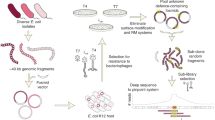
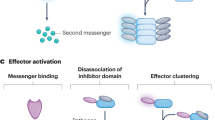
Abstract
Viruses and their hosts are engaged in a constant arms race leading to the evolution of antiviral defence mechanisms. Recent studies have revealed that the immune arsenal of bacteria against bacteriophages is much more diverse than previously envisioned. These discoveries have led to seemingly contradictory observations: on one hand, individual microorganisms often encode multiple distinct defence systems, some of which are acquired by horizontal gene transfer, alluding to their fitness benefit. On the other hand, defence systems are frequently lost from prokaryotic genomes on short evolutionary time scales, suggesting that they impose a fitness cost. In this Perspective article, we present the ‘pan-immune system’ model in which we suggest that, although a single strain cannot carry all possible defence systems owing to their burden on fitness, it can employ horizontal gene transfer to access immune defence mechanisms encoded by closely related strains. Thus, the ‘effective’ immune system is not the one encoded by the genome of a single microorganism but rather by its pan-genome, comprising the sum of all immune systems available for a microorganism to horizontally acquire and use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roux, S., Hallam, S. J., Woyke, T. & Sullivan, M. B. Viral dark matter and virus–host interactions resolved from publicly available microbial genomes. eLife 4, e08490 (2015).
Touchon, M., Bernheim, A. & Rocha, E. P. Genetic and life-history traits associated with the distribution of prophages in bacteria. ISME J. 10, 2744–2754 (2016).
Koonin, E. V., Senkevich, T. G. & Dolja, V. V. The ancient virus world and evolution of cells. Biol. Direct 1, 29 (2006).
Kronheim, S. et al. A chemical defence against phage infection. Nature 564, 283–286 (2018).
Niewoehner, O. et al. Type III CRISPR–Cas systems generate cyclic oligoadenylate second messengers to activate Csm6 RNases. Nature 548, 543–548 (2017).
Kazlauskiene, M., Kostiuk, G., Venclovas, C., Tamulaitis, G. & Siksnys, V. A cyclic oligonucleotide signaling pathway in type III CRISPR–Cas systems. Science 357, 605–609 (2017).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 (2018).
Koonin, E. V., Makarova, K. S. & Wolf, Y. I. Evolutionary genomics of defense systems in archaea and bacteria. Annu. Rev. Microbiol. 71, 233–261 (2017).
van Houte, S., Buckling, A. & Westra, E. R. Evolutionary ecology of prokaryotic immune mechanisms. Microbiol. Mol. Biol. Rev. 80, 745–763 (2016).
Rostøl, J. T. & Marraffini, L. (Ph)ighting phages: how bacteria resist their parasites. Cell Host Microbe 25, 184–194 (2019).
Dy, R. L., Richter, C., Salmond, G. P. C. & Fineran, P. C. Remarkable mechanisms in microbes to resist phage infections. Annu. Rev. Virol. 1, 307–331 (2014).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 (2010).
Borges, A. L., Davidson, A. R. & Bondy-denomy, J. The discovery, mechanisms, and evolutionary impact of anti-CRISPRs. Annu. Rev. Virol. 4, 37–59 (2017).
Samson, J. E., Magadán, A. H., Sabri, M. & Moineau, S. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–687 (2013).
Oliveira, P. H., Touchon, M. & Rocha, E. P. C. The interplay of restriction-modification systems with mobile genetic elements and their prokaryotic hosts. Nucleic Acids Res. 42, 10618–10631 (2014).
Hille, F. et al. The biology of CRISPR–Cas: backward and forward. Cell 172, 1239–1259 (2018).
Tock, M. R. & Dryden, D. T. F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8, 466–472 (2005).
Wang, L. et al. Phosphorothioation of DNA in bacteria by dnd genes. Nat. Chem. Biol. 3, 709–710 (2007).
Thiaville, J. J. et al. Novel genomic island modifies DNA with 7-deazaguanine derivatives. Proc. Natl Acad. Sci. USA 113, E1452–E1459 (2016).
Goldfarb, T. et al. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 34, 169–183 (2015).
Ofir, G. et al. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 3, 90–98 (2018).
Amitai, G. & Sorek, R. CRISPR–Cas adaptation: new insights into the mechanism of action. Nat. Rev. Microbiol. 14, 67–76 (2016).
Makarova, K. S. et al. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Makarova, K. S., Wolf, Y. I. & Koonin, E. V. Classification and nomenclature of CRISPR–Cas systems: where from here? CRISPR J. 1, 325–336 (2018).
Malina, A. et al. PAM multiplicity marks genomic target sites as inhibitory to CRISPR–Cas9 editing. Nat. Commun. 6, 10124 (2015).
Hegge, J. W., Swarts, D. C. & Van Der Oost, J. Prokaryotic argonaute proteins: novel genome-editing tools? Nat. Rev. Microbiol. 16, 5–11 (2018).
Swarts, D. C. et al. DNA-guided DNA interference by a prokaryotic argonaute. Nature 507, 258–261 (2014).
Bingham, R., Ekunwe, S. I. N., Falk, S., Snyder, L. & Kleanthous, C. The major head protein of bacteriophage T4 binds specifically to elongation factor Tu. J. Biol. Chem. 275, 23219–23226 (2000).
Kaufmann, G. Anticodon nucleases. Trends Biochem. Sci. 25, 70–74 (2000).
Durmaz, E. & Klaenhammer, T. R. Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189, 1417–1425 (2007).
Chopin, M. C., Chopin, A. & Bidnenko, E. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8, 473–479 (2005).
Depardieu, F. et al. A eukaryotic-like serine/threonine kinase protects staphylococci against phages. Cell Host Microbe 20, 471–481 (2016).
Harms, A., Brodersen, D. E., Mitarai, N. & Gerdes, K. Toxins, targets, and triggers: an overview of toxin-antitoxin biology. Mol. Cell 70, 768–784 (2018).
Cohen, D. et al. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature https://doi.org/10.1038/s41586-019-1605-5 (2019).
Bair, C. L. & Black, L. W. A type IV modification dependent restriction nuclease that targets glucosylated hydroxymethyl cytosine modified DNAs. J. Mol. Biol. 366, 768–778 (2007).
Bryson, A. et al. Covalent modification of bacteriophage T4 DNA inhibits CRISPR–Cas9. mBio 6, e00648 (2015).
Deveau, H. et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400 (2008).
Pawluk, A., Davidson, A. R. & Maxwell, K. L. Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol. 16, 12–17 (2017).
Knott, G. J. et al. Broad-spectrum enzymatic inhibition of CRISPR-Cas12a. Nat. Struct. Mol. Biol. 26, 315–321 (2019).
Dong, L. et al. An anti-CRISPR protein disables type V Cas12a by acetylation. Nat. Struct. Mol. Biol. 26, 308–314 (2019).
Bair, C. L., Rifat, D. & Black, L. W. Exclusion of glucosyl-hydroxymethylcytosine DNA containing bacteriophages is overcome by the injected protein inhibitor IPI*. J. Mol. Biol. 366, 779–789 (2007).
Iida, S., Streiff, M. B., Bickle, T. A. & Arber, W. Two DNA antirestriction systems of bacteriophage P1, darA, and darB: characterization of darA-phages. Virology 157, 156–166 (1987).
Godde, J. S. & Bickerton, A. The repetitive DNA elements called CRISPRs and their associated genes: evidence of horizontal transfer among prokaryotes. J. Mol. Evol. 62, 718–729 (2006).
Makarova, K. S., Wolf, Y. I. & Koonin, E. V. Comparative genomics of defense systems in archaea and bacteria. Nucleic Acids Res. 41, 4360–4377 (2013).
Peters, J. E., Makarova, K. S., Shmakov, S. & Koonin, E. V. Recruitment of CRISPR–Cas systems by Tn7-like transposons. Proc. Natl Acad. Sci. USA. 114, E7358–E7366 (2017).
Seed, K. D., Lazinski, D. W., Calderwood, S. B. & Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494, 489–491 (2013).
Faure, G. et al. CRISPR–Cas in mobile genetic elements: counter-defence and beyond. Nat. Rev. Microbiol. 17, 513–525 (2019).
Makarova, K. S., Wolf, Y. I., Snir, S. & Koonin, E. V. Defense islands in bacterial and archaeal genomes and prediction of novel defense systems. J. Bacteriol. 193, 6039–6056 (2011).
Stern, A., Keren, L., Wurtzel, O., Amitai, G. & Sorek, R. Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet. 26, 335–340 (2010).
Heussler, G. E. & O’Toole, G. A. Friendly fire: biological functions and consequences of chromosomal-targeting by CRISPR–Cas systems. J. Bacteriol. 198, 1481–1486 (2016).
Vercoe, R. B. et al. Cytotoxic chromosomal targeting by CRISPR/Cas systems can reshape bacterial genomes and expel or remodel pathogenicity islands. PLOS Genet. 9, e1003454 (2013).
Cui, L. & Bikard, D. Consequences of Cas9 cleavage in the chromosome of Escherichia coli. Nucleic Acids Res. 44, 4243–4251 (2016).
Pleška, M. et al. Bacterial autoimmunity due to a restriction-modification system. Curr. Biol. 26, 404–409 (2016).
Berngruber, T. W., Lion, S. & Gandon, S. Evolution of suicide as a defence strategy against pathogens in a spatially structured environment. Ecol. Lett. 16, 446–453 (2013).
Seidel, R., Bloom, J. G. P., Dekker, C. & Szczelkun, M. D. Motor step size and ATP coupling efficiency of the dsDNA translocase EcoR124I. EMBO J. 27, 1388–1398 (2008).
Vale, P. F. et al. Costs of CRISPR–Cas-mediated resistance in Streptococcus thermophilus. Proc. Biol. Sci. 282, 20151270 (2015).
Jiang, W. et al. Dealing with the evolutionary downside of CRISPR immunity: bacteria and beneficial plasmids. PLOS Genet. 9, e1003844 (2013).
Bikard, D., Hatoum-Aslan, A., Mucida, D. & Marraffini, L. A. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection. Cell Host Microbe 12, 177–186 (2012).
Puigbò, P., Lobkovsky, A. E., Kristensen, D. M., Wolf, Y. I. & Koonin, E. V. Genomes in turmoil: quantification of genome dynamics in prokaryote supergenomes. BMC Biol. 12, 66 (2014).
Puigbò, P., Makarova, K. S., Kristensen, D. M., Wolf, Y. I. & Koonin, E. V. Reconstruction of the evolution of microbial defense systems. BMC Evol. Biol. 17, 94 (2017).
Childs, L. M., England, W. E., Young, M. J., Weitz, J. S. & Whitaker, R. J. CRISPR-induced distributed immunity in microbial populations. PLOS ONE 9, e101710 (2014).
Levin, B. R., Moineau, S., Bushman, M. & Barrangou, R. The population and evolutionary dynamics of phage and bacteria with CRISPR-mediated immunity. PLOS Genet. 9, e1003312 (2013).
Houte, S. van et al. The diversity-generating benefits of a prokaryotic adaptive immune system. Nature 532, 385–388 (2016).
Hyman, P. & Abedon, S. T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 70, 217–248 (2010).
de Jonge, P. A., Nobrega, F. L., Brouns, S. J. J. & Dutilh, B. E. Molecular and evolutionary determinants of bacteriophage host range. Trends Microbiol. 27, 51–63 (2019).
Miller, E. S. et al. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67, 86–156 (2003).
Molineux, I. in TheBacteriophagesVol. 2 Ch. 20 (ed. Calender, R.) (Oxford University Press, 2005).
Levy, A. et al. CRISPR adaptation biases explain preference for acquisition of foreign DNA. Nature 520, 505–510 (2015).
Bernheim, A. et al. Inhibition of NHEJ repair by type II-A CRISPR–Cas systems. Nat. Commun. 8, 170647 (2017).
Bernheim, A., Bikard, D., Touchon, M. & Rocha, E. P. C. A matter of background: DNA repair pathways as a possible cause for the sparse distribution of CRISPR–Cas systems in bacteria. Philos. Trans. R. Soc. B Biol. Sci. 374, 20180088 (2019).
Dupuis, M.-È., Villion, M., Magadán, A. H. & Moineau, S. CRISPR–Cas and restriction-modification systems are compatible and increase phage resistance. Nat. Commun. 4, 2087 (2013).
Hynes, A. P., Villion, M. & Moineau, S. Adaptation in bacterial CRISPR–Cas immunity can be driven by defective phages. Nat. Commun. 5, 4399 (2014).
Silas, S. et al. Type III CRISPR–Cas systems can provide redundancy to counteract viral escape from type I systems. eLife 6, e27601 (2017).
Amitai, G. & Sorek, R. Intracellular signaling in CRISPR–Cas defense. Science 357, 550–551 (2017).
Rehman, S., Ali, Z., Khan, M., Bostan, N. & Naseem, S. The dawn of phage therapy. Rev. Med. Virol. 29, e2041 (2019).
Abedon, S. T., García, P., Mullany, P. & Aminov, R. Editorial: phage therapy: past, present and future. Front. Microbiol. 8, 981 (2017).
Oliveira, P. H., Touchon, M., Cury, J. & Rocha, E. P. C. The chromosomal organization of horizontal gene transfer in bacteria. Nat. Commun. 8, 841 (2017).
Couvin, D. et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 46, W246–W251 (2018).
Oliveira, P. H., Touchon, M. & Rocha, E. P. C. Regulation of genetic flux between bacteria by restriction modification systems. Proc. Natl Acad. Sci. USA. 113, 5658–5663 (2016).
Acknowledgements
The authors thank S. Doron and A. Millman for their help in the analysis presented in Box 1 and Figure 2, M. Voichek and N. Tal for their helpful comments on the figures in this study, and J. Cury and the Sorek laboratory members for comments on earlier versions of this manuscript. A.B. is the recipient of a European Molecular Biology Organization (EMBO) Long Term Fellowship (EMBO ALTF 186-2018). R.S. was supported, in part, by the Israel Science Foundation (personal grant 1360/16), the European Research Council (grant ERC-CoG 681203), the German Research Council (DFG) priority programme SPP 2002 (grant SO 1611/1-1), the Ernest and Bonnie Beutler Research Program of Excellence in Genomic Medicine and the Knell Family Center for Microbiology.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.S. is a scientific cofounder and consultant of BiomX Ltd, Pantheon Ltd and EcoPhage Ltd. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Microbiology thanks E. Koonin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bernheim, A., Sorek, R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat Rev Microbiol 18, 113–119 (2020). https://doi.org/10.1038/s41579-019-0278-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-019-0278-2