Abstract
This study explored the effect of geographical and floral origins on the antioxidant activities of Saudi honey samples related to their content of short peptides originated from honeybee proteins. The studied antioxidants were the total protein concentration, catalase activity, phenolic acids and flavonoids. The antioxidant activity assays included were the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, the ferric reducing antioxidant power (FRAP) assay and Ascorbic acid Equivalent Antioxidant Capacity (AEAC). The studied honey samples were obtained from the southwestern region of Saudi Arabia, namely Asir (65) and Jazan (25). The floral origins of the honey samples were Acacia (51), Ziziphus (4) and polyfloral (35). The LC/MS technique was used to detect the short peptides and the mascot database was used to identify the short peptides, their precursor proteins and the protease enzymes that produce them. Jazan honey was characterized by high number of short peptides. The short peptides were originated from honeybee proteins by the action of proteases from the honeybees and bacteria. The antioxidant activity of the honey samples increase with the increase of their content of short peptides and proteins. The amino acids type and sequence of the short peptides qualify them to act as antioxidant, antimicrobial, anti-diabetic, anti-hypertension, immunomodulatory and cholesterol lowering peptides.
Similar content being viewed by others
Introduction
Honey is one of the honeybee natural products with sweet nature. To produce honey, the honeybees depend on natural feed or supplemental nutrition. The natural feed of honeybees include, the nectar of the plants, secretions of living parts of the plants or excretion of plant sucking insects (Hemiptera). Supplemental food of honeybees are made from mixtures of different diets including, Yeasts, egg white and yolk, milk (whole and skimmed), corn, soybean and/or wheat flour, fish, linseed and/or peanut meal and potato or sweet potato powder. The honeybees supplemental meals contains variable constituents such as the pollens, sucrose syrup, invert sugar syrup, proteins, amino acids, lipids and vitamins1,2,3. Honey is classified to two classes depending on the natural food source of honeybees; nectar or blossom honey and honeydew honey. Nectar honey is produced by the honeybees depending on the plants nectar as feed and it is rich in pollens. Honeydew honey is produced when honeybees depend on the secretions of living parts of the plants or on the excretions of Hemiptera insects. The honey is composed of major and minor components. The major components are the sugars and water while the minor components include, acids, proteins, amino acids, enzymes, flavonoids, phenolic acids, minerals and vitamins1,4,5. Beside the nutritional value of honey, it is used in the treatment and control of throat infection, bronchial asthma, eye diseases, hepatitis, constipation, eczema and healing of ulcers, wounds and burns. The minor components of honey act as antioxidants, antimicrobials, anticancer, anti-metastasis and anti-inflammatory6.
Proteins are polymers of L-α-amino acids bound by peptide bonds. The concentration of proteins in honey is very small (0.2–0.5%) but with vital biological functions. The proteins of honey are originated from the honeybees, pollens or the nectar of the plants. The dominant proteins in honey are the Major Royal Jelly Proteins (MRJP). The MRJP proteins are classified to nine groups (MRJP-1 to MRJP-9) with antioxidant and antimicrobial activities7. Other proteins found in honey are the definsin-1that contributes to the antibacterial activity of honey and enzymes such as the diastase, invertase, glucose oxidase, catalase and the serine proteases8,9.
Short peptides (oligopeptides) are small proteins composed of small number of L-α-amino acids (less than 30 or 50 amino acids) bound by peptide bonds. Short peptides containing up to seven amino acids are famous as ultra-short peptides. The short peptides are introduced as promising molecules in therapeutics and medicine. They have antioxidant, antibacterial, antiviral, anti-hypertension and anti-formation of renal stones9,10. The short peptides are formed in honey because the honey contains digestive enzymes from the honeybees gut such as the trypsin and pepsin. The digestive enzymes are endopeptidases which degrade the honey proteins to produce ultra-short peptides11. Short peptides in honey samples from Asir region were previously reported in a pilot study published by Alaerjani et al. (2021)11.
Phenolic acids are carboxylic acids arisen from benezoic and cinnamic acids basic structure. They are of two types; hydroxybenzoic acid hydroxycinnamic acids. Examples of hydroxybezoic acid like gallic, salicylic acids, vanillic acid, syringic acid and protocatechuic acid while hydroxycinnamic acids include cinnamic, rosmarinic, caffeic, p-coumaric, ferulic, and chlorogenic acid12. Phenolic acids act as antioxidants and there is inverse relationship between them and cancer, cardiovascular diseases, hypertension, metabolic syndrome, fatty liver (non-alcoholic) and type-2 diabetes milletus12,13. Rich sources of phenolic acids include leaf of vegetables, skin of fruits and seeds. Regarding the content of phenolic acids in honey, it contains, gallic acid, protocatechuic acid, ellagic acid, 2,3,4-trihydroxybenzoic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, caffeic acid, chlorogenic, p-coumaric acid, genistic acid, caffeic acid and ferulic acid14,15,16.
Flavonoids are polyphenols containing skeleton nucleus of C6-C3-C6 structure or phenyl-ether ring-phenyl. They are classified to seven classes according to the chemical modification on the basic structure of its skeleton. The seven classes are flavones, flavonols, flavanols, flavanones, isoflavones, anthocyanins and chalcones. Rich sources of flavonoids include vegetables, fruits and grains. Flavonoids are synthesized from phenylalanine and malonylCoA and are a family of more than 8000 members. Flavonoids are known to act as antioxidant molecules and to be associated with decreased rates of metabolic diseases and decreased complications of cardiovascular diseases17,18. Flavonoids which are reported to be present in honey include Apigenin, Catechin, Chrysin, Galangin, Genistein, Isorhamnetin, Kaempferol, Luteolin, Myricetin, Pinobanksin, Pinocembrin, Quercetin and Rutin16,19.
Antioxidant activity of honey is its ability to remove oxidant molecules which are of radical and non-radical nature. Oxidant molecules are classified to reactive oxygen species, reactive nitrogen species, reactive chlorine species and reactive sulfur species. All of the reactive species have radical and non-radical molecules20,21. Examples of antioxidant molecules include the phenolic acids, flavonoids, proteins and short peptides, amino acids, vitamins and enzymes21. Honey contains variable antioxidant molecules such as the phenolic acids, flavonoids, organic acids, vitamins, trace elements, amino acids, enzymes (catalase), proteins, short peptides and products of Maillard reaction9,11,14,15,16,19,22. The antioxidant activity of honey is majorly due to their content of phenolic acids and flavonoids because the concentration of the other antioxidant compounds is trace23.
The physicochemical properties, chemical composition and the biological activities of honey are affected by variable factors including the plant origin and its related factors such as the soil composition, geographical or climate origin, honeybee associated factors including honeybee species, health and nutrition beside the honey associated factors such as the honey harvest, processing, storage, transport and the effect of the honey constituents on each other24,25,26.
This article was designed to investigate the antioxidant activity of honey samples from Asir and Jazan regions at the southwestern part of Saudi Arabia related to their short peptides originated from honeybees and the concentration of total proteins, phenolic acids, flavonoids and the activity of catalase enzyme.
This study was carried out because there is rare information about the antioxidant activities, short peptides, proteins concentration and their types in honey samples from Asir and Jazan regions. The uniqueness of this study is the investigation of presence of short peptides, their precursor proteins and the enzymes that produce them. The findings of this study would potentiate the nutritional and medicinal values of Asir and Jazan honey samples and may suggest markers for them.
Material and methods
Study area and sample collection
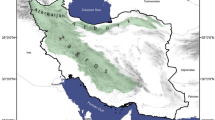
The region of Asir is located in the southwestern Saudi Arabia at 41–45°E longitude and 17–21°N latitude (Fig. 1). It is found on a highland that contains the Saudi’s highest mountains. The altitude of Asir region rises from the sea level up to 3,000 m et al.-souda Mountains. The mountains of Asir region has a foggy climate which leads to the dominance of coniferous trees and dense forests. The temperature in this region is low (12–35 °C) and the rainfall is more (300 mm mean amount annually) compared to the other regions of Saudi Arabia27,28.
Jazan is a southwestern region of Saudi Arabia located at 42° 33′ 4″ E longitude and 16° 53′ 21″ N latitude. It is bordered by Asir region on the North and the Red Sea on the West. The Jazan region is the richest agricultural part of Saudi Arabia because it contains different environments such as the agricultural lands, mountains, valleys, deserts and semi deserts. It has a 300 km red sea coast and over 100 islands. The Jazan region is well known by its hot desert climate with an average temperature of 30 °C and a rainfall range between 70 and 270 mm annually27,28,29.
The samples were collected, directly, from the bee farms and in their hives to avoid adulteration. The honey was extracted by pressing the hives at room temperature.
Confirmation of floral origin
The method of Louveaux et al. (1978)30 was followed to determine the floral origin of the studied honey samples was. Three to seven milliliters (ml) of the honey samples were transferred in a 15 ml falcon tube and diluted with distilled water to reach a total volume of 15 ml. The diluted honey samples were centrifuged for about 30 min at 6000 round per minutes (rpm) and the top layer was removed by decantation. The pellet was mounted on a microscope slide and the pollens were investigated under a microscope. The honey was considered as monofloral if one type of pollens is dominant (> 45%). If there was no dominant pollen, the honey was considered as polyfloral. The percentage of the pollen in the honey sample was determined following the bellow equation
Determination of moisture percentages
The moisture and total sugars percentages of the honey samples were determined using the refractometer according to the methods of international honey commission31.
Determination of pH and acidity
The pH of the honey samples was determined in 13.3% (W/V) honey solution using HANNA portable meter (Hanna HI9811-5 Portable pH/EC/TDS/°C Meter, Phoenix, AZ, USA). The meter was calibrated before the measurement using two buffers of pH 4.0 and 7.0. The concentration of the acids was measured by titration with 0.1M NaOH solution. The end of the titration was taken when the pH of the honey samples reached 8.30 according to the methods of international honey commission. The acidity of honey is expressed in milli-equivalent (meq) of acid per kg of honey according to the equation bellow32.
Measurement of electrical conductivity
The EC of the honey samples was determined in 20% (W/V) honey solution using the HANNA portable meter (Hanna HI9811-5 Portable pH/EC/TDS/°C Meter, Phoenix, AZ, USA). The meter was calibrated with standard KCl solution with a conductivity of 1413 μS/cm. The measurement was carried out following the instructions of the international honey commission33.
Measurement of fructose, glucose and sucrose
The sugars are measured according to the method of the international honey commission using the HPLC-RID34.
Quantification of total proteins
The spectrophotometric method of Bradford35 was applied for the purpose of determining the total protein concentration in the studied honey samples. The honey samples were diluted with distilled water (50% W/V) and 100 µL of the diluted honey samples were reacted with 5 mL of coomassie brilliant blue (The coomassie blue reagent was prepared as follows: 100 mg of Coomassie Brilliant Blue G-250 was dissolved in 50 mL of 95% ethanol, 100 mL of 85% (W/V) phosphoric acid was added and finally, the solution was diluted to one liter using deionized water and filtered) and the absorbance was recorded at the wavelength of 595 nm. A standard curve was created using the Albumin with serial dilutions in the range of (0–800 µg/mL).
Determination of catalase activity
CAT damages the H2O2 to H2O and O2. The activity of CAT was measured depending on the decrease in H2O2 concentration of the honey sample after being incubated with a standard solution of H2O2. The measurement was done according to the instructions of the Megazyme international company kit (CAT kit number: 200206-6). The kit is composed of two reactions: (1) honey samples (CAT) is incubated with a standard solution of H2O2 for 5 min. Then, the enzyme is stopped by adding sodium azide solution. (2) The remaining H2O2 is broken-down by peroxidase (POD) with the addition of 3,5-dichloro-2-hydroxy-benzenesulfonic acid (DHBS) and 4-aminoantipyrine (AAP) to produce a complex dye (quinoneimine), which is determined at the wavelength of 520 nm36,37. The CAT kit contains five bottles: bottle 1) 1.5 M Potassium phosphate buffer (30 mL, pH 7.0) and sodium azide (0.02% w/v), bottle 2) POD and AAP, bottle 3) DHBS solution (20 mL) and sodium azide (0.02% w/v), bottle 4) H2O2 standard solution (10 mL, ~ 1.3 M) and bottle 5) Catalase Standard (4g)36.
Preparation of the CAT assay reagents
To prepare the peroxidase solution, the contents of bottle 1 (phosphate buffer) were solubilized in 250 mL distilled water. 10 mL of the diluted phosphate buffer were used to solubilize the contents of bottle 2 (POD and AAP). After that, the solubilized POD and AAP were transferred to diluted phosphate buffer (POD solution). The contents of bottle 3 (DHBS solution) were transferred to the POD solution prepared in the previous step and the pH was adjusted to 7.0 using 1 M HCl. The mixture of bottle 1, 2 and 3 is the colorimetric reagent solution which was divided to several aliquots and kept bellow -10 °C.
For the H2O2 substrate Solution, 0.1 mL of bottle 4 (H2O2 standard solution) was diluted with 1 mL assay buffer (150 mM Potassium phosphate, pH 7.0) to obtain a final concentration of 130 mM36.
To prepare the Catalase Control Standard, 100 mg of bottle 5 (Catalase standard) were dissolved in 20 mL of the assay buffer35.
The 150 mM Potassium phosphate buffer, pH 7.0 was prepared by dissolving 13.06 g of potassium phosphate dibasic (K2HPO4 in 300 mL of distilled water and the pH was adjusted to 7.0 with 1 M HCl36.
CAT assay procedure
From each honey sample one gram was diluted in 1 mL of buffer solution. 0.05 mL from the treated honey sample was transferred to 0.05 mL of the H2O2 solution in a test tube and preserved at 25 °C for 5 min. To stop the reaction, 15 mM sodium azide solution (0.9 mL) was added. Upon the stop of the previous reaction, 0.04 mL of the mentioned tube was pipetted in to a cuvette of spectrophotometer. 3 mL of peroxidase mixture (POD + DHB + AAP) were transferred to the cuvette and the contents were mixed and incubated for 15 min at 25 °C. At the end, the absorbance of the cuvette content was read at 520 nm against a blank tube. In state of the honey sample, the blank tube was containing 0.05 mL of phosphate buffer (150 mM Potassium phosphate, pH 7.0)37.
In order to calculate the CAT activity in the studied honey samples, a standard curve was derived using variable activities of the CAT (0.0, 0.4, 0.8, 1.6, 3.2 and 6.4 U/0.05 mL). The same procedure of the honey and blank samples analysis was followed. A line equation was derived by the usage of the Excel program. The CAT activities were obtained according to the result of the line equation and multiplied by 2 (dilution factor) to obtain the activity of CAT in U/g of honey. The equation bellow was used to calculate the activity of the catalase in U/g:
where; 20: to convert the 0.05 mL to 1 mL. 2: Dilution factor.
To obtain the activity in Kg of honey the results were divided by 1000.
Detection of short peptides
The chemical composition of the honey samples was scanned using the LC-ESI-QTOF-MS. The separation of the components was carried out using the HPLC Reverse phase elution with Electrospray Ionization (ESI) (Waters Symmetry LC18 column 250 × 4.6 mm, 5 µm) and the masses were identified by the usage of SCIEX 500 Series Accurate-Mass Quadrupole Time-of Flight (Q-TOF; Agilent CA, USA). The Mass Hunter software of Agilent technologies was used to analyze the mass so as to identify the molecular formula. The specifications of the LC–MS system with Agilent 1200 Series Diode Array Detector are (module G1315B; detection type: 1,024-element photodiode array; light source: deuterium and tungsten lamps; wavelength range 190–950 nm). The mobile phase was composed of (A) formic acid (0.1%, v/v); (B) acetonitrile + 0.1% formic acid; gradient (in solvent B): (i) 20%, from 0 to 20 min, (ii) 95%, from 20 to 27 min, and (iii) 35%, at 27–30 min of total run time; flow rate was 0.2 mL/min; temperature was 35˚C and injection volume was 3 L. The ESI parameters were both negative and positive ion modes, mass range 100–1,200 m/z, spray voltage 4 kV, gas temperature 325 °C, gas flow 10 L/min, and Nebulizer was 40 psi. According to the manufacturer instructions, tuning and optimization are carried out before any run and on each single day11.
The precursor masses obtained from the LC–MS which possess molecular formula of short peptides (at least 2 Nitrogen and 3 oxygen atoms) were searched in the mascot search engine through the ms/ms ions search (https://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=MIS). Swiss Prot was chosen as a database to be searched and the other metazo was used as a taxonomy option while the enzymes were chosen one by one till a protein from the Apis species was obtained.
Measurement of total phenolic acids
The total phenolic acids were measured by reacting them with Folin–Ciocalteu reagent. 10 mL of 2N Folin–Ciocalteu reagent (47641-100ML-F; Sigma-Aldrich; MI, USA) were diluted with 90 mL distilled water. One mL of honey sample (0.1g/ mL) was transferred to a test tube containing 5 mL of the diluted Folin–Ciocalteu reagent, shaken for five minutes and 4 mL of 7.5% (w/v) solution of sodium carbonate (13418-1KG-R; Sigma Aldrich; MI, USA) was added. The test tube was incubated for one and a half hour at room temperature covered with silver foil. Finally, the absorbance was read in spectrophotometer at the wavelength of 760 nm against a blank tube containing one mL of distilled water instead of the honey sample. The analysis of each honey sample was carried out in triplicate and the mean of the readings was taken as the result of the total phenolic acids in the samples. The concentration of the total phenolic acids was expressed as gallic acid equivalent (GAE) per 100 g of honey38.
Different concentrations of gallic acid (G7384-250G; Sigma-Aldrich; MI, USA) were used to create standard curve so as to calculate the concentration of phenolic acids in the honey samples. A stock solution of gallic acid (5 mg/mL) was prepared by dissolving 0.5 g in 100 mL of distilled water: methanol solution (1:1). The different concentrations of gallic acids were 0.00, 0.001, 0.002, 0.004, 0.008 and 0.016 mg/ mL. straight line equation was derived using the EXCEL program and the concentration of the phenolic acids was calculated accordingly. The result was multiplied by 1000 to convert the sample weight from 0.1 g to 100 g.
Measurement of flavonoids
2 mL of 2% methanolic aluminium chloride (237051-500G; SigmaAldrich; MI, USA) solution was added to 2 mL of honey solution (2 mg/mL), incubated at room temperature for 30 min and the absorbance was measured at the wavelength of 415 nm against a blank tube composed of a honey solution and methanol without aluminium chloride39.
The total flavonoids concentration in the honey samples was determined depending on a quercetin calibration curve and the concentration was expressed as mg of quercetin equivalent per 100 g of honey (mg QE/ 100 g). Different concentrations of quercetin (Q4951-100G; Sigma-Aldrich; MI, USA) were prepared in methanol. The concentrations of quercetin were 0.00005, 0.001, 0.002, 0.005, 0.01, 0.02, 0.025 and 0.05 mg/ mL. Straight line equation was derived using the EXCEL program and used for the determination of the flavonoids concentration in the 4 mg of honey (2 mL). The result derived from the straight line equation was multiplied by 250 to obtain the concentration in one gram and after that multiplied by 100 to have the concentration in 100g.
Antioxidant activity
Antioxidant activity assays are classified to two types according to the nature of the oxidant molecules: 1) Lipid peroxidation assays which includes thiobarbituric acid assay (TBA), malonaldehyde/ high-performance liquid chromatography (MA/HPLC) assay, malonaldehyde/ gas chromatography (MA/GC) assay, β-carotene bleaching assay, and conjugated diene assay40. 2) Free radical scavenging such as the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay, ferric reducing/antioxidant power (FRAP) assay, ferrous oxidation-xylenol orange (FOX) assay, ferric thiocyanate (FTC) assay, and aldehyde/carboxylic acid (ACA) assay40.
Moreover, the antioxidant assays are classified to three classes according to their mechanism of action; Hydrogen Atom Transfer (HAT), Single Electron Transfer (SET) and combined HAT and SET. HAT antioxidant assays remove the oxidant molecules through donating hydrogen atom while SET antioxidant assays remove the oxidant molecules through donating a single electron41.
Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The basic principle of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay involves the addition of specific amount of the DPPH to the honey sample. The antioxidants in honey deplete the DPPH leading to reduction in its absorption. The DPPH assay is classified as HAT and SET antioxidant assay40. The absorption reduction is measured as reduction percentage as follows:
Firstly, the DPPH was prepared in concentration of 0.1mM in methanol. Secondly, two grams of each honey sample were dissolved in 10 mL of distilled water. Thirdly, 1.8 mL from the DPPH solution was taken and added to 0.2 mL of honey and the mixture was kept at room temperature in darkness for one hour. Fourthly, the absorbance was read at the wavelength of 517 nm40. The scavenging activity of the radical (A%) was determined according to the equation bellow40:
where A%: Reduction percentage; Aa: absorbance of the honey sample and A0: absorbance of standard sample.
Ferric reducing antioxidant power (FRAP)
The major idea of the FRAP assay is the acceptance of electron from the antioxidant molecules in the honey samples (SET antioxidant assay). The assay involves the reduction of ferric-tripyridyltriazine [FeIII(TPTZ)] (colorless) to ferrous-tripyridyltriazine [FeII(TPTZ)] (blue colour)42.
The first step was the preparation of the FRAP reagent which is prepared by mixing of three solutions: 1) 0.3 M acetate buffer (10 mL); 2) 0.01 M TPTZ in 0.04 M HCl (1 mL); and 3) 0.02 M FeCl3. 6H2O (1 mL). The second step was the creation of standard curve using ferrous sulfate with concentration range of (4.8–0.6 mg/mL). The third step was the sample treatment which was carried out by dissolving one gram of each honey in 5mL methanol (6 times dilution). The fourth step involved pipette of 200 µL from the different standard solutions or the diluted honey sample in a test tube, addition of 1.5 mL of the FRAP reagent to it and incubation of the mixtures at 37 °C for 4 min. The fifth step was the measurement of the absorbance at 593 nm after calibrating the spectrophotometer using 200 µL of distilled water in state of the honey sample42. Finally, the results obtained from the straight line equation was multiplied by 5 (5 mL) to obtain the activity in one gram and multiplied by 100 to have the activity in 100 g of honey.
Ascorbic acid equivalent antioxidant capacity (AEAC)
The ascorbic acid equivalent antioxidant capacity assay uses vitamin C as scavenger of DPPH to create standard curve and the antioxidant activity is expressed as mg of Ascorbic acid Equivalent Antioxidant Capacity per 100 g of honey (mg AEAC/100 g honey sample)43. The assay was carried out according to the following order: Firstly, the standard curve was generated using different concentrations of ascorbic acid (0.0001, 0.001, 0.01, 0.02, 0.03, 0.04, 0.05, and 0.06 (mg/mL). The ascorbic acid standard solutions were prepared from stock solution with a concentration of 0.1 mg/mL. Secondly, one gram of each honey sample was dissolved in 5 mL of distilled water (0.2g/ mL). Thirdly, To measure the absorbance of each standard solution or sample, 0.5 mL of each ascorbic acid standard or sample was mixed with 2.5 mL of methanol and 2.5 mL of the DPPH solution as prepared in the DPPH assay. Fourthly, the mixture was incubated for 15 min at darkroom temperature. Fifthly, the absorbance of these solutions was determined at 517 nm. Finally, the antioxidant content of the honey sample was calculated using the equation of the standard curve, multiplied by 5 to get the activity in one gram and multiplied by 100 to have the activity in 100 g of honey expressed as (mg AEAC/100 g honey sample)43.
Statistical analysis
The obtained mean values of the studied parameters were compared using the independent t-test and the analysis of variance (ANOVA) of the Statistical Package for Social Sciences (SPSS). The correlations of the studied parameters were examined using the Pearson’s correlation. Moreover, the Pearson correlations between the studied parameters were investigated with specific consideration for the short peptides and the antioxidant activities. The significance level was set at ≤ 0.05.
Results and discussion
Floral and geographical origin of the honey samples
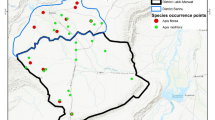
The microscopic analysis showed that the honey samples were of three floral origins; Acacia, Ziziphus and polyfloral (Fig. 1) and (Table 1).
The major dominant honeys in Saudi Arabia are the Acacia, Ziziphus, Lavender (lavandula dentate) and Hypoestes forskaolii (Majra)44. The Acacia honey of Saudi Arabia includes Acacia tortilis (Samar), Acacia hamulosa (Gatada), Acacia gerrardii / Acacia origena (Taleh), Acacia ehrenbergiana (Salm), and Acacia asak (Dahiana). The Ziziphus honey is of two types (Ziziphus spina Christi and Ziziphus nummularia)44,45,46.
Quality parameters
The results of the honey quality parameters and total sugars are presented in (Table 2) including the mean, standard deviation (SD), minimum and maximum values of each parameter. All the values of the studied quality parameters comply with the values of the Codex and Saudi Food and Drug Authority (SFDA) standards1,47.
The geographical origin of the honey samples significantly affected the moisture percentage of the Acacia honey and the EC of the polyfloral honey.
The geographical origin had significant effect on the moisture percentage and HMF of the Acacia honey and the EC of the polyfloral honey. The floral origin had significant effect on the pH, moisture and HMF of the honey samples from Jazan while it had significant effect on the glucose and the sum of fructose and glucose of Asir honey (Table 2) and (Table 3). Many of the previous studies concluded that the floral and geographical origin had significant effects of the quality parameters of honey48,49,50. In a conference abstract published by the journal of biological chemistry, we reported that the geographical origin had insignificant effect on some studied honey quality parameters including the moisture, acidity and electrical conductivity48. Moreover, the same abstract concluded that the floral origin from one geographical origin (hot climate) had significant effects on the moisture, pH and total sugars51.
Total proteins
The line equation of the standard curve of the protein assay was Y = 0.0011X + 0.0205 and the R2 was equal to 0.98 (Fig. 2).
The mean, SD, minimum and maximum values of the total protein concentration in Acacia, Ziziphus and polyfloral honey samples of Asir region were (667.17, 210.36, 416.0, 1172.0 µg/g), (610.67, 3.06, 608.0, 614.0 µg/g) and (915.48, 198.28, 450.0–1260.0 µg/g), respectively. The protein results of the Acacia, Ziziphus and polyfloral honey samples of Jazan region were (642.06, 186.39, 345.0, 991.0 µg/g), (737.5, 280.72, 539.0, 936.0 µg/g) and (645.8, 49.97, 571.0, 725.00 µg/g), respectively (Fig. 3).
The geographical region significantly affected the total protein concentration of Acacia honey (p-value = 0.044) and polyfloral honey (p-value ≤ 0.001). With regard to the effect of the floral origin, the mean total protein concentration of the polyflroal honey of Asir region was significantly more than the protein concentration in the Acacia and Ziziphus honeys (p-value = 0.004 and p-value = 0.011, respectively). The floral origin of Jazan region had insignificant effect on the total protein concentration. The comparison between the mean values of the total protein in the different honey samples showed the significant effects of the geographical and botanical origins on the total protein concentration.
The ranges of total proteins in Asir and Jazan honey samples irrespective of their floral origins were (416.20–1260.0 µg/g) and (345.0–991.0 µg/g), respectively. The protein content range of the studied honey samples is different than the range of our pilot study results (345.0–1260.0 µg/g) compared to (222.54–560.5 µg/g)11. Azeredo et al. (2003) measured the protein concentration in honey samples of different botanical origin and a reported high concentration of proteins in two of their honey samples (2236 and 2212 µg/g)35. This study reported significant effects of the geographical and floral origins on the protein concentration of the studied honey samples (Table 3). In our published abstract in the Journal of biological chemistry, we stated that the floral and geographical origins of honey samples had significant effect on the total concentration of honey proteins51. The previous studies proved that the physicochemical properties and chemical composition of honey are affected by the floral and geographical origins of honey43,45,48,49,50,51,52. It has been stated that the protein concentration in honey is impacted by the honeybee species and the honey type53.
Catalase
The standard curve of the catalase is presented in (Fig. 2). There was inverse relationship between the absorbance and the catalase activity and the equation was y = -0.3806x + 0.5837 and R2 = 0.9733.
The mean ± SD and range of CAT activity of the Acacia honey of Asir region were 168.70 U/kg ± 76.04 and (52.16–382.89 U/kg), of the Ziziphus honey of Asir were 122.18 U/kg ± 86.07 and (63.33–250.06 U/kg) and the results of Asir polyfloral honey were (162.05 U/kg ± 67.14 and (19.68–238.41 U/kg). The results of the CAT of Asir region honeys were more than those of Jazan region. The results of the CAT activities of the Acacia honey from Jazan were 80.38 U/kg ± 54.84 and (13.24–125.64 U/kg), of the Ziziphus honey from Jazan were 102.68 U/kg ± 2.21 and (101.11–104.24 U/kg) and the results of the Jazan polyfloral honey were 130.66 U/kg ± 36.45 and (65.13–203.66 U/kg) (Fig. 3).
The Acacia honey of Asir region had significantly increased mean value of CAT activity compared to the Acacia honey of Jazan (P-value = 0.015). As Asir region has a cold climate, it can be concluded that the catalase activity increases in cold climates. The conclusion is that the geographical origin had significant effect on the CAT activity of the Acacia honey while the floral origin had insignificant effect.
It is known that honey catalase is mainly derived from the nectar of the plants and the microorganisms in honey and it is affected by the other components in the nectar of the plants. Similar to our findings one of the literature mentioned that wheat catalase increases in low temperature environment9,54. As a honey component, the catalase is affected by the floral origin, nectar constituents, climate conditions, processing and storage environment9,52,54. Moreover, the results of this study are very low compared to Brazilian honey samples which were with a range of 9970–99070 U/g (9.97–99.07 U/mg)55.
Short peptides
Short peptides in the honey samples from Asir region
Nine precursor masses from Asir region honey samples were found to be short peptides from Apis mellifera and Apis florea proteins (Table 4). The two honeybee species were present in the study area. According to the mascot search engine, the enzymes that produced the short peptides were trypsin, chymotrypsin, pepsin A, Clostripain (Arg-C) and V8-DE. The trypsin, chymotrypsin and pepsin are produced by the honeybees9 while the Clostripain and V8-DE are of bacterial origin.
Clostripain is a cysteine endopeptidase that cleaves peptide bonds from the carboxyl end of Arginine residues. The clostripain is produced by the Clostridium histolyticum bacterium which is found in the feces of animals56. The V8 is a protease is produced by Staphylococcus aureus and it cleaves peptide bonds from the carboxyl ends of glutamate and aspartate residues57.
Presence of short peptides digested by enzymes of bacterial origin in honey suggests presence of these bacteria in the intestinal flora of the honeybees. However, some researchers reported that honey contains some Clostridium species such as the Clostridium botuinum and Clostridium perfringens58,59,60. Presence of short peptides in honey due to the action of clostripain need to be investigated since the Clostridium histolyticum is not present in the flora of the honeybees. Moreover, It is reported by Anjum et al. (2021)58 that staphylococcus species are present in the gut of Apis mellifera.
Regarding the honey samples from Asir region, seven short peptides were detected in Acacia honey and two were found in polyfloral honey while no short peptide was detected in the Ziziphus honey samples. The precursor masses that correspond to the short peptides in the Acacia honey were 635.3990, 519.3547, 573.4010, 622.3807, 521.3077, 620.3872 and 393.2230, whereas, the precursor masses corresponding to the short peptides of the polyfloral honeys were 519.3506 and 650.3761 (Table 4).
The mascot search showed that the precursor mass of 635.3990 is either a trypsin digest of hunchback protein of Apis mellifera (YCHSL KLHLR; Tyr-Cys-His-Ser-Leu-Lys-Leu-His-Leu-Arg) or a trypsin digest of Tertiapin of Apis mellifera (IIIPHMCWKK; Ile-Ile-Ile-Prl-His-Met-Cys-Trp-Lys-Lys) (Table 4). The hunchback protein is an insect transcription factor that activates of repress gene expression associated with embryo development61. Short peptides containing histidine, tyrosine, phenylalanine, cysteine, lysine and valine are well known by their antioxidant activity63,64. The trypsin digests of the hunchback protein in the honey samples can act as antioxidants. Short peptides containing tyrosine, leucine isoleucine, arginine, phenylalanine, tryptophan, proline and lysine at their carboxyl or amino terminals act as antihypertensive medicine through inhibiting the Angiotensin Converting Enzyme (ACE)65. The two short peptides of the trypsin digest of the hunchback protein contain tyrosine and lysine at their N-terminals and contain leucine and arginine at their C-terminals. i.e. the two short peptides are qualified to inhibit the ACE and act as antihypertensive peptides. The Tertiapin is a protein neurotoxin and is a minor component of the honeybee venom66. The trypsin digest of the Tertiapin (IIIPHMCWKK) has three isoleucine residues at its N-terminal and two lysine residues at its C-terminal which qualifies it to act as antihypertensive medicine65. Moreover, presence of methionine in the structure of the trypsin digest of Tertiapin qualifies it to act as antioxidant beside its content of histidine, cysteine and lysine63,64,67. The presence of proline and tryptophan in the sequence of the trypsin digest of Tertiapin suggests its antimicrobial activity68.
The second precursor mass was for chymotrypsin digest with the value of 519.3547 (Table 4). It was a digestion product of Apidermin-2 of Acacia honey samples produced by Apis mellifera. Apidermin-2 is a circular structural protein with antibacterial and antifungal activities69. The Chymotrypsin digest of Apidermin-2 had the sequence of AAVPLAPTIAL (Ala-Ala-Val-Pro-Leu-Ala-Pro-Thr-Ile-Ala-Leu) which can be classified as alanine rich short peptide. Alanine rich peptides are known by their antimicrobial activity70.
The third precursor mass (573.4010) was from Acacia honey and it was corresponding to two Clostripain digests for Myosuppressin and orcokinin proteins of Apis mellifera (Table 4). Myosuppressin is an insect neuropeptide famous by its activity as visceral muscle inhibitor such as the muscles of the gastro intestinal tract and heart70. Orcokinin is a neuropeptide in insects and crustaceans that enhance the frequency of gastrointestinal tract muscle contraction72. The clostrpain digests of Myosuppressin and Orcokinin were IRKVCVALSR (Ile-Arg-Lys-Val-Cys-Val-Ala-Ser-Arg) and TAFDNFFKR (Thr-Ala-Phe-Asp-Asn-Phe-Phe-Lys-Arg). The clostripain digest of Myosuppressin can act as antimicrobial (contains repetitive Arginine and valine residues)73 and antihypertensive (contains isoleucine at its amino terminal and arginine at its carboxyl terminal)65. The clostrpain digest of Orocokinin can act as antimicrobial since it is rich in phenylalanine (33%)74.
The fourth precursor mass was due to the activity of the clostripain with the value of 622.3807 and it is for the pheromone biosynthesis activation neuropeptide (PBAN-type neuropeptide) (Table 4). It is a pheromone with variable functions including pheromone synthesis muscle contraction and diapause induction and termination75. The clostripain digest of the PBAN-type neuropeptide has the sequence of IGFAVFSSFNR (Ile-Gly-Phe-Ala-Val-Phe-Ser-Ser-Phe-Asn-Arg). The clostripain digest can act as anti-hypertensive because it has isoleucine at its amino terminal and Arg at its carboxyl terminal65. Moreover, the digest contains three phenyalanine residues out of eleven amino acids which qualifies it to act as antimicrobial71.
650.3761 was the fifth precursor mass and it was pepsin A digest product in a polyfloral honey sample. The mascot search showed that this pepsin A digest is either from Melittin or Homeobox protein H40 (Fragment) of Apis mellifera (Table 4). Melittin is the major bee venom protein that constitutes about 52% of bee venom dry weight. Melittin has promising future as a medicine for the treatment of leukemia76. Generally, Homeobox proteins are transcription factors that play roles in the morphogenesis and differentiation of cells74. The pepsin A digest of melittin had the sequence of ISWIKNKRKQ (Ile-Ser-Trp-Ile-Lys-Asn-Lys-Arg-Lys-Gln) which is lysine rich peptide. Lysine rich peptides containing tryptophan and or aliphatic amino acids act as hypo-cholesterolemic peptides beside their role as antimicrobial and antioxidants63,64,74. The Homeobox 40 digest of the pepsin A was lysine and threonine rich polypeptide (ENKFKTTRYL; Glu-Asn-Lys-Phe-Lys-Thr-Thr-Arg-Tyr-Leu) which is qualified to act as antimicrobial and antioxidant63,64,73,74.
The sixth precursor mass was (519.3506) and it was for Apidermin-2 (circular structural protein) of a polyfloral honey sample produced by pepsin A (Table 4). The short peptide was rich in alanine and proline (AAVPLAPTIAL; Ala-Ala-Val-Pro-Leu-Ala-Pro-thr-Ile-Ala-Leu). The alanine and proline rich peptides act as antimicrobials68,70. Proline rich short peptides are reported to act as anti-diabetics78.
The seventh precursor mass was (521.3077) obtained from Acacia honey and produced by pepsin A. It was either for a digest from Apamin or Mast cell degranulating peptide of Apis mellifera (Table 4). Apamin is a bee venom neurotoxin protein responsible for the regulation of the gene expression associated with cell development. Pharmacologically, it irreversibly blocks the potassium channels that depend on Ca2+79. Mast cell degranulating peptide is a bee venom peptide strongly de-granulates the mast cells leading to the release of histamine. It acts as anti-inflammatory and blocks potassium channels leading to low blood pressure80. The pepsin A digest of the Apamin and the Mast cell degranulating peptide had the sequence of (SVILITSYF; Ser-Val-Ile-Leu-Ile-Thr-Ser-Tyr-Phe). Many short peptides that contain serine, isoleucine, leucine, valine and threonine possess antioxidant activity64. Furthermore, repetitive amino acids is a character of antimicrobial peptides74.
The eights precursor mass (620.3872) was due to the activity pepsin A in Acacia honey sample. The mascot search showed that the mentioned pepsin A digest was either a product of Abaecin or Homeobox protein H17 (Fragment) peptides (Table 4). Abaecin is a proline rich protein secreted by the Apis mellifera in response to bacterial infection81. The Homeobox protein H17 (Fragment) is a transcription factor associated with the morphogenesis and differentiation of insects cell77. The pepsin A digest of Abaecin is an alanine rich peptide (ATICAAFAYVPL; Ala-Thr-Ile-Cys-Ala-Ala-Phe-Ala-Tyr-Val-Pro-Leu) which can act as antimicrobial peptide70. The pepsin A digest of Abaecin can be classified as antioxidant peptide since it contains cysteine, tyrosine, valine, isoleucine, leucine and phenylalanine63,64. The pepsin A digest of the Homeobox H17 was rich in Arginine, Lysine and alanine (QNRRAKAKRL; Gln-Asn-Arg-Arg-Ala-Lys-Ala-Lys-Arg-Leu). Arginine or lysine rich peptides have the ability of penetrating cell membranes which suggests them to be used in drug delivery82,83. Moreover, the pepsin A digest of the Homeobox H17 can act as antimicrobial and hypocholesterolemic peptide because it is alanine and glycine rich, respectively70,74.
The ninth precursor mass (393.2230) was produced by the V8 protease of the Staphylococcus spp of an Acacia honey sample. The V8 digest was either for the Brain peptide MVPVPVHHMADELLRNGPDTVI or the Homeobox H90 (Table 4). The brain peptide MVPV is one of the peptide that controls the behavior of bees when foraging for the collection of nectar or pollens84. As a homeobox, the homeobox H90 is a transcription factor involved in the regulation of gene expression associated with the cell differentiation and insect morphogenesis74. The V8 digest of the MVPV brain peptide is (LLRNGPD; Leu-Leu-Arg-Asn-Gly-Pro-Asp) which is rich in leucine. Short peptide with multiple leucine residues regulates the intracellular phosphorylation which is involved in the regulation of many metabolic processes85. The V8 digest of the Homeobox H90 was (LSHCVPE; leu-Ser-Cys-Val-Pro-Glu) which is suitable to act as antioxidant peptide63,64. The short peptides produced by the V8 of the Staphylococcus aureus reflect the usefulness of honey proteomics in detecting the contamination of honey by pathogenic bacteria.
Short peptides of the Jazan honey samples
Short peptides in Jazan honey samples were detected in the mascot search engine after choosing the trypsin, chymotrypsin, TrypChymo, pepsin, Arg-C (Clostripain), Lysyl-endopeptidase (Lys-C and Lys-N), flavastacin (Asp-N). As mentioned above the trypsin, chymotrypsin and pepsin are secreted by the honeybees while the clostripain is normally isolated from the Clostridium histolyticum. The lysyl enopeptidases are produced by the lysobacter strains which are reported to be present in honey86. The flavastacin cleaves peptide bonds from the amino terminal of Aspartate and it is isolated from Flavobacterium meningosepticum bacterium87. Presence of short peptides in honey due to flavastacin action may be due to contamination of honey by the water borne Flavobacterium meningosepticum. However, the microorganisms are considered as a major source of enzymes in honey9.
This study reported that twenty seven precursor masses from the Jazan honeys are for enzymes digests of variable proteins. The first precursor mass was (416.1897) in a polyfloral honey produced by the trypsin enzyme. The mascot search showed that the mentioned trypsin digest is for the diuretic hormone class 2 of Apis mellifera (Table 5). The diuretic hormones of insects induce diuresis in response to energy mobilization due to crop draining into the gut or due to environmental stresses88. The sequence of the obtained trypsin digest of the Diuretic hormone class-2 was (GLDLGLSR; Gly-leu-Asp-Leu-Ser-Arg) (Table 5). Leucine rich short peptides act as regulatory molecules for metabolism and presence of the amino acids of this short peptide is an indicator of its antimicrobial activity85,89. Decker et al. (2022) stated that antimicrobial peptides contain frequent amino acids (Alanine, Cysteine, Glycine, Histidine, Isoleucine, Lysine, Leucine, Proline, Arginine, and Valine) and occasionally (Aspartate, Glutamate, Phenylalanine, Serine, Threonine, Tryptophan, and Tyrosine) while three amino acids are not found in the structure of the antimicrobial peptides (Asparagine, glutamine and Methionine)89.
The second precursor mass (280.0965) was from polyfloral honey for clostripain (Arg-C) action. The mascot search showed that this clostripain was for either Melittin of Apis dorsata with the sequence of (KRQE; Lys-Arg-Gln-Glu) or two homeobox proteins E30 or E60 with one sequence (RVKR; Arg-Val-Lys-Arg) (Table 5). The clostripain digest of the Apis dorsata melittin contains arginine and lysine which are character of the peptides that can penetrate cell membranes and act as drug delivery vehicle82,83. The clostripain digest of the homeoboxes (E30 or E60) contains 2 Arg, one Lys and one Val which propose it to act as a drug delivery molecule and as anti ACE because it contains Arg at its N-terminal and Arg at its C-terminal65,82,83.
The third precursor mass was from poyfloral honey sample produced by Apis florea with the value of (458.2721) and it a product of chymotrypsin activity. The chymotrypsin digest was for the melittin according to the mascot search result with sequence of (IKNKRKQ; Ile-Lys-Asn-Lys-Arg-Lys-Gln) (Table 5). The chymotrypsin digest of the melittin is rich in Lysine and contains arginine which a feature of cell penetrating peptides according to Ma et al. (2023)82. Chymotrypsin digest of Melittin can act as antioxidant because it contains isoleucine and Lysine63,64. Moreover, Lysine or arginine rich peptides are proved to have antimicrobial activity without mentioning that if they contain glutamine, asparagine or methionine or not89,90.
The fourth short peptide had the precursor mass of (510.2713) and it was a clostripain product of a polyfloral honey sample produced by Apis mellifera. The short peptide was digested from Pro-corazonin protein. Corazonin is a neuropeptide hormone of insects which has effects on visceral muscles and regulates coloration91. The clostripain digest of the Pro-corazonin had the sequence of (QPAPTNNNY; Gln-Pro-Ala-Pro-Thr-Asn-Asn-Asn-Tyr) (Table 5). Short peptides containing sequences of QP, PT, PA and AP are reported to have anti-diabetic activity92. Furthermore, glutamine/ asparagine rich peptides play role in the control of epigenetic (staple and inheritable changes at chromosomal level)93.
The fifth precursor mass (427.2311) was for trypsin digest either for Larval-specific very high density lipoprotein, diuretic hormone class-2 or chymotrypsin inhibitor in a polyfloral honey sample with sequences of (VAPFPHGK; Val-Ala-Pro-Phe-Pro-His-Gly-Lys), (GFSGSQAAK; Gly-Phe-Ser-Gly-Ser-Gln-Ala-Ala-Lys) and (ICTMQCR; Ile-Cys-Thr-Met-Gln-Cys-Arg), respectively (Table 5). Larval-specific very high density lipoprotein is responsible for the transport and storage of lipids through the hemolymph during metamorphosis. Metamorphosis is the active holometabolism during the development through the four stages of honeybees (egg, larva, pupa and adult)94. The chymotrypsin inhibitor is a 56 amino acid protein which inhibits both chymotrypsin and cathepsin G95. The trypsin digest of the Larval-specific very high density lipoprotein is proline rich peptide containing valine and alanine which is a character of anti-diabetic peptides beside its antioxidant activity because it contains valine, pheylalanine, histidine and lysine63,64,92. The trypsin digest of the diuretic hormone class-2 (GFSGSQAAK; Gly-Phe-Ser-Gly-Ser-Gln-Ala-Ala-Lys) is glycine, serine and alanine rich peptide which is proposed to act as neuroprotective and suggested for the treatment of Alzheimer disease96. The trypsin digest of the chymotrypsin inhibitor can act as anti-hypertension since it contains isoleucine at its N-terminal and arginine at its C-terminal65. Furthermore, it can act as antioxidant because of its content of isoleucine, cysteine, methionine and threonine63,64,67.
The sixth precursor mass (608.3546) was Apisimin digest of a chymotrypsin in a polyforal honey sample. Apisimin is a royal jelly protein with 54 amino acids that do not contain sulfur containing amino acids, arginine, histidine, tyrosine, tryptphan and proline97. The sequence of the amino acids of the chymotrypsin Apisimin digest was (SKIVAVVVLAAF; Ser-Lys-Ile-Val-Ala-Val-Val-Val-Leu-Ala-Ala-Phe) (Table 5). Alanine, arginine and valine rich peptides are reported to act as antimicrobial molecules70,73.
The seventh precursor mass (658.4379) was a digest product of the bacterial enzyme lysylyl endopeptidase (Lys-C) with the sequence of (AKRLQEAEIEK; Ala-Lys-Arg-Leu-Gln-Glu-Ala-Glu-Ile-Gu-Lys). The mascot search showed that this sequence is for homeobox H 17 protein (Table 5). Due to its repetitive sequence of alanine and glutamate, it may act as antioxidant as stated by Chen et al. (2015)92. Chen research group found that short peptides form walnut proteins containing repetitive sequences of Alanine and glutamate (ADIYTEEAGR) has antioxidant capacity98.
The eights precursor mass (520.3415) was obtained from a polyfloral honey sample by the action of the Lys-C bacterial endopeptidase. The mascot search showed that the precursor mass was for a short peptide from the venom phospholipase A2 of Apis dorsata. The Lys-C digest of the phospholipase A2 is (LEHPVTGCGK; leu-Glu-His-Pro-Val-Thr-Gly-Cys-Gly-Lys). As beekeeping in Saudi Arabia does not involve Apis dorsata, we can conclude that this sample is either imported from outside Saudi Arabia or mixed with honey from abroad. The digest of the phospholipase A2 is expected to have antioxidant activity because it contains leucine, histidine, valine, threonine, cysteine and lysine63,64. Furthermore, the phospholipase A2 can act as inhibitor of ACE (anti-hypertension) because it contains leucine at its N-terminal and lysine at its C-terminal65.
The ninth precursor mass (493.3322) was for the brain peptide MVPV due to the activity of the bacteria flavastacin enzyme. The sequence of the flavastacin digest was (VPVPVHHMA; Val-Pro-Val-Pro-Val-His-His-Met-Ala) (Table 5). Because of its amino acid content and sequence, the flavastacin digest of the brain peptide is expected to act as anti-diabetic (VPVPV), antioxidant due to its content of valine, histidine and methionine and antimicrobial because of the poly valine residues63,64,73,92.
The tenth precursor mass (357.2246) was a Clostripain digest for the histone 4 of Apis mellifera. It has the sequence of (TLYGFGG; Thr-Lys-Tyr-Gly-Phe-Gly-Gly) (Table 5). Histone 4 (H4) is one of the histone proteins (H2A, H2B, H3 and H4) that form nucleosome with DNA strands. Histone proteins are major regualtors of gene expression (replication, transcription, translation)99. The Clostripain digest of H4 is qualified to act as antioxidant due its content of threonine, lysine, tyrosine, phenylalanine63,64 and antimicrobial because it is a glycine rich short peptide100.
The eleventh precursor mass (328.2059) was a Clostripain digest of definsin-1 of Apis mellifera carnica or Apis mellifera with the sequence of (ADRHR; Ala-Asp-Arg-His-Arg) (Table 5). Definsin-1 is an antimicrobial peptide that is secreted by the salivary gland and it is associated with social immunity101. The Clostripain digest of Definsin-1 can penetrate the cell membrane because it is Arginine rich and it can act as antimicrobial73,82.
The twelfth precursor mass (389.2388) was obtained from a polyfloral honey due to the action of the bacterial lysylyl peptidase (Lys-N) on the Abaecin antibacterial peptide (Table 5). The Lys-N digest sequence is (KWPQGY; Lys-Trp-Pro-Gln-Gly-Tyr) which can act as antioxidant due its content of lysine and tyrosine63 and antimicrobial because of the tryptophan and proline68.
The flavastacin was the endopeptidase that produced the thirteenth precursor mass (519.3292) in a polyfloral honey sample. The flavastacin digest was from the definsin-1 of Apis mellfiera and it has the sequence of (DLWDKRFG; Asp-Leu-Trp-Asp-Lys-Arg-Phe-Gly) (Table 5). Presence of leucine, lysine, and phenylalanine may increase the antioxidant activity of this flavastacin digest63,64. The flavastacin digest short peptides may act as antimicrobial because its sequence is composed of amino acids well known to be in the structure of antimicrobial peptides89,102. Moreover, this flavastacin digest contains leucine, arginine and lysine which is a character of the short peptides that modulate the immune system activity92,103.
The fourteenth precursor mass (622.3818) was for a short peptide (IGFAVFSSFNR; Ile-Gly-Phe-Ala-Val-Phe-Ser-Ser-Phe-Asn-Arg) produced by the action of the clostripain on the PBAN-type neuropeptide of Apis mellifera (Table 5). Phenylalanine rich peptides are well known by their antimicrobial activity70,104. Furthermore, the short peptide of the PBAN-type neuropeptide contains isoleucine at its N-terminal and arginine at it C-terminal which may qualify it to act as anti-hypertension beside its activity as antioxidant because of its content of isoleucine, phenylalanine, valine and serine63,64,65.
The fifteenth precursor mass (517.3110) was for a short peptide (QILIDANVF;Gln-Ile-Leu-Ile-Asp-Ala-Asn-Val-Phe) produced by the action of the chymotrypsin on the Apisimin (royal jelly protein of Apis mellifera) (Table 5). The chymotrypsin digest of the Apisimin contains all the essential branched chain amino acids (leucine, isoleucine and valine) which reduces the risk of branched chain amino acids related metabolic syndromes105. Because the chymotrypsin digest of Apisimin contains multiple iselucine, leucine, valine and phenylalanine, it can act as antioxidant and antimicrobial63,64,89.
The precursor mass (367.1107) was the sixteenth one which is a product of the lysylyl-endopeptidse (Lys-N) in a polyfloral honey sample. The Lys-N digest was for ATP synthase protein 8 of the Apis mellifera ligustica with the sequence (PQMMPM; Pro-Gln-Met-Met-Pro-Met). The Apis mellifera ligustica is not within the beekeeping species in Saudi Arabia and presence of its protein in a local honey may reflect that the honey was mixed with an imported honey (Table 5). The Lys-N digest is a methionine rich peptide that is known to act as an antioxidant67. Furthermore, the Lys-N contains sequences of PQ and MP which is reported to act as anti-diabetic92.
The seventeenth precursor mass (380.2157) was for the short peptide (GRENQR; Gly-Arg-Glu-Asn-Gln-Arg) which was produced by the action of Clostripain on the Orcokinin peptide (neuropeptide associated with visceral muscle contraction) of the Apis mellifera (Table 5). The Clostripain digest of the orcokinin is an arginine rich peptide which is expected to be capable of penetrating cell membranes and act as antimicrobial73,82.
The mascot search showed that the eighteenth precursor mass (302.0782) was for the short peptide (TKINK; Thr-Lys-Ile-Asn-Lys) that is produced by the trypsin from the ATP synthase protein 8 of Apis mellifera (Table 5). The trypsin digest of the ATP synthase is a lysine rich peptide that is capable of penetrating the cell membranes, act as antimicrobial and antioxidant63,64,89. Moreover, the trypsin digest of the ATP synthase may act as cholesterol lowering peptide because it is rich in lysine and contains isoleucine73.
The nineteenth short peptide (VAPFPHG; Val-Ala-Pro-Phe-Pro-His-Gly) had the precursor mass of (363.1751). Lys-N was the endopeptidase that produced the short peptide from the Larval-specific very high density lipoprotein of the Apis mellifera (Table 5). Due to its content of phenylalanine, valine and histidine, the Lys-N digest may act as antioxidant and because it is rich in proline it is suggested to have antimicrobial activity63,64,68.
The twentieth short peptide (DRHRRVTC; Asp-Arg-His-Arg-Arg-Val-Thr-Cys) was obtained from definsin-1 by the action of the flavastacin endopeptidase (Asp-N). The precursor mass corresponding to the Asp-N digest was (521.3077) (Table 5). The Asp-N digest of the definsin-1 may act as antimicrobial and has the capability of invading the cell membranes because it is as arginine rich peptide73,74,82. Also, this short peptide may act as antioxidant due to its content of histidine, valine, threonine and cysteine63,64.
The mascot search showed that the twenty one precursor mass (455.3233) was for the short peptide (KKWNWF; Lys-Lys-Trp-Asn-Trp-Phe) which was produced by the action of pepsin from the ATP synthase protein 8 of the Apis mellifera ligustica (Table 5). The pepsin digest of the ATP synthase is lysine and tryptophan rich peptide which may qualify it to have antimicrobial activity with the ability of penetrating the cell membranes81,105. Also, this pepsin digest can act as hypocholesterolemic peptide because it is rich in lysine and contains tryptophan74.
The mascot search for the precursor mass (620.3872) showed that it is a pepsin digest for either Abaecin or homeobox protein H17 of Apis mellifera. The short peptide of abaecin is (ATICAAFAYVPL; Ala-Thr-Ile-Cys-Ala-Ala-Phe-Ala-Tyr-Val-Pro-Leu) while that of homeobox H17 is (QNRRAKAKRL; Gln-Asn-Arg-Arg-Ala-Lys-Ala-Lys-Arg-Leu) (Table 5). The pepsin digest of abaecin is alanine rich which may qualify it act as antimicrobial70, and it may be with antioxidant activity because it contains threonine, isoleucine, cysteine, phenalalnine, tyrosine, valine and leucine63,64. The pepsin digest of the homeobox H17 is rich in alanine, arginine and lysine which qualifies it to act as antimicrobial and anti-hypercholesterolemia70,73,74,89.
The short peptide number 23 has the sequence of (VSSIVSGANVSAVLL; Val-Ser-Ser-Ile-Val-Ser-Gly-Ala-Asn-Val-Ser-Ala-Val-Leu-Leu) with the precursor mass of (708.4044) and it is produced as a result of the pepsin action on the royal jelly protein (Apisimin) of Apis mellifera (Table 5). The pepsin digest of apisimin is rich in valine (4), serine (4), alanine (2) and leucine (2) which is a character of antimicrobial peptides89. Leucine rich short peptides are reported to regulate metabolic pathways85.
The twenty fourth precursor mass (446.2507) was for the trypsin digest of abaecin (bee venom protein) with sequence of (IKWPQGY; Ile-Lys-Trp-Pro-Gln-Gly-Tyr) (Table 5). Short peptides containing tryptophan and proline are reported to have antimicrobial activity and short peptides that contains a sequence of proline and glutamine have anti-diabetic activity68,92. Gua et al. (2009) stated that short peptides from royal jelly protein containing tyrosine at their C-terminal have potent antioxidant activity107.
The twenty fifth precursor mass (587.3875) was for a sequence of amino acids (DHHLKTHMR; Asp-His-His-Leu-Lys-Thr-His-Met-Arg) produced from the Protein krueppel of the Apis mellifera by the action of the trypsin (Table 5). The Krueppel protein is a juvenile hormone responsible for the regulation of metamorphosis in insects. Metamorphosis is the transformation from bee animal stages egg, larva, pupa and adult108. Histidine rich peptides are reported to have antimicrobial and cell penetrating activities109. The trypsin digest of the Krueppel protein may be with antioxidant activity because it contains histidine, leucine, threonine and methionine63,64,67.
The mascot search for the twenty six precursor mass (597.3459) showed it is for the Lys-C digest of the Hymenoptaecin of the Apis mellifera with the sequence of (SRPSLDIDYK; Ser-Arg-Pro-Ser-Leu-Asp-Ile-Asp-Tyr-Lys) (Table 5). The Hymenoptaecin is an antibacterial peptide secreted by the honeybees in response to infection110. The Lys-C digest of the hymenoptaecin may act as antioxidant because it contains serine, leucine, isoleucine, tyrosine and lysine63,64. Short peptides rich in acidic amino acids such as aspartic and glutamic acids are stated to be with antimicrobial activities100.
The last precursor mass (383.1745) was for a clostripain digest of the definsin-2 with the sequence of (NGVCICR; Asn-Gly-Val-Cys-Ile-Cys-Arg) (Table 5). Definsin-2 is an antibacterial peptide secreted form the lymph for the individual immunity101. The clostripain digest of definsin-2 is expected to have antioxidant activity due to its content of valine, cysteine and isoleucine64. Moreover, cysteine rich peptides are proved to have antimicrobial activity111.
Total phenolic acids
The equation of the standard curve of the total phenolic acids was Y = 53.947X- 0.0061 and the R2 = 0.9997 (Fig. 2).
The high concentration of phenolic acids was reported for the Jazan honey samples as follows: Acacia Jazan (504.1 ± 195.41 mg GA/100g), Ziziphus Jazan (658.35 ± 159.45 mg GA/100g) and polfloral Jazan (476.49 ± 111.99 mg GA/100g) compared to Acacia Asir (198.20 ± 279.16 mg GA/100g), Ziziphus Asir (96.91 ± 11.00 mg GA/100g) and polyfloral Asir (179.24 ± 72.58 mg GA/100g) (Fig. 4). The honeys form Jazan region had significantly high concentration of phenolic acids compared to those from Asir region with p-values ≤ 0.005 (Table 3). The floral origin had insignificant effect on the concentration of the total phenolic acids either in Jazan or Asir region.
Suleiman et al. (2020) reported that Acacia and Ziziphus honey samples from different altitudes in Asir region had mean concentrations of total phenolic acids of (67.18 ± 2.39 and 91.83 ± 1.47mg GA/100g) and (81.37 ± 2 and 96.99 ± 1.59 GA/100g), respectively112. Mohammed et al. (2020)113 found mean concentrations of total phenolic acids in Acacia honeys from Asir region were (81.47 ± 1.25 and 91.33 ± 0.96 mg GA/100g). However, this study found that the mean values of total phenolic acids in Acacia and Zziphus honey samples from Asir region were comparable to the previous studies with the mean values of 198.20 ± 279.16 mg GA/100g and 96.91 ± 11.00 mg GA/100g, respectively. Vazquez et al. (2021) reported a range of (48–203 mg GA/100g) for total phenolic acids in variable honey samples of different floral origins (chestnut, black berry, eucalyptus and heather) from Galicia in the Northwest region of Spain114. This study reported a wider range of total phenolic acids concentration in the studied honey samples (16.6–1890.4 mg GA/100g). The wider range of total phenolic acids may be due to the different geographical and floral origins. Thirty Croatian Acacia honey samples were reported to be with mean and range concentration of total phenolic acids of (43.66 ± 6.45 mg GA/kg) and (31.72–80.11 mg GA/Kg), respectively115. A Malaysian study reported that the concentration of total phenolic acids in Acacia honey depends on the harvest season and that the range of total phenolic acids was from 129.16 to 341.67 mg GA/Kg (12.916–34.167 mg GA/100g)116. The mean and range of total phenolic acids of the Croatian and Malaysian studies in the Acacia honey were very low compared to the results of this study which may be because of the different sub species of the Acacia plant and the different geographical origin52.
Acacia tortilis honey samples from Oman were reported to be with a mean value of total phenolic acids of 2236 mg GA/kg (223.6mg GA/100g)117, which is high compared to the mean value Acacia honey of Asir (198.20 ± 279.16 mg GA/100g) and lower than the mean value of Acacia honey from Jazan region (504.1 ± 195.41 mg GA/100g). The same study117, registered an average concentration of phenolic acids (1279mg GA/Kg = 127 mg/100g) in Omani Ziziphus honey. This study reported high mean concentration of phenolic acids in the Ziziphus honey from Jazan region (658.35 ± 159.45 mg GA/100g) compared to the Omani Ziziphus honey and comparable mean value of the Ziziphus honey from Asir region (96.91 ± 11.00 mg GA/100g). The Omani study found that the mean value of the phenolic acids in multifloral honeys was (1066 mg GA/Kg = 106.6 mg GA/100g)117. The mean values of the multifloral honeys from Oman were low compared to the mean value of the phenolic acids of the Jazan polyfloral honeys (476.49 ± 111.99 mg GA/100g) and Asir polyfloral honeys (179.24 ± 72.58 mg GA/100g).
Flavonoids
The standard curve equation for the flavonoids was (Y = 38.417 + 0.1102) while the R2 was 0.964 (Fig. 2). The results of the flavonoids concentration in the honey samples from Asir and Jazan regions are comparable with values (mg QE/ 100g) of Acacia Asir (5.03 ± 5.81), Acacia Jazan (3.99 ± 3.20), Ziziphus Asir (1.27 ± 0.05), Ziziphus Jazan (1.85 ± 0.78), Polyfloral Asir (4.07 ± 2.63) and Polyfloral Jazan (5.00 ± 2.12) (Fig. 4). The floral and geographical origins of the honey samples had insignificant effect on the concentration of flavonoids.
A Previous study on Acacia and Ziziphus honey samples from Asir region showed that our results are lower than the flavonoid mean concentration in Acacia and Ziziphus honey samples from variable altitudes in Asir region112. Suleiman et al. (2020)112 reported mean flavonoid values of (2.02 ± 0.87 and 8.24 ± 0.49 mg QE/100g) for Acacia honey and (9.15 ± 0.56 and 5.43 ± 0.49 mg QE/100g) for Ziziphus honey. Moreover, Mohammed et al. (2020)112 reported high level of flavonoids in Acacia honey from Asir region with the mean values of 8.78 ± 0.23 and 10.63 ± 0.53 mg QE/100g.
Studies from Romania and Italy reported decreased ranges of flavonoids in honey compared to the results of this study. The ranges of flavonoids were as follows; Romania (0.91 to 2.42 mg QE/100g)118 and Italy (0.45 to 1.01 mg QE/100 g)119.
Al-Farsi et al. (2018)117 reported very high concentration of flavonoids in Omani honey compared to the findings of this study as follows; Acacia tortilis (2143 mg QE/Kg = 214.3 mg QE/ 100g), Ziziphus (848 mg QE/Kg = 84.8 mg QE/ 100g) and multifloral (925 mg QE/Kg = 92.5 mg QE/ 100g).
DPPH
The ability of the honey samples from Asir and Jazan regions to scavenge the DPPH are comparable. The reduction percentage (%) were as follows: Acacia Asir (31.23 ± 16.43), Acacia Jazan (35.03 ± 16.95), Ziziphus Asir (59.03 ± 15.88), Ziziphus Jazan (38.95 ± 32.6), Polyfloral Asir (49.45 ± 26.0) and Polyfloral Jazan (53.4 ± 49.66) (Fig. 5). the geographical origin had insignificant effect on the reduction percentage of the studied honey samples while the floral origin of Asir regin had significant effect (Acacia Asir Vs Polyfloral Asir) (Table 3).
Alshammari et al. (2022) found that Saudi Acacia gerrardii Benth honey and Ziziphus spina Christi had the highest DPPH reduction percentage of 66.4% and 32.6%, respectively119. The DPPH scavenging activity of Jazan Ziziphus honey samples of this study and that of Alshammari et al. (2022) are comparable but the result of Asir Ziziphus honey is almost double that of Alshammari120. The mean DPPH scavenging activity of Asir Acacia honey of this study is very low compared to the scavenging activity of the Acacia gerrardii Benth.
Moniruzzaman et al. (2013) studied Malaysian honey samples produced by three different honeybee species and three floral origins and concluded that the highest DPPH scavenging activity was reported for the Tualang honey with a reduction percentage of (59.89%) and the Acacia honey was characterized by the lowest reduction percentage121. Serbian polyfloral honeys were stated to be with radical scavenging activity ranging from 1.31% up to 25.61%122, which is very low compared to result of the polyfloral honeys of this study. Ethiopian honeys from different geographical origins were with DPPH reduction range of (23.74–40.11%)43.
FRAP
The FRAP standard curve had the equation of (Y = -0.1403 + 1.3557) and the R2 of 0.9869 (Fig. 2). The results of the FRAP assay of the studied Asir and Jazan honey samples were comparable except for the Ziziphus honey which significantly increased in the Asir honey compared to Jazan honey (p-value ≤ 0.001). The floral origin of Asir honey had significant effect on the FRAP assay (Table 3). The results of the FRAP assay (mg of Fe3+/100g) for the studied honeys were Acacia Asir (205.77 ± 113.29), Acacia Jazan (240.92 ± 75.17), Ziziphus Asir (580.20 ± 112.28), Ziziphus Jazan (142.20 ± 83.16), Polyfloral Asir (233.04 + 163.13) and Polyfloral Jazan (304.26 ± 157.47) (Fig. 6).
Al-Hindi et al. (2011)123 studied the antioxidant and antibacterial activities of Saudi unifloral (including Acacia and Ziziphus) and multifloral honey samples and found that the FRAP activity of Acacia honey was 0.4833 ± 0.011 mg of Fe3+/2g of honey (24.165 mg of Fe3+/100g) while the FRAP activity of the two Ziziphus honeys were 0.5958 ± 0.01 and 0.7837 ± 0.009 mg of Fe3+/2g of honey (29.79 and 39.185 mg of Fe3+/100g). A Malaysian Acacia honey was the FRAP activity of 82.386 ± 5.930 mg TE/100g of honey which is very low compared to the findings of this study which may be due to the different geographical origin and the Acacia subspecies124. The antioxidant activities of honey samples depend on thier geographical and floral origins51,52,125.
AEAC
The straight line equation of the AEAC assay was (Y = -458.98X + 1.3234) and the R2 was 0.9922 (Fig. 2). The mean and the Standards deviation of the AEAC (mg of VitC/ 100g) were as follows: Acacia Asir (308.519 ± 172.47), Acacia Jazan (323.5369 ± 82.24), Ziziphus Asir (363.20 ± 340.18), Ziziphus Jazan (135.42 ± 99.53), Polyfloral Asir (485.97 ± 312.14) and Polyfloral Jazan (330.703 ± 88.31) (Fig. 6). The level of vitamin C equivalent antioxidant capacity was significantly increased in the Polyfloral honey of Asir region compared to the Acacia honey of Asir region and to the Polyfloral honey of Jazan region (P-value ≤ 0.05) (Table 3).
Honey samples from different geographical origins and an unidentified floral origins in Ethiopia were with Ascorbic acid equivalent capacity ranging from 16.23 to 26.59 mg VitC/ 100g43. Acacia and multifloral honey samples from Burkina Faso were stated to have an AEAC of 17.50 ± 0.05 mg/100g and (from 10.20 ± 0.59 to 37.87 ± 0.26 mg/100g), respectively126. Similar to our findings that polyfloral honey samples were with the highest AEAC, Islam et al. (2012)127 stated that multifloral honey samples from Bangladesh had the highest AEAC compared to monofloral honeys (34.1 ± 1.4mg/100 g). German Acacia honey and Pakistani Ziziphus jujube scored AEAC of 31.09 ± 0.008 and 26.66 ± 0.71 mg/100g, respectively128.
Antioxidant Short peptides of the honey samples and their antioxidant activity
Honey samples containing short peptides with possible antioxidant activity because of their amino acid content and sequence were with DPPH radical scavenging, FRAP and AEAC. The antioxidant power increased with the increased number of the antioxidant short peptides (Fig. 7). The antioxidant activity of the short peptides is referred to their content and sequence of amino acids and their mechanism is through proton electron donation, metal chelating and regulation of the activity of antioxidant enzymes129,130. The antioxidant activity of the short peptides is studied by many previous studies63,64,67.
Pearson’s correlations
The Pearson’s correlation showed that there were significant correlations between the DPPH radical scavenging and the FRAP, AEAC and the total protein. The AEAC was significantly correlated to the DPPH and total proteins. The FRAP was significantly correlated to the DPPH and total phenolic acids (Table 6). The results showed the capability of the proteins to act as radical scavenger and as detoxifier through proton donation. Many previous studies proved the role of proteins as antioxidant molecules131,132,133,134.
Limitations of the study
The limitations of this study include the : 1) small number of samples and 2) other factors known to affect the chemical composition and biological activities of honey are not considered such as honeybee species, nutrition and health, bee keeping practices and soil composition.
Conclusions
-
1.
Jazan honey samples were characterized by high number of short peptides compared to the samples from Asir region.
-
2.
Short peptides were produced in the honey samples as a result for the action of proteases on honeybee proteins. The proteases are produced by the honeybees (pepsin, trypsin and chymotrypsin) or bacteria (clostripian or Arg-C, flavastascin or Asp-N, Lysylyl endopeptidase or Lys-C and peptidyl lysine metalloendopeptidase or Lys-N).
-
3.
The short peptides were produced from honeybee proteins including antibacterial proteins (Definsin-1, Definsin-2, Apidermin-2, Abaecin and Hymenoptaecin), neuropeptides (PBAN-type neuropeptide, Mysosuppresin, Orocokinin, Pro-corazonin, and Brain peptide MVPVP), transcription factors (Hunchback protein and Homeobox proteins H4, H17, H90, E30 and E60), royal jelly proteins (Apisimin), hormones (Diuretic hormone class-2 and Protein krueppel), Lipoproteins (Larval specific very high density lipoprotein), enzyme inhibitors (Chymotrypsin inhibitor) and enzymes (ATP synthase).
-
4.
The antioxidant activity of the honey samples increases with the increase of the number of the antioxidant short peptide they contain.
-
5.
The total protein concentration was directly and significantly correlated to the antioxidant activity including the radical scavenging (DPPH) and proton donation (AEAC).
Data availability
The raw data associated with this study are available upon request from the corresponding author at: mohammedelimam@yahoo.com.
References
Codex Alimentarius. Standard for honey. Adopted in 1981. Revised in 1987, 2001. Amended in 2019, 2022. Available at: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252Fcxs_012e.pdf.
Ricigliano, V. A., Williams, S. T. & Oliver, R. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 18, 52 (2022).
Paray, B. A. et al. Honeybee nutrition and pollen substitutes: A review. Saudi J. Biol. Sci. 28, 1167–1176 (2021).
Khan, S. U. et al. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 25, 320–325 (2018).
Pita-Calvo, C. & Vázquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 59, 79–87 (2017).
Samarghandian, S., Farkhondeh, T. & Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 9, 121–127 (2017).
Ibrahim, H. R., Nanbu, F. & Miyata, T. Potent antioxidant peptides derived from honey major protein enhance tolerance of eukaryotic cells toward oxidative stress. Food Prod. Process. Nutr. 3, 11 (2021).
Kwakman, P. H. et al. How honey kills bacteria. FASEB J. 24, 2576–2582 (2010).
Alaerjani, W. M. A. et al. Biochemical reactions and their biological contributions in honey. Molecules 27, 4719 (2022).
Apostolopoulos, V. et al. A global review on short peptides: Frontiers and perspectives. Molecules 26, 430 (2021).
ALaerjani, W. et al. Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values. Open Chem. 19, 1171–1182 (2021).
Godos, J. et al. Dietary phenolic acids and their major food sources are associated with cognitive status in older Italian adults. Antioxidants 10, 700 (2021).
Kumar, N. & Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 24, e00370 (2019).
Pereira, D. M., Valentão, P., Pereira, J. A. & Andrade, P. B. Phenolics: From chemistry to biology. Molecules 14, 2202–2211 (2009).
Cheung, Y., Meenu, M., Yu, X. & Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 22, 290–308 (2019).
Cianciosi, D. et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 23, 2322 (2018).
Safe, S. et al. Flavonoids: structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 37, 147–162 (2021).
Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 63, 1035–1042 (2000).
Pyrzynska, K. & Biesaga, M. Analysis of phenolic acids and flavonoids in honey. Trends Anal. Chem. 28, 893–902 (2009).
Ali, S. S., Ahsan, H., Zia, M. K., Siddiqui, T. & Khan, F. H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 44, e13145 (2020).
Flieger, J., Flieger, W., Baj, J. & Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 14, 4135 (2021).
Bogdanov, S., Jurendic, T., Sieber, R. & Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 27, 677–689 (2008).
Becerril-Sánchez, A. L., Quintero-Salazar, B., Dublán-García, O. & Escalona-Buendía, H. B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 10, 1700 (2021).
Taleuzzaman, M., Kala, C. & Gilani, S. J. Validation, chemical composition, and stability of honey from Indian Himalayas. In Therapeutic Applications of Honey and its Phytochemicals (eds Rehman, M. U. & Majid, S.) (Springer, Singapore, 2020). https://doi.org/10.1007/978-981-15-6799-5_5.
Wang, H. et al. (2023), Composition, functional properties and safety of honey: A review. J. Sci. Food Agric. 103, 6767–6779 (2023).
Solayman, M. et al. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 15, 219–233 (2016).
DeNicola, E., Aburizaiza, O. S., Siddique, A., Khwaja, H. & Carpenter, D. O. Climate change and water scarcity: The case of Saudi Arabia. Ann. Global Health 81, 342–353 (2015).
Mahmoud, S. H., Alazba, A. A. & Amin, M. T. Identification of potential sites for groundwater recharge using a GIS-based decision support system in Jazan Region-Saudi Arabia. Water Resour. Manag. 28, 3319–3340 (2014).
Youssef, A. M., Pradhan, B., Sabtan, A. A. & El-Harbi, H. M. Coupling of remote sensing data aided with field investigations for geological hazards assessment in Jazan area, Kingdom of Saudi Arabia. Environ. Earth Sci. 65, 119–130 (2012).
Louveaux, J., Maurizio, A. & Vorwohl, G. Methods of melissopalynology. Bee World 59, 139–154 (1978).
Bogdanov, S. Harmonized methods of the International Honey Commission. Determination of moisture refractometric method. International Honey Commission, 2009, pp.10–15. Available at: https://ihc-platform.net/ihcmethods2009.pdf.
Bogdanov, S. Harmonized methods of the International Honey Commission. Determination of pH and Free acidity. International Honey Commission, 2009, pp. 21–25. Available at: https://ihc-platform.net/ihcmethods2009.pdf.
Bogdanov, S. Harmonized methods of the International Honey Commission. Determination of electrical conductivity. International Honey Commission, 2009, pp.16–18. Available at: https://ihc-platform.net/ihcmethods2009.pdf.
Bogdanov, S. Harmonized methods of the International Honey Commission. Determination of sugars by HPLC. International Honey Commission, 2009, pp. 46–48. Available at: https://ihc-platform.net/ihcmethods2009.pdf.
Azeredo, L. D. A. C., Azeredo, M. A. A., de Souza, S. R. & Dutra, V. M. L. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 80, 249–254 (2003).
Megazyme. Catalase assay procedure. 2019. Available at: https://www.megazyme.com/documents/Assay_Protocol/K-CATAL_DATA.pdf.
Neogen and Megazyme. Catalase assay kit. Available at: https://www.megazyme.com/Catalase-Assay-Kit. Accessed on 25th July 2023.
Agbor, G. A., Vinson, J. A. & Donnelly, P. E. Folin-Ciocalteau reagent for polyphenolic assay. Int. J. Food Sci. Nutr. Diet. 3, 147–156 (2014).
Meda, A., Lamien, C. E., Romito, M., Millogo, J. & Nacoulm, O. G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91, 571–577 (2005).
Moon, J. K. & Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 57, 1655–1666 (2009).
Munteanu, I. G. & Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 22, 3380 (2021).
Ahmed, M. et al. Physiochemical, biochemical, minerals content analysis, and antioxidant potential of national and international honeys in Pakistan. J. Chem. 2016, 8072305 (2016).
Yayinie, M. et al. Polyphenols, flavonoids, and antioxidant content of honey coupled with chemometric method: Geographical origin classification from Amhara region, Ethiopia. Int. J. Food Prop. 25, 76–92 (2022).
Al-Ghamdi, A. A. & Ansari, M. J. Biological and therapeutic roles of Saudi Arabian honey: A comparative review. J. King Saud Univ. Sci. 33, 101329 (2021).
Ansari, M. J. et al. Validation of botanical origins and geographical sources of some Saudi honeys using ultraviolet spectroscopy and chemometric analysis. Saudi J. Biol. Sci. 25, 377–382 (2018).
Al-Ghamdi, A. A., Tadesse, Y. & Adgaba, N. Evaluation of major Acacia species in the nursery towards apicultural landscape restoration around Southwestern Saudi Arabia. Saudi J. Biol. Sci. 27, 3385–3389 (2020).
Saudi Food and Drug Authority. Guide to the Production, Trading and Import of Honey and Bee Products; Saudi Food and Drug Authority Publications: Riyadh, Saudi Arabia, 2021; pp. 14–18. Available at: https://sfda.gov.sa/sites/default/files/2021-03/%D8%AF%D9%84%D9%8A%D9%84%20%D8%A7%D9%84%D8%B9%D8%B3%D9%84%202021.pdf.
El Sohaimy, S. A., Masry, S. H. D. & Shehata, M. G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 60, 279–287 (2015).
Scholz, M. B. D. S. et al. Indication of the geographical origin of honey using its physicochemical characteristics and multivariate analysis. J. Food Sci. Technol. 57, 1896–1903 (2020).
Schiassi, M. C. E. V. et al. Quality of honeys from different botanical origins. J. Food Sci. Technol. 58, 4167–4177 (2021).
Alaerjani, W., Abu Melha, S. & Mohammed, M. Abstract 1029 Studies on honey samples from the southwestern region of Saudi Arabia: Their antioxidant activities related to their content of essential amino acids and proteins. J. Biol. Chem. 300, 105966 (2024).
Mohammed, M. E. A. Factors affecting the physicochemical properties and chemical composition of bee’s honey. Food Rev. Int. 38, 1330–1341 (2022).
Bocian, A., Buczkowicz, J., Jaromin, M., Hus, K. K. & Legáth, J. An effective method of isolating honey proteins. Molecules 24, 2399 (2019).
Nikolić, T. V. et al. Environmental effects on superoxide dismutase and catalase activity and expression in honey bee. Arch. Insect Biochem. Physiol. 90, 181–194 (2015).
Игнaтeнкo, A. A., Peпкинa, H. C. & Taлaнoвa, B. B. Catalase and peroxidase contribution to promoting cold tolerance in wheat. Proc. Karelian Res. Centre Russ. Acad. Sci. 4, 74–83 (2018).
Franchini, R. A. A., Matos, M. A. C. & Matos, R. C. Amperometric determination of catalase in Brazilian commercial honeys. Anal. Lett. 44, 232–240 (2011).
Labrou, N. E. & Rigden, D. J. The structure–function relationship in the clostripain family of peptidases. Eur. J. Biochem. 271, 983–992 (2004).
Prasad, L., Leduc, Y., Hayakawa, K. & Delbaere, L. T. J. The structure of a universally employed enzyme: V8 protease from Staphylococcus aureus. Acta Cryst. D, 256–259 (2004).
Grenda, T. et al. Clostridium botulinum spores in Polish honey samples. J. Vet. Sci. 19, 635–642 (2018).
Nevas, M., Lindström, M., Hautamäki, K., Puoskari, S. & Korkeala, H. Prevalence and diversity of Clostridium botulinum types A, B, E and F in honey produced in the Nordic countries. Int. J. Food Microbiol. 105, 145–151 (2005).
Al-Waili, N., Salom, K., Al-Ghamdi, A. & Ansari, M. J. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci. World J. 2012, 930849 (2012).
Anjum, S. I. et al. Honey bee gut an unexpected niche of human pathogen. J. King Saud Univ. Sci. 33, 101247 (2021).
Perry, M. W., Bothma, J. P., Luu, R. D. & Levine, M. Precision of hunchback expression in the Drosophila embryo. Curr. Biol. 22, 2247–2252 (2012).
Jiang, B., Zhang, X., Yuan, Y., Qu, Y. & Feng, Z. Separation of Antioxidant Peptides from Pepsin Hydrolysate of Whey Protein Isolate by ATPS of EOPO Co-polymer (UCON)/Phosphate. Sci. Rep. 7, 13320 (2017).
Peighambardoust, S. H., Karami, Z., Pateiro, M. & Lorenzo, J. M. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules 11, 631 (2021).
Daskaya-Dikmen, C., Yucetepe, A., Karbancioglu-Guler, F., Daskaya, H. & Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 9, 316 (2017).
El Mehdi, I. et al. Analytical methods for honeybee venom characterization. J. Adv. Pharm. Technol. Res. 13, 154–160 (2022).
Aledo, J. C. Methionine in proteins: The Cinderella of the proteinogenic amino acids. Protein Sci. 28, 1785–1796 (2019).
Mishra, A. K., Choi, J., Moon, E. & Baek, K. H. Tryptophan-rich and proline-rich antimicrobial peptides. Molecules 23, 815 (2018).
Kim, B. Y. et al. Antimicrobial Activity of Apidermin 2 from the Honeybee Apis mellifera. Insects 13, 958 (2022).
Teixeira, L. D., Silva, O. N., Migliolo, L., Fensterseifer, I. C. M. & Franco, O. L. In vivo antimicrobial evaluation of an alanine-rich peptide derived from Pleuronectes americanus. Peptides 42, 144–148 (2013).
Marciniak, P., Kuczer, M. & Rosinski, G. New physiological activities of myosuppressin, sulfakinin and NVP-like peptide in Zophobas atratus beetle. J. Comp. Physiol. B 181, 721–730 (2011).
Stangier, J., Hilbich, C., Burdzik, S. & Keller, R. Orcokinin: A novel myotropic peptide from the nervous system of the crayfish, Orconectes limosus. Peptides 13, 859–864 (1992).
Dong, N. et al. Strand length-dependent antimicrobial activity and membrane-active mechanism of arginine- and valine-rich β-hairpin-like antimicrobial peptides. Antimicrob. Agents Chemother. 56, 2994–3003 (2012).
van Kan, E. J., Demel, R. A., van der Bent, A. & de Kruijff, B. The role of the abundant phenylalanines in the mode of action of the antimicrobial peptide clavanin. Biochim. Biophys. Acta 1615, 84–92 (2003).
Choi, M. Y. & Vander Meer, R. K. Identification of a new member of the PBAN family of neuropeptides from the fire ant, Solenopsis invicta. Insect Mol Biol. 18, 161–169 (2009).
Ceremuga, M. et al. Melittin-A natural peptide from bee venom which induces apoptosis in human leukaemia cells. Biomolecules 10, 247 (2020).
Mark, M., Rijli, F. & Chambon, P. Homeobox genes in embryogenesis and pathogenesis. Pediatr. Res. 42, 421–429 (1997).
Akbarian, M., Khani, A., Eghbalpour, S. & Uversky, V. N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 23, 1445 (2022).
Gu, H., Han, S. M. & Park, K. K. Therapeutic effects of apamin as a bee venom component for non-neoplastic disease. Toxins 12, 195 (2020).
Ziai, M. R., Russek, S., Wang, H. C., Beer, B. & Blume, A. J. Mast cell degranulating peptide: A multi-functional neurotoxin. J. Pharm. Pharmacol. 42, 457–461 (1990).
Casteels, P. et al. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera). Eur. J. Biochem. 187, 381–386 (1990).
Ma, M., Zhao, R., Li, X., Jing, M., Song, R., Fan, J. Biological properties of arginine-rich peptides and their application in cargo delivery to cancer. Curr. Drug Deliv. 2023.
Liu, B. R., Chiou, S. H., Huang, Y. W. & Lee, H. J. Bio-membrane internalization mechanisms of arginine-rich cell-penetrating peptides in various species. Membranes 12, 88 (2022).
Brockmann, A. et al. Quantitative peptidomics reveal brain peptide signatures of behavior. Proc. Natl. Acad. Sci. U.S.A. 106, 2383–2388 (2009).
Schaefer, L. & Iozzo, R. V. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 283, 21305–21309 (2008).
Stavropoulou, E. et al. Microbial community structure among honey samples of different pollen origin. Antibiotics 12, 101 (2023).
Tarentino, A.L. Flavastacin. Editor(s): Alan J. Barrett, Neil D. Rawlings, J. Fred Woessner. Handbook of Proteolytic Enzymes (Second Edition) (Academic Press, 2004), pp 631–632.
Even, N., Devaud, J. M. & Barron, A. B. General stress responses in the honey bee. Insects 3, 1271–1298 (2012).
Decker, A. P., Mechesso, A. F. & Wang, G. Expanding the landscape of amino acid-rich antimicrobial peptides: Definition, deployment in nature, implications for peptide design and therapeutic potential. Int. J. Mol. Sci. 23, 12874 (2022).
Adriano, M. et al. Arginine- and lysine-rich peptides: Synthesis, characterization and antimicrobial activity. Lett. Drug Des. Discov. 15(3), 1341 (2018).
Predel, R., Neupert, S., Russell, W. K., Scheibner, O. & Nachman, R. J. Corazonin in insects. Peptides 28, 3–10 (2007).
Daliri, E. B., Oh, D. H. & Lee, B. H. Bioactive peptides. Foods 6, 32 (2017).
Osherovich, L. Z. & Weissman, J. S. Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI(+)] prion. Cell 106, 183–194 (2001).
Shipman, B. A., Ryan, R. O., Schmidt, J. O. & Law, J. H. Purification and properties of a very high density lipoprotein from the hemolymph of the honeybee Apis mellifera. Biochemistry 26, 1885–1889 (1987).
Cierpicki, T., Bania, J. & Otlewski, J. NMR solution structure of Apis mellifera chymotrypsin/cathepsin G inhibitor-1 (AMCI-1): Structural similarity with Ascaris protease inhibitors. Protein Sci. 9, 976–984 (2000).
Yao, S. Y. et al. A peptide rich in glycine-serine-alanine repeats ameliorates Alzheimer-type neurodegeneration. Br. J. Pharmacol. 180, 1878–1896 (2023).
Bíliková, K. et al. Apisimin, a new serine-valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: Purification and molecular characterization. FEBS Lett. 528, 125–129 (2002).
Chen, H. et al. Identification of antioxidative peptides from defatted walnut meal hydrolysate with potential for improving learning and memory. Food Res. Int. 78, 216–223 (2015).
Kumar, K., Moirangthem, R. & Kaur, R. Genome protection: Histone H4 and beyond. Curr. Genet. 66, 945–950 (2020).
Huan, Y., Kong, Q., Mou, H. & Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 11, 582779 (2020).
Il’iasov, R. A., Gaĭfullina, L. R., Saltykova, E. S., Poskriakov, A. V. & Nikolenko, A. G. Defensins in the honeybee antinfectious protection] [Abstract]. Zh. Evol. Biokhim. Fiziol. 48, 425–432 (2012).
Miller, A. et al. Zn-enhanced Asp-rich antimicrobial peptides: N-terminal coordination by Zn(II) and Cu(II), which distinguishes Cu(II) binding to different peptides. Int. J. Mol. Sci. 22, 6971 (2021).
Rahiman, S. S. F., Morgan, M., Gray, P., Shaw, P. N. & Cabot, P. J. Inhibitory effects of dynorphin 3–14 on the lipopolysaccharide-induced toll-like receptor 4 signalling pathway. Peptides 90, 48–54 (2017).
Dini, I., De Biasi, M. G. & Mancusi, A. An overview of the potentialities of antimicrobial peptides derived from natural sources. Antibiotics 11, 1483 (2022).
Kawaguchi, T. et al. Wheat-bran autolytic peptides containing a branched-chain amino acid attenuate non-alcoholic steatohepatitis via the suppression of oxidative stress and the upregulation of AMPK/ACC in high-fat diet-fed mice. Int. J. Mol. Med. 39, 407–414 (2017).
Gopal, R., Seo, C. H., Song, P. I. & Park, Y. Effect of repetitive lysine-tryptophan motifs on the bactericidal activity of antimicrobial peptides. Amino Acids 44, 645–660 (2013).
Guo, H., Kouzuma, Y. & Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 113, 238–249 (2009).
Sun, Y., Fu, D., Liu, B., Wang, L. & Chen, H. Roles of Krüppel Homolog 1 and broad-complex in the development of Dendroctonus armandi (Coleoptera: Scolytinae). Front. Physiol. 13, 865442 (2022).
Lointier, M., Dussouillez, C., Glattard, E., Kichler, A. & Bechinger, B. Different biological activities of histidine-rich peptides are favored by variations in their design. Toxins 13, 363 (2021).
Casteels, P., Ampe, C., Jacobs, F. & Tempst, P. Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J. Biol. Chem. 268, 7044–7045 (1993).
Ma, H. et al. Evolution of antimicrobial cysteine-rich peptides in plants. Plant Cell Rep. 42, 1517–1527 (2023).
Suleiman, M. H. A., ALaerjani, W. M. A. & Mohamed, M. E. A. Influence of altitudinal variation on the total phenolic and flavonoid content of Acacia and Ziziphus honey. Int. J. Food Prop. 23, 2077–2086 (2020).
Mohammed, M. E. A. et al. Acacia honey from different altitudes: Total phenols and flavonoids, laser-induced fluorescence (LIF) spectra, and anticancer activity. J. Int. Med. Res. 48, 300060520943451 (2020).
Vazquez, L., Armada, D., Celeiro, M., Dagnac, T. & Llompart, M. Evaluating the presence and contents of phytochemicals in honey samples: Phenolic compounds as indicators to identify their botanical origin. Foods 10, 2616 (2021).
Krpan, M. et al. Antioxidant activities and total phenolics of acacia honey. Czech J. Food Sci. 27, S245–S247 (2009).
Moniruzzaman, M., Sulaiman, S. A., Azlan, S. A. M. & Gan, S. H. Two-year variations of phenolics, flavonoids and antioxidant contents in acacia honey. Molecules 18, 14694–14710 (2013).
Al-Farsi, M., Al-Amri, A., Al-Hadhrami, A. & Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 4, e00874 (2018).
Al, M. L. et al. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 112, 863–867 (2009).
Blasa, M. et al. Raw millefiorihoney is packed full of antioxidants. Food Chem. 97, 217–222 (2006).
Alshammari, G. M. et al. Phenolic compounds, antioxidant activity, ascorbic acid, and sugars in honey from ingenious Hail Province of Saudi Arabia. Appl. Sci. 12, 8334 (2022).
Moniruzzaman, M., Khalil, M. I., Sulaiman, S. A. & Gan, S. H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 13, 43 (2013).
Gašić, U. et al. Phenolic profile and antioxidant activity of Serbian polyfloral honeys. Food Chem. 145, 599–607 (2014).
Al-Hindi, R. R., Bin-Masalam, M. S. & El-Shahawi, M. S. Antioxidant and antibacterial characteristics of phenolic extracts of locally produced honey in Saudi Arabia. Int. J. Food Sci. Nutr. 62, 513–517 (2011).
Chua, L. S., Rahaman, N. L. A., Adnan, N. A. & Tan, T. T. E. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 313798 (2013).
Mărgăoan, R. et al. Monofloral honeys as a potential source of natural antioxidants, minerals and medicine. Antioxidants 10, 1023 (2021).
Meda, A., Lamien, C. E., Romito, M., Millogo, J. & Nacoulma, O. G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91, 571–577 (2005).
Islam, A. et al. Physicochemical and antioxidant properties of Bangladeshi honeys stored for more than one year. BMC Complement. Altern. Med. 12, 177 (2012).
Zou, T. B., He, T. P., Li, H. B., Tang, H. W. & Xia, E. Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 21, 72 (2016).
Esfandi, R., Walters, M. E. & Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 5, e01538 (2019).
Ahmed, M. et al. Physiochemical, biochemical, minerals content analysis, and antioxidant potential of national and international honeys in Pakistan. J. Chem. 2016, 8072305 (2016).
Irato, P. & Santovito, G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 10, 579 (2021).
Zhu, Y., Lao, F., Pan, X. & Wu, J. Food protein-derived antioxidant peptides: Molecular mechanism, stability and bioavailability. Biomolecules 12, 1622 (2022).
Feng, P. D. H., Lin, H. & Chen, W. A. O. D. The antioxidant protein database. Sci. Rep. 7, 7449 (2017).
Funding
The authors extended their appreciation to the Deanship of Scientific Research and Graduate Studies at King Khalid University for funding this work through the Large Research Project under the grant number RGP.2/377/44. Moreover, this research was logistically supported by the Saudi Aramco.
Author information
Authors and Affiliations
Contributions
Alaerjani suggested the research subject; Mohammed and Alaerjani designed the research; Alaerjani did all the practical activities. Mohammed did the statistical analysis; Alaerjani drafted the manuscript. Mohammed revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alarjani, W.M.A., Mohammed, M.E.A. Antioxidant activities of Saudi honey samples related to their content of short peptides. Sci Rep 14, 24318 (2024). https://doi.org/10.1038/s41598-024-74824-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74824-4