a failure, and perspectives for the future. Hepatobiliary Surg. Nutr. 7, 487–489 (2018).
Salem, R. et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 68, 1429–1440 (2018).
Biederman, D. M. et al. Outcomes of radioembolization in the treatment of hepatocellular carcinoma with portal vein invasion: resin versus glass microspheres. J. Vasc. Interv. Radiol. 27, 812–821.e812 (2016).
Garin, E. et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: a new personalized promising concept. Eur. J. Nucl. Med. Mol. Imaging 40, 1057–1068 (2013).
Garin, E. et al. Major impact of personalized dosimetry using 90Y loaded glass microspheres SIRT in HCC: Final overall survival analysis of a multicenter randomized phase II study (DOSISPHERE-01). J. Clin. Oncol. 38, 516–516 (2020).
Spreafico, C. et al. Development of a prognostic score to predict response to yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J. Hepatol. 68, 724–732 (2018).
Mosconi, C., Cucchetti, A., Pettinato, C., Golfieri, R. & Cappelli, A. Validation of response to yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J. Hepatol. 69, 259–260 (2018).
Lee, J., Shin, I. S., Yoon, W. S., Koom, W. S. & Rim, C. H. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: meta-analyses and a systematic review. Radiother. Oncol. 145, 63–70 (2020).
Meyer, T. Stereotactic body radiotherapy for hepatocellular carcinoma – still searching for a role. J. Hepatol. 73, 15–16 (2020).
Bang, A. & Dawson, L. A. Radiotherapy for HCC: ready for prime time? JHEP Rep. 1, 131–137 (2019).
Shen, P. C. et al. Comparison of stereotactic body radiation therapy and transarterial chemoembolization for unresectable medium-sized hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 105, 307–318 (2019).
Sapir, E. et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 100, 122–130 (2018).
Ashamalla, H., Manzerova, J., Soni, V., Kodiyan, J. & Guirguis, A. Patterns of care and effectiveness of stereotactic body radiation therapy and yttrium-90 radioembolization in unresected hepatocellular carcinoma: an NCDB analysis. Int. J. Radiat. Oncol. Biol. Phys. 102, e63 (2018).
Yoon, S. M. et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 4, 661–669 (2018).
Duan, J. et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol. 6, 375–384 (2020).
Greten, T. F., Duffy, A. G. & Korangy, F. Hepatocellular carcinoma from an immunologic perspective. Clin. Cancer Res. 19, 6678–6685 (2013).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20, 1239–1251 (2019).
Topalian, S. L. et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 5, 1411–1420 (2019).
Sutherland, L. M. et al. Radiofrequency ablation of liver tumors: a systematic review. Arch. Surg. 141, 181–190 (2006).
Kaseb, A. O. et al. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma. Cancer Immunol. Res. 7, 1390–1395 (2019).
Ayaru, L. et al. Unmasking of alpha-fetoprotein-specific CD4+ T cell responses in hepatocellular carcinoma patients undergoing embolization. J. Immunol. 178, 1914–1922 (2007).
Hiroishi, K. et al. Strong CD8+ T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J. Gastroenterol. 45, 451–458 (2010).
Mizukoshi, E. et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 57, 1448–1457 (2013).
Nobuoka, D. et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 40, 63–70 (2012).
Zerbini, A. et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 66, 1139–1146 (2006).
Zerbini, A. et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 138, 1931–1942 (2010).
Chu, K. F. & Dupuy, D. E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer 14, 199–208 (2014).
Haen, S. P. et al. Elevated serum levels of heat shock protein 70 can be detected after radiofrequency ablation. Cell Stress Chaperones 16, 495–504 (2011).
Ahmed, M. et al. Radiofrequency ablation (RFA)-induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins. Int. J. Hyperthermia 34, 934–942 (2018).
Kalbasi, A. & Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Guan, Q. et al. Correlation between vascular endothelial growth factor levels and prognosis of hepatocellular carcinoma patients receiving radiofrequency ablation. Biotechnol. Biotechnol. Equip. 29, 119–123 (2015).
Petrillo, M. et al. Hypoxia and tumor angiogenesis in the era of hepatocellular carcinoma transarterial loco-regional treatments. Future Oncol. 14, 2957–2967 (2018).
Yang, J., Yan, J. & Liu, B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 9, 978 (2018).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Khan, K. A. & Kerbel, R. S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 15, 310–324 (2018).
Rahma, O. E. & Hodi, F. S. The Intersection between tumor angiogenesis and immune suppression. Clin. Cancer Res. 25, 5449–5457 (2019).
Missiaen, R., Mazzone, M. & Bergers, G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin. Cancer Biol. 52, 107–116 (2018).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015).
Shi, L. et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 10, 5421 (2019).
Marelli, L. et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc. Intervent. Radiol. 30, 6–25 (2007).
Llovet, J. M., Montal, R. & Villanueva, A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J. Hepatol. 70, 1262–1277 (2019).
Kudo, M. et al. Response evaluation criteria in cancer of the liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 revised version). Hepatol. Res. 40, 686–692 (2010).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Forner, A. et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 115, 616–623 (2009).
Llovet, J. M. et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl Cancer Inst. 100, 698–711 (2008).
Georgiades, C. et al. Lack of response after initial chemoembolization for hepatocellular carcinoma: does it predict failure of subsequent treatment? Radiology 265, 115–123 (2012).
Bruix, J. et al. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology 54, 2238–2244 (2011).
Lencioni, R. New data supporting modified RECIST (mRECIST) for hepatocellular carcinoma. Clin. Cancer Res. 19, 1312–1314 (2013).
Seyal, A. R. et al. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology 62, 1111–1121 (2015).
Salem, R. & Lewandowski, R. J. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 11, 604–e44 (2013).
Acknowledgements
J.M.L is supported by European Commission (EC)/Horizon 2020 Program (HEPCAR, Ref. 667273-2), EIT Health (CRISH2, Ref. 18053), Accelerator Award (CRUCK, AEEC, AIRC) (HUNTER, Ref. C9380/A26813), National Cancer Institute (P30-CA196521), U.S. Department of Defense (CA150272P3), Samuel Waxman Cancer Research Foundation, Spanish National Health Institute (SAF2016-76390) and the Generalitat de Catalunya/AGAUR (SGR-1358). P.K.H. is supported by the fellowship grant of the German Research Foundation (DFG HA 8754/1-1). T.F.G. is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. T.M. is supported by the NIHR UCLH Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.M.L. receives research support from Bayer HealthCare Pharmaceuticals, Boehringer-Ingelheim, Bristol-Myers Squibb, Eisai Inc. and Ipsen. He has also received consulting fees from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Can-Fite, Celsion Corporation, Eisai Inc., Eli Lilly, Glycotest, Ipsen, Merck, Nucleix, Roche and Sirtex. T.D.B. has received research support from Boston-Scientific Galil, and Terumo, and advisory board fees from AstraZeneca, Boston-Scientific, Esai, Guerbet and Terumo. L.K. receives consulting fees from Bayer, Eisai, Exelixis, Gilead and Merck, and research funding from Glycotest and TARGET-HCC. T.M. has received advisory board fees from AstraZeneca, BTG, Eisai, Ipsen, Roche and Tarveda. The remaining co-authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks E. Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
U.S. National Library of Medicine Clinical Trials.gov: https://clinicaltrials.gov/
Rights and permissions
About this article
Cite this article
Llovet, J.M., De Baere, T., Kulik, L. et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 18, 293–313 (2021). https://doi.org/10.1038/s41575-020-00395-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-00395-0
This article is cited by
-
Effectiveness and safety of transarterial chemoembolization combined with PD-1 inhibitors and lenvatinib for unresectable intrahepatic cholangiocarcinoma
European Radiology Experimental (2025)
-
Multiparametric MRI-based radiomics and clinical nomogram predicts the recurrence of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization
BMC Cancer (2025)
-
Donafenib versus sorafenib in triple therapy for unresectable hepatocellular carcinoma: a propensity score-matched multicenter analysis
World Journal of Surgical Oncology (2025)
-
Microalgae-based biodegradable embolic agent for the treatment of hepatocellular carcinoma through transarterial embolization
Journal of Nanobiotechnology (2025)
-
PD-1 inhibitors improve the efficacy of transcatheter arterial chemoembolization combined with apatinib in advanced hepatocellular carcinoma: a meta-analysis and trial sequential analysis
BMC Cancer (2025)