Abstract
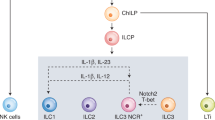
More than a decade ago, type 2 innate lymphoid cells (ILC2s) were discovered to be members of a family of innate immune cells consisting of five subsets that form a first line of defence against infections before the recruitment of adaptive immune cells. Initially, ILC2s were implicated in the early immune response to parasitic infections, but it is now clear that ILC2s are highly diverse and have crucial roles in the regulation of tissue homeostasis and repair. ILC2s can also regulate the functions of other type 2 immune cells, including T helper 2 cells, type 2 macrophages and eosinophils. Dysregulation of ILC2s contributes to type 2-mediated pathology in a wide variety of diseases, potentially making ILC2s attractive targets for therapeutic interventions. In this Review, we focus on the spectrum of ILC2 phenotypes that have been described across different tissues and disease states with an emphasis on human ILC2s. We discuss recent insights in ILC2 biology and suggest how this knowledge might be used for novel disease treatments and improved human health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Mjosberg, J. M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12, 1055–1062 (2011).
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011).
Fort, M. M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Mazzurana, L. et al. Tissue-specific transcriptional imprinting and heterogeneity in human innate lymphoid cells revealed by full-length single-cell RNA-sequencing. Cell Res. 31, 554–568 (2021).
Bal, S. M., Golebski, K. & Spits, H. Plasticity of innate lymphoid cell subsets. Nat. Rev. Immunol. 20, 552–565 (2020).
Seehus, C. R. et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat. Commun. 8, 1900 (2017).
Golebski, K. et al. IL-1beta, IL-23, and TGF-beta drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat. Commun. 10, 2162 (2019).
Morita, H. et al. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J. Allergy Clin. Immunol. 143, 2190–2201.e9 (2019).
Golebski, K. et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 54, 291–307.e7 (2021). This study describes the differentiation of human IL-10-producing ‘regulatory’ ILC2s and their role in allergen immunotherapy.
Huang, Y. et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119 (2018). The first description of the gut–lung axis of migratory ILC2s in helminth infection in mice.
van der Ploeg, E. K. et al. Steroid-resistant human inflammatory ILC2s are marked by CD45RO and elevated in type 2 respiratory diseases. Sci. Immunol. 6, eabd3489 (2021). This study describes activated and steroid-resistant CD45R0+ ILC2s in patients with asthma and chronic rhinosinusitis with nasal polyps.
Oyesola, O. O. et al. The prostaglandin D2 receptor CRTH2 promotes IL-33-induced ILC2 accumulation in the lung. J. Immunol. 204, 1001–1011 (2020).
Wojno, E. D. et al. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 8, 1313–1323 (2015).
Nagasawa, M. et al. KLRG1 and NKp46 discriminate subpopulations of human CD117+CRTH2- ILCs biased toward ILC2 or ILC3. J. Exp. Med. 216, 1762–1776 (2019).
Liu, S. et al. Optimal identification of human conventional and nonconventional (CRTH2-IL7Ralpha-) ILC2s using additional surface markers. J. Allergy Clin. Immunol. 146, 390–405 (2020).
Nagasawa, M., Germar, K., Blom, B. & Spits, H. Human CD5+ innate lymphoid cells are functionally immature and their development from CD34+ progenitor cells is regulated by Id2. Front. Immunol. 8, 1047 (2017).
Hernandez, D. C. et al. An in vitro platform supports generation of human innate lymphoid cells from CD34+ hematopoietic progenitors that recapitulate ex vivo identity. Immunity 54, 2417–2432.e5 (2021).
Alisjahbana, A. et al. CD5 surface expression marks intravascular human innate lymphoid cells that have a distinct ontogeny and migrate to the lung. Front. Immunol. 12, 752104 (2021).
Maric, J. et al. Cytokine-induced endogenous production of prostaglandin D2 is essential for human group 2 innate lymphoid cell activation. J. Allergy Clin. Immunol. 143, 2202–2214.e5 (2019).
Hochdorfer, T., Winkler, C., Pardali, K. & Mjosberg, J. Expression of c-Kit discriminates between two functionally distinct subsets of human type 2 innate lymphoid cells. Eur. J. Immunol. 49, 884–893 (2019).
Bernink, J. H. et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 20, 992–1003 (2019).
Bal, S. M. et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 17, 636–645 (2016).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Bernink, J. H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Lim, A. I. et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J. Exp. Med. 213, 569–583 (2016).
Ohne, Y. et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat. Immunol. 17, 646–655 (2016).
Silver, J. S. et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat. Immunol. 17, 626–635 (2016).
Almeida, F. F. & Belz, G. T. Innate lymphoid cells: models of plasticity for immune homeostasis and rapid responsiveness in protection. Mucosal Immunol. 9, 1103–1112 (2016).
Jegatheeswaran, S., Mathews, J. A. & Crome, S. Q. Searching for the elusive regulatory innate lymphoid cell. J. Immunol. 207, 1949–1957 (2021).
Bando, J. K. et al. ILC2s are the predominant source of intestinal ILC-derived IL-10. J. Exp. Med. 217, e20191520 (2020).
Chantveerawong, T. et al. Increased circulating CRTH2+ Tregs are associated with asthma control and exacerbation. Allergy 77, 681–685 (2021).
Boonpiyathad, T. et al. IL-10-producing innate lymphoid cells increased in patients with house dust mite allergic rhinitis following immunotherapy. J. Allergy Clin. Immunol. 147, 1507–1510.e8 (2021).
Howard, E. et al. IL-10 production by ILC2s requires Blimp-1 and cMaf, modulates cellular metabolism, and ameliorates airway hyperreactivity. J. Allergy Clin. Immunol. 147, 1281–1295.e5 (2021).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Ricardo-Gonzalez, R. R. et al. Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J. Exp. Med. 217, e20191172 (2020). This study tracks the origin of circulating ILC2s in mice to conclude that tissue-specific activation of ILC2s contributes to systemic type 2 immunity.
Hurrell, B. P. et al. Distinct roles of LFA-1 and ICAM-1 on ILC2s control lung infiltration, effector functions, and development of airway hyperreactivity. Front. Immunol. 11, 542818 (2020).
Zhang, S. et al. Neuropilin-1 mediates lung tissue-specific control of ILC2s function in type 2 immunity. Nat. Immunol. 23, 237–250 (2021).
Zeis, P. et al. In situ maturation and tissue adaptation of type 2 innate lymphoid cell progenitors. Immunity 53, 775–792.e9 (2020).
Campbell, L. et al. ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J. Exp. Med. 216, 2714–2723 (2019).
Fonseca, R. et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 21, 412–421 (2020).
Forkel, M. et al. Distinct alterations in the composition of mucosal innate lymphoid cells in newly diagnosed and established Crohn’s disease and ulcerative colitis. J. Crohns Colitis 13, 67–78 (2019).
Pu, Q. et al. Gut microbiota regulate gut-lung axis inflammatory responses by mediating ILC2 compartmental migration. J. Immunol. 207, 257–267 (2021).
Hepworth, M. R. et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 348, 1031–1035 (2015).
Xiao, S. Y., Li, Y. & Chen, W. F. Kinetics of thymocyte developmental process in fetal and neonatal mice. Cell Res. 13, 265–273 (2003).
Schneider, C. et al. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 174, 271–284.e14 (2018).
Li, N. et al. Memory CD4+ T cells are generated in the human fetal intestine. Nat. Immunol. 20, 301–312 (2019).
Schreurs, R. et al. Human fetal TNF-alpha-cytokine-producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 50, 462–476.e8 (2019).
Mao, K. et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554, 255–259 (2018).
Chen, R. et al. Allergen-induced increases in sputum levels of group 2 innate lymphoid cells in subjects with asthma. Am. J. Respir. Crit. Care Med. 196, 700–712 (2017).
Winkler, C. et al. Activation of group 2 innate lymphoid cells after allergen challenge in asthmatic patients. J. Allergy Clin. Immunol. 144, 61–69.e7 (2019). This study describes activated ILC2s in blood and BAL fluid following lung segmental allergen provocation in patients with allergic asthma.
Miller, M. M. et al. BATF acts as an essential regulator of IL-25-responsive migratory ILC2 cell fate and function. Sci. Immunol. 5, eaay3994 (2020).
Puttur, F. et al. Pulmonary environmental cues drive group 2 innate lymphoid cell dynamics in mice and humans. Sci. Immunol. 4, eaav7638 (2019).
Ercolano, G., Falquet, M., Vanoni, G., Trabanelli, S. & Jandus, C. ILC2s: new actors in tumor immunity. Front. Immunol. 10, 2801 (2019).
Xu, X. et al. Group-2 innate lymphoid cells promote HCC progression through CXCL2-neutrophil-induced immunosuppression. Hepatology 74, 2526–2543 (2021).
Heinrich, B. et al. The tumour microenvironment shapes innate lymphoid cells in patients with hepatocellular carcinoma. Gut https://doi.org/10.1136/gutjnl-2021-325288 (2021).
Jacquelot, N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 22, 851–864 (2021).
Moral, J. A. et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 579, 130–135 (2020). The first description of the role of ILC2s in mediating the effects of anti-PD1 cancer immunotherapy in mice.
Qi, J. et al. Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell Rep. Med. 2, 100353 (2021).
Goc, J. et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 184, 5015–5030.e16 (2021).
Ercolano, G. et al. PPAR drives IL-33-dependent ILC2 pro-tumoral functions. Nat. Commun. 12, 2538 (2021).
Huang, Q. et al. Type 2 innate lymphoid cells protect against colorectal cancer progression and predict improved patient survival. Cancers 13, 559 (2021).
Trabanelli, S. et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 8, 593 (2017).
Wu, L. et al. Mesenchymal PGD2 activates an ILC2-Treg axis to promote proliferation of normal and malignant HSPCs. Leukemia 34, 3028–3041 (2020).
Schuijs, M. J. et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 21, 998–1009 (2020).
Batyrova, B. et al. PD-1 expression affects cytokine production by ILC2 and is influenced by peroxisome proliferator-activated receptor-gamma. Immun. Inflamm. Dis. 8, 8–23 (2020).
Wang, S. et al. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 30, 610–622 (2020).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–1099 (2018).
Schneider, C. et al. Tissue-resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity 50, 1425–1438.e5 (2019).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Lee, M. W. et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015).
Rana, B. M. J. et al. A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J. Exp. Med. 216, 1999–2009 (2019).
Koga, S. et al. Peripheral PDGFRalpha+gp38+ mesenchymal cells support the differentiation of fetal liver-derived ILC2. J. Exp. Med. 215, 1609–1626 (2018).
Shan, B. et al. Cold-responsive adipocyte progenitors couple adrenergic signaling to immune cell activation to promote beige adipocyte accrual. Genes Dev. 35, 1333–1338 (2021).
Cardoso, F. et al. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature 597, 410–414 (2021). Describes ILC2s as effectors in the neuronal regulation of adipose tissue metabolism.
Galle-Treger, L. et al. Costimulation of type-2 innate lymphoid cells by GITR promotes effector function and ameliorates type 2 diabetes. Nat. Commun. 10, 713 (2019).
Rodriguez-Rodriguez, N., Gogoi, M. & McKenzie, A. N. J. Group 2 innate lymphoid cells: team players in regulating asthma. Annu. Rev. Immunol. 39, 167–198 (2021).
He, J. et al. Bilirubin represents a negative regulator of ILC2 in allergic airway inflammation. Mucosal Immunol. https://doi.org/10.1038/s41385-021-00460-0 (2021).
Karagiannis, F. et al. Lipid-droplet formation drives pathogenic group 2 innate lymphoid cells in airway inflammation. Immunity 52, 620–634.e6 (2020).
Thio, C. L., Chi, P. Y., Lai, A. C. & Chang, Y. J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 142, 1867–1883.e12 (2018).
Surace, L. et al. Dichotomous metabolic networks govern human ILC2 proliferation and function. Nat. Immunol. 22, 1367–1374 (2021). The first report of the metabolic requirements for maintenance, proliferation and effector cytokine production of human ILC2s.
Wallrapp, A. et al. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 51, 709–723.e6 (2019).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 (2017).
Nagashima, H. et al. Neuropeptide CGRP limits group 2 innate lymphoid cell responses and constrains type 2 inflammation. Immunity 51, 682–695.e6 (2019).
Sui, P. et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360, eaan8546 (2018).
Mayer, J. U. et al. Homeostatic IL-13 in healthy skin directs dendritic cell differentiation to promote TH2 and inhibit TH17 cell polarization. Nat. Immunol. 22, 1538–1550 (2021).
Kim, B. S. et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170ra116 (2013).
Bielecki, P. et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 592, 128–132 (2021).
Alkon, N. et al. Single-cell analysis reveals innate lymphoid cell lineage infidelity in atopic dermatitis. J. Allergy Clin. Immunol. 149, 624–639 (2021).
Teunissen, M. B. M. et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR+ ILC3 in lesional skin and blood of psoriasis patients. J. Invest. Dermatol. 134, 2351–2360 (2014).
Kloverpris, H. N. et al. Innate lymphoid cells are depleted irreversibly during acute HIV-1 infection in the absence of viral suppression. Immunity 44, 391–405 (2016).
Warren, K. J. et al. Neutralization of IL-33 modifies the type 2 and type 3 inflammatory signature of viral induced asthma exacerbation. Respir. Res. 22, 206 (2021).
Fonseca, W. et al. Uric acid pathway activation during respiratory virus infection promotes Th2 immune response via innate cytokine production and ILC2 accumulation. Mucosal Immunol. 13, 691–701 (2020).
Vu, L. D. et al. Elevated levels of type 2 respiratory innate lymphoid cells in human infants with severe respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 200, 1414–1423 (2019).
Fonseca, W., Lukacs, N. W., Elesela, S. & Malinczak, C. A. Role of ILC2 in viral-induced lung pathogenesis. Front. Immunol. 12, 675169 (2021).
Rajput, C. et al. Rhinovirus C infection induces type 2 innate lymphoid cell expansion and eosinophilic airway inflammation. Front. Immunol. 12, 649520 (2021). Demonstrates that the accumulation of bronchial ILC2s during in vivo rhinovirus infection correlates with respiratory symptoms in humans.
Dhariwal, J. et al. Pulmonary innate lymphoid cell responses during rhinovirus-induced asthma exacerbations in vivo: a clinical trial. Am. J. Respir. Crit. Care Med. 204, 1259–1273 (2021).
Satoh-Takayama, N. et al. Bacteria-induced group 2 innate lymphoid cells in the stomach provide immune protection through induction of IgA. Immunity 52, 635–649.e4 (2020).
Kramer, B. et al. Compartment-specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLoS Pathog. 13, e1006373 (2017).
Ordovas-Montanes, J. et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 560, 649–654 (2018).
Saco, T., Ugalde, I. C., Cardet, J. C. & Casale, T. B. Strategies for choosing a biologic for your patient with allergy or asthma. Ann. Allergy Asthma Immunol. 127, 627–637 (2021).
Bacharier, L. B. et al. Dupilumab in children with uncontrolled moderate-to-severe asthma. N. Engl. J. Med. 385, 2230–2240 (2021).
Bruce, D. W. et al. Third party type 2 innate lymphoid cells prevent and treat GI tract GvHD. Blood Adv. 5, 4578–4589 (2021).
Huang, Q. et al. IL-10 producing type 2 innate lymphoid cells prolong islet allograft survival. EMBO Mol. Med. 12, e12305 (2020).
DuPage, M. & Bluestone, J. A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat. Rev. Immunol. 16, 149–163 (2016).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Garcia, M. et al. Innate lymphoid cell composition associates with COVID-19 disease severity. Clin. Transl. Immunol. 9, e1224 (2020).
Gomez-Cadena, A. et al. Severe COVID-19 patients exhibit an ILC2 NKG2D+ population in their impaired ILC compartment. Cell. Mol. Immunol. 18, 484–486 (2021).
Schulz-Kuhnt, A. et al. ILC2 lung-homing in cystic fibrosis patients: functional involvement of CCR6 and impact on respiratory failure. Front. Immunol. 11, 691 (2020).
Acknowledgements
The authors thank J. Bernink, K. Golabski and R. Stadhouders for helpful input on the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
H.S. is a consultant for GlaxoSmithKline. J.M. declares no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks G. Eberl, N. Satoh-Takayama and R. Locksley for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Alarmin
-
A type of damage-associated molecular pattern composed of endogenously produced proteins or peptides, including IL-33, that are released upon immune activation, degranulation, cell injury or death.
- Chronic rhinosinusitis with nasal polyps
-
(CRSwNP). A chronic inflammation of sinonasal tissues, resulting in the formation of inflammatory lesions and polyps, and manifested as rhinorrhoea, nasal congestion, and facial pressure and/or pain.
- Beiging
-
The process by which white adipose tissue is converted to a phenotype more similar to that of brown adipose tissue, thereby increasing energy expenditure by reducing lipids stored within the adipose tissue.
- Enkephalins
-
Small peptides that function as neurotransmitters and that bind to opioid receptors.
- Metabolic syndrome
-
A combination of conditions, including high blood pressure, high blood glucose levels, high levels of waist body fat, and high cholesterol or triglyceride levels, that predisposes for development of heart disease and type 2 diabetes.
- Ketogenic diet
-
Dietary intake of high levels of fat and low levels of carbohydrate.
Rights and permissions
About this article
Cite this article
Spits, H., Mjösberg, J. Heterogeneity of type 2 innate lymphoid cells. Nat Rev Immunol 22, 701–712 (2022). https://doi.org/10.1038/s41577-022-00704-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-022-00704-5
This article is cited by
-
Reinventing type 2 immunity in cancer
Nature (2025)
-
The immunology of asthma and chronic rhinosinusitis
Nature Reviews Immunology (2025)
-
Aggravating mechanisms from COVID-19
Virology Journal (2024)
-
Exogenous IL-33 promotes tumor immunity via macroscopic regulation of ILC2s
Scientific Reports (2024)
-
Tissue microenvironment induces tissue specificity of ILC2
Cell Death Discovery (2024)