Abstract
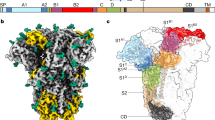
Coronaviruses cause respiratory tract infections in humans and outbreaks of deadly pneumonia worldwide. Infections are initiated by the transmembrane spike (S) glycoprotein, which binds to host receptors and fuses the viral and cellular membranes. To understand the molecular basis of coronavirus attachment to oligosaccharide receptors, we determined cryo-EM structures of coronavirus OC43 S glycoprotein trimer in isolation and in complex with a 9-O-acetylated sialic acid. We show that the ligand binds with fast kinetics to a surface-exposed groove and that interactions at the identified site are essential for S-mediated viral entry into host cells, but free monosaccharide does not trigger fusogenic conformational changes. The receptor-interacting site is conserved in all coronavirus S glycoproteins that engage 9-O-acetyl-sialogycans, with an architecture similar to those of the ligand-binding pockets of coronavirus hemagglutinin esterases and influenza virus C/D hemagglutinin-esterase fusion glycoproteins. Our results demonstrate these viruses evolved similar strategies to engage sialoglycans at the surface of target cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ge, X. Y. et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503, 535–538 (2013).
Haagmans, B. L. et al. Middle east respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 14, 140–145 (2014).
Su, S. et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 24, 490–502 (2016).
Isaacs, D., Flowers, D., Clarke, J. R., Valman, H. B. & MacNaughton, M. R. Epidemiology of coronavirus respiratory infections. Arch. Dis. Child 58, 500–503 (1983).
Menachery, V. D. et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 21, 1508–1513 (2015).
Menachery, V. D. et al. SARS-like WIV1-CoV poised for human emergence. Proc. Natl Acad. Sci. USA 113, 3048–3053 (2016).
Hu, B. et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 13, e1006698 (2017).
Bosch, B. J., van der Zee, R., de Haan, C. A. & Rottier, P. J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 77, 8801–8811 (2003).
Walls, A. C. et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature 531, 114–117 (2016).
Millet, J. K. & Whittaker, G. R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 202, 120–134 (2015).
Belouzard, S., Chu, V. C. & Whittaker, G. R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl Acad. Sci. USA 106, 5871–5876 (2009).
Millet, J. K. & Whittaker, G. R. Host cell entry of middle east respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl Acad. Sci. USA 111, 15214–15219 (2014).
Walls, A. C. et al. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl Acad. Sci. 114, 11157–11162 (2017).
Burkard, C. et al. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 10, e1004502 (2014).
Walls, A. et al. Crucial steps in the structure determination of a coronavirus spike glycoprotein using cryo-electron microscopy. Protein Sci. 26, 113–121 (2017).
Walls, A. C. et al. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 23, 899–905 (2016).
Xiong, X. et al. Glycan shield and fusion activation of a deltacoronavirus spike glycoprotein fine-tuned for enteric infections. J. Virol. 92, e01628-17 (2018).
Kirchdoerfer, R. N. et al. Pre-fusion structure of a human coronavirus spike protein. Nature 531, 118–121 (2016).
Kirchdoerfer, R. N. et al. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 8, 15701 (2018).
Pallesen, J. et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl Acad. Sci. USA 114, E7348–E7357 (2017).
Gui, M. et al. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 27, 119–129 (2017).
Shang, J. et al. Cryo-EM structure of infectious bronchitis coronavirus spike protein reveals structural and functional evolution of coronavirus spike proteins. PLoS Pathog. 14, e1007009 (2018).
Song, W., Gui, M., Wang, X. & Xiang, Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 14, e1007236 (2018).
Shang, J.et al. Cryo-EM structure of porcine delta coronavirus spike protein in the pre-fusion state. J. Virol. https://doi.org/10.1128/JVI.01556-17 (2017).
Walls, A. C. et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion.Cell 176, 1026–1039.e15 (2019).
Vijgen, L. et al. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 79, 1595–1604 (2005).
Lau, S. K. et al. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J. Virol. 85, 11325–11337 (2011).
Vlasak, R., Luytjes, W., Spaan, W. & Palese, P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl Acad. Sci. USA 85, 4526–4529 (1988).
Huang, X. et al. Human coronavirus HKU1 spike protein uses O-acetylated sialic acid as an attachment receptor determinant and employs hemagglutinin-esterase protein as a receptor-destroying enzyme. J. Virol. 89, 7202–7213 (2015).
de Groot, R. J. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj. J. 23, 59–72 (2006).
Desforges, M., Desjardins, J., Zhang, C. & Talbot, P. J. The acetyl-esterase activity of the hemagglutinin-esterase protein of human coronavirus OC43 strongly enhances the production of infectious virus. J. Virol. 87, 3097–3107 (2013).
Bakkers, M. J. et al. Betacoronavirus adaptation to humans involved progressive loss of hemagglutinin-esterase lectin activity. Cell Host Microbe 21, 356–366 (2017).
Zeng, Q., Langereis, M. A., van Vliet, A. L., Huizinga, E. G. & de Groot, R. J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc. Natl Acad. Sci. USA 105, 9065–9069 (2008).
Rosenthal, P. B. et al. Structure of the haemagglutinin-esterase-fusion glycoprotein of influenza C virus. Nature 396, 92–96 (1998).
Song, H. et al. An open receptor-binding cavity of hemagglutinin-esterase-fusion glycoprotein from newly-identified influenza D virus: basis for its broad cell tropism. PLoS Pathog. 12, e1005411 (2016).
Herrler, G. et al. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 4, 1503–1506 (1985).
Stencel-Baerenwald, J. E., Reiss, K., Reiter, D. M., Stehle, T. & Dermody, T. S. The sweet spot: defining virus-sialic acid interactions. Nat. Rev. Microbiol 12, 739–749 (2014).
Neu, U., Bauer, J. & Stehle, T. Viruses and sialic acids: rules of engagement. Curr. Opin. Struct. Biol. 21, 610–618 (2011).
Schauer, R. & Kamerling, J. P. Exploration of the sialic acid world. Adv. Carbohydr. Chem. Biochem 75, 1–213 (2018).
Peng, G. et al. Crystal structure of bovine coronavirus spike protein lectin domain. J. Biol. Chem. 287, 41931–41938 (2012).
Peng, G. et al. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl Acad. Sci. USA 108, 10696–10701 (2011).
Li, W. et al. Identification of sialic acid-binding function for the middle east respiratory syndrome coronavirus spike glycoprotein. Proc. Natl Acad. Sci. USA 114, E8508–E8517 (2017).
Hulswit, R. J. G. et al. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc. Natl Acad. Sci. USA 116, 2681–2690 (2019).
Collins, A. R. HLA class I antigen serves as a receptor for human coronavirus OC43. Immunol. Invest 22, 95–103 (1993).
Langereis, M. A. et al. Complexity and diversity of the mammalian sialome revealed by nidovirus virolectins. Cell Rep. 11, 1966–1978 (2015).
Bakkers, M. J. et al. Coronavirus receptor switch explained from the stereochemistry of protein-carbohydrate interactions and a single mutation. Proc. Natl Acad. Sci. USA 113, E3111–E3119 (2016).
Li, F., Li, W., Farzan, M. & Harrison, S. C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309, 1864–1868 (2005).
Lu, G. et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 500, 227–231 (2013).
Ou, X. et al. Crystal structure of the receptor binding domain of the spike glycoprotein of human betacoronavirus HKU1. Nat. Commun. 8, 15216 (2017).
Yuan, Y. et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 8, 15092 (2017).
Campbell, M. G., Veesler, D., Cheng, A., Potter, C. S. & Carragher, B. A resolution reconstruction of the Thermoplasma acidophilum 20S proteasome using cryo-electron microscopy. eLife 4, e06380 (2015).
Seetharaman, J. et al. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J. Biol. Chem. 273, 13047–13052 (1998).
Dormitzer, P. R., Sun, Z. Y., Wagner, G. & Harrison, S. C. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21, 885–897 (2002).
Langereis, M. A., Zeng, Q., Heesters, B. A., Huizinga, E. G. & de Groot, R. J. The murine coronavirus hemagglutinin-esterase receptor-binding site: a major shift in ligand specificity through modest changes in architecture. PLoS Pathog. 8, e1002492 (2012).
Kunkel, F. & Herrler, G. Structural and functional analysis of the surface protein of human coronavirus OC43. Virology 195, 195–202 (1993).
Fei, Y. et al. Characterization of receptor binding profiles of influenza a viruses using an ellipsometry-based label-free glycan microarray assay platform. Biomolecules 5, 1480–1498 (2015).
Xiong, X. et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature 497, 392–396 (2013).
Guo, H. et al. Kinetic analysis of the influenza A virus HA/NA balance reveals contribution of NA to virus-receptor binding and NA-dependent rolling on receptor-containing surfaces. PLoS Pathog. 14, e1007233 (2018).
Sakai, T., Takagi, H., Muraki, Y. & Saito, M. Unique directional motility of influenza C Virus controlled by its filamentous morphology and short-range motions. J. Virol. https://doi.org/10.1128/JVI.01522-17 (2018).
Sakai, T., Nishimura, S. I., Naito, T. & Saito, M. Influenza A virus hemagglutinin and neuraminidase act as novel motile machinery. Sci. Rep. 7, 45043 (2017).
Mukherjee, S. et al. Mechanism and significance of cell type-dependent neutralization of flaviviruses. J. Virol. 88, 7210–7220 (2014).
Park, J. E. et al. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl Acad. Sci. USA 113, 12262–12267 (2016).
Owczarek, K. et al. Early events during human coronavirus OC43 entry to the cell. Sci. Rep. 8, 7124 (2018).
Wickramasinghe, I. N., de Vries, R. P., Grone, A., de Haan, C. A. & Verheije, M. H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 85, 8903–8912 (2011).
Liu, C. et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 89, 6121–6125 (2015).
Schultze, B. et al. Transmissible gastroenteritis coronavirus, but not the related porcine respiratory coronavirus, has a sialic acid (N-glycolylneuraminic acid) binding activity. J. Virol. 70, 5634–5637 (1996).
Nilsson, E. C. et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med 17, 105–109 (2011).
Reiter, D. M. et al. Crystal structure of reovirus attachment protein sigma1 in complex with sialylated oligosaccharides. PLoS Pathog. 7, e1002166 (2011).
Ito, T. et al. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 227, 493–499 (1997).
Raj, V. S. et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495, 251–254 (2013).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Tegunov, D. C., P. Real-time cryo-EM data pre-processing with Warp. Preprint at: https://www.biorxiv.org/content/10.1101/338558v1 (2018).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zivanov, J., Nakane, T. & Scheres, S.H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Goddard, T. D., Huang, C. C. & Ferrin, T. E. Visualizing density maps with UCSF Chimera. J. Struct. Biol. 157, 281–287 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Wang, R. Y. et al. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. eLife 5, e17219 (2016).
Frenz, B. D. R. et al. Automatically fixing errors in glycoprotein structures with Rosetta. Structure 27, 1–6 (2019).
DiMaio, F., Leaver-Fay, A., Bradley, P., Baker, D. & Andre, I. Modeling symmetric macromolecular structures in Rosetta3. PLoS One 6, e20450 (2011).
DiMaio, F. et al. Atomic-accuracy models from 4.5-A cryo-electron microscopy data with density-guided iterative local refinement. Nat. Methods 12, 361–365 (2015).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D. Biol. Crystallogr 66, 12–21 (2010).
Agirre, J. et al. Privateer: software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 22, 833–834 (2015).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Goddard, T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res 32, W665–W667 (2004).
Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001).
Kaname, Y. et al. Acquisition of complement resistance through incorporation of CD55/decay-accelerating factor into viral particles bearing baculovirus GP64. J. Virol. 84, 3210–3219 (2010).
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences (R01GM120553 to D.V.), a Pew Biomedical Scholars Award (D.V.) and an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund (D.V.), the Zoonoses Anticipation and Preparedness Initiative (IMI115760, F.A.R. and B.-J.B.), the Pasteur Institute (M.A.T. and F.A.R.), the Centre National de la Recherche Scientifique (F.A.R.), the LabEx Integrative Biology of Emerging Infectious Diseases (F.A.R.), the Netherlands Organization for Scientific Research (NWO TOP-PUNT 718.015.003, G.-J.B.) and CSC grant 2014-03250042 (Y.L.). This work was also partly supported by the Arnold and Mabel Beckman cryo-EM center and the Proteomics Resource (UWPR95794) at the University of Washington, and the Electron Imaging Center for NanoMachines supported by the NIH (1U24GM116792, 1S10RR23057 and 1S10OD018111), NSF (DBI-1338135) and CNSI at UCLA. We thank Y. Matsuura (Osaka University, Japan) for provision of the VSV-G pseudotyped VSVΔG/Fluc plasmids.
Author information
Authors and Affiliations
Contributions
M.A.T., A.C.W., Y.L., R.J.d.G. and D.V. designed the experiments. C.W. and B.-J.B. designed and cloned the protein constructs. M.A.T. and C.W. carried out protein expression and purification. Z.L. and G.-J.B. provided key reagents; M.A.T. and A.C.W. performed cryo-EM sample preparation and data collection. M.A.T., A.C.W. and D.V. processed the cryo-EM data. M.A.T. and D.V. built and refined the atomic models. Y.L. and D.K. carried out the binding and pseudovirus assays. M.A.T., A.C.W., Y.L., R.J.d.G., F.A.R. and D.V. analyzed the data. M.A.T., A.C.W. and D.V. prepared the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 CryoEM analysis of apo and holo HCoV-OC43 S glycoprotein.
a, c, Representative electron micrographs (defocus: 1.4 μm and 1.2 µm) and class averages of apo (a) and holo (c) HCoV-OC43 S trimer embedded in vitreous ice. Scale bars: 100 nm (micrograph) and 200 nm Å (class averages). b, d Gold-standard (blue) and model/map (red) Fourier shell correlation curves. The resolution was determined to 2.9 Å and 2.8 Å for the apo and holo HCoV-OC43 S glycoprotein maps, respectively. The 0.143 and 0.5 cut-off values are indicated by horizontal dashed lines.
Supplementary Figure 2 Carbohydrate ligands are recognized by distinct regions of the HCoV-OC43 S, human galectin-3 or rotavirus VP8* β-sandwich.
(a-c) Ribbon diagrams in two orthogonal orientations showing 9-O-Ac-Me-Sia bound to the HCoV-OC43 S domain A (a), galactose bound to human galectin-3 (b, PDB 1A3K) and sialic acid bound to rotavirus VP8* (c, PDB 1KQR). Each domain is shown in the same two orientations for comparison.
Supplementary Figure 3 Zoomed-in view of the sialoglycan-binding site in the holo-HCoV-OC43 S glycoprotein structure.
The A domain is rendered as a ribbon diagram with the side chains of key ligand-interacting residues shown as sticks and the corresponding cryoEM density shown as a blue mesh. The ligand is rendered as sticks with atoms colored by elements (carbon: grey, nitrogen: blue, oxygen: red).
Supplementary Figure 4 Free 9-O-Ac-Me-Sia receptor does not trigger HCoV-OC43 S fusogenic conformational changes.
(a) SDS-PAGE. (b) Purified wild-type HCoV-OC43 S ectodomain trimer in the prefusion conformation. (c-f) The wild-type HCoV-OC43 S ectodomain trimer remained in the prefusion conformation after cleavage with 28 µg.mL−1 trypsin (c), incubation with 100 mM 9-O-Ac-Me-Sia of pre-cleaved trimer (d) or trypsin cleavage of 9-O-Ac-Me-Sia-bound trimer (e), cleavage with 28 µg.mL−1 trypsin and incubation at pH 4.5 for 1 hour at room temperature (f), as visualized by single-particle electron microscopy of negatively stained samples. The wild-type HCoV-OC43 S ectodomain trimer was cleaved with 28 µg.mL−1 trypsin and heated for 20 minutes at 50 °C in the absence (g) or presence (h) of 10% isopropanol (used to dissolve the trypsin inhibitor added to stop the proteolytic reaction). Only the latter condition led to the formation of postfusion rosettes. Scale bars: 200 nm.
Supplementary Figure 5 Coronavirus S galectin-like A domains have a conserved architecture.
(a–d) Ribbon diagrams of the HCoV-OC43 (a), BCoV (b, PDB 4H14), PHEV (c, PDB 6QFY) and HCoV-HKU1 (d, PDB 5I08) A domains.
Supplementary information
Supplementary Information
Supplementary Figures 1–5
Rights and permissions
About this article
Cite this article
Tortorici, M.A., Walls, A.C., Lang, Y. et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol 26, 481–489 (2019). https://doi.org/10.1038/s41594-019-0233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-019-0233-y
This article is cited by
-
Molecular basis of host recognition of human coronavirus 229E
Nature Communications (2025)
-
A novel neutralizing monoclonal antibody recognizes a linear antigenic epitope of the spike protein of swine acute diarrhoea syndrome coronavirus
Virology Journal (2024)
-
Discordant phylodynamic and spatiotemporal transmission patterns driving the long-term persistence and evolution of human coronaviruses
npj Viruses (2024)
-
Immunoinformatics design of a structural proteins driven multi-epitope candidate vaccine against different SARS-CoV-2 variants based on fynomer
Scientific Reports (2024)
-
A broadly generalizable stabilization strategy for sarbecovirus fusion machinery vaccines
Nature Communications (2024)