Abstract
To describe the long-term health outcomes of patients with COVID-19 and investigate the potential risk factors. Clinical data during hospitalization and at a mean (SD) day of 249 (15) days after discharge from 40 survivors with confirmed COVID-19 (including 25 severe cases) were collected and analyzed retrospectively. At follow-up, severe cases had higher incidences of persistent symptoms, DLCO impairment, and higher abnormal CT score as compared with mild cases. CT score at follow-up was positively correlated with age, LDH level, cumulative days of oxygen treatment, total dosage of glucocorticoids used, and CT peak score during hospitalization. DLCO% at follow-up was negatively correlated with cumulative days of oxygen treatment during hospitalization. DLCO/VA% at follow-up was positively correlated with BMI, and TNF-α level. Among the three groups categorized as survivors with normal DLCO, abnormal DLCO but normal DLCO/VA, and abnormal DLCO and DLCO/VA, survivors with abnormal DLCO and DLCO/VA had the lowest serum IL-2R, IL-8, and TNF-α level, while the survivors with abnormal DLCO but normal DLCO/VA had the highest levels of inflammatory cytokines during hospitalization. Altogether, COVID-19 had a greater long-term impact on the lung physiology of severe cases. The long-term radiological abnormality maybe relate to old age and the severity of COVID-19. Either absent or excess of inflammation during COVID-19 course would lead to the impairment of pulmonary diffusion function.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) is a recently emerged infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1. As of December 31, 2020, accumulative 81,475,053 confirmed cases and 1,798,050 confirmed deaths were reported globally. During the outbreak period, researchers mainly focus on the epidemiological characteristics, infection and pathophysiological mechanisms, as well as treatment methods of this disease. Previous studies have shown that COVID-19 involves multiple organs, and the lung is one of the most important organs involved.
Among patients with COVID-19, about 14% cases were severe and 5% cases were critical2, and the overall case-fatality rate (CFR) was 5.0% (4788 total deaths/96,673 total confirmed cases) in China, based on data up to December 31, 2020, from Chinese Center for Disease Control and Prevention. Although the overall CFR of COVID-19 is lower than that of severe acute respiratory syndrome (SARS) (9.6%) and Middle East respiratory syndrome (MERS) (34.4%)2, radiology and lung function abnormalities can be found in a considerable proportion of COVID-19 survivors at time of hospital discharge, in early convalescence phase, and even at three and six months after discharge3,4,5,6,7.Severe patients had a higher incidence of diffusion capacity of the lung for carbon monoxide (DLCO) impairment and encountered more total lung capacity (TLC) decrease and six-minute walk distance (6MWD) decline compared with non-severe patients at 30 days after discharged4. While there is lack of clinical evidence for the long-term follow up of pulmonary function and physiological disorder in severe COVID-19 patients.
Thus, we retrospectively collected and analyzed the clinical data during hospitalization and at eight months after discharge to investigate the long-term impact of severe COVID-19 on pulmonary function, chest high-resolution computed tomography (HRCT) pictures, and related physiological characteristics and try to find out the potential risk factors.
Results
Subjects’ characteristics
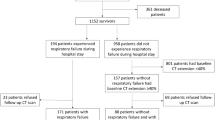
We recruited 21 women and 19 men with a media (IQR) age of 57 (40–68) years and a mean ± SD body mass index (BMI) of 25.47 ± 4.22 kg/m2 including 25 severe cases. Common comorbidities included hypertension (18 cases, 45.0%), diabetes mellitus (six cases, 15.0%), coronary heart disease (four cases, 10.0%), chronic obstructive pulmonary disease (COPD) (two cases, 5.0%), and asthma (one case, 2.5%). Eight (20.0%) patients were smokers, but only one was current smoker on admission. During the period having COVID-19, 32 (80.0%) patients received oxygen treatment and two of them required additional noninvasive positive pressure ventilation (NIPPV) treatment and ICU admission; 24 (60.0%) patients received glucocorticoids and five of them required high-dose glucocorticoids.
The follow-up was obtained on an average ± SD of 249 ± 15 days after discharge. At eight months 22 (55.0%) patients still had persistent physical and (or) psychological symptoms, and nine (22.5%) patients still suffered from different degrees of limitations in daily life. Although all patients had normal FVC, one (2.5%), 13 (32.5%), and nine (22.5%) patients had TLC, DLCO, and DLCO/VA below 80% of predicted values, respectively. Eight (20.0%) patients had FEV1/FVC below 70% of predicted values, and two of them had history of COPD with significant history of cigarette smoking, the other six had no history of COPD, asthma, or cigarette smoking. Twenty-two (55.0%) patients had at least two of the three indexes of MEF50, MEF25, and MMEF75/25 below 65% of predicted values, which indicates small airway dysfunction. The chest HRCT of 28 (70.0%) patients were normal or basically normal (CT score < 5), whereas the remaining 12 patients had an abnormal CT at eight months after discharge. However, CT abnormalities and pulmonary function impairments were not completely consistent on all of patients. Follow-up CT images of two severe COVID-19 patients with abnormal CT but different pulmonary diffusion function status at eight months after discharge were presented in Fig. 1. Most common abnormal CT patterns were ground glass opacity (GGO) (21 cases, 52.5%), irregular lines (19 cases, 47.5%), subpleural line (two cases, 5.0%), and reticular pattern (two cases, 5.0%) (Fig. 2).
Serial HRCTs of two severe COVID-19 with different pulmonary diffusion function status. (a–d) Serial HRCTs of a 65-year-old COVID-19 female patient with normal pulmonary diffusion function at eight months post discharge: (e–h) Serial HRCTs of a 71-year-old COVID-19 female patient with abnormal pulmonary diffusion function at eight months post discharge.
Abnormal CT patterns of severe COVID-19 patient at follow-up of eight months post discharge. The abnormal CT patterns are indicated by arrows in (a) ground glass opacities, (b) irregular lines, (c) subpleural line, and (d) reticular pattern which could still be seen on some of COVID-19 patients in convalescence of eight months post discharge.
Subgroup analyses
Patients were divided into two groups according to severity of COVID-19
In compared with mild cases, severe cases had higher CT peak score (p < 0.001), higher rate of receiving oxygen treatment (p = 0.007), longer cumulative days of oxygen treatment (p < 0.001), higher maximum inhaled oxygen concentration (p = 0.004), and longer cumulative days of receiving glucocorticoids during hospitalization (p = 0.020), a trend of higher rate of receiving glucocorticoids (p = 0.094), and a trend of higher total dosage of glucocorticoids used during hospitalization (p = 0.054). More details were summarized in Table 1.
At eight months after discharge, more patients in severe cases group had persistent physical and (or) psychological symptoms (p = 0.009). Severe cases had higher score in PF (p = 0.043), higher rate of abnormal DLCO (p = 0.013), higher rate of small airway dysfunction (p = 0.050), lower FEV1% predicted (p = 0.008), lower FVC% predicted (p = 0.042), lower MEF50% predicted (p = 0.023), lower MVV% predicted (p = 0.032), higher Z at 5 Hz% predicted (p = 0.020), which indicated higher total respiratory impedance, higher Rperipheral (p = 0.015), which indicated higher resistance in the peripheral airways, lower DLCO% predicted (p = 0.023), and higher CT score (p = 0.009) in compared with mild cases. More details were summarized in Table 2.
Patients were divided into two groups according to CT score at follow up
As compared with patients with normal or basically normal CT (CT score < 5) at eight months, patients with abnormal CT (CT score ≥ 5) had higher CT peak score (p < 0.001), lower lymphocyte count (p = 0.003), higher procalcitonin (PCT) level (p = 0.038), higher lactate dehydrogenase (LDH) level (p = 0.021), higher D-dimer level (p = 0.031), lower albumin level (p = 0.002), higher aspartate aminotransferase (AST) level (p = 0.032), longer cumulative days of oxygen treatment (p < 0.001), higher maximum inhaled oxygen concentration (p = 0.010), longer cumulative days of receiving glucocorticoids (p = 0.007), and higher total dosage of glucocorticoids used during hospitalization (p = 0.024).
At eight months after discharge, patients with abnormal CT had higher BMI (p = 0.019), lower TLC% predicted (p = 0.005), lower RV% predicted (p = 0.007), and higher rate of abnormal DLCO (p = 0.032). For abnormal CT patterns, patients with abnormal CT were more likely to have residual GGO (p = 0.002) and irregular lines (p = 0.005). More details were summarized in Table 3 and Supplementary Table S1.
Patients were divided into two groups according to DLCO at the follow up
As compared with patients with normal DLCO at eight months, patients with abnormal DLCO had longer cumulative days of oxygen treatment (p = 0.005), higher rate of receiving glucocorticoids (p = 0.040), longer cumulative days of receiving glucocorticoids (p = 0.020), and higher total dosage of glucocorticoids used (p = 0.031) during hospitalization. At eight months after discharge, patients with abnormal DLCO had higher CT score (p = 0.046), and lower MVV% predicted (p = 0.019). More details were summarized in Supplementary Table S2.
According to DLCO and DLCO/VA, patients were categorized as group A with DLCO ≥ 80% predicted (n = 27), group B with DLCO < 80% predicted but DLCO/VA ≥ 80% predicted (n = 5), and group C with DLCO and DLCO/VA both < 80% predicted (n = 8). The serum interleukin (IL)-2R, IL-8, and tumor necrosis factor (TNF)-α levels during hospitalization in group C patients were the lowest among the three groups (p = 0.023, 0.009, and 0.022, respectively). The TLC% predicted and RV% predicted in group B were lower than those of other two groups (p < 0.001, and p = 0.010, respectively). Patients in group B had lower X at 5 Hz [− 0.08 ± 0.04 (group A) vs − 0.10 ± 0.02 (group B) vs − 0.05 ± 0.03 (group C), p = 0.027], which indicated higher elastic recoil of the peripheral airways respectively. More details were summarized in Table 4 and Supplementary Table S3.
Explore the association between abnormal CT patterns and worse symptoms
To find specific abnormal CT patterns associated to worse symptoms, patients were divided into two groups according to Post-COVID-19 Functional Status (PCFS) scale grade. However, no matter the cut-off value of grouping was set as PCFS scale grade ≥ 1 or 2, there was no significant difference in abnormal CT patterns at eight months after discharge between the two groups (Supplementary Table S4).
Correlation analyses
Then we analyzed correlations among clinical data during the period having COVID-19, pulmonary function, CT score, and related physiological characteristics at follow up (Supplementary Table S5). CT score after discharge was positively correlated with age (R = 0.417, p = 0.008), BMI (R = 0.373, p = 0.018), CT peak score (R = 0.769, p < 0.001), PCT level (R = 0.367, p = 0.030), LDH level (R = 0.371, p = 0.024), D-dimer level (R = 0.482, p = 0.004), cumulative days of oxygen treatment (R = 0.541, p < 0.001), maximum inhaled oxygen concentration (R = 0.623, p < 0.001) , cumulative days of receiving glucocorticoids (R = 0.426, p = 0.007), and total dosage of glucocorticoids used (R = 0.423, p = 0.007) during hospitalization, negatively correlated with lymphocyte count (R = -0.508, p = 0.001), and albumin level during hospitalization (R = − 0.515, p = 0.001). DLCO% of predicted values was negatively correlated with cumulative days of oxygen treatment (R = − 0.335, p = 0.040). DLCO/VA% of predicted values was positively correlated with BMI (R = 0.378, p = 0.016), and serum TNF-α level during hospitalization (R = 0.422, p = 0.028). The 6MWD was negatively correlated with age (R = − 0.484, p = 0.002), BMI (R = − 0.366, p = 0.020), PCT level (R = − 0.414, p = 0.013), cumulative days of oxygen treatment (R = − 0.377, p = 0.020), and maximum inhaled oxygen concentration (R = − 0.377, p = 0.026) during hospitalization.
Logistic regression analyses
Based on univariate analyses, variables with significant differences between patients with normal and abnormal CT (or DLCO) were further analyzed by logistic regression analyses using the forward: LR method. It was found that the increase of CT peak score during hospitalization was the independent risk factor associated with abnormal CT at eight months after discharge (p = 0.006, OR 1.370, 95% CI 1.092 to 1.719, Table 5), and longer cumulative days of oxygen treatment was associated with abnormal DLCO at eight months after discharge (p = 0.009, OR 1.085, 95% CI 1.021 to 1.154, Table 5).
Discussion
Since December 2019 a novel coronavirus, now named as SARS-CoV-2, has caused a global COVID-19 pandemic. Although most of the infected persons are asymptomatic or mild patients8,9, COVID-19 has led to large number of severe cases due to the huge number of confirmed cases. According to previous reports, anomalies of pulmonary ventilation and diffusion function were noted in a considerable proportion of COVID-19 patients at the time of hospital discharge, especially in patients with severe disease3. At 30 days after discharge severe patients still had a higher incidence of DLCO impairment and lower TLC compared with non-severe patients4, which indicated that severe patients may need more time to recover and further long time follow-up studies are necessary. At three months after discharge 39 patients (70.9%) still had abnormal CT manifestation and nine patients (16.4%) had impaired DLCO6, but only four severe cases were entered in this study. In our study, 25 severe cases were enrolled, a considerable proportion (48%) of severe COVID-19 patients still had abnormalities on DLCO at eight months after discharge. The proportion was higher than that of patients at severity scale 4 (29%) but lower than that of patients at severity scale 5–6 (56%) reported by Cao et al.7 at 6 months after discharge. The median CT score of severe cases was 3 (IQR 2–10) at 8 months after discharge which was higher than that of mild cases. Only four severe COVID-19 patients (16%) had completely normal CT, which the proportion was lower than 29.1% reported by Zhao et al.6 at three months after discharge. Ten severe COVID-19 patients (40%) still had abnormal CT score above 5. To our knowledge, this is the first study that reported the proportion of patients with abnormal CT on severe COVID-19 cases at over a half of year after discharge. Even at eight months after discharge, severe cases still had a higher incidence of DLCO impairment and lower TLC compared with mild patients. For all patients, patients with abnormal CT had lower TLC% predicted, RV% predicted, higher rate of abnormal DLCO, and a trend of lower 6MWD, indicating that this kind of patients might have potential restrictive ventilation dysfunction, pulmonary diffusion function impairment, and poor exercise tolerance.
Viral pneumonia images are the most common feature of chest CT in patients with COVID-19, mainly present as GGO and consolidative pulmonary opacities10,11,12, as similar as SARS and MERS13,14. However, lesions of COVID-19 are more likely to impact bilateral pulmonary and multiple lobs than those of SARS. Previous studies have been reported that SARS have long-term effects on lung function, chest CT scans, and related physiological characteristics in part of survivors, even at one year after discharge15,16,17,18.
In our study, two of severe cases received N-acetylcysteine (NAC) therapy after discharge from hospital. One of them was treated with NAC for two weeks after discharge, another patient received long-term NAC, and combination inhaled corticosteroids plus long-acting β-agonists (ICS/LABA) therapy due to the comorbidity with COPD. One patient with severe COVID-19 received long-term ICS/LABA therapy due to the comorbidity with asthma, and was planning to receive pirfenidone therapy because of residual abnormal CT manifestation, abnormal DLCO, and abnormal DLCO/VA. Evidence has shown the efficacy of corticosteroid in reducing 28-day mortality in critically ill patients with COVID-1919. However, no evidence currently supports or refutes the benefits of corticosteroid or anti-fibrotic agents for patients with persistent symptoms or abnormal CT/DLCO. The efficacy of anti-fibrotic agent, such as pirfenidone, in patients with COVID-19 is mainly speculated based on the pharmacological mechanism and the pathophysiology of COVID-19. Unlike idiopathic pulmonary fibrosis (IPF) or pulmonary fibrosis secondary to autoimmune causes, fibrosis seen in some COVID-19 survivors may not progressive. Therefore, regular follow-up to evaluate the residual pulmonary deficits and the scope of fibrosis is essential to determine the necessity of anti-fibrotic treatment.
Even 12 (48%) severe cases in this study still had abnormal DLCO at over a half of year after discharge, which the proportion was lower than 76.5% reported by Huang et al.4 in severe cases at 30 days after discharged, and other lung function indexes, such as FVC and TLC, had the same trends. These results indicated that lung injury caused by COVID-19 in severe cases may have ability to self-rehabilitation, similar to SARS16.
However, the rehabilitation process of patients with different severity was variable. Even for two severe cases which had been treated with NIPPV and ICU admission, one of them had basically normal CT and normal DLCO, the other had abnormal CT and DLCO at eight months after discharge (Supplementary Fig. S1). The underlined mechanism is still not well known. As shown in our results, in compared with patients with normal or basically normal CT, patients with abnormal CT had higher CT peak score, higher PCT level, higher LDH level, higher AST level, and longer cumulative days of receiving glucocorticoids and oxygen treatment. Correlation analyses indicated that, CT scores after discharge was positively correlated with age. DLCO% predicted was negatively correlated with cumulative days of oxygen treatment. Logistic regression analyses indicated that the increase of CT peak score was the independent risk factor associated with residual abnormal CT, and longer cumulative days of oxygen treatment was associated with abnormal DLCO at follow-up. These results indicated that COVID-19 had a greater long-term impact on the lung physiology of patients who were older, more severe, and more complicated at the acute phase.
Interestingly, the correlation of BMI with CT abnormalities and pulmonary diffusion function at follow-up was not consistent. Patients with abnormal CT had higher BMI, and BMI positively correlated with CT score, consistent with the finding that obese patients are more likely to develop into severe COVID-1920. BMI positively correlated with DLCO/VA% predicted, and had a trend of positive correlation with DLCO% predicted after discharge in this study. In previous studies, the relationship between BMI with pulmonary diffusion function and pulmonary fibrosis has not been sufficiently understood. A study of African Americans with no cardiopulmonary or chest wall disease showed that BMI was negatively correlated with DLCO21. Another study on patients with COPD from China showed that BMI was positively correlated with DLCO and DLCO/VA22.A series of clinical studies on IPF showed that lower BMI is associated with a poorer outcome of IPF23,24,25, but indexes of pulmonary diffusion function were not observed in these studies. We speculate that better nutritional status may promote the recovery of lung injury from severe COVID-19, as BMI can be considered as an indicator of nutrition.
Surprisingly, six (15%) patients without obvious CT abnormalities also presented an abnormal of DLCO. We think this phenomenon might be caused by microthrombus formation which has been confirmed by the autopsy findings in patients dying of COVID-1926. The other option is they may already have abnormal DLCO before COVID-19 disease.
As DLCO could not sufficiently reflect the gas exchange capacity. Then we divided patients into three groups according to DLCO and DLCO/VA. As shown in Table 4, patients with abnormal DLCO but normal DLCO/VA had the lowest TLC% predicted and RV% predicted, markers of restrictive ventilation dysfunction, indicating that for this group of patients the decrease of DLCO was mainly caused by reduced alveolar volume. But pulmonary interstitial or vascular abnormalities caused by COVID-19 might also exist and contribute to the abnormality of DLCO in patients with abnormal DLCO but normal DLCO/VA, because this group of patients had a trend of lower DLCO/VA as compared with patients with normal DLCO (p = 0.0502). Surprisingly, we found that patients with abnormal DLCO and DLCO/VA had lowest serum IL-2R, IL-8, and TNF-α levels as compared with the other two groups, although the difference was only significant as compared with patients with abnormal DLCO but normal DLCO/VA. Correlation analyses indicated that DLCO/VA% of predicted values was positively correlated with serum IL-2R and TNF-α level, and had a trend of positive correlation with serum IL-6 level. Previous studies indicated that cytokine storm may contribute to the severity and mortality of COVID-19. In the other hand, previous study had demonstrated that IL-2-deficient mice have an impaired viral clearance capacity in lymphocytic choriomeningitis virus infection27. TNF-α has the ability to against virus infection28. Animal and vitro experiments indicated that IL-2, IL-2R, IL-8, and IL-6 are involved in the repair process in different tissues after damage29,30,31,32. All of these indicated that inflammation can be a double-edged sword: inflammation plays an essential role in viral clearance and initiating repair process33,34,but hyperinflammation may lead to organs injury and severity of disease. According to the results from this study, we speculate that both hyperinflammation and absent of inflammation could lead to the impairment of pulmonary diffusion function in the recovery stage of COVID-19.
Of note, only 40 patients were recruited. A limited number of cases may lead to biased results, especially in those with strong subjectivity such as SF-36. The limited sample size may also lead us to be unable to find specific abnormal CT patterns associated to worse symptoms and DLCO at follow-up, although our results indicated that abnormal CT was related to abnormal DLCO. We also note that pulmonary function and chest HRCT before SARS-CoV-2 infection are not known, which make it inappropriate to simply attribute abnormal pulmonary function and CT scans to COVID-19. Finally, asymptomatic patients and patients with intubation were not enrolled and only two patients required additional NIPPV treatment were enrolled in this study. The long-term impact of COVID-19 on asymptomatic and critically ill cases could not conclude from this study.
Conclusions
In conclusion, our study has demonstrated that survivors with severe COVID-19 had higher incidences of DLCO impairment, persistent symptoms in daily life, and higher abnormal CT score as compared with mild cases. The long-term radiological abnormality may relate to old age and the severity of COVID-19. Either absent or excess of inflammation reaction during COVID-19 course would lead to the impairment of pulmonary diffusion function at the recovery stage.
Methods
Subjects
In this study, all participants were confirmed by positive SARS-CoV-2 nucleic acid testing results by real-time reverse transcriptase polymerase chain-reaction from January 27, 2020 to March 13, 2020 in Wuhan, China. Severe COVID-19 cases were defined as meeting any of the following2: (1) shortness of breath, respiratory rate ≥ 30 times/min; (2) oxygen saturation ≤ 93% at a rest state; (3) partial arterial oxygen pressure/fraction of inspiration O2 ≤ 300 mmmHg; (4) the severity of clinical symptoms was aggravating progressively, > 50% lesions progression within 24 to 48 h in lung imaging. Otherwise were defined as mild cases. Exclusion criteria: (1) died before the eight months follow-up visit; (2) unable to follow up because of various serious psychological or physical disorders; (3) missing any of results on chest HRCT, pulmonary function, clinical questionnaires, or 6MWD at the eight months follow-up visit; (4) poor cooperation so that the pulmonary function test results are inaccurate; (5) uncontactable or declined to participate. Finally, our study enrolled 25 survivors with severe COVID-19 and 15 survivors with mild COVID-19.
We collected the clinical data from the onset of COVID-19 and throughout the hospital stay (including epidemiological, demographic, comorbidities characteristics, laboratory data, chest CT scans, and treatment details), and the clinical data at about eight months after discharge (including chest CT scans, characteristics of pulmonary function, and health-related quality of life) of all participants. All the clinical data were checked by two physicians.
All participants were given a written informed consent prior to inclusion. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB ID: TJ-IRB20210115). All methods were carried out in compliance with the Declaration of Helsinki.
Chest HRCT and image quantification
All participants underwent chest CT scan during the follow-up period. Refer to the method described previously, a radiologic scoring system was used to quantify lung lesions11. Briefly, each of the five lung lobes was reviewed for the lesions, such as ground-glass opacification (GGO), interstitial thickening, consolidation, bronchiectasis, irregular interfaces, and so on. According to the percentage area occupied, each lobe was evaluated 0–5 points represent normal performance, lesions involving < 5% of lobe, lesions involving 5–25% of lobe, lesions involving 25–50% of lobe, lesions involving 50–75% of lobe, and lesions involving > 75% of lobe respectively. Then the CT score was reached by summing individual segmental scores.
Pulmonary function testing
All participants underwent pulmonary function tests in the Pulmonary Function laboratory, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology followed by American Thoracic Society/European Respiratory Society guidelines35.
The assessment included: forced expiratory volume in one second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), maximal expiratory flow at 50% of FVC (MEF50), maximal expiratory flow at 25% of FVC (MEF25), maximal midexpiratory flow between 75 and 25% of FVC (MMEF75/25), maximum voluntary ventilation (MVV), TLC, residual volume (RV), DLCO, and ratio of carbon monoxide diffusion capacity to alveolar ventilation (DLCO/VA) measured by means of the single-breath test. The hemoglobin value was also taken for correcting the DLCO.
Impulse oscillation system was used to measure the following parameters: impedance at 5 Hz (Z at 5 Hz), which indicates the total respiratory impedance; resistance in the peripheral airways (Rperipheral); and reactance at 5 Hz corrected by predicted value (X at 5 Hz), which inversely indicates the elastic recoil of the peripheral airways.
Clinical questionnaires and six-minute walk test (6MWT)
At eight months after discharge all participants answered the Post-COVID-19 Functional Status (PCFS) scale and the MOS 36-item Short-Form Health Survey (SF-36) to assess the impact of severe COVID-19 on health-related quality of life.
PCFS is a simple tool to describe the impact of residual symptoms of COVID-19 on functional status of patients after discharge, which classify patients into 0 to 4 grades. Briefly, grade 0 reflects the absence of any residual symptoms and functional limitation, grade 1 reflects the patient still has persistent physical and (or) psychological symptoms but only has negligible limitations in daily life. From grade 2, residual symptoms and functional limitations are present to an increasing degree. Grade 4 represents the patients suffering from severe functional limitations and requiring assistance from another person due to residual symptoms36.
SF-36 consisting of 11 questions and a total of 36 responses that assess physical functioning (PE), role limitation due to physical problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitation due to emotional problems (RE), mental health (MH), and reported health transition (HT). Scores for each item domain range from 0 (worst) to 100 (best). These subscales were then combined to form the physical component summary (PCS) and mental component summary (MCS) scores37.
All patients underwent 6MWT without supplemental oxygen, oxygen saturation on pulse oximeter (SpO2) and Borg-scale before and after exercise, and 6MWD were measured as previously described38.
Statistical analyses
Data were expressed as means ± standard deviation (SD) when data were normally distributed or medians with interquartile range (IQR) when data were not normally distributed. Comparisons were determined by Student’s t-test, Mann–Whitney U test or Fisher exact tests as appropriate. Multiple groups were compared using one-way analysis of variance (ANOVA) with a Bonferroni correction (normal data) or a Kruskal–Wallis test with a Dunn intergroup comparison (non-normal data). Spearman’s rank correlation coefficient was used for correlation analyses. We used logistic regression analysis to explore the independent risk factor associated with abnormal CT or DLCO. Statistical significance was considered as p < 0.05. SPSS software V.25.0 was used for analyses.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- CFR:
-
Case-fatality rate
- SARS:
-
Severe acute respiratory syndrome
- MERS:
-
Middle East respiratory syndrome
- DLCO:
-
Diffusion capacity of the lung for carbon monoxide
- TLC:
-
Total lung capacity
- 6MWD:
-
Six-minute walk distance
- HRCT:
-
High-resolution computed tomography
- GGO:
-
Ground-glass opacification
- FEV1 :
-
Forced expiratory volume in one second
- FVC:
-
Forced vital capacity
- PEF:
-
Peak expiratory flow
- MEF50:
-
Maximal expiratory flow at 50% of FVC
- MEF25:
-
Maximal expiratory flow at 25% of FVC
- MMEF75/25:
-
Maximal midexpiratory flow between 75 and 25% of FVC
- MVV:
-
Maximum voluntary ventilation
- RV:
-
Residual volume
- DLCO/VA:
-
Ratio of carbon monoxide diffusion capacity to alveolar ventilation
- Z at 5 Hz:
-
Impedance at 5 Hz
- Rperipheral:
-
Resistance in the peripheral airways
- X at 5 Hz:
-
Reactance at 5 Hz corrected by predicted value
- 6MWT:
-
Six-minute walk test
- PCFS:
-
The Post-COVID-19 Functional Status
- SF-36:
-
The MOS 36-item Short-Form Health Survey
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- ANOVA:
-
One-way analysis of variance
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- NIPPV:
-
Noninvasive positive pressure ventilation
- LDH:
-
Lactate dehydrogenase
- PCT:
-
Procalcitonin
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- NAC:
-
N-acetylcysteine
- ICS/LABA:
-
Combination inhaled corticosteroids plus long-acting β-agonists
- IPF:
-
Idiopathic pulmonary fibrosis
References
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395, 565–574. https://doi.org/10.1016/S0140-6736(20)30251-8 (2020).
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242. https://doi.org/10.1001/jama.2020.2648 (2020).
Mo, X. et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. https://doi.org/10.1183/13993003.01217-2020 (2020).
Huang, Y. et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 21, 163. https://doi.org/10.1186/s12931-020-01429-6 (2020).
Daher, A. et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 174, 106197. https://doi.org/10.1016/j.rmed.2020.106197 (2020).
Zhao, Y. M. et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 25, 100463. https://doi.org/10.1016/j.eclinm.2020.100463 (2020).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet https://doi.org/10.1016/S0140-6736(20)32656-8 (2021).
Sakurai, A. et al. Natural history of asymptomatic SARS-CoV-2 infection. N. Engl. J. Med. 383, 885–886. https://doi.org/10.1056/NEJMc2013020 (2020).
Tabata, S. et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: A retrospective analysis. Lancet Infect. Dis. 20, 1043–1050. https://doi.org/10.1016/S1473-3099(20)30482-5 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5 (2020).
Chung, M. et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295, 202–207. https://doi.org/10.1148/radiol.2020200230 (2020).
Ai, T. et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 296, E32–E40. https://doi.org/10.1148/radiol.2020200642 (2020).
Ooi, G. C. & Daqing, M. SARS: Radiological features. Respirology 8(Suppl), S15-19. https://doi.org/10.1046/j.1440-1843.2003.00519.x (2003).
Ajlan, A. M., Ahyad, R. A., Jamjoom, L. G., Alharthy, A. & Madani, T. A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: Chest CT findings. AJR Am. J. Roentgenol. 203, 782–787. https://doi.org/10.2214/AJR.14.13021 (2014).
Hui, D. S. et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 60, 401–409. https://doi.org/10.1136/thx.2004.030205 (2005).
Xie, L. et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest 127, 2119–2124. https://doi.org/10.1378/chest.127.6.2119 (2005).
Ong, K. C. et al. 1-year pulmonary function and health status in survivors of severe acute respiratory syndrome. Chest 128, 1393–1400. https://doi.org/10.1378/chest.128.3.1393 (2005).
Hui, D. S. et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 128, 2247–2261. https://doi.org/10.1378/chest.128.4.2247 (2005).
Group et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: A meta-analysis. JAMA 324, 1330–1341. https://doi.org/10.1001/jama.2020.17023 (2020).
Stefan, N., Birkenfeld, A. L., Schulze, M. B. & Ludwig, D. S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 16, 341–342. https://doi.org/10.1038/s41574-020-0364-6 (2020).
Mehari, A. et al. Obesity and pulmonary function in African Americans. PLoS ONE 10, e0140610. https://doi.org/10.1371/journal.pone.0140610 (2015).
Wu, Z. et al. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: A retrospective real world research. J. Thorac. Dis. 10, 5086–5099. https://doi.org/10.21037/jtd.2018.08.67 (2018).
Jouneau, S. et al. Analysis of body mass index, weight loss and progression of idiopathic pulmonary fibrosis. Respir. Res. 21, 312. https://doi.org/10.1186/s12931-020-01528-4 (2020).
Nakatsuka, Y. et al. The clinical significance of body weight loss in idiopathic pulmonary fibrosis patients. Respiration 96, 338–347. https://doi.org/10.1159/000490355 (2018).
Simon, M., Paulo, G. C. F. & Marostica, J. C. Body mass index and albumin levels are associated with pulmonary function parameters in pediatric subjects with cystic fibrosis. Rev. Paul Pediatr. 37, 414–418. https://doi.org/10.1590/1984-0462/;2019;37;4;00016 (2019).
Wichmann, D. et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann. Intern. Med. 173, 268–277. https://doi.org/10.7326/M20-2003 (2020).
Cousens, L. P., Orange, J. S. & Biron, C. A. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J. Immunol. 155, 5690–5699 (1995).
Horiuchi, T., Mitoma, H., Harashima, S., Tsukamoto, H. & Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 49, 1215–1228. https://doi.org/10.1093/rheumatology/keq031 (2010).
Cho, H. S., Reboldi, A., Hall, J. A. & Berg, L. J. The Tec kinase ITK is essential for ILC2 survival and epithelial integrity in the intestine. Nat. Commun. 10, 784. https://doi.org/10.1038/s41467-019-08699-9 (2019).
Seraphim, P. M. et al. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 254, 117813. https://doi.org/10.1016/j.lfs.2020.117813 (2020).
Maybin, J. A., Hirani, N., Jabbour, H. N. & Critchley, H. O. Novel roles for hypoxia and prostaglandin E2 in the regulation of IL-8 during endometrial repair. Am. J. Pathol. 178, 1245–1256. https://doi.org/10.1016/j.ajpath.2010.11.070 (2011).
Yang, M. L. et al. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 7, 43829. https://doi.org/10.1038/srep43829 (2017).
Newton, A. H., Cardani, A. & Braciale, T. J. The host immune response in respiratory virus infection: Balancing virus clearance and immunopathology. Semin. Immunopathol. 38, 471–482. https://doi.org/10.1007/s00281-016-0558-0 (2016).
Karin, M. & Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315. https://doi.org/10.1038/nature17039 (2016).
Graham, B. L. et al. Standardization of spirometry 2019. Update an official American Thoracic Society and European respiratory society technical statement. Am. J. Respir. Crit. Care Med. 200, e70–e88. https://doi.org/10.1164/rccm.201908-1590ST (2019).
Klok, F. A. et al. The post-COVID-19 functional status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. https://doi.org/10.1183/13993003.01494-2020 (2020).
McHorney, C. A., Ware, J. E., Lu, J. F. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 32, 40–66. https://doi.org/10.1097/00005650-199401000-00004 (1994).
Casanova, C. et al. The 6-min walk distance in healthy subjects: Reference standards from seven countries. Eur. Respir. J. 37, 150–156. https://doi.org/10.1183/09031936.00194909 (2011).
Author information
Authors and Affiliations
Contributions
M.X., W.N., and S.Z. contributed to study design and had full access to all data in the study. All authors contributed to data collection. S.Z., W.N., and M.X. contributed substantially to the data analysis and interpretation. S.Z. and M.X. drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, S., Bai, W., Yue, J. et al. Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep 11, 13854 (2021). https://doi.org/10.1038/s41598-021-93191-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93191-y
This article is cited by
-
Impact of COVID infection on lung function test and quality of life
Scientific Reports (2023)
-
Long COVID in autoimmune rheumatic diseases
Rheumatology International (2023)
-
Solanum nigrum L. in COVID-19 and post-COVID complications: a propitious candidate
Molecular and Cellular Biochemistry (2023)
-
1-year quality of life and health-outcomes in patients hospitalised with COVID-19: a longitudinal cohort study
Respiratory Research (2022)
-
“Long COVID” results after hospitalization for SARS-CoV-2 infection
Scientific Reports (2022)