Abstract
For patients with epilepsy, one of the biggest problems is the unpredictability of the time when the next seizure will occur. Interestingly, some epileptic patients experience a sensory sensation preceding seizures, called aura, which helps them move to safety before a seizure. Here, we describe the development of the first animal model of auras, which could allow for a more detailed study of this phenomenon. Specifically, in mice, we presented sensory stimuli (sound and light cues) a few seconds before kindling an animal to induce seizures. Animals were kindled by electrical stimulation in the basolateral amygdalar nucleus. Over the course of stimulation sessions, animals started showing progressively stronger freezing behavior to sensory cues preceding kindling. Interestingly, seizures are known to cause retrograde amnesia, thus it was surprising that the association between seizures and preceding sensory cues developed in all experimental animals. In summary, our experiments show that similarly to auras, a sensory sensation can be associated with incoming generalized seizures and is not erased by retrograde amnesia.
Similar content being viewed by others
Introduction
Epilepsy is a serious brain condition, affecting over 70 million people worldwide with the highest prevalence in infants and older age groups1. Epilepsy is a brain disorder, resulting in excessive and hypersynchronous neuronal activity leading to ‘seizures’2. Seizures are often spontaneous and usually sudden events involving rapid onset of symptoms that can range from dramatic convulsive activity to subjective experiences or sensations that individuals perceive or encounter. During the initial part of certain seizures, a phenomenon known as "auras" can take place, wherein individuals retain consciousness and subsequently recall this as sensory sensations preceding behavioral seizures3. Auras are typically focal seizures manifested as ‘subjective’, internal events often described by patients as viscerosensory, somatic sensations, visions or related to autonomic functions such as flushing, sweating, piloerection4.
Mesial temporal lobe epilepsy (mTLE), the most common example of drug-resistant adult epilepsy, is clinically identified by prominent auras followed by episodes of staring, behavioral arrest, and frequent oro-facial automatisms5,6,7. Of the diverse manifestations of auras, those classified as viscerosensorial and affective are frequently linked to temporal lobe seizures8. Visceral sensations encompass bodily perceptions from the thorax, stomach, or bladder, including heaviness, constriction, ascending sensations, pain, flushing, nausea, tachycardia, and dyspnea9. Within the realm of affective auras, the sensation of fear stands out as the most prevalent symptom associated with epileptic discharges originating from the mesial temporal lobe8,10,11.
Fear auras, unlike the sensation of fear, are not associated with any perceived threats, including the imminent threat of a seizure. They are sudden in onset, occurring for a short duration with the individual being conscious during this ictal period. During fear sensation, an immediate threat activates the lateral nucleus of the amygdala leading to the central nucleus initiating defensive physiological reactions such as freezing12,13. Fear conditioning experiments suggest that the ventral hippocampus is involved in the manifestation of fear and anxiety, whereas the dorsal hippocampus is responsible for processing the temporal aspects and contextual cues associated with the fear response14,15,16. The hippocampus integrates contextual cues conveyed by the anterior cingulate cortex retro-splenial cortex (RSC), and post-rhinal cortex, subsequently influencing the anterior hypothalamus to generate an appropriate response, as extensively documented in the fear conditioning literature17. It has been postulated that in mTLE patients with amygdalar and hippocampal sclerosis, the occurrence of seizures with fear auras may stem from epileptic activity occurring in the brain circuits involved in defensive behavior8,18. In a stimulation study assessing visceral and emotional responses in drug-refractory epilepsy patients during pre-surgical evaluation, the amygdala and hippocampus emerged as the structures most frequently associated with these responses9. Despite the etiological distinctions between fear sensations and fear auras, there exists a similarity in the brain networks responsible for these disparate behaviors.
In our experimental study, we hypothesize that pairing auditory and visual cues with seizures originating from the amygdala in a rodent model of mTLE may allow for the identification of the associated aural phenomenon. Our study shows mice conditioned to auditory and light cues preceding seizures exhibiting a freezing response upon cue exposure. Notably, these associations develop despite seizure-induced retrograde amnesia19,20, resembling patient experiences of auras preceding generalized seizures.
Materials and methods
Subjects
All experiments were carried out in accordance with protocols approved by the Animal Welfare Committees of the University of Lethbridge and followed the guidelines established by the Canadian Council on Animal Care. This study adheres to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for comprehensive reporting of animal research. The study cohort comprised of twelve male C57BL/6 J mice procured from Jackson Laboratories (Bar Harbor, USA). The mice were aged 6 months at the time the experiments started. The animals weighed between 25 and 35 g at the beginning of the experiment. They were housed in transparent plastic cages and kept in a room with a controlled environment, specific-pathogen free, and maintained on a 12-h light/dark cycle with lights on at 07:30 h. The animal cohort was categorized into two groups: the Light and Auditory Tone Conditioned (LAC) group (N = 6), and the Unconditioned (UC) control group (N = 6) (see Experimental Design “Study design”.
Surgery
The mice were anaesthetized with 3% isoflurane and maintained between 1 and 2% for the duration of surgery. Lidocaine (2%) was administered subcutaneously at the incision site. The electrodes were chronically implanted under stereotaxic control. Bipolar electrodes were implanted bilaterally in the basolateral amygdalar nucleus, and a unipolar electrode was inserted into the dorsal hippocampus. In addition, a ground electrode was placed on the cerebellar surface which also served as reference. The inferior tips of the amygdalar electrodes were located at coordinates 1.3 mm posterior to bregma, 3.0 mm lateral to the midline, and 4.6 mm ventral to the skull. The hippocampal electrode was placed at coordinates 2.3 mm posterior to bregma, 1.5 mm lateral to the midline, and 1.3 mm ventral to the skull. In addition, in three out of six LAC mice (experimental results shown in supplementary “Introduction” and figure S1 in Supplementary Information) bipolar electrodes were also implanted in the retrosplenial cortex, 3 mm posterior to the bregma, 0.3 mm lateral to the midline and 0.5 mm ventral to the cortical surface. The electrodes were constructed from PFA (perfluoroalkoxy) coated, stainless steel wire, 0.114 mm (0.0045 inches) in diameter (A-M Systems, Carlsborg, WA). In our experiments, we used unipolar and bipolar electrodes with a tip separation of 500 microns. The unipolar served only as a recording electrode while the bipolar was used for both stimulation and recording by using a switch. To ensure reliable connections, the wire ends were carefully stripped of Teflon insulation and securely attached to gold-plated stainless-steel female contact pins (Plastics1) to form the recording and stimulating electrodes. These contact pins were affixed to a 6-channel electrode pedestal implant which served as a stable base. During recording and stimulation, the pedestal implant was linked to a mating plug, which was connected to the pre-amplifier and than to the amplifier.
Recording
For electrophysiological recordings of local field potential (LFP) we used the hippocampal electrode, contralateral amygdalar electrode, and the stimulating electrode in the ipsilateral amygdala (which also served to record brain activity by a switch). All the electrodes were referenced to the cerebellar ground electrode. By referencing the hippocampal electrode to the cerebellum, we were able to reduce external electrical noise, which affects both locations equally. The rationale for using two electrodes at different depths in the amygdala was to achieve a more local signal and use these electrodes for stimulation. One of the tips of the bipolar amygdalar electrode was also referenced to the cerebellar electrode while recording to reduce the noise mentioned above from the recorded signal. The electrographic data was fed into a unitary gain preamplifier (Grass instruments 7P122 adapter), which then passed the signal into an analog amplifier (Grass Technologies 7P122G Low Level D.C. Amplifier). The signal was then digitized at a sampling rate of 2 kHz through Axon Digidata 1550A Low-Noise Digitizer and saved on the recording computer using Axoscope software.
We also video recorded the mice (Fig. 1) to measure freezing behavior and assess the severity of the behavioral seizures using the Racine scale21. The video data was acquired at 30 frames/second through a Raspberry Pi 5-MP (mega-pixel) Camera connected to Raspberry Pi 3 Model B (Pi). The Pi also generated a one-second auditory tone at 12 kHz and activated light-emitting diodes (LEDs) for one second. Additionally, the Pi triggered the Master 8 stimulator to generate the kindling stimulus. To ensure time synchronization between the video data and electrophysiological signals, a custom Python script was used to send signals through the general-purpose input/output (GPIO) pins of the Pi to the electrophysiology recording system for each frame captured by the Raspberry Pi camera. Time stamps were also sent upon generation of the auditory tone, LED flash, and electrical kindling stimulus through the GPIOs.
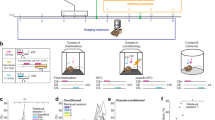
Experimental apparatus. Raspberry Pi camera was positioned on the roof of the cage to capture the mouse's behavior during the session. The cage was equipped with a pair of LED bulbs placed on opposite walls, while speakers producing auditory tones were positioned outside the other two walls. A small hole in the roof of the cage allowed for the transmission of wired electrophysiology signals from the mouse's brain to an amplifier. The same wire was also used to deliver the electrical kindling stimulus to the animal’s brain.
Experimental design
Kindling protocol
After 10 days of habituating mice to the recording apparatus, all the animals were kindled once daily to evoke seizures (Fig. 2). One of the amygdalar electrodes was electrically ‘kindled’ with 1 s train of 1 ms biphasic pulses at 60 Hz using a Master 8 Pulse Stimulator. The Master 8 produced a constant voltage stimulus, which was then converted to a constant current via a Stimulus isolator unit, A-385 (from World Precision Instruments). The current amplitude of stimulation was started at 50 microamperes and then increased by the same amount till seizures were evoked (electrographic seizures or afterdischarges of 5 s duration)22. Upon reaching this current amplitude, all animals received this stimulation for all subsequent training sessions.
Experimental design and timeline. (a) Timeline of the LAC group. In the light and auditory tone conditioned (LAC) group, the training sessions consisted of tone and LED flash presentations before a kindling stimulation. (b) Timeline of the UC group. In the unconditioned (UC) control group, the training days consisted of kindling stimulation alone. All other experimental procedures: surgery, recovery, habituation, and testing were similar for the two groups.
Sensory cue pairing—Auditory and visual stimulation
For sensory cue pairing with the seizures in the LAC animals, the kindling stimulus was preceded by the auditory tone (presented 4 s before kindling) and LED flash (3 s before kindling onset) (Fig. 2a and 3a). The auditory tone was 12 kHz in frequency for one second duration generated by a custom Python code output by 5 V twin speakers (1.2 watts per speaker) with a frequency range of 80 Hz to 20 kHz. The LED flash, lasting for one second, was produced by two LED bulbs triggered by the GPIO pins of a Raspberry Pi through the same code which produced the audio output. The UC animals only received the electrical kindling stimulus (without preceding sensory cues) (Fig. 2b).
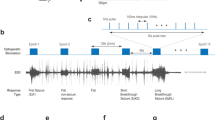
Analysis of electrophysiology signal. (a) Representative LFP signal from a LAC animal. The vertical bars represent the time stamps for the auditory stimulus and LED flash respectively. The lightning bolt indicates kindling stimulation following the sound and light presentation. (b) Measuring the duration of evoked seizures by thresholding the LFP amplitude. The last ictal spike with amplitude exceeding 10 standard deviations above the baseline activity (before kindling onset) was used to mark the end of seizures (green line). (c) Time frequency analysis. Continuous wavelet transform (CWT) of the LFP signal was used to identify the evoked seizures characterized by the increase in power of higher frequencies relative to the baseline activity.
Study design
The experimental sessions were divided into training (kindling) and testing sessions for the LAC and UC animal cohorts. To assess the progression of freezing behavior in the LAC animals across training sessions, where sensory cues (auditory cue followed by visual cue) were paired with amygdalar kindling-induced seizures, movement was quantified following the presentation of the auditory cue. Further details on the video analysis procedures used to quantify movement and movement changes across sessions can be found in “Video analysis”. We quantified this change across the training sessions (n = 18) for each LAC animal. The training sessions (n = 18) of the UC animals comprised only the kindling stimulus without any pairing with cues. Assessing freezing behavior across the training sessions enables the measurement of changes over time and allows for the consideration of any potential impact stemming from surgical procedures. Starting from no electrographic or behavioral seizures and progressing to advanced seizures with serial kindling, this evaluation measures the freezing effect attributable to cue pairing during evolving seizure activity in the LAC animals.
Furthermore, to assess the impact of the sensory cue association, we compared the freezing response of LAC animals with that of the UC group across two test sessions, which were interspersed between the training sessions. During these test sessions, animals were subjected solely to sensory cues. The test sessions aimed to determine whether the observed freezing behavior resulted from the pairing of cues with seizures (LAC) or if the repeated seizure exposure (LAC and UC) made the animals more fearful, causing them to freeze in response to sensory cues.
Analysis methods
Local field potential—Quantifying the duration of evoked seizures
The electrophysiology data was first normalized by z-scoring the data and then filtered using a Butterworth filter to remove low-frequency movement artifacts and highlight the slow-frequency band of evoked seizures (2–12 Hz)23,24. To identify periods of ictal activity, first we selected 60 s of baseline activity before sensory stimulation was applied (Fig. 3b). As seizures are characterized by a heightened amplitude of local field potentials25, we use a threshold of 10 standard deviations calculated from the baseline period to detect ictal spikes during seizure. Once the kindling stimulus is presented, any electrical activity exceeding that threshold was identified as an electrographic seizure. The duration of the seizure was determined as time elapsed from the delivery of the electrical stimulus to the last ictal spike which exceeds the threshold level (Fig. 3b).
To confirm that the duration of seizures was correctly marked, we also use spectrograms as illustrated in Fig. 3c. For spectral analysis of the LFP signal, we applied a Butterworth filter with a high-pass frequency of 2 Hz to the normalized the signal, and used continuous wavelet transform (CWT) using complex valued Morse wavelets. As we were interested only in the power, we took the absolute value of the complex signal to measure the magnitude of a specific frequency at a particular time. We used CWT instead of the standard Fourier spectrogram because CWT is more suited for shorter time signals as in our case.
Video analysis
For the animal movement analysis, video frames were extracted 60 s before and after the auditory stimulus. Each frame was converted from red, green and blue (RGB) to grayscale and a thresholding was applied to detect the mouse in each frame, as illustrated in Fig. 4a. For detecting movement, video was down sampled to 10 frames per second and then consecutive frames were subtracted from each other (Fig. 4a, right panel). The difference in the number of pixels between consecutive frames was used as a measure of the mouse’s movement. To normalize this movement data, we z-scored each individual session. Following this, we convolved the z-scored data with a 500-ms duration Gaussian window and rescaled the resulting values to a range between zero and ten. Note that when mouse does not move then the number of pixels different between frames is close to zero. The freezing duration was determined by setting a threshold near zero (specifically, 1 on the rescaled movement values). A sample of movement measure during two session is presented in Figs. 4b. We quantified changes in movement during the session by calculating the mean movement and freezing duration within a three-second window before and after the auditory stimulus presentation. The differences between post-cue and pre-cue mean movements, as well as post-cue and pre-cue freezing durations, were then used for statistical analysis. Due to technical problems, we missed data from few sessions for two animals, which resulted in a smaller number of points for those sessions in our plots.
Video analysis to quantify freezing behavior. (a) Video data pre-processing. The RGB frames were converted to grayscale and a thresholding was applied to detect the mice. Next, we did frame-differencing to quantify movement of mice between frames (b). Movement analysis of a LAC mouse during a training (kindling) session. This displays the mouse movement during a kindling evoked seizure paired with cues (auditory tone and LED flash). Post-cue freezing, represented by a red line, occurs in the three-second window after presentation of the auditory tone and before the kindling stimulus. After the kindling stimulus, the animal’s behavior progresses to a Racine Stage 5 seizure (see Video 1 in the supplementary materials showing animal during this session).
Results
Kindling led to advanced seizures in all animals
Representative electrographic seizures recorded from the dorsal hippocampus (from the hemisphere ipsilateral to the stimulating amygdalar electrodes) in the LAC and UC animals are shown in Fig. 5a,b respectively. In both groups of animals, the kindling procedure evoked seizures, with the duration of seizures increasing over stimulation sessions (Fig. 6a). The duration plotted in Fig. 6 represents electrographic data recorded from the vicinity of the stimulating electrodes implanted in the amygdala. A Pearson correlation coefficient between the sessions and seizure duration was 0.446 with p-value = 3.207e-10. The rate of change in seizure duration was quantified by a linear regression analysis giving a positive slope of 0.978 between the sessions and the duration of the evoked seizures (Fig. 6a). Across days, seizures developed similarly in both LAC and UC groups (see Video 1 in the supplementary material showing a representative LAC animal during the kindling sessions with behavioral seizures with Racine stage 5 (rearing and falling)21). The Anova analysis did not reveal any difference in the seizure duration between LAC and UC groups as evidenced by a p-value of 0.181 (Fig. 6b), and neither a difference between groups across days (p-value = 0.159). These results show that in both LAC and UC groups there was a similar progressive increase in the duration of seizures over days, thus providing validation that UC animals can be used as a proper control group for LAC animals.
Examples of recorded seizures. (a). Time frequency analysis of a training session for a LAC (auditory tone – LED – Kindling stimulus) animal. (b). Time frequency analysis of a training session for a UC (kindling stimulus alone) animal. Figure 5a and 5b show local field potentials (LFPs) and continuous wavelet transform (CWT) analyses for representative LAC (a) and UC animals (b) respectively. In the top panels of both (a) and (b), LFP recordings were thresholded at 10 standard deviations above baseline (see also Fig. 3) to measure the duration of evoked seizures following kindling. The wavelet analysis in the bottom panels shows seizure activity, as indicated by an increase in high-frequency power relative to baseline activity in both animal groups.
Progression of seizure duration across kindling sessions by group. (a). Each data point represents the seizure duration in a single animal during a single session. Linear regression highlights the positive correlation between session progression and seizure duration. (b) Boxplot comparing seizure durations between the LAC and UC groups. Statistical analysis using Anova shows no significant difference in seizure duration between the two groups.
Behavioral changes in the LAC group after sensory cue presentation
Using video analyses as described in the Methods section, we quantified the amount of change in animal movement following the presentation of sensory cues as a function of the kindling training sessions. The animals in the LAC group exhibited a reduction in post-cue movement as the kindling stimulation continued over sessions (Fig. 7a). This change was quantified by measuring mean movement within a three-second window following the auditory cue presentation and subtracting the mean movement within the same period preceding the cue presentation (see Fig. 4a). To examine the relationship between this difference in mean movement and the number of sessions, we calculated a squared correlation coefficient (R-squared = 0.165, Pearson’s correlation coefficient = -0.406). This R-squared value means that approximately 16.5% of the variance in the movement can be attributed to the number of training sessions (with cue exposure paired with kindling). Furthermore, we also calculated the F-statistic (18.8; p-value = 0.000036***) confirming that this effect was statistically significant (Fig. 7a). The slope of the linear regression model that fit the data had a negative slope (regression coefficient = -0.1594) which provides information on how quickly the animal’s movement decreased after cue presentation across sessions.
Behavioral change in animals due to cue association with seizures. (a) Regression showing an association between sessions and post-cue movement in LAC animals. The progressive change in the difference in mean movement following sensory cue presentation across kindling sessions is plotted. The regression analysis illustrates a negative relation between the amount of movement and the session number. (b) Individual LAC Movement. Data for individual animals is shown here, where the progression of the change in movement following cue presentation from the initial stages of kindling (first three training sessions) to the last three training sessions are compared. The negative slope for all animals shows that movement reduction was consistent across all LAC animals. (c) Regression showing an association between sessions and post-cue freezing in LAC animals. Plot illustrating the increase in freezing behavior across sessions. Freezing duration was defined as time without movement within 3 s before or after cue presentation (Methods Sect. 2.5.2 and Fig. 4). (d) Group comparison of movement change due to cue association. Comparison of the difference in mean movement following cue presentation between LAC and UC groups during the test sessions (only cues). Animals in LAC group showed significantly fewer movements as compared to the control UC group in which kindling was not associated with sensory cues.
The decrease in movement after cue presentation was observed in all LAC animals. This is illustrated in Fig. 7b, showing average post-cue movement difference during the first three training sessions and during the last three training sessions for each animal. We tested for statistical significance in post-cue movement difference across sessions by comparing these first three and last three training sessions, using a one-way Anova. The Anova analysis showed a significant F-statistic of 16.55 with a p-value of 0.000266(***), indicating a significant difference in movement patterns. Additionally, the Paired t-test was used, which also showed a significant t-statistic of 3.531 with p-value of 0.000257(***), further supporting the presence of an association between cues and seizures as evidenced by a behavioral alteration. In addition, a non-parametric test, namely Kolmogorov–Smirnov (KS) test was conducted. The D-statistic, which is the maximum diagonal distance between the first and last three training sessions, was 0.556 and the p-value was 0.0067 demonstrating significant statistical difference. All these analyses of the training sessions support the hypothesis that LAC animals learned to associate sensory cues with incoming seizure.
To ensure that our analyses are not sensitive to a specific definition of movement, we repeated analyses using animal freezing duration. The freezing duration was defined as the amount of time within 3 s after cue presentation, when movement was smaller than 1 unit (see Methods). We observed a positive relation between sessions and the difference in freezing duration after cue presentation (Fig. 7c) which was consistent with our prior finding (Fig. 7a). The squared correlation coefficient between freezing duration and session number: R-squared was 0.108 (Pearson’s correlation coefficient = 0.3279), showing that approximately 10.8% of the variation in freezing duration can be explained by the number of cue exposure sessions. Moreover, the linear regression model was found to be statistically significant, as evidenced by the F-statistic of 11.4 and a p-value of 0.00104. Thus, all the above analyses showed that pairing sensory cues with incoming seizures resulted in a significant change in behaviour in LAC animals.
We further verified this effect by comparing movement after cue presentation between LAC and UC groups during the test sessions. Note that in UC animals’ sensory cues were not presented during kindling sessions, however, after animals developed seizures, we had test sessions, where animals were only presented with sensory cues (not followed by electrical stimulation; Fig. 2). Similarly, in LAC animals, after seizure developed, we had test sessions where we presented only sensory cues. Out of the four sessions two were selected where the animal had previously experienced a generalized seizure in the training sessions. This choice was made so that test sessions with a similar seizure stage in all animals were used for making the comparison. Representative videos from such test sessions are included in Supplementary Materials: Video 2 for the LAC group and Video 3 for the UC group. For this comparison between LAC and UC group, we calculated the mean movement before and after the cue presentation over a longer time window: 20 s time window. We then performed a one-way Anova test on the difference between post-cue mean movement and pre-cue mean movement. The test yielded a F-value of 28.95 and a p-value of 0.000025 (Fig. 7d). To ensure the robustness of our statistical findings, we employed the non-parametric Kolmogorov–Smirnov test. The obtained D-statistic of 0.75, accompanied by a p-value of 0.00078, supports the statistical significance of our results. Those results are consistent with the results presented in Fig. 7a,b, confirming the behavioral changes in LAC animals due to association of sensory cues with seizures. Note that using UC animals as a control group, it allowed us to test that kindled animals are not becoming just more ‘fearful’ to sensory stimuli due to experiencing seizures, but probably because of the sensory cues being conditioned to seizure phenomenology.
In three out of these six LAC animals following the sensory cue pairing with evoked seizures, retrosplenial cortex (RSC) stimulation was paired with the evoked seizures (Supplementary Sect. 1 and Figure S1 in Supplementary Information). All of these animals exhibited a progressive increase in freezing behavior following RSC stimulation across sessions where RSC stimulation was paired with the evoked seizures (RSC training sessions). For statistical analysis, refer to Supplementary Sect. 1.
We repeated our LAC experiments in a set of nine animals, where we paired the auditory and visual cues in a similar fashion (Supplementary Sect. 2 and figure S2 in Supplementary Information). Here we observed a similar trend in the animals’ behavior, with the animals progressively freezing due to the pairing of sensory cues with evoked seizures across sessions displaying reproducibility of our experimental results. For further details refer to supplementary Sect. 2 (Supplementary Information).
Discussion
In our experiments, continual temporal pairing of sensory cues with evoked seizures over several sessions in an animal model of temporal lobe epilepsy resulted in a progressive change in the animals' behavior. This change was quantified as an increase in freezing behavior across the sessions. These findings are interesting because amnesia is associated with seizures, specifically amnesia for events just preceding the seizure onset20,26,27,28. The ability of LAC animals to recall sensory cues presented prior to seizures raises an important question: how do they remember these cues?
A cardinal feature of generalized seizures, distinguished by abnormal synchronous activity in both cerebral hemispheres, is loss of consciousness followed by recovery with the individual having no memory of the episode29. This amnestic event is particularly prevalent in cases of secondarily generalized tonic–clonic seizures originating from the temporal lobe30. In a clinical study of mTLE patients, high seizure frequency was shown to be associated with greater deficits in autobiographical memory, relating to one’s own experiences, as reported in anterograde memory tests31. Clinical studies evaluating memory deficits in mTLE patients revealed amnesia for events preceding up to two years32,33,34. In many of these instances, patients were conscious during the seizure's onset and even made efforts to seek assistance from a nurse. However, they subsequently fail to recall the events associated with the ictal episode and even their attempt to seek help30.
Electroconvulsive therapy (ECT), a treatment inducing generalized tonic–clonic convulsions recommended for medically intractable affective disorders, usually causes retrograde amnesia affecting both episodic and semantic memory19,35,36,37,38,39. In an experimental study, mice subjected to pentylenetetrazole-induced seizure immediately after being trained on a spatial memory task demonstrated a notable decline in their subsequent performance on the following day, indicating that seizures induce retrograde amnesia20. This is further supported by a study in which rats were unable to create memories for a spatial task after being subjected to electroconvulsive seizures suggesting that seizures cause a deficit similar to bilateral hippocampal lesions40. Both the above-mentioned studies also found a correlation between impaired task performance and the effects of seizures on long-term potentiation (LTP)20,40.
However, there are also studies providing evidence of the epileptic subjects having some degree of recall regarding events associated with the epileptic attack. A study testing the association of environmental cues with amygdalar kindling displayed rats developing a preference for regions in the experimental apparatus where seizures were not evoked41. Building upon the previous study, further investigations were conducted by the same group to explore the conditioning effects to other cues associated with kindling, specifically the consumption of flavored solutions42. Their study revealed that the association of kindling with flavored solutions resulted in an aversive response in the rats. It is relevant to mention that in these studies, kindling brain structures in the limbic cortex, namely the basolateral amygdala and dorsal hippocampus were more strongly associated with the behavioral changes when compared to the effects observed due to kindling non-limbic brain areas.
A similar change in behavior is seen in our current experiments, where mice exposed to an auditory tone and a LED flash seconds before the electrical kindling (to evoke seizures), were able to remember this association as demonstrated by freezing behavior. This freezing behavior increased in intensity across the training sessions. We propose that as in classical conditioning, the sensory cues (conditioned stimulus) associate with the aural stage of seizures (unconditioned response) during which the mice retain consciousness and have memory preservation43,44,45. Consequently, the LAC animals demonstrated an ability to retain these cues in memory, leading to the development of an associated conditioned response that could potentially activate the brain networks associated with the epileptic circuit46. Even if sensory cues do not cause the entire seizure epoch as during kindling, our experiments suggest conditioning to the aural event.
The experiments described in Supplementary Sect. 1 (Supplementary Information), where RSC stimulation paired with evoked seizures led to progressive freezing behavior, have interesting implications. Initially, these animals had already experienced sensory cue pairing with seizures, which also resulted in progressive freezing. The RSC encodes contextual information and integrates various sensory inputs, including visual, auditory, and somatosensory stimuli47,48,49. Because RSC stimulation occurred in the same environment where cue-seizure pairing previously took place, it suggests that this stimulation might activate associated sensory networks, potentially eliciting sensations without distinct sensory percepts. Additionally, these interoceptive sensations, if present, paired with evoked seizures, precede the behavioral responses, resembling an aura-like phenomenon. However, since this phenomenon was observed in a limited sample set, it is important to replicate the experiment with a larger cohort of animals.
In summary, our experiments provide an important advancement toward developing an animal model of auras. Firstly, like auras, sensory sensation take place before the start of behavioral seizures. Secondly, analogous to clinical auras, our experimental animals remembered sensory sensations as demonstrated by freezing response. Thirdly, supplementing our experiments by additional electrical stimulation to sensory association cortex (RSC) led to a similar freezing response, which could be due to a phenomenon resembling clinical auras.
Establishing an animal model of auras can lead to multiple benefits. Firstly, seizures are unpredictable events, thus being able to study auras in more details could help to more reliably identify incoming seizure onset50. Secondly, model of auras could allow us to test new treatments to interrupt epileptic networks exactly before seizure onset. Thirdly, auras help to localize the epileptogenic zone51 and we could use single neuron recordings and 2-photon imaging in rodents to better understand this concept. The identification of the epileptogenic zone is extremely crucial in drug resistant seizures where surgical resection is the gold standard. Finally, the presence of specific auras serves as prognostic markers for the surgical outcome in epileptic patients with drug resistant epilepsy52. Combining electrophysiology with imaging in animal models to identify brain activity associated with auras could help detect epileptogenic regions often overlooked during resection surgeries, thereby significantly improving patient prognosis.
Data availability
All data supporting described findings can be obtained from the corresponding authors (R.D. and A.L.) upon reasonable request, and sample videos used for our analysis are provided in Supplementary Materials.
References
Thijs, R. D., Surges, R., O’Brien, T. J. & Sander, J. W. Epilepsy in adults. Lancet 393, 689–701 (2019).
Blume, W. T. et al. Glossary of descriptive terminology for ictal semiology: Report of the ILAE task force on classification and terminology. Epilepsia 42, 1212–1218 (2001).
Gupta, A. K., Jeavons, P. M., Hughes, R. C. & Covanis, A. Aura in temporal lobe epilepsy: Clinical and electroencephalographic correlation. J. Neurol. Neurosurg. Psychiatry 46, 1079–1083 (1983).
Lowenstein, D. H. Seizures and Epilepsy. In Harrison’s Principles of Internal Medicine, 20e (ed. Lowenstein, D. H.) (McGraw-Hill Education, 2018).
Engel, J. A Proposed Diagnostic Scheme for People with Epileptic Seizures and with Epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia 42, 796–803 (2001).
Engel, J. Introduction to temporal lobe epilepsy. Epilepsy Res. 26, 141–150 (1996).
Engel, J. Mesial Temporal Lobe Epilepsy: What Have We Learned?. Neurosci. 7, 340–352 (2001).
Cendes, F. et al. Relationship between atrophy of the amygdala and ictal fear in temporal lobe epilepsy. Brain 117, 739–746 (1994).
Soulier, H. et al. Visceral and emotional responses to direct electrical stimulations of the cortex. Ann. Clin. Transl. Neurol. 10, 5–17 (2023).
Fernández-Torre, J. L. Epileptic auras: classification, pathophysiology, practical usefulness, differential diagnosis and controversials. Rev. Neurol. 34, 977–983 (2002).
Macrae, D. Isolated Fear. Neurology 4, 497 (1954).
LeDoux, J. E. & Pine, D. S. Using neuroscience to help understand fear and anxiety: A two-system framework. Am. J. Psychiatry 173, 1083–1093 (2016).
LeDoux, J. E. Brain mechanisms of emotion and emotional learning. Curr. Opin Neurobiol. 2, 191–197 (1992).
Esclassan, F., Coutureau, E., Di Scala, G. & Marchand, A. R. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus 19, 33–44 (2009).
Kjelstrup, K. G. et al. Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. 99, 10825–10830 (2002).
McHugh, S. B., Deacon, R. M. J., Rawlins, J. N. P. & Bannerman, D. M. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav. Neurosci. 118, 63–78 (2004).
Gross, C. T. & Canteras, N. S. The many paths to fear. Nat. Rev. Neurosci. 13, 651–658 (2012).
Leal, R. B. et al. Amygdala levels of the GluA1 subunit of glutamate receptors and its phosphorylation state at serine 845 in the anterior hippocampus are biomarkers of ictal fear but not anxiety. Mol. Psychiatry 25, 655–665 (2020).
Squire, L. R., Chace, P. M. & Slater, P. C. Retrograde amnesia following electroconvulsive therapy. Nature 260, 775–777 (1976).
Naik, A. A. et al. Mechanism of seizure-induced retrograde amnesia. Prog. Neurobiol. 200, 101984 (2021).
Racine, R. J. Modification of seizure activity by electrical stimulation: II Motor seizure. Electroencephalogr. Clin. Neurophysiol. 32, 281–294 (1972).
Bawden, H. N. & Racine, R. J. Effects of bilateral kindling or bilateral sub-threshold stimulation of the amygdala or septum on muricide, ranacide, intraspecific aggression and passive avoidance in the rat. Physiol. Behav. 22, 115–123 (1979).
Leung, L.-W.S. Hippocampal electrical activity following local tetanization. I. Afterdischarges. Brain Res. 419, 173–187 (1987).
Bragin, A., Penttonen, M. & Buzsáki, G. Termination of Epileptic Afterdischarge in the Hippocampus. J. Neurosci. 17, 2567–2579 (1997).
Teskey, G. C. Kindling. in Oxford Research Encyclopedia of Psychology (Oxford University Press, 2020).
Duncan, C. P. The retroactive effect of electroshock on learning. J. Comp. Physiol. Psychol. 42, 32–44 (1949).
Jus, A. & Jus, K. Retrograde amnesia in petit mal. Arch. Gen. Psychiatry 6, 163–167 (1962).
McGaugh, J. L. Memory–A century of consolidation. Science 1979(287), 248–251 (2000).
Kammerman, S. & Wasserman, L. Seizure disorders. West. J. Med. 175, 184 (2001).
Blum, D. E., Eskola, J., Bortz, J. J. & Fisher, R. S. Patient awareness of seizures. Neurology https://doi.org/10.1212/WNL.47.1.260 (1996).
Voltzenlogel, V., Vignal, J.-P., Hirsch, E. & Manning, L. The influence of seizure frequency on anterograde and remote memory in mesial temporal lobe epilepsy. Seizure 23, 792–798 (2014).
Ahern, G. L. et al. Paraneoplastic temporal lobe epilepsy with testicular neoplasm and atypical amnesia. Neurology https://doi.org/10.1212/WNL.44.7.1270 (1994).
Kapur, N. et al. Very Long-Term Amnesia in Association with Temporal Lobe Epilepsy: Evidence for Multiple-Stage Consolidation Processes. Brain Cogn. https://doi.org/10.1006/brcg.1997.0927 (1997).
Baker, J. et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. https://doi.org/10.1093/braincomms/fcab038 (2021).
Enev, M. et al. Imaging Onset and Propagation of ECT-induced Seizures. Epilepsia 48, 238–244 (2007).
Landry, M., Moreno, A., Patry, S., Potvin, S. & Lemasson, M. Current practices of electroconvulsive therapy in mental disorders. J. ECT 37, 119–127 (2021).
Meechan, C. F., Laws, K. R., Young, A. H., McLoughlin, D. M. & Jauhar, S. A critique of narrative reviews of the evidence-base for ECT in depression. Epidemiol. Psychiatr. Sci. 31, e10 (2022).
Meeter, M., Murre, J. M. J., Janssen, S. M. J., Birkenhager, T. & van den Broek, W. W. Retrograde amnesia after electroconvulsive therapy: A temporary effect?. J. Affect. Disord. 132, 216–222 (2011).
Nikolin, S. et al. Comparative efficacy, cognitive effects and acceptability of electroconvulsive therapies for the treatment of depression: protocol for a systematic review and network meta-analysis. BMJ Open 12, e068313 (2022).
Reid, I. C. & Stewart, C. A. Seizures, memory and synaptic plasticity. Seizure 6, 351–359 (1997).
Barnes, S. J., Pinel, J. P. J., Francis, L. H. & Wig, G. S. Conditioning of ictal and interictal behaviors in rats by amygdala kindling: Context as the conditional stimulus. Behav. Neurosci. 115, 1065 (2001).
Wig, G. S., Barnes, S. J. & Pinel, J. P. J. Conditioning of a flavor aversion in rats by amygdala kindling. Behav. Neurosci. 116, 347 (2002).
Bouton, M. E. & Moody, E. W. Memory processes in classical conditioning. Neurosci. Biobehav. Rev. 28, 663–674 (2004).
Rehman, I., Mahabadi, N., Sanvictores, T. & Rehman, C. I. Classical Conditioning (StatPearls Publishing, 2022).
Clark, R. E. & Squire, L. R. Classical Conditioning and Brain Systems: The Role of Awareness. Science 1979(280), 77–81 (1998).
Janowsky, J. S., Laxer, K. D. & Rushmer, D. S. Classical Conditioning of Kindled Seizures. Epilepsia 21, 393–398 (1980).
Todd, T. P., Fournier, D. I. & Bucci, D. J. Retrosplenial cortex and its role in cue-specific learning and memory. Neurosci. Biobehav. Rev. 107, 713–728 (2019).
Hindley, E. L., Nelson, A. J. D., Aggleton, J. P. & Vann, S. D. The rat retrosplenial cortex is required when visual cues are used flexibly to determine location. Behav. Brain Res. 263, 98–107 (2014).
Miller, A. M. P., Vedder, L. C., Law, L. M. & Smith, D. M. Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition. Front Hum. Neurosci. https://doi.org/10.3389/fnhum.2014.00586 (2014).
Schulze-Bonhage, A. & Haut, S. Premonitory features and seizure self-prediction: Artifact or real?. Epilepsy Res. 97, 231–235 (2011).
Foldvary-Schaefer, N. & Unnwongse, K. Localizing and lateralizing features of auras and seizures. Epilepsy Behav. 20, 160–166 (2011).
Ashalatha, R. et al. Do auras predict seizure outcome after temporal lobe epilepsy surgery?. Epilepsy Res. 147, 109–114 (2018).
Acknowledgements
The authors thank I Esteves, H Chang, R Pais, D Pahwa, A Neumann, Y Kaushik, M Ello, R Singh, J Higham, M Pearson, B Ponech, T Miller and K Iyer for help on this project. We also thank Ian Q. Whishaw, Bryan Kolb, Bruce L. McNaughton, and G. Campbell (Cam) Teskey for inspiring discussion on the relation between seizures and physiological brain functions.
Funding
This work was supported by a CIHR Project grant and NSERC DG to AL and Alberta Innovates –Data Enabled Innovations Graduate Student Scholarship awarded to RD.
Author information
Authors and Affiliations
Contributions
R.D. and A.L. designed the experiment. R.D., A.M. and C.H. conducted the experiments and collected the data. R.D. and C.H. performed the surgeries. V.L., A.M., and C.H. performed the histology for the brain sections. R.D. and A.L. analyzed the data and wrote the manuscript, which all authors helped to revised.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Das, R., Howey, C., McFetridge, A. et al. Associating sensory cues with incoming seizures: developing an animal model of auras. Sci Rep 14, 20881 (2024). https://doi.org/10.1038/s41598-024-71885-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71885-3