Abstract
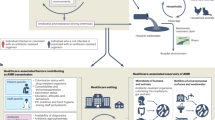
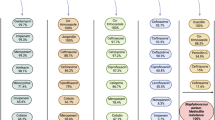
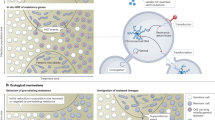
The use of antibiotics has enabled the successful treatment of bacterial infections, saving the lives and improving the health of many patients worldwide. However, the emergence and spread of antimicrobial resistance (AMR) has been highlighted as a global threat by different health organizations, and pathogens resistant to antimicrobials cause substantial morbidity and death. As resistance to multiple drugs increases, novel and effective therapies as well as prevention strategies are needed. In this Review, we discuss evidence that vaccines can have a major role in fighting AMR. Vaccines are used prophylactically, decreasing the number of infectious disease cases, and thus antibiotic use and the emergence and spread of AMR. We also describe the current state of development of vaccines against resistant bacterial pathogens that cause a substantial disease burden both in high-income countries and in low- and medium-income countries, discuss possible obstacles that hinder progress in vaccine development and speculate on the impact of next-generation vaccines against bacterial infectious diseases on AMR.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kapoor, G., Saigal, S. & Elongavan, A. Action and resistance mechanisms of antibiotics: a guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 33, 300–305 (2017).
Mladenovic-Antic, S. et al. Correlation between antimicrobial consumption and antimicrobial resistance of Pseudomonas aeruginosa in a hospital setting: a 10-year study. J. Clin. Pharm. Ther. 41, 532–537 (2016).
Hay, S. I. et al. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 16, 78 (2018).
IACG No Time To Wait: Securing The Future From Drug-Resistant Infections (2019).
United Nations. Political Declaration of the High-Level Meeting of the General Assembly on Antimicrobial Resistance, A/71/L.2. 22 (2016).
Aslam, B. et al. Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 11, 1645–1658 (2018).
Rappuoli, R., Santoni, A. & Mantovani, A. Vaccines: an achievement of civilization, a human right, our health insurance for the future. J. Exp. Med. 216, 7–9 (2019).
Kennedy, D. A. & Read, A. F. Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance. Proc. Natl Acad. Sci. USA 115, 12878–12886 (2018). This review compares emergence of drug resistance and vaccine resistance and explains why vaccine resistance is not a major concern.
Baker, S. J., Payne, D. J., Rappuoli, R. & De Gregorio, E. Technologies to address antimicrobial resistance. Proc. Natl Acad. Sci. USA 115, 12887–12895 (2018). This review describes how innovative vaccine technologies have the potential to boost the development of vaccines targeting several classes of multidrug-resistant bacteria.
Klugman, K. P. & Black, S. Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects. Proc. Natl Acad. Sci. USA 115, 12896–12901 (2018).
Jansen, K. U., Knirsch, C. & Anderson, A. S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 24, 10–19 (2018). This review highlights the value of vaccines as one of the modalities to combat AMR globally, using select examples.
Cassini, A. et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 19, 56–66 (2019).
CDC. Antibiotic Resistance Threats in the United States, 2019. https://www.cdc.gov/DrugResistance/Biggest-Threats.html (Department of Health and Human Services, 2019). This is a CDC report with the latest national death and infection estimates that underscore the continued threat of antibiotic resistance in the USA.
World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 (2017). This WHO document reports a list of antibiotic-resistant bacteria to help in prioritizing research and development investments for new and effective interventions
CDC, Healthcare-associated infections, https://www.cdc.gov/hai/patientsafety/ar-hospitals.html
Hersh, A. L., Chambers, H. F., Maselli, J. H. & Gonzales, R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Intern. Med. 168, 1585–1591 (2008).
Prosperi, M. et al. Molecular epidemiology of community-associated methicillin-resistant Staphylococcus aureus in the genomic era: a cross-sectional study. Sci. Rep. 3, 1902 (2013).
Khan, M. S. et al. ‘LMICs as reservoirs of AMR’: a comparative analysis of policy discourse on antimicrobial resistance with reference to Pakistan. Health Policy Plan. 34, 178–187 (2019).
FAO Global Forum on Food Security and Nutrition, http://www.fao.org/3/cb0863en/cb0863en.pdf
Founou, R. C., Founou, L. L. & Essack, S. Y. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS ONE 12, e0189621 (2017). This study analyses the published literature on the clinical and economic implications of AMR in developing countries.
Ayukekbong, J. A., Ntemgwa, M. & Atabe, A. N. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control. 6, 47 (2017).
Bagnoli, F. & Payne, D. J. Reaction: alternative modalities to address antibiotic-resistant pathogens. Chem 3, 369–372 (2017).
Romanò, L. et al. Hepatitis B vaccination. Hum. Vaccin. Immunother. 11, 53–57 (2015).
Zanetti, A. R., Van Damme, P. & Shouval, D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 26, 6266–6273 (2008).
Octavia, S. et al. Newly emerging clones of Bordetella pertussis carrying prn2 and ptxP3 alleles implicated in Australian pertussis epidemic in 2008-2010. J. Infect. Dis. 205, 1220–1224 (2012).
Cherry, J. D. Epidemic pertussis in 2012–the resurgence of a vaccine-preventable disease. N. Engl. J. Med. 367, 785–787 (2012).
Mooi, F. R. et al. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in the Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect. Immun. 66, 670–675 (1998).
Mishra, R. P., Oviedo-Orta, E., Prachi, P., Rappuoli, R. & Bagnoli, F. Vaccines and antibiotic resistance. Curr. Opin. Microbiol. 15, 596–602 (2012).
Miller, E., Andrews, N. J., Waight, P. A., Slack, M. P. & George, R. C. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect. Dis. 11, 760–768 (2011).
Moore, M. R. et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197, 1016–1027 (2008).
Lipsitch, M. & Siber, G. R. How can vaccines contribute to solving the antimicrobial resistance problem? mBio 7, e00428-16 (2016). This minireview describes the significant contributions of current vaccines and the potential of future vaccines in controlling AMR and elucidates the mechanisms by which this can occur.
Sihvonen, R., Siira, L., Toropainen, M., Kuusela, P. & Patari-Sampo, A. Streptococcus pneumoniae antimicrobial resistance decreased in the Helsinki Metropolitan Area after routine 10-valent pneumococcal conjugate vaccination of infants in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 36, 2109–2116 (2017).
John, T. J., Cherian, T. & Raghupathy, P. Haemophilus influenzae disease in children in India: a hospital perspective. Pediatr. Infect. Dis. J. 17, S169–S171 (1998).
Oliver, S. P., Murinda, S. E. & Jayarao, B. M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodborne Pathog. Dis. 8, 337–355 (2011).
No authors. Standing up to antimicrobial resistance. Nat Rev Microbiol 8, 836 (2010).
McEwen, S. A. & Fedorka-Cray, P. J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 34 (Suppl. 3), S93–S106 (2002).
Hoelzer, K. et al. Vaccines as alternatives to antibiotics for food producing animals. Part 1: challenges and needs. Vet. Res. 49, 64 (2018).
Tekle, Y. I. et al. Controlling antimicrobial resistance through targeted, vaccine-induced replacement of strains. PLoS ONE 7, e50688 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01887912 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03090191?term=vaccine&cond=clostridium+difficile&rank=7 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02316470?term=vaccine (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04026009 (2019).
Pechine, S., Bruxelle, J. F., Janoir, C. & Collignon, A. Targeting clostridium difficile surface components to develop immunotherapeutic strategies against Clostridium difficile infection. Front. Microbiol. 9, 1009 (2018).
Starks, C. M. et al. Optimization and qualification of an assay that demonstrates that a FimH vaccine induces functional antibody responses in women with histories of urinary tract infections. Hum. Vaccin. Immunother. https://doi.org/10.1080/21645515.2020.1770034 (2020).
Huttner, A. & Gambillara, V. The development and early clinical testing of the ExPEC4V conjugate vaccine against uropathogenic Escherichia coli. Clin. Microbiol. Infect. 24, 1046–1050 (2018).
Huttner, A. et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 17, 528–537 (2017).
Fattom, A. et al. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: phase III randomized study. Hum. Vaccin. Immunother. 11, 632–641 (2015).
Fowler, V. G. et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309, 1368–1378 (2013).
Pozzi, C. et al. Vaccines for Staphylococcus aureus and target populations. Curr. Top. Microbiol. Immunol. 409, 491–528 (2017).
Edwards, J. L., Jennings, M. P. & Seib, K. L. Neisseria gonorrhoeae vaccine development: hope on the horizon? Curr. Opin. Infect. Dis. 31, 246–250 (2018).
Vincent, L. R. & Jerse, A. E. Biological feasibility and importance of a gonorrhea vaccine for global public health. Vaccine 37, 7419–7426 (2018).
Petousis-Harris, H. et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 390, 1603–1610 (2017).
Craig, A. P. et al. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 33, 4520–4525 (2015).
Petousis-Harris, H. & Radcliff, F. J. Exploitation of Neisseria meningitidis group B OMV vaccines against N. gonorrhoeae to inform the development and deployment of effective gonorrhea vaccines. Front. Immunol. 10, 683 (2019).
University of Alabama at Birmingham NIH study to explore vaccine for gonorrhea prevention. https://www.uab.edu/news/health/item/10852-nih-study-to-explore-vaccine-for-gonorrhea-prevention (2019).
Raterman, E. L. & Jerse, A. E. Female mouse model of Neisseria gonorrhoeae infection. Methods Mol. Biol. 1997, 413–429 (2019).
Edwards, J. L. & Apicella, M. A. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17, 965–981 (2004).
The Boston Consulting Group. Vaccines to tackle drug resistant infections. An evaluation of R&D opportunities (2018). This report evaluates the development potential of vaccines against pathogens with high levels of AMR.
Priebe, G. P. & Goldberg, J. B. Vaccines for Pseudomonas aeruginosa: a long and winding road. Expert Rev. Vaccines 13, 507–519 (2014).
Doring, G., Meisner, C. & Stern, M. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc. Natl Acad. Sci. USA 104, 11020–11025 (2007).
Pier, G. B. et al. Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect. Immun. 62, 3972–3979 (1994).
Rello, J. et al. A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients. Crit. Care 21, 22 (2017).
Bianconi, I. et al. Genome-based approach delivers vaccine candidates against Pseudomonas aeruginosa. Front. Immunol. 9, 3021 (2018).
Choi, M., Tennant, S. M., Simon, R. & Cross, A. S. Progress towards the development of Klebsiella vaccines. Expert Rev. Vaccines 18, 681–691 (2019).
Seeberger, P. H. et al. A semi-synthetic glycoconjugate vaccine candidate for carbapenem-resistant Klebsiella pneumoniae. Angew. Chem. Int. Ed. 56, 13973–13978 (2017).
Zigterman, J. W. et al. Immunogenic properties of octasaccharide-protein conjugates derived from Klebsiella serotype 11 capsular polysaccharide. Infect. Immun. 47, 421–428 (1985).
Feldman, M. F. et al. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl Acad. Sci. USA 116, 18655–18663 (2019).
Follador, R. et al. The diversity of Klebsiella pneumoniae surface polysaccharides. Microb. Genom. 2, e000073 (2016).
Hegerle, N. et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS ONE 13, e0203143 (2018).
Lee, W. H. et al. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 47, e183 (2015).
Martin, R. M. et al. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere https://doi.org/10.1128/mSphere.00261-16 (2016).
World Health Organization. Typhoid vaccines: WHO position paper, March 2018 - recommendations. Vaccine 37, 214–216 (2019).
MacLennan, C. A., Martin, L. B. & Micoli, F. Vaccines against invasive Salmonella disease: current status and future directions. Hum. Vaccin. Immunother. 10, 1478–1493 (2014).
Lin, F. Y. et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med. 344, 1263–1269 (2001).
Wahid, R., Salerno-Goncalves, R., Tacket, C. O., Levine, M. M. & Sztein, M. B. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine 25, 1416–1425 (2007).
Wahid, R. et al. Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+ IgD- S. Typhi-specific IgA and IgG B memory cells in humans. Clin. Immunol. 138, 187–200 (2011).
Jin, C. et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet 390, 2472–2480 (2017).
Martin, L. B. et al. Status of paratyphoid fever vaccine research and development. Vaccine 34, 2900–2902 (2016).
Gat, O. et al. Cell-associated flagella enhance the protection conferred by mucosally-administered attenuated Salmonella Paratyphi A vaccines. PLoS Negl. Trop. Dis. 5, e1373 (2011).
Micoli, F. et al. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS ONE 7, e47039 (2012).
Tennant, S. M., MacLennan, C. A., Simon, R., Martin, L. B. & Khan, M. I. Nontyphoidal salmonella disease: current status of vaccine research and development. Vaccine 34, 2907–2910 (2016).
Lanzilao, L. et al. Strain selection for generation of O-antigen-based glycoconjugate vaccines against invasive nontyphoidal salmonella disease. PLoS ONE 10, e0139847 (2015).
Watson, D. C., Robbins, J. B. & Szu, S. C. Protection of mice against Salmonella typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect. Immun. 60, 4679–4686 (1992).
Simon, R. et al. Sustained protection in mice immunized with fractional doses of Salmonella Enteritidis core and O polysaccharide-flagellin glycoconjugates. PLoS ONE 8, e64680 (2013).
Micoli, F. et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl Acad. Sci. USA 115, 10428–10433 (2018).
Mani, S., Wierzba, T. & Walker, R. I. Status of vaccine research and development for Shigella. Vaccine 34, 2887–2894 (2016).
van der Put, R. M. et al. A synthetic carbohydrate conjugate vaccine candidate against shigellosis: improved bioconjugation and impact of alum on immunogenicity. Bioconjug. Chem. 27, 883–892 (2016).
Hatz, C. F. et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: a single blind, partially randomized phase I study. Vaccine 33, 4594–4601 (2015).
Riddle, M. S. et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized phase I study. Clin. Vaccine Immunol. 23, 908–917 (2016).
Obiero, C. W. et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front. Immunol. 8, 1884 (2017).
Micoli, F. & MacLennan, C. A. Outer membrane vesicle vaccines. Semin. Immunol. 25, 87–88 (2020).
Martinez-Becerra, F. J. et al. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect. Immun. 80, 1222–1231 (2012).
Pore, D. & Chakrabarti, M. K. Outer membrane protein A (OmpA) from Shigella flexneri 2a: a promising subunit vaccine candidate. Vaccine 31, 3644–3650 (2013).
Steer, A. C. et al. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine 34, 2953–2958 (2016).
McNeil, S. A. et al. Safety and immunogenicity of 26-valent group A Streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41, 1114–1122 (2005).
Dale, J. B., Penfound, T. A., Chiang, E. Y. & Walton, W. J. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 29, 8175–8178 (2011).
Steer, A. C., Law, I., Matatolu, L., Beall, B. W. & Carapetis, J. R. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect. Dis. 9, 611–616 (2009).
Batzloff, M. R. et al. Protection against group A streptococcus by immunization with J8-diphtheria toxoid: contribution of J8- and diphtheria toxoid-specific antibodies to protection. J. Infect. Dis. 187, 1598–1608 (2003).
Guilherme, L. et al. Towards a vaccine against rheumatic fever. Clin. Dev. Immunol. 13, 125–132 (2006).
Sheel, M., Moreland, N. J., Fraser, J. D. & Carapetis, J. Development of group A streptococcal vaccines: an unmet global health need. Expert Rev. Vaccines 15, 227–238 (2016).
Pandey, M. et al. A synthetic M protein peptide synergizes with a CXC chemokine protease to induce vaccine-mediated protection against virulent streptococcal pyoderma and bacteremia. J. Immunol. 194, 5915–5925 (2015).
Bensi, G. et al. Multi high-throughput approach for highly selective identification of vaccine candidates: the group A Streptococcus case. Mol Cell Proteomics 11, M111.015693 (2012).
Rivera-Hernandez, T. et al. An experimental group a streptococcus vaccine that reduces pharyngitis and tonsillitis in a nonhuman primate model. mBio https://doi.org/10.1128/mBio.00693-19 (2019).
Kabanova, A. et al. Evaluation of a group A Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine 29, 104–114 (2010).
Henningham, A. et al. Virulence role of the GlcNAc side chain of the Lancefield cell wall carbohydrate antigen in non-M1-serotype group A Streptococcus. mBio https://doi.org/10.1128/mBio.02294-17 (2018).
Schodel, F. et al. Clinical development strategy for a candidate group A streptococcal vaccine. Vaccine 35, 2007–2014 (2017).
Gamez-Gonzalez, L. B., Hamada, H., Llamas-Guillen, B. A., Ruiz-Fernandez, M. & Yamazaki-Nakashimada, M. BCG and Kawasaki disease in Mexico and Japan. Hum. Vaccin. Immunother. 13, 1091–1093 (2017).
Mangtani, P. et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 58, 470–480 (2014).
Nemes, E. et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N. Engl. J. Med. 379, 138–149 (2018).
Tait, D. R. et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 381, 2429–2439 (2019).
Van Der Meeren, O. et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 379, 1621–1634 (2018).
Vekemans, J., O’Brien, K. L. & Farrar, J. Tuberculosis vaccines: rising opportunities. PLoS Med. 16, e1002791 (2019).
FAO Global Forum on Food Security and Nutrition, http://www.fao.org/3/cb0863en/cb0863en.pdf
Cabral, M. P. et al. Design of live attenuated bacterial vaccines based on D-glutamate auxotrophy. Nat. Commun. 8, 15480 (2017).
Micoli, F. et al. Glycoconjugate vaccines: current approaches towards faster vaccine design. Expert Rev. Vaccines 18, 881–895 (2019).
Krammer, F. SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020).
Sevilla, J. P., Bloom, D. E., Cadarette, D., Jit, M. & Lipsitch, M. Toward economic evaluation of the value of vaccines and other health technologies in addressing AMR. Proc. Natl Acad. Sci. USA 115, 12911–12919 (2018).
Roope, L. S. J. et al. The challenge of antimicrobial resistance: what economics can contribute. Science 364, eaau4679 (2019). This article highlights prospects for future research on the economics of AMR.
Bloom, D. E., Black, S., Salisbury, D. & Rappuoli, R. Antimicrobial resistance and the role of vaccines. Proc. Natl Acad. Sci. USA 115, 12868–12871 (2018). This article highlights that an integrated strategy that includes antibiotics, vaccines, diagnostic tools, antibodies and new tools targeting the host or the microbiome or delivered by bacteriophages is required to fight AMR effectively.
Balsells, E. et al. Infection prevention and control of Clostridium difficile: a global review of guidelines, strategies, and recommendations. J. Glob. Health 6, 020410 (2016).
Peng, Z. et al. Update on antimicrobial resistance in clostridium difficile: resistance mechanisms and antimicrobial susceptibility testing. J. Clin. Microbiol. 55, 1998–2008 (2017).
Asadi Karam, M. R., Habibi, M. & Bouzari, S. Urinary tract infection: pathogenicity, antibiotic resistance and development of effective vaccines against uropathogenic Escherichia coli. Mol. Immunol. 108, 56–67 (2019).
Kourtis, A. P. et al. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections - United States. MMWR Morb. Mortal. Wkly. Rep. 68, 214–219 (2019).
Unemo, M., Del Rio, C. & Shafer, W. M. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.EI10-0009-2015 (2016).
El Zowalaty, M. E. et al. Pseudomonas aeruginosa: arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol. 10, 1683–1706 (2015).
Munoz-Price, L. S. et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13, 785–796 (2013).
Dyson, Z. A., Klemm, E. J., Palmer, S. & Dougan, G. Antibiotic resistance and typhoid. Clin. Infect. Dis. 68, S165–s170 (2019).
Qamar, F. N., Azmatullah, A., Kazi, A. M., Khan, E. & Zaidi, A. K. A three-year review of antimicrobial resistance of Salmonella enterica serovars Typhi and Paratyphi A in Pakistan. J. Infect. Dev. Ctries. 8, 981–986 (2014).
Kariuki, S., Gordon, M. A., Feasey, N. & Parry, C. M. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 33, C21–C29 (2015).
Gordon, M. A. et al. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin. Infect. Dis. 46, 963–969 (2008).
Puzari, M., Sharma, M. & Chetia, P. Emergence of antibiotic resistant Shigella species: a matter of concern. J. Infect. Public Health 11, 451–454 (2018).
Zafar, A. et al. Antibiotic susceptibility in Streptococcus pneumoniae, Haemophilus influenzae and Streptococcus pyogenes in Pakistan: a review of results from the Survey of Antibiotic Resistance (SOAR) 2002-15. J. Antimicrob. Chemother. 71, i103–i109 (2016).
US Department of Health and Human Services. Antibiotic resistance threats in the United States. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (2013).
Gandhi, N. R. et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375, 1830–1843 (2010).
Nguyen, L. Antibiotic resistance mechanisms in M. tuberculosis: an update. Arch. Toxicol. 90, 1585–1604 (2016).
Marston, H. D., Paules, C. I. & Fauci, A. S. Monoclonal antibodies for emerging infectious diseases - borrowing from history. N. Engl. J. Med. 378, 1469–1472 (2018).
McConnell, M. J. Where are we with monoclonal antibodies for multidrug-resistant infections? Drug Discov. Today 24, 1132–1138 (2019).
DiGiandomenico, A. et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 6, 262ra155 (2014).
Lowy, I. et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362, 197–205 (2010).
Gulati, S. et al. Complement alone drives efficacy of a chimeric antigonococcal monoclonal antibody. PLoS Biol. 17, e3000323 (2019).
Fischetti, V. A. Development of phage lysins as novel therapeutics: a historical perspective. Viruses 10, 310 (2018).
Merabishvili, M. et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS ONE 4, e4944 (2009).
Sahota, J. S. et al. Bacteriophage delivery by nebulization and efficacy against phenotypically diverse Pseudomonas aeruginosa from cystic fibrosis patients. J. Aerosol Med. Pulm. Drug Deliv. 28, 353–360 (2015).
Nale, J. Y. et al. Bacteriophage combinations significantly reduce Clostridium difficile growth in vitro and proliferation in vivo. Antimicrob. Agents Chemother. 60, 968–981 (2016).
Lima, R., Del Fiol, F. S. & Balcao, V. M. Prospects for the use of new technologies to combat multidrug-resistant bacteria. Front. Pharmacol. 10, 692 (2019).
Relman, D. A. & Lipsitch, M. Microbiome as a tool and a target in the effort to address antimicrobial resistance. Proc. Natl Acad. Sci. USA 115, 12902–12910 (2018).
Khanna, S. et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J. Infect. Dis. 214, 173–181 (2016).
Gerding, D. N. et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA 313, 1719–1727 (2015).
Kim, W. J. et al. Commensal Neisseria kill Neisseria gonorrhoeae through a DNA-dependent mechanism. Cell Host Microbe 26, 228–239.e8 (2019).
Idelevich, E. A. & Becker, K. How to accelerate antimicrobial susceptibility testing. Clin. Microbiol. Infect. 25, 1347–1355 (2019).
van Belkum, A. et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 17, 51–62 (2019).
Li, W. et al. Rapid identification and antimicrobial susceptibility testing for urinary tract pathogens by direct analysis of urine samples using a MALDI-TOF MS-based combined protocol. Front. Microbiol. 10, 1182 (2019).
Rappuoli, R. Glycoconjugate vaccines: principles and mechanisms. Sci. Transl. Med. 10, eaat4615 (2018).
Delany, I., Rappuoli, R. & De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 6, 708–720 (2014).
Serruto, D., Serino, L., Masignani, V. & Pizza, M. Genome-based approaches to develop vaccines against bacterial pathogens. Vaccine 27, 3245–3250 (2009).
Serruto, D., Bottomley, M. J., Ram, S., Giuliani, M. M. & Rappuoli, R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30, B87–B97 (2012).
Moriel, D. G. et al. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc. Natl Acad. Sci. USA 107, 9072–9077 (2010).
Rappuoli, R., Bottomley, M. J., D’Oro, U., Finco, O. & De Gregorio, E. Reverse vaccinology 2.0: human immunology instructs vaccine antigen design. J. Exp. Med. 213, 469–481 (2016).
Crank, M. C. et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 365, 505–509 (2019).
Scarselli, M. et al. Rational design of a meningococcal antigen inducing broad protective immunity. Sci. Transl. Med. 3, 91ra62 (2011).
Gnopo, Y. M. D., Watkins, H. C., Stevenson, T. C., DeLisa, M. P. & Putnam, D. Designer outer membrane vesicles as immunomodulatory systems-reprogramming bacteria for vaccine delivery. Adv. Drug Deliv. Rev. 114, 132–142 (2017).
Gerke, C. et al. Production of a shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS ONE 10, e0134478 (2015).
Launay, O. et al. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine 22, 164–172 (2017).
Launay, O. et al. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: results from a phase 1 clinical trial. Front. Immunol. 10, 335 (2019).
Wacker, M. et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298, 1790–1793 (2002).
Kampf, M. M. et al. In vivo production of a novel glycoconjugate vaccine against Shigella flexneri 2a in recombinant Escherichia coli: identification of stimulating factors for in vivo glycosylation. Microb. Cell Fact. 14, 12 (2015).
Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 19, 1597–1608 (2013).
Otieno, L. et al. Safety and immunogenicity of RTS,S/AS01 malaria vaccine in infants and children with WHO stage 1 or 2 HIV disease: a randomised, double-blind, controlled trial. Lancet Infect. Dis. 16, 1134–1144 (2016).
Cunningham, A. L. et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 375, 1019–1032 (2016).
Author information
Authors and Affiliations
Contributions
F.M., F.B. and D.S. researched data for the article and wrote the article. All authors substantially contributed to the discussion of the content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F.M., F.B., R.R. and D.S. are employees of the GSK group of companies, which is involved in the discovery and commercialization of vaccines and therapeutics against bacterial infections.
Additional information
Peer review information
Nature Reviews Microbiology thanks K. Atkins and S. Vong for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
AMR Action Fund: https://amractionfund.com
CDC: https://www.cdc.gov/hai/patientsafety/ar-hospitals.html
FAO Global Forum on Food Security and Nutrition: http://www.fao.org/3/cb0863en/cb0863en.pdf
Institute for Health Metrics and Evaluation: http://www.healthdata.org/gbd
Supplementary information
Rights and permissions
About this article
Cite this article
Micoli, F., Bagnoli, F., Rappuoli, R. et al. The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol 19, 287–302 (2021). https://doi.org/10.1038/s41579-020-00506-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-020-00506-3
This article is cited by
-
Epitope mapping of recombinant Salmonella enterica serotype Heidelberg flagellar hook-associated protein by in silico and in vivo approaches
BMC Veterinary Research (2025)
-
Enhancing vaccine clinical trials participation among elderly: challenges and strategies
Trials (2025)
-
Farmer field schools as interventions to reduce the need for antimicrobials in agrifood systems: a longitudinal analysis of layer farmer field school graduates and non-graduates in Kenya
Antimicrobial Resistance & Infection Control (2025)
-
Intranasal delivery of mRNA expressing newly identified Acinetobacter baumannii antigens protects against bacterial lung disease
npj Vaccines (2025)
-
Changing climate and socioeconomic factors contribute to global antimicrobial resistance
Nature Medicine (2025)