Abstract
Kadsura heteroclita (Roxb) Craib also named Xuetong in Tujia ethnomedicine in China, has been traditionally employed in rheumatoid arthritis (RA) treatment. Our preceding investigations have elucidated that Xuetongsu (XTS), a triterpenoid compound predominant in Xuetong, showed excellent anti-RA-fibroblast-like synoviocytes (RAFLS) proliferation effect. However, XTS belongs to the trace components of the Xuetong plant, which poses certain limitations to the research. In this study, we designed a method that enhanced the extraction yield of XTS and explored the mechanism of its inhibition of RAFLS cell proliferation and migration in the treatment of RA. The results displayed that XTS reduced RAFLS cell proliferation, with an IC50 value of 4.68 ± 0.65 µM. A series of experimental techniques were utilized to show that XTS induce apoptosis in RAFLS cells at concentrations ranging from 4.5 to 18 µM, including wound healing assay, flow cytometry, and western blot analysis. Moreover, XTS at dosages of 0.42–0.84 mg/kg markedly attenuated paw swelling and synovial hyperplasia in arthritic rats, primarily through the inhibition of RAFLS migration and promotion of RAFLS apoptosis via High mobility group box 1 (HMGB-1)/Matrix metalloproteinase-9 (MMP-9)/MMP-13 signaling pathway and Bcl-2/Bax/Caspase-3 signaling pathway, respectively.
Similar content being viewed by others
Introduction
Kadsura heteroclita (Roxb) Craib, also called as Xuetong in Chinese Tujia ethnomedicine, possesses an edible fruit1,2 and is utilized for its medicinal properties, including promoting blood circulation, dispelling “wind evil”, and relieving “damp evil”. It has been used in the treatment of rheumatism and arthralgia, as well as rheumatoid arthritis (RA), within Tujia ethnomedicinal practices. The anti-inflammatory and analgesic effects of Xuetong has been studied in our previous research1,3. Based on these, we isolated several triterpenoids and lignan compounds from Xuetong, among which Xuetongsu (XTS) was identified as promising. It demonstrated a significant inhibitory effect on the proliferation of RA-fibroblastoid synovial (RAFLS) cells, indicating potential as a valuable compound for anti-RA drug development4,5,6,7. However, the current extraction yield of XTS from Xuetong is a mere 0.036%, a rate that is insufficient to meet the demands of research and development. Therefore, enhancing the extraction efficiency of XTS is important for securing a substantial quantity of this pharmacologically active compound, which could enable more extensive and detailed investigations into its anti-inflammatory mechanisms8.
RA is an inflammatory disease characterized by aggressive synovial hyperplasia and progressive bone destruction, manifesting as symmetrical pain, swelling, and redness in the afflicted joints9. Synovial inflammation is a primary pathological feature in the early stages of RA. As RA advances, it may precipitate cartilage damage and bone erosion, potentially culminating in irreversible disability in extreme cases10,11. Importantly, the inhibition of early proliferation of RAFLS plays a crucial role in mitigating subsequent cartilage and bone destruction11. RAFLS cells are the main cellular component of the synovial membrane lining in joint cavity. The aggressive proliferation of RAFLS is characteristic of their ability to invade the joint, culminating in synovial inflammation and consequent joint damage10,12. In the context of RA pathogenesis, RAFLS cells exhibit a high level of activation, paralleling tumor cells with their pronounced inflammatory proliferation and resistance to programmed cell death, primarily driving synovial hyperplasia13,14. These cells are capable of invading and dismantling the cartilage matrix, thereby facilitating joint destruction15. High mobility group box 1 (HMGB-1), matrix metalloproteinase-9 (MMP-9) and MMP-13 have been found with high level expression in proliferating synovial tissue of RA patients16,17. Moreover, the synovial tissue displays a dysregulation of pro-apoptotic and anti-apoptotic molecules in RAFLS cells, which results in apoptotic resistance and is often evidenced by over-expressed anti-apoptotic mediators, such as Bcl-2 and Bcl-xl, and the low-expressed pro-apoptotic proteins, including Bax, Bid Bad, Caspase-8 and Caspase-3, in RAFLS cells18. Therefore, there is an urgent need to seek a rational treatment approach to identify effective treatment strategies that alleviate synovial inflammation and inhibit synovial hyperplasia, particularly for managing late-stage RA.
Currently, the primary strategy of current anti-RA therapies focuses on attenuating the inflammatory response mediated by macrophages to reduce synovial inflammation, encompassing anti-inflammatory drugs (NSAIDs) and biologic agents19,20,21. Nonetheless, these medications are relevant to severe adverse effects, including gastrointestinal disturbances, hepatorenal toxicity, osteoporosis, and hyperglycemia22. Given the pathological mechanisms of RA, promoting apoptosis in RAFLS cells emerges as a viable approach for developing safe and efficacious RA treatments. Our studies have demonstrated that XTS markedly suppresses the proliferation of RAFLS cells, potentially through the facilitation of apoptotic processes within these cells. Consequently, XTS represents a promising candidate for RA therapy. One important aspect that needs to be addressed is improving the extraction rate of XTS from Xuetong, as this has significant implications for further research4,5,6,7.
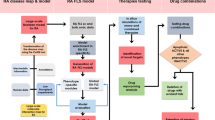
In general, XTS is a triterpenoid compound in Xuetong, which can inhibit the invasive proliferation of RAFLS and is a very promising anti-RA drug. But the extraction rate of XTS from Xuetong is only 0.036%. In addition, the pathological feature of RA is invasive synovial hyperplasia, and the current drugs for RA are suboptimal and mixed (Scheme 1). Therefore, it is of great significance to develop new, effective and safe anti-RA drugs. In this study, we optimized the extraction process of XTS from Xuetong, achieving improved extraction efficiency. Furthermore, we investigated the role of XTS in counteracting RA and discovered that XTS regulates migration and apoptosis in RAFLS cells. This effect is mediated by inhibiting cell migration molecules such as Cdc42, Rho A, Rac1, HMGB-1, MMP-9 and MMP-13 and activating Bcl-2/Bax/Caspase-3 apoptotic pathway, achieving the suppression of invasive synovial hyperplasia. This study laid a foundation for the development of anti-RA Chinese and innovative drugs.
Results
Structure elucidation of XTS
XTS was obtained in the form of a white amorphous powder, exhibiting an optical rotation of [α]\(\:\frac{20}{\text{D}}\): 35.4 (c 0.20, in CH3COCH3). The molecular formula of XTS was determined to be C30H44O4 based on the HR-ESI-MS ion at m/z 467.6660[M-H]− (calculated for 467.6662), indicative of 9 double bond equivalents (DBEs). The UV spectrum of XTS exhibited a peak absorption at λmax 210 nm. By analyzing the NMR data, including DEPT and HSQC spectra, it was determined that there were three alene proton signals present at [δH 6.61 (1 H, dt, J = 6.7 Hz, 1.8 Hz H-24), 4.81 (1 H, m, H-28), 4.74 (1 H, m, H-28)] and 5 methyl hydrogen signals delta [ δH 0.93 (3 H, s, H3-18), 0.97 (3 H, d, J = 6.7 Hz, H3-21), 1.92 (3 H, d, J = 1.7 Hz, H3-27), 1.68 (3 H, s, H3-29). 1.00 (3 H, s, H3-30)] (Fig. S1, S3 and S5). The13C NMR spectra (Fig. S2) suggested that there was one carboxyl carbon signal at [δC 178.2 (C-3)], one carbonyl carbon signal at [δC 166.8 (C-26)], 4 olefine carbon signals at [δC 149.4 C (C-4), 139.6 (C-24), 128.4 (C-25), 111.8 (C-28)], and 5 methyl carbon signals [δC 19.9 (C-18), 13.2 (C-21), 17.2 (C-27), 19.6 (C-29), 18.0 (C-30)]1. H NMR (600 MHz, CDCl3) and13C NMR (150 MHz, CDCl3) (Table S1). The NMR data suggested that XTS is a triterpene compound.
The analysis of1H-1H COSY spectrum for XTS revealed four distinct spin systems: H-1/H-2, H-6/H-7/H-8, H-11/H-12, and H-15/H-16/H-17/H-20/H-21/H-22/H-23/H-24 (Fig. S4). HMBC correlations were observed from H-1 to C-2, C-3; from H-5 to C-1, C-4, C-6, C-7, C-28, C-29; from H-11 to C-9, C-13, C-19; from H-19 to C-8, C-9, C-10; and from H-24 to C-22, C-23, C-26, C-27 (Fig. S6). Thus, the planar structure of XTS was established (Fig. 1).
Further structural elucidation through ROESY correlations indicated α-orientation for the protons H-17 and H-30, while the β-orientation was assigned to protons H-5 with H-28 and H-18 with H-9 and H-20, thus defining the three-dimensional configuration of XTS (Fig. S7).
Plant resources and chemical characterization of Xuetong and its extract, XTS. (A), The stems and leaves of Xuetong plants. (B), The white crystalline powder of XTS. (C), The chemical formula of XTS. (D), Key 1H-1H COSY, HMBC, and ROESY correlations of XTS. (E), The X-ray crystallographic structure of XTS.
Qualitative and quantitative analysis
After structural identification, the enriched XTS were evaluated qualitatively and quantitatively. As illustrated in Fig. S8B, the results revealed a retention time for the XTS standard of 14.6 min, which was identical to the sample’s retention time. The retention time for both the standard and the sample of XTS was recorded at 14.6 min. Quantitative analysis, supported by the standard curve exhibiting a correlation coefficient of 1 (Fig. S8), determined the XTS concentration in the sample to be 24.2 µg/mL, indicating that the initial 2.5 mg of XTS extract powder contained 2.42 mg of XTS, achieving a purity of 96.8%, which exceeds the minimum purity criterion of 95%. The optimized industrial process outlined in this study facilitated the extraction and isolation of 26.22 g of XTS from 13.2 kg of Kadsura heteroclita powdered stems, corresponding to an extraction yield of 0.1986%. This yield represents a 5.52-fold increase over the yield achieved with the previous extraction method, conclusively demonstrating the efficacy of the new optimization technique in enhancing XTS extraction from Xuetong.
IC50 assay and the inhibitory effect of XTS on RAFLS cell viability
The Cell Counting Kit-8 (CCK-8) assay results demonstrated that conventional anti-RA medications, including indomethacin (Indo), methotrexate (MTX) and XTS, significantly reduced the viability of RAFLS cells at concentrations ranging from 2.5 to 20 µM. In contrast, the group treated with sinomenine (SIN, extracted from the Sinomenium actum Rehd. et Wils, a traditional anti-RA herbal remedy) did not show a notable difference in cytotoxicity compared to the untreated control group. The IC50 values for XTS, Indo, MTX, and SIN were calculated to be 4.68 ± 0.65 µM, 3.81 ± 0.77 µM, 2.95 ± 0.63 µM, and 62.87 ± 5.79 µM, respectively, indicating the concentration required to inhibit 50% of RAFLS cell proliferation (Fig. 2A). Furthermore, exposing RAFLS cells to 4.5, 9.0, and 18.0 µM of XTS for 48 h decreased cell viabilities to 57.69 ± 6.37%, 33.81 ± 5.73%, and 15.58 ± 2.26%, respectively (Fig. 2B).
XTS inhibited the migration of RAFLS cells
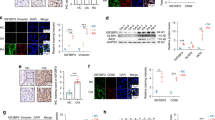
Uncontrolled migration and invasion of RAFLS cells play a crucial role in the synovial hyperplasia process of RA, which is closely associated with the elevated expression levels of MMPs and HMGB-1 in synovial tissue. According to the findings of the wound healing assay, treatment with XTS resulted in a reduction of migration and invasion capabilities of RAFLS cells, showing a dose-dependent response. Remarkable suppression was observed at XTS concentrations of 9.0 and 18 µM (Fig. 3A). Furthermore, activity of cell migration molecules such as Cdc42, Rho A and Rac1 are significantly downgraded in group of XTS in 18.0 µM (Fig. 3B,C). Treatment with XTS at concentrations of 9.0 and 18.0 µM resulted in a significant decrease in the expression of MMP-9, MMP-13 and HMGB-1 proteins in RAFLS cells (Fig. 3D, E). These results strongly support the potential of XTS to inhibit cell migration and invasion processes by effectively reducing the expression of MMP-9, MMP-13 and HMGB-1 proteins in RAFLS cells.
XTS inhibited RAFLS cell migration.(A), XTS inhibited RAFLS cell migration and invasion at concentrations of 4.5, 9.0, and 18.0 μM. (B), The expression level of cell migration molecules Rac1, Rho A and Cdc42 in RAFLS cells after XTS treatment. (C), Gray value of Rac1, Rho A and Cdc42 in RAFLS cells after XTS treatment. (D),The expression level of MMP-13, MMP-9 and HMGB-1 proteins in RAFLS cells after XTS treatment. E, Gray value of MMP-13, MMP-9 and HMGB-1 proteins in RAFLS cells after XTS treatment.
XTS triggered RAFLS cell apoptosis
To assess the effect of XTS on inducing apoptosis in RAFLS cells, an apoptosis detection kit was utilized, and apoptosis rates were measured through flow cytometry. Compared to a positive drug Indo (5 µM), XTS treatment significantly induced apoptosis in RAFLS cells after 48 h (Fig. 4A and B). Further analysis of the total apoptotic cell ratio, encompassing both early and late stages of apoptosis, underscored XTS’s efficacy in prompting apoptosis in these cells. Additionally, the impact of XTS on pivotal proteins associated with the mitochondrial apoptosis pathway was investigated via western blot analysis. The analysis revealed that XTS treatment led to an upregulation of the pro-apoptotic proteins Caspase-8, Caspase-3, Bad, Bid, and Bax while the expression of the anti-apoptotic protein Bcl-2 and Bcl-xl were downregulated (Fig. 4C, D). These results strongly indicate the involvement of Caspase-8, Caspase-3, Bad, Bid, Bax, Bcl-2 and Bcl-xl in XTS-mediated apoptosis.
XTS triggered RAFLS cell apoptosis.(A), Flow cytometry analysis of RAFLS cells after treatment with XTS (0, 4.5, 9, 18 μM) for 48 h. (B), The apoptosis percentage of RAFLS cells after treatment with Indo (5 μM) and XTS (0, 4.5, 9, 18 μM). (C), Representative western blot assay of Caspase-3, Bax, and Bcl-2 proteins in RAFLS cells after treatment with XTS (0, 4.5, 9, 18 μM). (D), Gray value of Caspase-8, Caspase-3, Bad, Bid, Bax, Bcl-2 and Bcl-xl proteins in RAFLS cells after treatment with Indo (5 μM) and XTS (0, 4.5, 9, 18 μM).
Biocompatibility of XTS
To assess the in vivo safety of injectable XTS, a hemolysis test was performed. As depicted in Fig. 5A, the positive group exhibited a hemolysis rate of 100%. In contrast, the XTS samples at concentrations ranging from 0.21 to 3.36 mg/mL exhibited minimal hemolytic activity (hemolysis rate below 2%), indicating that XTS has excellent biocompatibility and high biosafety in vivo. Furthermore, the cytotoxicity test revealed that XTS exhibited minimal cytotoxicity on human fibroblast-like synoviocytes (HFLS), NIH-3T3, and RAW264.7 cells. MTX at 5 µM inhibited cell viability in the three cell types to less than 40%, whereas XTS maintained the cell viability of 100% even at 18 µM (Fig. S9, S10, and S11).
XTS inhibited paw swelling and synovial hyperplasia in adjuvant-induced arthritis (AIA) rats
Following AIA immunization, rats in the model group displayed evident paw swelling. However, when compared to the model group, intravenous injection of XTS (0.21, 0.42, and 0.84 mg/kg) significantly suppressed paw swelling in AIA rats via a dosage-dependent manner (Fig. 5B). Additionally, XTS exerted notable inhibition of RA severity, as evidenced by a reduction in the arthritis index starting from day 15 after complete Freunds’ adjuvant (CFA) injection (Fig. 5D). Starting from day 10 post-modeling, the model group displayed a notable reduction in weight gain compared to the normal group. Notably, treatment with XTS (0.21, 0.42, and 0.84 mg/kg) showed a gradual weight gain beginning on day 12 after CFA injection (Fig. 5C). These findings suggest that XTS could improve the life of AIA rats by elevating weight and associated symptoms.
Hematoxylin and eosin (H&E) staining revealed that AIA rats, in the absence of any treatment, exhibited severe synovial hyperplasia, infiltration of inflammatory cells and narrowing of the joint space. However, intriguingly, XTS treatment significantly ameliorated the pathological conditions in AIA rats. After treatment with XTS, a reduction in synovial tissue hyperplasia was observed, along with normal joint spacing. The joint surfaces between cartilage and bone appeared smooth, particularly in rats treated with high doses of XTS (Fig. 5E). Furthermore, micro-CT imaging demonstrated evident erosion of the articular cartilage and bone in AIA rats after 21 days of RA induction. However, XTS at dosages of 0.42 and 0.84 mg/kg effectively reversed this condition (Fig. 5F). These histopathological and radiological evaluation results further highlight the therapeutic potential of XTS in alleviating pathological and skeletal structural changes associated with arthritis.
The inhibitory effects of XTS on synovial hyperplasia and bone destruction in AIA rats. (A), The images and quantification analysis of hemolysis assay of different concentrations of XTS in red blood cells (RBCs). (B), The paw images of rats. (C), The body weight of rats. (D), Paw swelling thickness and arthritic score in rats. (E), H&E stain of rat paws. (F), Micro-CT of paw images in rats.
XTS inhibited the synovial proliferation of AIA rats by regulating apoptotic pathways
Dysregulations in pro-apoptotic and anti-apoptotic proteins in RAFLS cells from synovial tissue represent an important factor in RA progression. This disruption significantly contributes to the excessive synovial hyperplasia by inhibiting apoptosis of RAFLS cells, thereby playing an essential role. Consequently, there is a proliferation of RAFLS cells and their phenotypic transformation, further exacerbating the progression of RA.
To investigate whether XTS can regulate this balance, we compared the expression levels of key apoptotic proteins in different treatment groups of RAFLS cells. Western blot analysis revealed that treatment with XTS at a dosage of 0.21–0.84 mg/mL significantly increased the expression levels of Bax and Caspase-3 proteins, which are key regulators of apoptosis, with their upregulation suggesting a restoration of the apoptotic pathway. However, the expression level of Bcl-2 was reduced in rat paw tissues, further supporting the pro-apoptotic effects of XTS treatment (Fig. 6A−D). The findings indicate that treatment with XTS can modulate the Bcl-2/Bax/Caspase-3 signaling pathway, resulting in the suppression of synovial hyperplasia in AIA rats.
XTS reduced inflammatory factors in serum and paw tissue on AIA rat
In the pathological process of RA, inflammatory cytokines’ contributions cannot go unnoticed. To study the effect of XTS on the inflammatory factors in the paw tissue and serum of AIA rats, ELISA kits were used to measure the expression levels of tumor necrosis factor α (TNF-α), interleukin-2 (IL-2) and rheumatoid factor (RF) in the supernatant of the AIA rats` paw tissue and serum after XTS treatment. Figure 6E-G demonstrates that XTS reduces the expression of pro-inflammatory cytokines such as TNF-α, IL-2 and RF, suggesting anti-inflammatory effect of XTS.
XTS inhibited the synovial inflammatory proliferation by regulating apoptotic pathways and reduced inflammatory factors from AIA rat serum and paw tissue. (A), Representative western blot assay and grey value of Bax, Bcl-2, Caspase-3 proteins in rat paw tissue after treatment with various dosages of XTS. (B-D), Quantitative analysis of the gray values for Bcl-2, Bax, and Caspase-3 proteins. (E-G), Expression level of TNF-α, IL-2 and RF in serum and paw tissue on AIA rats
Discussion
Xuetong has been traditionally used in ethnomedicine for treating RA. Prior research has identified XTS, extracted from Xuetong stems, as possessing potent inhibitory effects on RAFLS cell proliferation4,5,6,7. However, the extraction efficiency of XTS from Xuetong was notably low at 0.036%8. To enhance the yield of XTS, the new extraction technique was conduct in this research, and this improved method significantly increased the extraction rate of XTS from Xuetong from 0.036 to 0.1986%. HPLC analysis confirmed the high purity of XTS at 96.8%, laying a solid foundation for further pharmacological exploration of XTS.
RA is classified by the World Health Organization (WHO) as one of the most debilitating diseases globally23. Its complex pathogenesis primarily involves chronic inflammation, synovial hyperplasia, and bone erosion24. However, the common treatments for RA, such as disease-modifying anti-rheumatic drugs (DMARDs) and NSAIDs, often have significant adverse effects that limit their clinical use based on the patient’s health condition25,26. This underscores the urgent need to develop more effective and safer therapeutic alternatives for the prevention and treatment of RA.
RAFLS cells, located within the synovial membrane of affected joints, are central to the pathogenesis of RA. These cells work to maintain the chronic inflammation and tissue degradation characteristic of RA. They secrete pro-inflammatory cytokines, enzymes, and other substances that facilitate the recruitment and activation of immune cells, leading to joint deterioration and discomfort. Furthermore, RAFLS cells shows the ability to invasively degrade cartilage and bone, accelerating the development of the pannus and joint damage27,28. The aberrant proliferation and diminished apoptosis of RAFLS cells underlie the pathogenesis of RA. The abnormal proliferation of synovial tissue is closely associated with the aberrant invasion and migration of RAFLS cells. Inhibiting the migration and invasive activities of RAFLS cells may contribute to preventing synovial hyperplasia in RA and protecting against joint damage27. Reports have identified an increase in anti-apoptotic molecules and a decrease in pro-apoptotic molecules within RAFLS cells in individuals with RA29. Thus, RAFLS cells exhibit a proliferative behavior similar to that of tumor cells30. Targeting and modulating the activity of RAFLS cells is a key therapeutic strategy for controlling RA and slowing its progression.
This study revealed that XTS effectively inhibits the proliferation of RAFLS cells. The IC50 value for XTS against RAFLS cell proliferation was determined to be 4.68 ± 0.65 µM. This IC50 value is substantially lower than the 62.87 ± 5.79 µM observed for RAFLS inhibition by SIN, demonstrating XTS’s superior efficacy in curtailing RAFLS proliferation. Furthermore, the IC50 values of MTX and Indo inhibiting RAFLS cell proliferation were 3.81 ± 0.77 µM and 2.95 ± 0.63 µM, respectively. At a concentration of 5 µM, MTX and Indo reduced the viability of HFLS to 28.8% and 52.5%, respectively, whereas HFLS cells treated with 18 µM XTS maintained 98% viability. While MTX and Indo are effective in inhibiting RAFLS proliferation, they exhibit considerable cytotoxicity towards HFLS compared to XTS. In contrast, XTS not only effectively inhibits the proliferation of RAFLS but also demonstrates minimal toxicity towards normal HFLS cells. Moreover, XTS promoted apoptosis in RAFLS cells, as determined through flow cytometry analysis. After XTS treatment, western blot analysis demonstrated a marked decrease in the protein expression of HMGB-1, MMP-9 and MMP-13. Furthermore, the upregulation of caspase-8, caspase-3, Bid, Bad and Bax, coupled with the downregulation of Bcl-2 and Bcl-xl, provided evidence supporting the inhibitory effects of XTS on RAFLS cell migration and the promotion of apoptosis31.
In vivo experiments demonstrated that XTS significantly reduced joint swelling in AIA rats32. H&E analysis showed that XTS treatment decreased pannus formation, fibrous tissue alterations, and bone erosion. Micro-CT imaging revealed that bone degradation in AIA rats was markedly ameliorated by XTS intervention. These results suggested that XTS effectively inhibits bone destruction and improves pathological abnormalities within joints on AIA rats. Consistent with these observations, western blot analysis of rats paw tissue revealed elevated levels of caspase-3 and Bax proteins, along with decreased levels of Bcl-2 protein, indicating that XTS may suppress synovial hyperplasia in RA by modulating apoptosis-related proteins within the apoptotic pathway.
Furthermore, these findings suggested that the potential of XTS to inhibit the migration and invasion of RAFLS cells by targeting the HMGB-1/MMP-9/MMP-13 pathway. XTS can activate the Bcl-2/Bax/Caspase-3 signaling pathway, regulate the expression of apoptotic proteins and balance between pro-apoptotic and anti-apoptotic factors, thus effectively suppresse synovial hyperplasia in RA. It is worth noting that this study primarily focuses on the inhibitory effect of XTS on synovial hyperplasia in RA, while the exploration of its additional protective effects is limited. For example, its impact on bone protection and inhibition of angiogenesis in RA treatment, as well as its potential to suppress the incidence of arthritis, have not been investigated in detail in this study. Therefore, future research could explore the ability of XTS to inhibit the incidence of RA and its more detailed mechanisms of action by conducting prophylactic administration and measuring indicators related to bone destruction and angiogenesis. Furthermore, it is necessary to further evaluate its clinical feasibility to fully understand the therapeutic potential of XTS for RA.
Conclusion
In summary, this study successfully optimized the extraction and separation efficiency of XTS in Xuetong. It significantly improved the extraction yield of XTS. The results demonstrate that XTS effectively inhibits the proliferation and migration of RAFLS cells while reducing the production of HMGB-1, MMP-9 and MMP-13. The activation of the apoptotic pathway in RAFLS cells plays a crucial role in XTS’s ability to suppress synovial hyperplasia in RA. Overall, these findings highlight the potential of XTS as a valuable option for the clinical treatment of synovial hyperplasia in RA.
Experimental sections
Materials and reagents
The Kadsura heteroclita dried stems were collected from Shimen City, Hunan Province, China. The collection of Kadsura heteroclita dried stems has been approved by the Agriculture and Rural Bureau of Shimen County, Changde City, Hunan Province and Hunan University of Chinese Medicine. The permissions for the collection of Kadsura heteroclita dried stems were obtained from the Agriculture and Rural Bureau of Shimen County, Changde City, Hunan Province and Hunan University of Chinese Medicine. The collection process of Kadsura heteroclita stem complies with the Plant Protection Law of the People’s Republic of China, and conforms to the standards of agricultural and rural plant collection of Shimen County. The Experiment complied with relevant institutional, national, and international guidelines and legislation. The plant materials of Kadsura heteroclita are stored in TCM and Ethnomedicine Innovation & Development International Laboratory, Innovative Materia Medica Research Institute, School of Pharmacy, Hunan University of Chinese Medicine, Changsha, 410,208, China. The specimen number is 2,020,081,501. The plant identification and the standard of XTS was conducted by Prof. Wang Wei. The CCK-8 kit, Apoptosis assay kit and protein extraction kit were purchased from SEVEN Co., Ltd (Beijing, China). Liquid paraffin (MKCM5905) and LPS (00497693) were obtained from Sigma-Aldrich Co., Ltd (MO, USA). Heat-inactivated Mycobacterium tuberculosis H37Ra (2216421) was obtained from (Becton, Dickinson and Company, Sparks, MD 21152, USA). The antibody of Rac1 (HA722225) was obtained from HuaBio Company (Hangzhou, China). The antibodies used, including β-actin (AF7018), Cdc42 (AF7828), RhoA (AF6352) MMP-9 (AF5228), MMP-13(AF5355), Bax (AF0120), Bid (DF6016), Bad (AF6471), Bcl-2 (AF6139), Bcl-xl (AF6414), Caspase-3 (AF7022), Caspase-8 (AF6442) and HMGB-1 (AF7020) were obtained from Affinity Co., Ltd (Jiangsu, China).
Extraction and isolation
It has been reported that 1.08 g XTS can be extracted and isolated from 3 kg Xuetong stems8. The optimized extraction process of XTS in this study is as follows: The air-dried Xuetong stem (13.2 kg) was extracted thrice using 90% ethyl alcohol (EtOH), each time for 1.5 h, with the assistance of ultrasound. Subsequently, the extracted solvents were evaporated under reduced pressure to yield a crude EtOH extract (3.2 kg). The crude extract was then suspended in water and sequentially partitioned with dichloromethane (DCM) and ethyl acetate (EtOAc), resulting in DCM-soluble and EtOAc-soluble fractions. The DCM fraction was further purified using silica gel column chromatography eluted with a PE-EtOAc gradient (from 1:0 to 0:1), producing six fractions (Fr. A-Fr. F). Subfraction Fr. B underwent further separation using silica gel column chromatography, Sephadex LH-20 gel chromatography, and recrystallization to yield XTS (26.22 g).
Structure characterization of XTS
The XTS spectra, including1H13, C1, H-1H COSY, HSQC, and HMBC, were acquired using a Bruker AV-600 spectrometer equipped with a single NMR probe operating at 600 MHz for1H and 150 MHz for13C in CDCl3. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) data were collected on a Waters UHPLC-H-CLASS/XEVO G2-XS Q-TOF mass spectrometer. UV-visible (UV-vis) spectral analysis was conducted using a SHIMADZU UV-vis spectrophotometer.
Qualitative and quantitative analysis
To perform a qualitative and quantitative assessment of the enriched XTS, we employed high-performance liquid chromatography (HPLC) to extract, identify, and quantify XTS. To prepare for analysis, a 2.5 mg sample of XTS was dissolved in 1 mL of DMSO solution. This solution was then further diluted 100-fold with methanol. The HPLC chromatogram was then generated to analyze XTS. The analysis was carried out utilizing an Agilent 1260 system featuring a diode array detector. A suitable Agilent TC-C18 column (5 μm, 150 mm × 4.6 mm) was selected and operated with a mobile phase consisting of an aqueous solution containing 0.1% phosphoric acid and 100% acetonitrile. A gradient elution protocol was followed: over 0–30 min, the acetonitrile concentration was adjusted from 65 to 85%. The optimized parameters consisted of a flow rate of 1 mL/min, a column temperature of 25 °C, an injection volume of 5 µL, and detection at a wavelength of 210 nm. The content of XTS was quantified employing the external standard method based on the standard concentration.
Cell culture
RAFLS and HFLS cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). RAW264.7 cells and NIH-3T3 cells were obtained from Procell Life Science & Technology Co., Ltd (Wuhan, China). These cells were cultured in DMEM/F-12 = 1:1 medium (RAFLS and HFLS cells) or DMEM medium (RAW264.7 cells and NIH-3T3 cells), supplemented with 10% FBS and 1% penicillin/streptomycin.
IC50 assay and cell viability assay
RAFLS cells were seeded in a 96-well plate at a density of 1.0 × 104 cells/well and can proliferate logarithmically. The cells were cultured in DMEM/F-12 = 1:1 medium supplemented with 1% FBS and 1% penicillin/streptomycin and treated with varying concentrations of XTS, SIN, Indo, and MTX (0, 2.5, 5, 7.5, 10, 15, 20 µM) for 48 h. Following that, the aforementioned medium was substituted with a medium supplemented with 10% CCK-8 solution. After a 30-minute incubation period, the optical density (OD) at 450 nm for each well was quantified using a microplate reader (Thermo Multiskan Sky, Waltham, MA, United States). Cell viability was determined using the following calculation:
Cell Viability = OD450 nm/sample/ OD450 nm/control× 100%.
RAFLS cells were treated with XTS at concentrations (0, 4.5, 9, 18 µM) or Indo (10 µM) for 48 h. Cell viability was assessed using the previously described method.
The cytotoxic effects of XTS were further evaluated by examining its impact on the viability of HFLS, NIH-3T3, and RAW264.7 cell lines. These cells were cultured with 1% FBS and 1% penicillin/streptomycin in corresponding medium, treated with different concentrations of XTS (0, 4.5, 9, 18 µM) or Indo (5.0 µM), MTX (5.0 µM), and SIN (5.0 µM) for 48 h. Cell viability was determined following the protocol mentioned above.
Wound healing assay
After treatment with various concentrations of XTS (0, 4.5, 9, 18 µM) for 48 h, a sterile plastic scraper was used to create a wound in the center of the RAFLS cell monolayer. The migratory capacity of RAFLS cells was assessed by measuring the wound area at 0, 12, 24, and 48 h post-injury. The percentage of the wound closure was determined by comparing the healed area at each time point to the initial wound area. The rate of cell migration was calculated using the formula:
Cell migration rate (%) = (Original wound distance-Residual wound distance) / Original wound distance × 100.
Apoptosis assay
Following a 48-hour treatment of RAFLS cells with various concentrations of XTS (0, 4.5, 9, 18 µM) and Indo (5 µM), the cells were collected into 1.5 mL Eppendorf (EP) tubes. Subsequently, they underwent three washes with pre-chilled phosphate-buffered saline (PBS), with each wash involving centrifugation at 800 rpm for 5 min at 4 °C. After the final wash, the cells were resuspended in 500 µL of 1× Binding Buffer. To each cell suspension, 5 µL of Annexin V-FITC and 5 µL of Propidium Iodide (PI) were added, and the mixture was incubated in the dark for 10 min. Flow cytometry was then employed to detect apoptotic cells, and the proportions (%) were measured using FlowJo 7.6 software.
Cell protein analysis by Western blot
RAFLS cells were seeded in a 6-well plate at a density of 1.0 × 106 cells/well and can proliferate logarithmically. All cells were divided into control and XTS low, medium and high dose groups (4.5, 9, 18 µM). In addition to the normal group, all groups were first induced by lipopolysaccharides (LPS, 100 ng/mL) for 24 h. Following 48-hour treatment with various concentrations of XTS (0, 4.5, 9, 18 µM), RAFLS cells were lysed in protein extract buffer on ice. The cells, comprising both the supernatant and adherent fractions, were collected through centrifugation at 800 rpm for 5 min. The protein concentrations were determined using the BCA assay. Subsequently, the proteins were separated by 10-12.5% SDS-PAGE gels and transferred to PVDF membranes. The membranes were then blocked with rapid sealing fluid at 25 °C for 10 min. After blocking, the membranes were incubated overnight at 4 °C with antibodies against Cdc42, Rho A, Rac1, MMP-9, MMP-13 and HMGB-1, which were diluted in TBST at a 1:1600 ratio. Following the incubation with secondary antibodies, the protein bands were visualized using enhanced chemiluminescence and quantified using Image J software.
RAFLS cells were seeded in a 6-well plate at a density of 1.0 × 106 cells/well and can proliferate logarithmically. All cells were divided into normal and model, Indo (5 µM) and XTS (4.5, 9, 18 µM) groups. Following 48-hour treatment with Indo (5 µM) and various concentrations of XTS (0, 4.5, 9, 18 µM), RAFLS cells were lysed in protein extract buffer on ice. The cells, comprising both the supernatant and adherent fractions, were collected through centrifugation at 800 rpm for 5 min. The protein concentrations were determined using the BCA assay. Subsequently, the proteins were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were then blocked with rapid sealing fluid at 25 °C for 10 min. After blocking, the membranes were incubated overnight at 4 °C with antibodies against Bcl-2, Bcl-xl, Bax, Bad, Bid, Caspase-3 and Caspase-8 which were diluted in TBST at a 1:1600 ratio. Following the incubation with secondary antibodies, the protein bands were visualized using enhanced chemiluminescence and quantified using Image J software.
Animals
Male SD rats weighing 70–90 g (Hunan SJA Animal Co., Ltd) were maintained under SPF conditions. All experiments were carried out in compliance with the ARRIVE guidelines. All methods were performed in accordance with the relevant guidelines and regulations. The animals were accommodated in a pathogen-free facility, with ambient temperature maintained at 23 ± 2 °C, and provided with ad libitum access to feed and water. The animal study was granted ethical approval by the ethics committee of Hunan University of Chinese Medicine (permit number: LL202103160001).
Hemolysis assay
Blood samples were obtained from the orbital venous plexus of rats, and thereafter, RBCs were isolated and washed with PBS buffer. A 40 µL aliquot of 2.5% RBCs suspended in PBS was mixed with 460 µL of XTS solutions at various concentrations (0.21, 0.42, 0.84, 1.68, 3.36 mg/mL), followed by incubation at 37 °C for 4 h. Concurrently, 40 µL of 2.5% RBCs were diluted in 460 µL of ultra-pure water to serve as a positive control. After incubation, samples were centrifuged at 3000 rpm for 5 min at 4 °C. A UV-vis spectrophotometer was used to check the absorbance of the supernatant 540 nm. Microscopic examination was conducted to assess the morphological alterations in RBCs. The percentage of hemolysis was determined using the following formula:
Hemolysis (%) = (I / I0) × 100%.
In the formula, “I” represents the absorbance of the supernatant after the addition of XTS at different concentrations to the red blood cell suspension, while “I0” represents the absorbance of complete hemolysis in pure water.
Induction of RA models and drug administration
To induce arthritis in Sprague Dawley (SD) rats, an AIA model was established by injecting 150 µL of CFA emulsion subcutaneously at the base of the tail. The CFA emulsion contained 2 mg/mL of heat-inactivated Mycobacterium tuberculosis (Mtb). The rats selected for this model weighed between 80 and 100 g. Following the induction of AIA, the rats were divided into several groups, which included a normal group, a model group (Model), an Indo (1 mg/kg) treatment group, and three groups receiving varying doses of XTS (0.21 mg/kg, 0.42 mg/kg, 0.84 mg/kg). From days 10 to 30 post-AIA immunization, all groups except the normal control were administered their respective treatments intravenously every 2 days. The XTS-treated groups received varying doses of XTS (0.21 mg/kg, 0.42 mg/kg, 0.84 mg/kg), the positive group received 1.0 mg/kg of Indo, while the model group injected an equivalent volume of saline.
Evaluation of arthritis index and radiologic analysis
After the AIA immunization, the body weight and hind paw swelling of each rat were recorded every three days using an electronic balance and a Vernier caliper, respectively. The degree of paw swelling was evaluated and scored based on the observed extent of swelling during these measurements. After a treatment period of 30 days, all rats were deeply anesthesia by 3% isoflurane and euthanized. Then their hind paws were severed using bone scissors for analysis. The excised paws were subjected to Micro-CT analysis (PerkinElmer-Caliper LS Quantum FX Demo, USA) to assess bone destruction.
Histopathological analysis
After 30 days of XTS treatment, the hind paws of rats were fixed in 4% paraformaldehyde for one week. This was followed by decalcification with 10% EDTA for 1–3 months. The decalcified hind paws were then embedded in hot melted paraffin wax, allowed to cool and solidify, and subsequently sectioned into thin slices. To visualize the tissue morphology, the sections were stained with H&E and examined under a light microscope.
Western blot analysis
Paw tissues were snap-frozen in liquid nitrogen to ensure their integrity before being pulverized. Proteins were then extracted using a lysis buffer supplemented with a protease inhibitor. The concentration of protein in each lysate was assessed using the BCA protein assay kit. Equal quantities of protein were loaded into the wells of an SDS-PAGE gel for electrophoresis. Following electrophoresis, the separated proteins were transferred onto a PVDF membrane. The PVDF membrane was blocked with rapid sealing fluid at 25 °C for 10 min, and then incubated overnight at 4 °C with the target antibody. The washed membrane was incubated with a secondary antibody for 1.5 h. The protein bands were visualized using an imaging system.
Inflammatory cytokine analysis about rats’ paw tissue and serum
After 30 days of XTS treatment, all rats were deeply anesthesia by 3% isoflurane and euthanized. Then their hind paws tissue and blood samples were collected. 1 g of hind paws tissue in 9 mL of PBS (pH = 7.4) was fully homogenized with a homogenizer. Centrifuge at 4 °C and 5,000 rpm for 5 min, and carefully collect the supernatants. Divide one to be tested and freeze the rest for later use. The collected blood was centrifuged at 4 °C and 3,500 rpm for 15 min. The upper serum was obtained. The concentration of factors in supernatants of paw tissue and serum, including TNF-α, IL-2 and RF, was determined using the ELISA kits.
Statistical analysis
The data are expressed as mean ± standard deviation (SD) and are derived from a minimum of three independent experiments. Statistical analyses were performed using SPSS version 22.0. Student’s t-test was employed for single comparisons, while one-way ANOVA was used for multiple comparisons. Statistical significance was defined as P < 0.05.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Wang, M. et al. A review of the phytochemistry and pharmacology of Kadsura Heteroclita, an important plant in Tujia ethnomedicine. J. Ethnopharmacol. 268, 113567 (2021).
Yu, H. et al. Kadsura heteroclita stem suppresses the onset and progression of adjuvant-induced arthritis in rats. Phytomedicine 58, 152876 (2019).
Yu, H. et al. Analgesic and anti-inflammatory effects and molecular mechanisms of Kadsura heteroclita stems, an anti-arthritic Chinese Tujia ethnomedicinal herb. J. Ethnopharmacol. 238, 111902 (2019).
Shehla, N. et al. Xuetonglactones A–F: highly oxidized lanostane and cycloartane triterpenoids from Kadsura Heteroclita Roxb. Craib. Front. Chem. 7, 935 (2020).
Yu, H. et al. Biomimetic Hybrid membrane-coated Xuetongsu assisted with laser irradiation for efficient rheumatoid arthritis therapy. ACS Nano 16, 502–521 (2022).
Wang, M. et al. Anti-RAFLS triterpenoids and Hepatoprotective Lignans from the leaves of Tujia Ethnomedicine Kadsura heteroclita (Xuetong). Front. Chem. 10, 878811 (2022).
Liu, R. et al. Pharmacokinetics, bioavailability, excretion, and metabolic analysis of Schisanlactone E, a bioactive ingredient from Kadsura heteroclita (Roxb) Craib, in rats by UHPLC–MS/MS and UHPLC-Q-Orbitrap HRMS. J. Pharm. Biomed. Anal. 177, 112875 (2020).
Wang, W. et al. New triterpenoids from Kadsura heteroclita and their cytotoxic activity. Planta Med. 72, 450–457 (2006).
Alivernini, S., Firestein, G. S. & McInnes, I. B. The pathogenesis of rheumatoid arthritis. Immunity https://doi.org/10.1016/j.immuni.2022.11.009 (2022).
Chu, C. Q. Fibroblasts in rheumatoid arthritis. N Engl. J. Med. 3 (2020).
Komatsu, N. & Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis — immune cell–fibroblast–bone interactions. Nat. Rev. Rheumatol. 18, 415–429 (2022).
Chau, M. et al. The synovial microenvironment suppresses chondrocyte hypertrophy and promotes articular chondrocyte differentiation. Npj Regen Med. 7, 51 (2022).
José Alcaraz, M. New potential therapeutic approaches targeting synovial fibroblasts in rheumatoid arthritis. Biochem. Pharmacol. 194, 114815 (2021).
Bustamante, M. F., Garcia-Carbonell, R., Whisenant, K. D. & Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 19, 110 (2017).
Colombo, F. et al. Targeting CD34 + cells of the inflamed synovial endothelium by guided nanoparticles for the treatment of rheumatoid arthritis. J. Autoimmun. 103, 102288 (2019).
Guo, H. F. et al. High mobility group box 1 induces synoviocyte proliferation in rheumatoid arthritis by activating the signal transducer and activator transcription signal pathway. Clin. Exp. Med. 11, 65–74 (2011).
Liu, Z. et al. Sinensetin attenuates IL-1β-induced cartilage damage and ameliorates osteoarthritis by regulating SERPINA3. Food Funct. 13, 9973–9987 (2022).
Deng, C. et al. Targeted apoptosis of macrophages and osteoclasts in arthritic joints is effective against advanced inflammatory arthritis. Nat. Commun. 12, 2174 (2021).
Zhang, W. et al. Emerging nanotherapeutics alleviating rheumatoid arthritis by readjusting the seeds and soils. J. Controlled Release 345, 851–879 (2022).
Moltó, A. & Dougados, M. Novel DMARD monotherapy in rheumatoid arthritis. Lancet 393, 2277–2278 (2019).
McInnes, I. B. & Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389, 2328–2337 (2017).
Friedman, B. & Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine 86, 301–307 (2019).
Wang, J. Y. et al. Comparative studies of different extracts from Eucommia ulmoides Oliv. Against Rheumatoid Arthritis in CIA rats. Evid. Based Complement. Alternat Med. https://doi.org/10.1155/2018/7379893 (2018).
Zhao, H., Lu, A. & He, X. Roles of MicroRNAs in Bone Destruction of Rheumatoid Arthritis. Front. Cell. Dev. Biol. https://doi.org/10.3389/fcell.2020.600867 (2020).
Roodenrijs, N. M. T. et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology 60, 3778–3788 (2021).
Pelechas, E. & Drosos, A. A. State-of-the-art glucocorticoid-targeted drug therapies for the treatment of rheumatoid arthritis. Expert Opin. Pharmacother 23, 703–711 (2022).
Xu, S. et al. Piperlongumine inhibits the proliferation, migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflamm. Res. 67, 233–243 (2018).
Vanniasinghe, A. S. et al. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin. Immunol. 151, 43–54 (2014).
Zhang, Q. et al. Guizhi-Shaoyao-Zhimu decoction possesses anti-arthritic effects on type II collagen-induced arthritis in rats via suppression of inflammatory reactions, inhibition of invasion & migration and induction of apoptosis in synovial fibroblasts. Biomed. Pharmacother 118, 109367 (2019).
Lin, J. et al. Iguratimod Inhibits the Aggressiveness of Rheumatoid Fibroblast-Like Synoviocytes. J. Immunol. Res. 1–11 (2019). (2019).
Zhang, C. et al. Extracellular HMGB-1 activates inflammatory signaling in tendon cells and tissues. Ther. Adv. Chronic Dis. 11, 204062232095642 (2020).
McInnes, I. et al. AB0160 effects of TOFACITINIB (CP-690,550) on lipid biomarkers in rat adjuvant-induced arthritis (AIA) model and in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 71, 646.13–646 (2013).
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (82204766, 82174078, 82304878); Natural Science Foundation of Hunan province (2023JJ40490, 2022JJ40318); Changjiang Scholars Program in Ministry Education, People’s Republic of China (T2019133); The Science and Technology Innovation Talents Program of Hunan Province(2024RC3201); Xiaohe Sci-Tech Talents Special Funding under Hunan Provincial Sci-Tech Talents Sponsorship Program (2023TJ-X71); Research Project of Hunan Administration of Traditional Chinese Medicine (B2023055); Changsha Outstanding innovative youth training program (kq2306021); Outstanding Youth Program of Hunan University of Chinese Medicine (202202); Open Foundation Project of Hunan International Joint Laboratory of Traditional Chinese Medicine (2022GJSYS02); Scientific Research Fund of Hunan University of Chinese Medicine (2021XJJJ006); Research Learning and Innovation Experimental Program for College Students of Hunan Province (S202210541050); Undergraduate Research and Innovation Foundation of Hunan University of Chinese Medicine (2023BKS097).
Author information
Authors and Affiliations
Contributions
Yasi Deng: Writing – original draft, Investigation, Data curation, Methodology. Hao Zheng: Software, Data curation. Shiqi Liu: Software, Data curation. Ying Deng: Software, Methodology. Yuxin Chen: Methodology. Mengyun Wang: Methodology. Ling Liang: Software, Methodology. Qingling Xie: Methodology, Validation. Yupei Yang: Methodology. Bin Li: Supervision, Resource. Juan Huang: Resource. Hanwen Yuan: Funding acquisition. Huanghe Yu: Supervision, Funding acquisition, Writing – review & editing. Wei Wang: Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The collection procedure of Kadsura heteroclita stems adheres to the guidelines set by the Plant Protection Law of the People’s Republic of China. It also conforms to the agricultural and rural plant collection standards of Shimen County and has received approval from the Agriculture and Rural Bureau of Shimen County, Changde City Hunan Province, as well as Hunan University of Chinese Medicine. The plant materials of Kadsura heteroclita are securely stored at the TCM and Ethnomedicine Innovation & Development International Laboratory, Innovative Materia Medica Research School of Pharmacy, Hunan University of Chinese Medicine, located in Institute. Changsha, China (Postal Code: 410208). These plant samples are permitted for experimental research. The study is reported in accordance with ARRIVE guidelines. The animal study was granted ethical approval by the ethics committee of Hunan University of Chinese Medicine (permit number: LL202103160001).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deng, Y., Zheng, H., Liu, S. et al. Effective extraction of Xuetongsu and its role in preventing RA synovial hyperplasia by targeting synovial cell migration and apoptosis. Sci Rep 14, 23345 (2024). https://doi.org/10.1038/s41598-024-73471-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73471-z