Abstract
Deficits in facial emotion recognition have frequently been established in temporal lobe epilepsy (TLE). However, static, rather than dynamic emotion recognition paradigms have been applied. Affective prosody has been insufficiently studied in TLE, and there is a lack of studies investigating associations between auditory and visual emotion recognition. We wished to investigate potential deficits in a dynamic morph task of facial emotion recognition and in an affective prosody recognition task, as well as associations between both tasks. 25 patients with TLE and 24 healthy controls (CG) performed a morph task with faces continuously changing in their emotional intensity. They had to press a button, as soon as they were able to recognize the emotion expressed, and label it accordingly. In the auditory task, subjects listened to neutral sentences spoken in varying emotional tones, and labeled the emotions. Correlation analyses were conducted across both tasks. TLE patients showed significantly reduced prosody recognition compared to CG, and in the morph task, there was a statistical trend towards significantly reduced performance for TLE. Recognition rates in both tasks were significantly associated. TLE patients show deficits in affective prosody recognition, and they may also be impaired in a morph task with dynamically changing facial expressions. Impairments in basic social-cognitive tasks in TLE seem to be modality-independent.
Similar content being viewed by others
Introduction
Temporal lobe epilepsy (TLE) is the most common type of focal epilepsy, comprising 2/3 of all focal epilepsies1. People with (focal) epilepsy (PW(F)E) suffer from neuropsychological deficits2. Intact cognitive abilities are highly relevant for everyday functioning and impairments can significantly reduce quality of life3,4,5,6,7,8. Deficits in social cognition may worsen social integration9, but are under-researched in PWFE to date.
Basic social cognition and the temporal lobes
The term “social cognition” subsumes perceptual and mental processes that are essential for successful social interactions, e.g., basic visual and auditory emotion recognition. The temporal lobes are important for basic visual and auditory emotion recognition10,11,12. Visual emotion recognition usually involves inferring emotional states from facial expressions, taking into account relevant facial regions, such as the eyes and the mouth. Mesiotemporal brain areas may be relevant to accurate facial emotion recognition13. The amygdala is a mesiotemporal structure which plays an important role in (facial) emotion processing14. There is evidence for the amygdala being involved if inferences to the emotional state of a person are to be derived from the eye region15. Neurological patients with uni- and especially bilateral amygdalar damage show more impaired facial emotion recognition, in particular where fear and other negative emotions are concerned, than patients with other kinds of brain damage or healthy subjects10,15,16. Accurate recognition of emotional signals is not only relevant concerning facial expressions, but also plays an essential role in verbal interactions where the auditory domain is important. The ability to understand spoken utterances and interpret their meaning, does not only rely on the syntactic structure of sentences or the semantic meaning of words, but also on the prosodic content of an utterance11. Prosody comprises modulation of speech through pitch, volume, tone and intonation, through which the semantic and affective content of speech can be inferred. The understanding of prosody seems to rely on an extensive neural network which comprises the posterior temporal lobe as well as subcortical structures11,12. Functional magnetic resonance imaging and transcranial magnet stimulation studies have provided evidence for the importance of the right hemisphere for processing affective prosody17,18,19. Right posterior lateral temporal lobe lesions may be associated with impairments in the comprehension of emotional prosody20. However, other studies cast doubt on the right-hemispheric lateralization of emotional prosody processing and point to the involvement of a complex network of left- and right-hemispheric areas in such processes21.
Basic emotion recognition in TLE
In TLE patients, seizure activity and—depending on individual etiology—structural lesions of the cortex can impair functioning of temporo-lateral as well as mesiotemporal areas including the amygdalae. Hence, deficits in basic facial emotion recognition have been frequently documented22, and evidence from eye-tracking studies suggests that during tasks of facial emotion recognition patients with TLE show abnormal eye-movement patterns, which may be associated with emotion recognition performance23. In such investigations, participants are usually presented with faces showing one of the six basic emotions. TLE patients tend to perform worse than healthy control subjects on this task24,25. Literature reviews suggest a more pronounced deficit for negative emotions in patients with TLE, perhaps especially for fear10,22. However, methodical issues in a number of studies render the assumption of such a specific deficit questionable. In the vast majority of prior research, static paradigms of facial emotion recognition have been applied. Using novel morph-tasks with slowly changing faces, from neutral to full emotional expressions26,27,28,29, allows not only for comparing correct answers between PWFE and healthy controls, but also the minimal degree of emotional expression which is needed for a correct response. Considering ecological validity, this is of high relevance for everyday social interactions, where people rarely show full facial expressions of basic emotions. Instead, subtle signals have to be decoded and interpreted correctly within a limited time frame.
For basic auditory emotion recognition, affective prosody recognition tasks can be applied. Affective prosody recognition can be assessed in a standardized manner by listening to spoken sentences that are semantically neutral. These are spoken with differing emotional prosody, and subjects are asked to identify the respective emotion11. Due to functional disturbances of relevant brain areas (see above), patients with TLE may also show deficits in recognizing affective prosodic content of acoustic stimuli 10,17. However, a very limited number of studies have tested affective prosody recognition with a standardized paradigm as described above, and included more than ten adult epilepsy patients11,19,30,31,32,33,34,35. Only some of these studies showed a deficit in prosodic emotion recognition in the included epilepsy patients. Whether a specific deficit for the recognition of fear or all negative emotions from prosody exists in patients with TLE, is questionable. For here, the same methodical concerns apply as in the studies on facial emotion recognition.
Even fewer studies have compared performance across the auditory and visual domain10,17. Three of these found cross-modal deficits30,31,33. Only one study including preoperative patients conducted a correlation analysis and reported a significant association between emotional prosody recognition and facial emotion recognition31. Hence, more research into cross-modal impairment of emotion recognition in patients with focal epilepsy is needed. So far, only tasks of prosody recognition of individual emotions have been applied in focal epilepsy research, but no tasks of prosody discrimination, which is a more basic process than emotion labelling.
The present study investigated facial emotion recognition in TLE patients with an animated morph task. Prosodic emotion recognition was also tested with a standardized paradigm, and we aimed to test the following hypotheses:
-
1.
Patients with TLE show a deficit in prosodic emotion recognition compared to healthy control subjects. The more basic process of affective prosody discrimination is not expected to differ between groups.
-
2.
Based on the literature on other patient groups suffering from structural and functional mesiotemporal abnormalities, it is expected that TLE patients also show a deficit in a dynamic facial emotion recognition task compared to healthy control subjects, i.e., average combined accuracy and speed indices are lower than those of healthy control subjects.
-
3.
Emotion recognition performance in TLE is expected to be cross-modal, i.e., performances in the auditory and visual domain are expected to be statistically related.
Methods
Subjects
Twenty-five patients with active TLE from the Epilepsy Center of Freiburg University Medical Center took part in the study. Parts of the sample were recruited from a cross-section study on social cognition in focal epilepsy36. Patients underwent interdisciplinary presurgical assessment. Diagnosis of TLE was based on data from video-EEG-monitoring, as well as MRI. Twenty-four healthy control subjects were recruited via advertisements on the Medical Center’s intranet pages. Exclusion criteria were: (i) severe psychiatric disorders, (ii) neurodegenerative disorders, (iii) other types of epilepsy, (iv) age below 18 years, (v) insufficient grasp of the German language, (vi) verbal intelligence: IQ ≥ 85.
One patient was excluded due to severe depression, three patients due to idiopathic generalized epilepsy or an extra-temporal epilepsy focus. One patient did not complete the animated morph task, but was not excluded from the study, as he completed all other assessments. Three patients had not experienced any seizures during the previous two months, but had experienced regular seizures beforehand. One patient did not receive anticonvulsive medication at the time of their neuropsychological evaluation. For reasons of matching, the two control subjects with the highest verbal IQ scores had to be excluded.
The study was approved by the Ethics Committee of Albert-Ludwigs-University Freiburg (No. 206/17) and conducted in accordance with the Declaration of Helsinki. All patients provided informed written consent before participation.
Instruments
Mehrfachwahl-Wortschatz-Intelligenztest (MWT-B)
Verbal-crystallized intelligence was estimated with a vocabulary test, the Mehrfachwahl-Wortschatz-Intelligenztest (MWT-B)37. Subjects are asked to recognize and mark each genuine German word among four distractors.
Beck Depression-Inventory, 2nd edition (BDI-II)
Depressive symptoms were assessed with the German version of the Beck Depression Inventory, 2nd edition38.
Affective prosody recognition
The prosody task was adapted from Nazarov and colleagues39.
Semantically neutral spoken sentences were presented in either an emotional (fearful, happy, sad, angry, disgusted) or neutral tone. Stimuli originate from the “Berlin Database of Emotional Speech.”40. In the recognition task, subjects had to choose which emotion was expressed out of a choice of six (see above). There was no time limit on this task. The recognition task included three practice trials and 72 experimental trials (12 repetitions of each emotion, 10 speakers). In a further task (emotion discrimination), the same sentence was spoken by two different persons, and the task was to state whether the emotional expressions are the same or different. The discrimination task included two practice trials and 20 experimental trials (10 trials with the same and 10 with different emotions, two different speakers on each trial). This task taps a more basic level of perception than identifying an emotion correctly from prosody.
Animated morph task
Facial emotion recognition was assessed with an animated morphing paradigm. The task consists of a series of computerized movies, depicting facial expressions changing successively from neutral (0%) to one of four emotional expressions (100%) in 2% increment steps. Digitized color photographs depicting four affective states (angry, happy, fearful, sad), selected from the Radboud Faces database (University of Nijmegen41), served as stimulus material for the experimental trials. Emotional expressions are altered using a morphing procedure (FantaMorph software, Abrosoft, Beijing, China). Every image is presented for 200 ms. The experiment consists of 40 emotion sequences (10 models (5 male/5 female) × 4 emotions) and takes about 20 min. to complete. Participants were asked to press a button as soon as they were able to detect an emotional expression. The sequence was then immediately stopped, the face disappeared, and the perceptual intensity as well as the selected emotion category were recorded. This task has been applied in previous studies of the work group26,28,42.
In order to assess performance on the morph task, the balanced integration score (BIS43) was applied, a composite performance score including accuracy of emotion identification as well as the speed of identification (expressed here as the grade of intensity needed for a correct identification). The BIS is the difference in standardized mean correct reaction times and the proportion of correct responses43.
Statistical analyses
For the main analyses, a one-way ANOVA was calculated for the morph task. For the prosody task and for secondary analyses, RM-ANOVAs were computed across prosody subtasks and the different emotions in both prosody recognition and the morph task, in order to explore whether potential deficits in the TLE group are diffuse or specific to individual emotions, i.e., whether an interaction between subject group and type of emotion appeared. The included variables were for (a) the prosody task: group status as a fixed factor and the two task types (prosody recognition and prosody discrimination) on the repeated measures factor, (b) the prosody recognition task: group status as a fixed factor and the five emotions (anger, fear, sadness, joy, disgust) plus the neutral expression on the repeated measures factor, and c) the morph task: group status as a fixed factor and the four emotions (anger, fear, sadness, joy) on the repeated measures factor. If applicable, post-hoc t-tests were computed.
Pearson correlations were computed in order to test for an association between auditory and visual emotion recognition accuracy, as well as the defined daily dose (DDD)44 of anticonvulsant medication and the measures of social cognition. Spearman’s rank correlations were computed in order to test for an association between depression scores (BDI-II) and auditory as well as visual emotion recognition.
Ethics approval
The study was approved by the Ethics Committee of Albert-Ludwigs-University Freiburg (No. 206/17).
Consent to participate
All participants gave informed written consent.
Results
Demographic and clinical variables
Table 1 shows clinical and demographic characteristics of TLE patients and demographic parameters for healthy control subjects. No significant group difference emerged between TLE patients and healthy controls for gender, age and verbal IQ (see Table 1). TLE patients as a group displayed significantly more depressive symptoms than the control group. More patients than healthy control subjects were currently unemployed or retired. Relationship status did not differ significantly between groups.
Facial emotion recognition (morph task)
The ANOVA showed a statistical trend towards a significant effect of group, i.e., in tendency, patients’ BIS, combined accuracy and speed, was lower than the control group’s mean BIS (see Table 2). Secondary RM-ANOVAs on accuracy scores revealed no significant interaction between group and type of emotion (F = 0.42, p = 0.65).
Emotion recognition from prosody (prosody task)
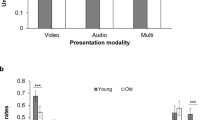
The RM-ANOVA revealed a significant effect of prosody task type (F = 67.7, p < 0.001), i.e., performance on prosody discrimination was superior to prosody recognition performance across groups. Furthermore, a significant interaction effect group x task emerged (F = 4.7, p = 0.04). Post-hoc t-Tests showed a significant difference between TLE patients and controls with the former performing significantly worse on prosody recognition (t = − 2.3, p = 0.03), i.e., on average, TLE patients were less accurate in identifying emotions from prosody than healthy controls (see Fig. 1), but not on prosody discrimination (t = − 0.2, p = 0.85; see Fig. S9 for an illustration of the interaction).
Secondary RM-ANOVAs revealed no significant interaction between group and type of emotion (F = 0.58, p = 0.67) for recognition accuracy.
Correlation analyses
The Pearson correlation coefficient for accuracy scores in affective prosody recognition and the morph task was significant (r = 0.41, p = 0.04, see Fig. 2). There were no significant associations between depression scores (BDI-II) or verbal IQ and either morph or prosody task performance (Spearman’s rank correlations for BDI-II and Pearson correlations for IQ), all coefficients r < 0.2, all p-values > 0.2). No significant correlations emerged for either the morph task or affective prosody recognition and drug-load (DDD) (Pearson correlations, all coefficients r < 0.2, all p-values > 0.4).
Additional analyses
In order to investigate whether lateralization had an effect on prosody and morph task performance, we compared patients with TLE in the dominant (N = 13) and in the non-dominant hemisphere (N = 10) on both tasks. No significant between-group differences were detected either for the prosody task (d = − 0.04, p = 0.92) or for the morph task (d = 0.69, p = 0.12).
Discussion
The present investigation shows a deficit in affective prosody recognition in patients with active TLE compared to a group of healthy control subjects. This finding is consistent with prior research showing deficits in prosodic emotion recognition in patients with TLE11,31,33 or after temporal lobectomy11,30,32, and adds to the modest number of studies investigating this aspect of emotion recognition in patients with TLE. Not all previous research has found impairment in recognizing emotional prosody in PWFE19,34,35. These negative results could be due to small subgroup sizes and differences in sampling, e.g., Adolphs and colleagues34 compared their patients to brain-damaged controls. Studies with larger samples or studies that included a comparison between their entire patient sample with a group of healthy control participants seem to have been more successful in discovering significant group differences favoring healthy controls11,30,31,33. TLE patients in the present study were also less accurate in identifying emotions from prosody than healthy control subjects. However, TLE patients did not differ from healthy controls regarding discrimination of emotional prosody. The RM-ANOVA revealed a significant group × task type interaction. Prior research in TLE has focused on active identification of emotions from prosody. Discrimination tasks have not been included, to our knowledge. Therefore, the present study has shown a dissociation between a comparison of prosodic information (prosody discrimination) and emotion labelling from prosody (emotional prosody recognition) in adult patients with TLE for the first time. This is interesting in so far as it shows that TLE patients’ impairment does not include the more basic process of prosodic discrimination, but merely the higher process of labelling the emotions expressed by prosodic content.
The present results show a trend towards reduced performance on a dynamic facial emotion recognition task in patients with TLE. Post-hoc power analyses showed that statistical power may have been too low to detect the medium-sized effect between the TLE and the control group (see “Limitations”). Previous research has focused on static, rather than dynamic paradigms of facial emotion recognition10,22. Deficits in static facial emotion recognition have been established in various studies including patients with TLE10,22,23. About half of the 31 studies included in Bora and Meletti’s systematic review22 only reported on postsurgical patients after temporal lobectomy. However, TLE patients were impaired regarding emotion recognition regardless of whether or not they had received surgery. Very few investigations have included dynamic tasks of facial emotion recognition: Tanaka and colleagues45 used a video-based task, in which the actors displayed an emotional expression from neutral for 2 s. and then returned to a neutral expression. The authors found a deficit in emotion recognition in the included patients with TLE and a further group with temporal lobectomy. A similar task was applied in a study by Young and colleagues46, producing similar results. Sedda and colleagues applied a task including morphed faces of varying degrees of emotional expression47. However, only one static face was shown at a time, and subjects had to label the shown emotion. Ammerlaan and colleagues48 used a task similar to the one in the present study, where faces displaying emotions gradually changed in emotional intensity. However, unlike the animated morph task in the present study, in the paradigm used by Ammerlaan and colleagues, no reaction times were measured, i.e., participants could simply watch the video until the final degree of intensity was reached and shown and then proceed with the task of labelling the displayed emotion. In contrast to this, during the morph task used in the present study, subjects were required to press a button, as soon as they believed they had recognized the displayed emotion. To our knowledge, the latter kind of animated morph task has not been applied in epilepsy research so far. The investigations cited above and the present results suggest a possible deficit for dynamic facial emotion recognition in TLE. TLE patients may show a deficit in a dynamic task of facial emotion recognition that combines the required intensity of facial expressions and correct emotion identifications. Such a task comes closer to everyday requirements in social cognition than a static emotion recognition task. In face-to-face interactions, we rarely see the full degree of an emotional expression in the person with whom we are interacting. Instead, we have to decode subtle emotional signals within a limited time frame and respond in an adequate manner. It will be important to investigate TLE patients’ deficits in such dynamic tasks more closely in the future.
Emotion recognition impairment discovered on the prosody task as well as the trend towards impaired performance on the morph task, was not confined to specific emotions. Instead, it can be hypothesized that patients show a more diffuse impairment across all emotions. Despite many previous studies having discovered that PWFE are impaired in recognizing fear, for example, there is no convincing evidence to suggest a specific deficit in fear recognition in this patient group22. Firstly, most studies have found deficits in recognizing other emotions as well10,22, secondly, repeated-measures analyses of variances have not routinely been conducted. Many studies seem to have conducted individual between-group comparisons for each emotion (with or without subsequent Bonferroni corrections) or a MANOVA (e.g.,30,31,33,49,50). This is problematic in so far, as only a repeated-measures analysis of variance allows for the detection of a significant interaction effect group × emotion, which is the precondition for subsequent individual comparisons51. If such an interaction effect cannot be shown, a pervasive deficit in TLE patients that is independent of the type of emotion seems more plausible.
Although this was not the main focus of the present study, we conducted secondary analyses, which showed the absence of an interaction effect between subject group and type of emotion concerning both, the visual and the auditory task. The lack of such an interaction effect points at an impairment across various emotions, rather than a specific deficit, but this notion needs to be explored further, as the lack of an interaction effect alone cannot be considered as evidence for a diffuse impairment. Therefore, it has to be concluded that we found no evidence for a specific deficit in emotion recognition in TLE in our sample. Bora and Meletti22 came to the following conclusion in their systematic review: As in comparison with healthy controls, TLE patients were significantly impaired on labelling fear, disgust, sadness, anger, surprise as well as happiness (even if the effect size for happiness was small (d = 0.2)), the authors concluded that chronic TLE leads to impaired recognition of all basic emotions.
Auditory and visual emotion recognition performance seems to be associated, as the significant correlation between the rates of correct identifications in both tasks shows. Although it is important to know whether PWFE show cross-modal deficits in emotion recognition, this issue has not been addressed sufficiently in prior research. In Monti and Meletti’s systematic review10, most of the included studies investigated either facial or prosodic emotion recognition, and out of the six studies exploring both modalities in more than one adult patient, four reported correlation analyses30,31,33,34. Out of these, three found significant correlations between auditory and visual measures of emotion recognition 30,31,33. These results show that the association between emotion recognition from prosody and from faces is under-researched, but taking into account present and past results, a significant association between the two modalities seems probable.
When patients with TLE in the dominant and non-dominant hemispheres were compared in our sample, there were no significant differences regarding performance on either of the tasks, which is consistent with most prior study results. Nevertheless, the analyses were underpowered due to the small N of the subgroups (see “limitatons”). Alba-Ferrara and colleagues17 postulate a key role of the right hemisphere in processing affective prosody. However, concerning lateralization, results across studies are less consistent than could be assumed from the results of functional imaging investigations in healthy subjects. None of the studies found performance impairment in left-sided TLE, and at least preliminary evidence for a relative deficit in right-sided TLE emerged in some studies33,52,53. However, most studies did not show a significant effect of lateralization, which may also be due to insufficient numbers of subjects in subgroup comparisons10,17. Another explanation for inconsistent results regarding lateralization could be the existence of reorganization processes and plasticity in PWFE, possibly via recruitment of contralateral cortical areas19.
Limitations
The included patient sample is of moderate size. Power may have been too low in order to detect between-group medium effect sizes on the morph task. Post-hoc power analyses revealed a power of 1 − β = 0.43. With a larger sample size, the group effect on the BIS for morphing may have become significant, rather than remaining a mere trend towards significance. Power was slightly higher for the prosody task (recognition) with 1 − β = 0.62, but low for the tiny effect on prosody discrimination (1 − β = 0.05). However, power was high for the interaction effect of the RM-ANOVA for the two prosody subtasks (1 − β = 0.99) as well as the interaction effects on the RM-ANOVAs for the individual emotions for morphing and prosody recognition (both 1 – β ≈ 0.8). The same holds true for the correlation between visual and auditory emotion recognition (1 − β = 0.85) so that these latter analyses should not have been impacted by the moderate sample size. However, analyses would have been underpowered to detect small, rather than medium correlations, such as were found in the remaining correlation analyses (r = 0.04 to 0.2). Sub-samples of patients with TLE in the dominant and non-dominant hemispheres were small. Therefore, subgroup comparisons according to lateralization were underpowered. Post-hoc power analyses revealed a power of 1 − β = 0.3 for medium effect sizes.
Our auditory and visual emotion recognition tasks were not parallel in design, i.e., only the morph task measured speed as well as accuracy, and both tasks did not include the same set of emotions. Therefore, we were unable to compare individual emotion recognition across tasks. Quasi-experimental studies are vulnerable to confounders. The participant groups included in the present study did not differ significantly in verbal IQ, age or gender. However, TLE patients displayed significantly increased symptoms of depression in comparison with the healthy control group. Nevertheless, correlation analyses showed that symptoms of depression were not significantly associated with our outcome parameters. Even though there were no significant group differences on verbal IQ, we conducted a correlation analyses between verbal IQ scores and social-cognitive parameters, which yet again did not reveal any significant associations. As anticonvulsive medication can result in adverse cognitive effects, the results of the present study could have been impacted by this. However, the lack of an association between DDD and the two measures of social cognition renders this bias less probable, although very small associations may not have been detected due to power restrictions (see above).
Summary and outlook
The present study shows impaired affective prosody recognition performance in patients with an active TLE. Furthermore, possibly, a deficit in dynamic facial emotion recognition may exist in this patient group. Even if the present study’s results merely provided a trend towards statistical significance for this measure, favoring the control group, dynamic facial emotion recognition paradigms appear to be a fruitful and somewhat more realistic approach of everyday demands on social cognition than static paradigms. As such, they should be researched in more detail and with larger patient cohorts. Furthermore, larger subgroups split by focus localization, lateralization and amygdalar involvement should be included. Studying patients with discrete lesions could increase our understanding of temporal lobe function in social cognition in general. In order to be able to examine deficits across modalities in more detail, auditory and visual tasks should employ a parallel design. Cross-modal social cognitive deficits in TLE may predict deficits in everyday social cognition, i.e., social functioning. Hence, the association between basic emotion recognition and social functioning needs to be explored further in future studies.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Semah, F. et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence?. Neurology 51, 1256–1262 (1998).
Wagner, K., Buschmann, F. & Metternich, B. Gedächtnis und epilepsie. Zeitschrift für Epileptol. 25, 243–246 (2012).
Chaplin, J. E., Wester, A. & Tomson, T. Factors associated with the employment problems of people with established epilepsy. Seizure 7, 299–303 (1998).
Gois, J. et al. Assessment of psychosocial adjustment in patients with temporal lobe epilepsy using a standard measure. Epilepsy Behav. 20, 89–94 (2011).
Meneses, R. F., Pais-Ribeiro, J. L., da Silva, A. M. & Giovagnoli, A. R. Neuropsychological predictors of quality of life in focal epilepsy. Seizure 18, 313–319 (2009).
Yogarajah, M. & Mula, M. Social cognition, psychiatric comorbidities, and quality of life in adults with epilepsy. Epilepsy Behav. 100, 106321 (2019).
Beghi, E. Social functions and socioeconomic vulnerability in epilepsy. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2019.05.051 (2019).
Steiger, B. K. & Jokeit, H. Why epilepsy challenges social life. Seizure 44, 194–198 (2017).
Szemere, E. & Jokeit, H. Quality of life is social—Towards an improvement of social abilities in patients with epilepsy. Seizure Eur. J. Epilepsy 26, 12–21 (2015).
Monti, G. & Meletti, S. Emotion recognition in temporal lobe epilepsy: A systematic review. Neurosci. Biobehav. Rev. 55, 280–293 (2015).
Kho, K. H. et al. Unimpaired sentence comprehension after anterior temporal cortex resection. Neuropsychologia 46, 1170–1178 (2008).
Baum, S. R. & Pell, M. D. The neural bases of prosody: Insights from lesion studies and neuroimaging. Aphasiology 13, 581–608 (1999).
Adolphs, R. et al. A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72 (2005).
Diamond, D. M., Campbell, A. M., Park, C. R., Halonen, J. & Zoladz, P. R. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007, 1–33 (2007).
Adolphs, R. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 15, 669–672 (1994).
Meletti, S. et al. Temporal lobe epilepsy and emotion recognition without amygdala: A case study of Urbach-Wiethe disease and review of the literature. Epileptic Disord. 16, 518–527 (2014).
Alba-Ferrara, L., Kochen, S. & Hausmann, M. Emotional prosody processing in epilepsy: Some insights on brain reorganization. Front. Hum. Neurosci. 12, 92 (2018).
Alba-Ferrara, L., Ellison, A. & Mitchell, R. Decoding emotional prosody : Resolving differences in functional neuroanatomy from fMRI and lesion studies using TMS. Brain Stimul. 5, 347–353 (2012).
An indicator of plasticity. Elizalde Acevedo, B. et al. Brain mapping of emotional prosody in patients with drug-resistant temporal epilepsy. Cortex 153, 97–109 (2022).
Ross, E. D. & Mesulam, M.-M. Dominant language functions of the right hemisphere?: Prosody and emotional gesturing. Arch. Neurol. 36, 144–148 (1979).
Kotz, S. A., Meyer, M. & Paulmann, S. Lateralization of emotional prosody in the brain: An overview and synopsis on the impact of study design. Prog. Brain Res. 156, 285–294 (2006).
Bora, E. & Meletti, S. Social cognition in temporal lobe epilepsy: A systematic review and meta-analysis. Epilepsy Behav. E&B 60, 50–57 (2016).
Metternich, B. et al. Eye-movement patterns during emotion recognition in focal epilepsy: An exploratory investigation. Seizure 100, 95–102 (2022).
Amlerova, J. et al. Emotion recognition and social cognition in temporal lobe epilepsy and the effect of epilepsy surgery. Epilepsy Behav. E&B 36, 86–89 (2014).
Edwards, M., Stewart, E., Palermo, R. & Lah, S. Facial emotion perception in patients with epilepsy: A systematic review with meta-analysis. Neurosci. Biobehav. Rev. 83, 212–225 (2017).
Schönenberg, M. et al. Epilepsy & Behavior Theory of mind abilities in patients with psychogenic nonepileptic seizures. Epilepsy Behav. 53, 20–24 (2015).
Schönenberg, M., Louis, K., Mayer, S. & Jusyte, A. Impaired identification of threat-related social information in male delinquents with antisocial personality disorder. J. Pers. Disord. 27, 496–505 (2013).
Schönenberg, M., Mayer, S. V., Christian, S., Louis, K. & Jusyte, A. Facial affect recognition in violent and non-violent antisocial behavior subtypes. J. Pers. Disord. 30, 708–719 (2016).
Schönenberg, M., Gausser, A. K., Mayer, S. V., Hautzinger, M. & Jusyte, A. Addressing perceptual insensitivity to facial affect in violent offenders: First evidence for the efficacy of a novel implicit training approach. Psychol. Med. 44, 1043–1052 (2014).
Brierley, B., Medford, N., Shaw, P. & David, A. S. Emotional memory and perception in temporal lobectomy patients with amygdala damage. J. Neurol. Neurosurg. Psychiatry 75, 593–599 (2004).
Bonora, A. et al. Recognition of emotions from faces and voices in medial temporal lobe epilepsy. Epilepsy Behav. 20, 648–654 (2011).
Dellacherie, D., Hasboun, D., Baulac, M., Belin, P. & Samson, S. Impaired recognition of fear in voices and reduced anxiety after unilateral temporal lobe resection. Neuropsychologia 49, 618–629 (2011).
Broicher, S. D., Kuchukhidze, G., Grunwald, T., Krämer, G. & Kurthen, M. “ Tell me how do I feel ”—Emotion recognition and theory of mind in symptomatic mesial temporal lobe epilepsy. Neuropsychologia 50, 118–128 (2012).
Adolphs, R., Tranel, D. & Damasio, H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology 15, 396–404 (2001).
Fowler, H. L. et al. Recognition of emotion with temporal lobe epilepsy and asymmetrical amygdala damage. Epilepsy Behav. E B 9, 164–172 (2006).
Metternich, B. et al. Affective empathy, theory of mind and social functioning in patients with focal epilepsy. Front. Psychiatry 13, 1–12 (2022).
Lehrl, S. Mehrfachwahl-Wortschatz- Intelligenztest MTW-B (Spitta-Verlag, 2005).
Hautzinger, M., Keller, F. & Kühner, C. Das Beck Depressions-Inventar BDI-II Deutsche Bearbeitung und Handbuch zum BDI II. (Harcourt Test Services, 2006).
Nazarov, A., Frewen, P., Oremus, C., Eg, S. & Mc, M. Comprehension of affective prosody in women with post-traumatic stress disorder related to childhood abuse. Acta Psychiatr. Scand. https://doi.org/10.1111/acps.12364 (2015).
Burkhardt, F., Paeschke, A., Rolfes, M., Sendlmeier, W. F. & Weiss, B. A database of German emotional speech. Interspeech 5, 1517–1520 (2005).
Langner, O. et al. Presentation and validation of the radboud faces database. Cogn. Emot. 24, 1377–1388 (2010).
Schönenberg, M., Wiedemann, E., Schneidt, A. & Jusyte, A. Processing of dynamic affective information in ADHD. J. Atten. Disord. 23, 32–39 (2015).
Liesefeld, H. R. & Janczyk, M. Combining speed and accuracy to control for speed-accuracy trade-offs (?). Behav. Res. Methods 51, 40–60 (2019).
WHO. ATC/DDD Index 2023.
Tanaka, A. et al. A more realistic approach, using dynamic stimuli, to test facial emotion recognition impairment in temporal lobe epilepsy. Epilepsy Behav. 28, 12–16 (2013).
Young, A. W., Hellawell, D. J., Van De Wal, C. & Johnson, M. Facial expression processing after amygdalotomy. Neuropsychologia 34, 31–39 (1996).
Sedda, A. et al. Ambiguous emotion recognition in temporal lobe epilepsy: The role of expression intensity. Cogn. Affect. Behav. Neurosci. 13, 452–463 (2013).
Ammerlaan, E. J. G., Hendriks, M. P. H., Colon, A. J. & Kessels, R. P. C. Emotion perception and interpersonal behavior in epilepsy patients after unilateral amygdalohippocampectomy. Acta Neurobiol. Exp. https://doi.org/10.55782/ane-2008-1690 (2008).
Meletti, S. et al. Epilepsy & behavior the affective value of faces in patients achieving long-term seizure freedom after temporal lobectomy. Epilepsy Behav. 36, 97–101 (2014).
Gomez-Ibanez, A., Urrestarazu, E. & Viteri, C. Recognition of facial emotions and identity in patients with mesial temporal lobe and idiopathic generalized epilepsy: An eye-tracking study. Seizure 23, 892–898 (2014).
Leppink, J., Sullivan, P. O. & Winston, K. Are differences between groups different at different occasions ?. Perspect. Med. Educ. 6, 413–417 (2017).
Adolphs, R. & Tranel, D. Intact recognition of emotional prosody following amygdala damage. Neuropsychologia 37, 1285–1292 (1999).
Sanz-Martin, A., Guevara, M. A., Corsi-Cabrera, M., Ondarza-Rovira, R. & Ramos-Loyo, J. Differential effect of left and right temporal lobectomy on emotional recognition and experience in patients with epilepsy. Rev. Neurol. 42, 391–398 (2006).
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by a research grant from the Research Committee of Albert-Ludwigs-University Freiburg, Germany. The article processing charge was funded by the Baden-Württemberg Ministry of Science, Research and Art and the University of Freiburg in the funding programme Open Access Publishing.
Author information
Authors and Affiliations
Contributions
B.M. and M.S. devised the project, N.G., M.S. and B.M. conceived and planned the experiments. B.M., M.J.G. and B.S. carried out the experiments. B.M. and N.G. carried out the statistical analyses. B.M., M.S., K.W., E.S. and A.S.-B. Contributed to the interpretation of the results. B.M. wrote the manuscript in consultation with M.J. B.S. prepared the tables. All authors provided critical feedback to the project and/or to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Metternich, B., Gehrer, N., Wagner, K. et al. Dynamic facial emotion recognition and affective prosody recognition are associated in patients with temporal lobe epilepsy. Sci Rep 14, 3935 (2024). https://doi.org/10.1038/s41598-024-53401-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53401-9