Abstract
Peritoneal fibrosis has been linked to hypoxia-inducible factor 1-alpha (HIF-1α) as well as O-linked-N-acetylglucosaminylation (O-GlcNAcylation) in peritoneal dialysis (PD). Genistein, recognized for its HIF-1α inhibitory and antifibrotic effects, presents a potential intervention against peritoneal mesothelial-mesenchymal transition (MMT) as well as fibrosis in PD. This study employed human peritoneal mesothelial cells (HPMCs) together with adenine-induced chronic kidney disease (CKD) rats undergoing peritoneal dialysis to explore Genistein’s role in high glucose-induced peritoneal MMT and fibrosis. Our findings reveal that Genistein exerts anti-MMT and anti-fibrotic effects by inhibiting HIF-1α in HPMCs under high glucose conditions. Genistein inhibited O-GlcNAcylation status of HIF-1α through the mTOR/O-GlcNAc transferase (OGT) pathway, promoting its ubiquitination as well as the subsequent proteasomal degradation. In adenine-induced CKD rats undergoing peritoneal dialysis, Genistein suppressed the mTOR/OGT expression and reduced the abundance of O-GlcNAcylation along with HIF-1α in the peritoneum. Additionally, Genistein protected against increased peritoneal thickness, fibrosis, and angiogenesis, while improving peritoneal function. Based on our results, it could be inferred that Genistein might inhibit the abundance of HIF-1α via the mTOR/OGT pathway, thereby ameliorating MMT as well as fibrosis in PD.
Similar content being viewed by others
Introduction
As a renal replacement technique, peritoneal dialysis (PD) is utilized for individuals suffering from end-stage renal disease. Chronic exposure to high glucose induces fibrosis and functional impairment of the peritoneum, compelling patients to transition towards hemodialysis or succumb to associated complications1. Glucose/hypoxia/glucose transporter 1 (GLUT-1) hypothesis, initially posited by Professor Krediet2,3, states that increased glucose absorption leads to an elevated intracellular NADH/NAD + ratio, termed pseudo-hypoxia. Consequently, this intracellular hypoxia upregulates the hypoxia-inducible factor-1 (HIF-1) gene, thereby facilitating the transcription of GLUT-1 and pro-fibrotic genes, contributing to peritoneal fibrosis (PF) and the development of ultrafiltration failure. HIF-1α is recognized as the primary response factor under hypoxic conditions4,5. Studies have confirmed the involvement of hypoxia as well as HIF-1α in the progression of PF. For instance, hypoxia increases HIF-1α’s abundance within human peritoneal mesothelial cells (HPMCs), promoting cellular mesothelial-to-mesenchymal transition (MMT) alongside fibrosis6. Intermittent hypoxia enhances rat peritoneal HIF-1α expression and increases peritoneal thickness7. Mitigation of PF has been observed through interventions aimed at impeding the hypoxic response or inhibiting HIF-1α8–10.
Post-translational modifications (PTMs) of proteins, involving structural adjustments mediated by diverse modifying enzymes, represent a crucial aspect influencing protein functionality. PTMs (like phosphorylation, acetylation, as well as ubiquitination) have attracted widespread attention due to their crucial roles in regulating cellular processes. O-linked-N-acetylglucosaminylation (O-GlcNAcylation), an atypical glycosylation, refers to the PTM where the attachment of a single O- linked N- -acetylglucosamine (O-GlcNAc) occurs on specific amino acid residues, including serine and threonine. This process is regulated through O-GlcNAc transferase (OGT) alongside O-GlcNAcase (OGA)11. O-GlcNAcylation exerts regulatory control over various aspects of protein functionality, including protein folding, subcellular localization, degradation, enzymatic activity, and the dynamic interplay among different PTMs12. Notably, elevated glucose concentrations have been reported to augment overall O-GlcNAcylation levels in HPMCs13. Based on our previous research, we have demonstrated that O-GlcNAcylation is crucial for the regulation of HIF-1α expression, and both of them induced MMT alongside fibrosis in HPMCs14. Therefore, HIF-1α and O-GlcNAcylation represent potential targets for preventing and treating PF.
Genistein, an isoflavone found in soybeans, exerts diverse molecular effects, including the suppression of inflammation, facilitation of apoptosis and metabolic pathways15. Notably, it demonstrates antifibrotic properties, attenuating renal fibrosis in a unilateral ureteral occlusion model16, improving pressure overload-induced cardiac dysfunction and mitigating interstitial fibrosis in mice17. Additionally, studies have reported that Genistein inhibits the protein expression of fibronectin as well as the connective-tissue growth factor in HPMCs stimulated by advanced glycation end products18. This suggests that Genistein may exhibit an anti-fibrotic effect on PD, while its exact role and mechanism are unclear. Previous studies have demonstrated that Genistein downregulates HIF-1α expression and inhibits the transcription of its downstream target genes19,20, we postulate that Genistein may ameliorate PF in PD by suppressing HIF-1α expression. Our preliminary experimental results indicate that OGT/O-GlcNAcylation regulates HIF-1α abundence14, and mTOR is involved in the regulation of OGT expression21,22,23, which is also modulated by Genistein via estrogen receptor24. Therefore, it is plausible that Genistein’s effect on PF may involve regulating HIF-1α through the mTOR/OGT signaling pathway.
Materials and methods
Cell culture
The HPMCs (HMrSV5 cell line, purchased from Shanghai Zishi Biotechnology Co., Ltd, China) were cultured in 1640 RPMI medium (containing 0.2% glucose), supplemented with fetal bovine serum (FBS, 10%) alongside penicillin/streptomycin (1%). The culturing process was conducted at a temperature of 37 °C in a 5% CO2 atmosphere. The control group was maintained in the complete medium, which was specifically referred to as low-glucose (LG) medium. Once the cells reached 3 to 5 generations and confluence, they were subjected to either LG medium or high-glucose (HG) medium with a glucose concentration of 2.5%. Various pharmacological agents, including FG-4592 (T2515, TargetMol), PUGNAc (ab144670, Abcam), MG-132 (T2154, TargetMol), MHY1485 (HY-B0795, MCE), ICI 182,780 (HY-13636, MCE) and Genistein (IG0110, Solarbio, China), were introduced into LG / HG medium at various concentrations, in accordance with the figure legends.
Cell viability and toxicity test
The HPMCs were plated in a 96-well plate and cultured in LG medium for a duration of 24 h, and then the wells were replaced with fresh LG medium with or without different concentrations of Genistein for a duration of 48 h. Subsequently, the assessment of cell viability was conducted using the Cell Counting Kit-8 reagent (CA1210, Solarbio, China), while cell toxicity was examined utilizing a cytotoxicity detection kit based on the release of lactate dehydrogenase (LDH) (C0016, Beyotime Biotechnology, China), following the guidelines provided by the manufacturer.
OGT overexpression through plasmid transient transfection
Upon reaching a confluence of 70–90%, HPMCs underwent transfection with OGT overexpression plasmids (GV657 vector, digested with BamHI/KpnI, obtained from Shanghai Jikai Gene Chemical Technology Co., Ltd, China) or OGT negative control plasmids utilizing Lipofectamine 2000 transfect reagent (Invitrogen), following the instructions provided by the manufacturer. Following 24 h of transfection, the medium was substituted. Cells were subjected to another culture for a duration of 48 h in LG / HG medium. Alternatively, cells underwent the treatment with Genistein (50 µM) and the incubation for an equivalent duration.
mRNA isolation as well as qRT-PCR
The extraction of cellular total RNA was conducted through a TRIzol Kit (10296028, ThermoFisher) complying with the instructions provided by the manufacturer. Subsequently, each sample underwent reverse transcription into cDNA utilizing a quantitative real-time PCR Kit (R233, Vazyme, China). The qRT-PCR were conducted using the QuantStudio 3 Real-Time PCR System from Thermo Fisher Scientific, adhering to the manufacturer’s instructions. The utilized primers were as follows: HIF-1α, F: ACTGCACAGGCCACATTCACG, R: AATCAGCACCAAGCAGGTCATAGG; β-Actin, F: GCTCACCATGGATGATGATATCGC; R: CACATAGGAATCCTTCTGACCCAT.
Extraction of proteins along with western blotting (WB)
Cells and rat peritoneum were lysed on ice using ristocetin-induced platelet aggregation (RIPA) buffer (R0010, Solarbio, China), supplemented with the inhibitors against protease alongside phosphatase. Following centrifugation at 14,000 g at the temperature of 4 °C for a duration of 5 min, we collected the lysates. Combined with SDS-PAGE loading buffer, the resulting supernatants were subjected to heating at 100 °C for a duration of 10 min. The protein samples were separated using SDS-PAGE on gradient gels, followed by transferring onto nitrocellulose membranes in equivalent amounts (Merck Millipore Ltd). Following blocking with nonfat milk (5%), the membranes were subjected to overnight incubation with primary antibodies, including Anti-O-GlcNAc (RL2, ab2739, Abcam), anti-OGT (F-12, sc-74546, Santa Cruz Biotechnology), anti- OGA (G-12, sc-376429, Santa Cruz Biotechnology), anti-HIF-1α (28b, sc-13515, Santa Cruz Biotechnology), anti-ubiquitin (P4D1, sc-8017, Santa Cruz Biotechnology), and anti-p-mTOR (AP0094, ABclonal, China), anti-mTOR (AF6308, Affinity, China), anti-fibronectin (A12932, Abclonal, China), anti-COL1A2 (A5786, Abclonal, China), anti-E-Cadherin (A11509, Abclonal, China), anti-α-SMA (A17910, Abclonal, China), and anti-β-Actin (AC038, Abclonal, China) at the temperature of 4 °C. Next, the membranes were subjected to incubation with secondary antibodies at room temperature (RT) for a duration of 1 h. Protein visualization was accomplished utilizing an ECL chemiluminescent substrate in conjunction with a GelView 6000 Pro system (BLT, China).
Immunoprecipitation
Cell lysis was carried out utilizing a RIPA lysis buffer (R0010, Solarbio, China) supplemented with inhibitors against proteases as well as phosphatases. The lysis process was conducted on ice. Subsequently, the lysates were acquired by subjecting them to centrifugation (14,000 g) at 4 °C for a duration of 5 min. The primary anti-HIF-1α antibody (28b, sc-13515, Santa Cruz Biotechnology) underwent incubation with Protein A/G Magnetic Beads (HY-K0202, MCE) on a rotary shaker for 30 min. Following this, magnetic beads were subjected to a meticulous washing process with phosphate-buffered saline containing 0.2% Tween (PBST) and then incubated with the obtained supernatants for an additional 30 min on a rotary shaker. After an additional wash, elution was performed using an SDS-PAGE loading buffer at the temperature of 100 °C for a duration of 10 min. Finally, the magnetic beads were subjected to boiling, and the resulting supernatant was obtained for subsequent detection.
Animal models and experimental groups
All animal-related experimental procedures conducted in this study adhere to the guidelines set forth by the National Institutes of Health (NIH) for the use of experimental animals. Additionally, the protocol for this study has been approved by the Ethics Committee of Chu Hsien-I Memorial Hospital at Tianjin Medical University (Approval Number: DXBYY-IACUC-2021042). The reporting of this study follows the ARRIVE guidelines. Forty male Sprague-Dawley rats weighing between 180 and 200 g were obtained from Spelford Biotechnology Co. (Beijing, China). These rats were kept in environments that were free of specific pathogens, where the temperature was maintained at 24 ± 2 °C alongside the humidity at 55 ± 5%. The rats were allowed ad libitum water together with a standard diet, following a light-dark (12 h:12 h) cycle. Following a one-week adaptive housing period, the rats were allocated into two groups in random: the CKD modeling group (n = 34) as well as the control group (n = 6). The CKD modeling group received 300 mg/Kg/d adenine (N0130, Shanghai Yuanju Bio-Tech Co., Ltd, China) through gavage for 30 days, while the control group received saline gavage alone. Both groups were observed for an additional 30 days. Throughout the modeling period, the rats’ body weight, urine volume, plasma creatinine, plasma BUN, and urine acid levels were measured biweekly, with hemoglobin levels assessed on day 0 and day 60. After 60 days of modeling, 18 rats with similar plasma creatinine were selected and randomly assigned to the CKD (n = 6), PD (n = 6), as well as Genistein (n = 6) groups. The PD group was intraperitoneally administered with peritoneal dialysate (20 ml, 4.25%) on a daily basis for a period of 5 weeks, while the Genistein group received intraperitoneal administration of Genistein (MB2170-2, Dalian Meilun Bio-Tech Co., Ltd, China) at 20 mg/kg based on PD. The Genistein dosage was determined with reference to researches by Jia et al.25 as well as Qin et al.17. At the conclusion of the five-week intraperitoneal injection period, evaluations of body weight, urine volume, plasma creatinine, plasma BUN, hemoglobin, and urine acid levels were conducted for all rats. Peritoneal function tests were performed, and subsequently, the animals were euthanized under isoflurane anesthesia. Tissues from the abdominal wall, and peritoneum outside the intraperitoneal injection area were collected for further analysis.
Peritoneal function test
Upon completion of the 5-week drug intervention, a two-day period of rest was instituted to facilitate absorption of residual fluid within the abdominal cavity. Subsequently, the peritoneal dialysate (20 ml, 4.25%) was introduced via intraperitoneal injection. Subsequently, after a two-hour interval, rats were anesthetized using isoflurane anesthesia. The midline of the abdominal cavity was carefully incised, allowing for drainage and collection of the peritoneal fluid. The ultrafiltration volume was determined by the difference between the drained peritoneal liquid volume and the initial 20 ml injection. The glucose and creatinine levels in the peritoneal outflow were tested.
Hematoxylin-eosin (HE) and Masson Trichrome staining
Samples from the abdominal wall were obtained and fixed in a 4% Paraformaldehyde solution for 48 h, after which they underwent paraffin embedding to produce sections. Subsequent staining was performed using the HE Stain Kit (G1120, Solarbio, China) as well as Masson’s Trichrome Stain Kit (G1340, Solarbio, China) in accordance with the manufacturer’s protocol. Stained sections were visualized utilizing a microscope (OLYMPUS IX83, Olympus Co., Japan). Subsequently, the OLYMPUS cellSens Standard 2.1 software (Olympus Co., Japan) was utilized to conduct the thickness measurements.
Peritoneal IF
After OCT embedding, frozen sections were prepared from the tissue of the abdominal wall. After fixation in 4% paraformaldehyde, the sections underwent 0.5% Triton X-100 penetration and 1% BAS sealing. Subsequently, the frozen sections were subjected to overnight incubation with primary antibodies, including anti-O-GlcNAc (RL2, ab2739, Abcam), anti-HIF-1α (28b, sc-13515, Santa Cruz Biotechnology), anti-OGT (F-12, sc-74546, Santa Cruz Biotechnology), and anti-p-mTOR (AP0094, ABclonal, China) at the temperature of 4 °C. Following this, the sections were incubated with TRITC or FITC secondary antibodies at 37 °C for a duration of 1 h. The sections were sealed using a sealing liquid that suppresses fluorescence interference along with DAPI (S2110, Solarbio, China), and subsequently visualized under a fluorescence microscope (OLYMPUS IX83, Olympus Co., Japan).
Immunohistochemistry (IHC) staining
The tissue sections embedded in paraffin were subjected to the processes of dewaxing as well as hydration prior to treatment with hydrogen peroxide (3%) for a duration of 10 min. Next, the sections underwent heat treatment using Tris-EDTA buffer for antigen retrieval, followed by blocking with BSA (1%) for a duration of 30 min at RT. The primary antibody anti-CD31 (AF6191, Affinity, China) was incubated overnight at the temperature of 4 °C. The next day, the sections were rinsed with PBS, and underwent an incubation for one hour at 37 °C with secondary antibody, specifically HRP-conjugated goat anti-rabbit IgG antibody. Color development was achieved through DAB staining for 1–10 min. Subsequent restaining with hematoxylin was performed. Observations and image capture were carried out utilizing a microscope (OLYMPUS IX83, Olympus Co., Japan).
Statistical analysis
Data analyses were achieved through either GraphPad Prism 8 or SPSS 23.0. All data were described with the mean ± standard error of the mean (SEM) following no less than three independent experiments. Besides, Student’s t-test was adopted for group comparisons, whereas ≥ 2 groups underwent one-way analysis of variance (ANOVA) and Tukey’s test. The statistical significance was set at P < 0.05.
Results
Effect of Genistein on O-GlcNAcylation alongside MMT and fibrosis of HPMCs induced by HG and HIF-1α
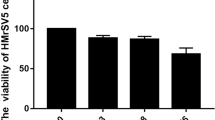
The CCK8 assay results indicated that Genistein inhibited cell proliferation in a concentration-dependent manner, while the LDH release assay showed that varying concentrations of Genistein did not lead to significant cell death toxicity (Fig. 1A). A concentration of 50 µM Genistein was selected, as it maintained over 80% cell viability without observable cytotoxic effects. Genistein is a natural phytoestrogen. An estrogen receptor antagonist was used to observe whether Genistein acts through the estrogen receptor. As shown in Fig. 1B, the estrogen receptor antagonist ICI 182,780 could reverse the inhibitory effect of Genistein on p-mTOR, which has been reported to regulate OGT expression. Figure 1C reveals that Genistein downregulated global O-GlcNAcylation and OGT expression, exhibiting no discernible impact on OGA. Additionally, we observed the effect of Genistein on HIF-1α and MMT. FG-4592 is an inhibitor of hypoxia-inducible factor prolyl hydroxylase, which can increase HIF-1α levels. WB showed that Genistein inhibited the expression levels of HIF-1α, α-SMA, fibronectin, as well as COL1A2 induced by HG conditions or FG-4592, increased the abundance of E-cadherin (Fig. 1D and E).
Effect of Genistein on O-GlcNAcylation and HPMCs’ MMT alongside fibrosis induced by HG and HIF-1α. (A) CCK-8 assay detecting cell viability and LDH-release assay measuring cell cytotoxicity under different concentrations of Genistein. (B) Western blots and measurements of p-mTOR/mTOR were conducted on cells exposed to HG media, with / without the addition of 50 µM Genistein or 100 nM ICI 182,780, for 48 h. (C) Western blots as well as measurements of global O-GlcNAcylation and O-GlcNAcylation enzymes within cells under the exposure of various glucose mediums with / without the addition of 50 µM Genistein for 48 h. (D, E) Western blots as well as measurements of HIF-1α’s abundance and proteins associated with MMT and fibrosis within cells under the exposure of mediums with various concentrations of glucose, with / without 50 µM Genistein or 25 µM FG-4592 addition for 48 h. *, P < 0.05; **, P < 0.001 vs. LG group. #, P < 0.05; ##, P < 0.001 vs. HG group. &, P < 0.05; &&, P < 0.001 vs. HG + FG-4592 group. $, P < 0.05 vs. HG + Genistein group. LG: control medium; HG: 2.5% glucose medium.
Effect of Genistein on HIF-1α abundance, HG-induced MMT alongside fibrosis of HPMCs through inhibiting the mTOR/OGT pathway
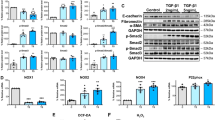
O-GlcNAcylation, a process primarily governed by OGT, plays a pivotal role in influencing cellular dynamics. Overexpression of OGT led to an augmentation in O-GlcNAcylation and HIF-1α expression, accompanied by elevated levels of fibrotic proteins and reduced expression of E-cadherin (Fig. 2A-C). Notably, the reversal of the inhibitory effects of Genistein on O-GlcNAcylation, HIF-1α, MMT alongside fibrosis in HPMCs was evident upon OGT overexpression (Fig. 2A-C). Studies have shown that mTOR can regulate the levels of OGT and O-GlcNAcylation. Whether Genistein regulates OGT and O-GlcNAcylation through mTOR is not clear. As shown in Fig. 2D, HG conditions increased the levels of p-mTOR/mTOR and OGT, while Genistein suppressed their expression. MHY1485, a mTOR agonist, increased the expression of p-mTOR alongside mTOR, while reversing the inhibition of Genistein on OGT, disclosing that Genistein regulates OGT via mTOR.
Effect of Genistein on HIF-1α’s abundance as well as HG-induced MMT and fibrosis of HPMCs through inhibiting the mTOR/OGT pathway. (A) Western blots as well as quantification of global O-GlcNAcylation under HG conditions, with or without OGT overexpression and with / without the addition of 50 µM Genistein for 48 h. (B, C) Western blots as well as measurements of OGT, HIF-1α, and proteins related with MMT alongside fibrosis under HG conditions, with / without OGT overexpression and with or without the addition of 50 µM Genistein for 48 h. (D) Western blots as well as quantification of p-mTOR/mTOR, OGT, HIF-1α, and proteins related with MMT alongside fibrosis under different glucose conditions, with / without the addition of 50 µM Genistein or 4 µM MHY1485 for a duration of 48 h. *, P < 0.05; **, P < 0.001 vs. LG group. #, P < 0.05; ##, P < 0.001 vs. HG group. $, P < 0.05; $$, P < 0.001 vs. OGT-NC group. LG: control medium; HG: 2.5% glucose medium.
Effect of Genistein on the O-GlcNAcylation status of HIF-1α, its ubiquitination as well as subsequent proteasomal degradation
Genistein inhibited the protein abundance of HIF-1α, but the mechanism remains unclear. Here, we investigated the effect of Genistein on the mRNA of HIF-1α and its protein ubiquitination degradation. Genistein did not reduce mRNA level of HIF-1α, but rather increased its expression (Fig. 3A). MG-132 is a potent proteasome inhibitor that prevents protein degradation, and PUGNAc is a specific inhibitor of OGA that prevents the removal of O-GlcNAc modifications from proteins, thereby increasing O-GlcNAcylation levels. As shown in Fig. 3B and C, the inclusion of MG-132 resulted in increased HIF-1α levels in the Genistein group, approaching those in the HG and LG + PUGNAc groups. Subsequent immunoprecipitation experiments confirmed that Genistein decreased the O-GlcNAcylation level of HIF-1α under HG conditions and concurrently increased its ubiquitination level (Fig. 3D and E). Collectively, these results elucidate that Genistein diminished HIF-1α abundance by inhibiting the O-GlcNAcylation status of HIF-1α and facilitating its ubiquitination along with the subsequent proteasomal degradation.
Effect of Genistein on the HIF-1α’s O-GlcNAcylation status, its ubiquitination along with proteasome-mediated degradation.(A) The mRNA expression level of HIF-1α following incubation with different concentrations of glucose and Genistein for a duration of 48 h. (B, C) Western blots as well as measurements of HIF-1α expression under different glucose concentrations, with / without 50 µΜ PUGNAc or 50 µM Genistein for 48 h. MG-132 (0.1 µΜ) was supplemented during the final 24 h. (D, E) Immunoprecipitated HIF-1α, western blots, as well as measurement of total, O-GlcNAcylated, and ubiquitinated HIF-1α under different glucose concentrations, with / without the addition of 50 µΜ PUGNAc or 50 µM Genistein for 48 h. *, P < 0.05; **, P < 0.001 vs. LG group. #, P < 0.05; ##, P < 0.001 vs. HG group. LG: control medium; HG: 2.5% glucose medium.
Effect of Genistein on peritoneal thickness and peritoneal function of CKD rats undergoing PD
An adenine-induced CKD model was employed to simulate human CKD, and the characteristics of different rat groups were recorded during CKD modeling and intraperitoneal injection of dialysate (Supplementary Table 1 and Supplementary Table 2). As shown in Fig. 4A–D, CKD rats did not exhibit obvious peritoneal thickening compared to the NC group. After five weeks of intraperitoneal dialysate injection, the PD group exhibited thickening of the visceral peritoneum alongside the parietal peritoneum from macroscopic observation, compared to CKD rats without intraperitoneal dialysate injection (Fig. 4A and B). Genistein administration preserved the morphology of both the visceral and parietal peritoneum, resembling the control and CKD groups (Fig. 4A and B). Histological analysis found that the peritoneum in the PD group exhibited significant thickening with increased collagen fiber deposition, as evidenced by HE staining and Masson staining (Fig. 4C and D). The membrane thickness of the PD group was approximately four times that of the CKD and control groups (Fig. 4E). Genistein treatment significantly prevented peritoneal thickening and reduced collagen fiber deposition. (Fig. 4C-E). Peritoneal fibrotic thickening is often associated with functional changes. In the PD group, the ultrafiltration function of the peritoneum decreased, and this impairment was effectively restored by Genistein treatment (Fig. 4F). Additionally, Genistein treatment reduced creatinine high transport and glucose absorption compared to PD group (Fig. 4G and H).
Effect of Genistein on peritoneal thickness, fibrosis, as well as peritoneal function of CKD rats undergoing peritoneal dialysis.(A) Morphology and thickness of the visceral peritoneum (omentum). (B) Morphology and thickness of the parietal peritoneum. (C) HE staining of the rat peritoneum. (D) Masson staining of the rat peritoneum. (E) Quantitative analysis of the thickness of the peritoneum within different group (n = 6 per group). (F-H) The volume of ultrafiltration, the dialysate to plasma (D/P) ratio of creatinine, and the 2-hour dialysate to 0-hour dialysate (D2/D0) ratio of glucose were measured in each group of rats before sacrifice (n = 6 per group). *, P < 0.05; **, P < 0.001 vs. CKD group. #, P < 0.05 vs. PD group.
Effect of Genistein on peritoneal fibrosis-related proteins and peritoneal angiogenesis in CKD rats undergoing PD
The upregulated α-SMA expression and the downregulated E-cadherin expression serve as indicators of MMT. WB analysis revealed that HG peritoneal dialysate induced an upregulated α-SMA expression together with a downregulated E-cadherin expression among the PD group compared to the CKD group. Notably, these alterations were reversed by Genistein treatment (Fig. 5A and B). There are no significant differences in MMT-related markers between CKD rats and NC rats. Genistein treatment further demonstrated inhibitory effects on the abundance of fibrotic proteins, including fibronectin and COL1A2 (Fig. 5A and B). Angiogenesis is pivotal for the progression of PF, with CD31 serving as a marker for endothelial cells within blood vessels. IHC was employed to assess CD31 expression, as shown in Fig. 5C and D, HG peritoneal dialysate resulted in an increase in the number of CD31-positive vessels within the peritoneum of the rats, whereas Genistein decreased the number of vessels.
Effect of Genistein on peritoneal fibrosis-related proteins and peritoneal angiogenesis in CKD rats undergoing PD. (A, B) Western blots as well as quantification of proteins associated with MMT and fibrosis in rat peritoneum (n = 6 per group). (C, D) Immunohistochemistry and quantification of CD31 in the rat peritoneum across various groups (n = 6 per group). *, P < 0.05 and **, P < 0.001 vs. CKD group. #, P < 0.05 and ##, P < 0.001 vs. PD group.
Effect of Genistein on the abundance of mTOR/OGT, O-GlcNAcylation, as well as HIF-1α within the peritoneum of CKD rats undergoing PD
Peritoneum protein was extracted and WB analysis revealed a substantial increase in O-GlcNAcylation abundance in the PD group, reaching approximately three times that of the CKD group. Notably, Genistein treatment effectively attenuated this increase, as illustrated in Fig. 6A. Genistein exhibited inhibitory effects on HIF-1α through the mTOR/OGT pathway in HPMCs. In line with in vitro results, the PD group displayed a significant elevation in the abundance of HIF-1α, p-mTOR / mTOR, as well as OGT, all of which were suppressed by Genistein treatment (Fig. 6B and C). Fluorescence intensity analysis demonstrated a substantial increase in the abundance of the aforementioned proteins in the peritoneum of the PD group in contrast with the control and CKD groups. Conversely, the Genistein group exhibited a decrease in fluorescence intensity, indicating the suppressive effect of Genistein on these proteins (Fig. 6D and E). Comparatively, the fluorescence intensity of proteins in the control and CKD groups was weak and displayed similar trends.
Effect of Genistein on the abundance of mTOR/OGT, O-GlcNAcylation alongside HIF-1α in the peritoneum of CKD rats undergoing peritoneal dialysis. (A-C) Western blots as well as quantification of global O-GlcNAcylation, p-mTOR/mTOR, OGT and HIF-1α expression in the rat peritoneum (n = 6 per group). (D, E) Immunofluorescence and quantification of p-mTOR, OGT, O-GlcNAcylation, and HIF-1α expression in the rat peritoneum (n = 6 per group). *, P < 0.05 and **, P < 0.001 vs. CKD group. #, P < 0.05 and ##, P < 0.001 vs. PD group.
Discussion
Genistein, a phytoestrogen, exerts estrogen-like effects by binding to estrogen receptors. Previous research has demonstrated its potential to inhibit HIF-1α expression19,20 and anti-fibrotic effects16,17. Our study specifically investigated the direct impact of Genistein on HG-induced peritoneal MMT and fibrosis. The experimental findings revealed a pronounced inhibition of MMT alongside fibrosis in HPMCs induced by HG and HIF-1α following Genistein treatment, corroborated by animal experiments that exhibited significant suppression of HIF-1α levels and concurrent antifibrotic effects in the peritoneum. Despite these promising results, the underlying mechanisms through which Genistein inhibits HIF-1α and its downstream molecules remained unclear. Our investigation sought to elucidate these mechanisms, and the results confirmed that Genistein exerted its regulatory impact on the abundance of HIF-1α protein, without affecting its mRNA expression. Genistein effectively reduces HIF-1α’s O-GlcNAcylation status, and promotes its ubiquitination as well as subsequent proteasomal degradation. Overexpression of OGT reversed the inhibitory effects of Genistein on O-GlcNAcylation, HIF-1α, as well as the MMT and fibrosis of HPMCs, indicating that Genistein’s inhibitory effect was achieved by OGT.
mTOR has been implicated in the modulation of OGT expression21,22,23. For instance, mTOR activation in breast cancer cells has been associated with increased cellular OGT and O-GlcNAcylation levels, concurrently inhibiting apoptosis21. Similarly, in colon cancer cells, both mTOR agonists and inhibitors have demonstrated regulatory effects on cellular OGT and O-GlcNAcylation expression22. In hepatocellular carcinoma cells, mTOR signaling has been identified as a crucial regulator of O-GlcNAcylation modification, affecting OGT stability within the cellular milieu23. Accordingly, we hypothesised that Genistein might modulate OGT expression through mTOR signaling pathways. Our cellular experiments substantiated this hypothesis, as the mTOR agonist, MHY1485, reversed the inhibitory impact of Genistein on OGT. In parallel, our rat experiments echoed these findings, revealing that Genistein attenuated the elevated levels of mTOR and OGT induced by peritoneal dialysate, consequently inhibiting the abundance of O-GlcNAcylation in the peritoneum. Interestingly, the pharmacological effects of Genistein bear resemblance to Rapamycin, a known mTOR inhibitor. Previous reports have indicated that rapamycin can decrease the protein abundance of HIF-1α without causing significant alterations in its mRNA levels9. Genistein has been documented to exhibit inhibitory effects on HIF-1α at both the protein and mRNA levels in hepatocellular carcinoma cells19, which is inconsistent with our findings. This discrepancy underscores potential variations in the regulatory and feedback mechanisms governing HIF-1α mRNA transcription across diverse cell types and under varying intervention conditions.
Peritoneal microvessels play a crucial role in the exchange of essential substances, including oxygen, hormones, water, nutrients, antibodies, and inflammatory mediators, between the peritoneum and the abdominal cavity26. Augmented angiogenesis amplifies the effective surface area for exchange, resulting in an increased peritoneal uptake of glucose and thereby contributing to the failure of peritoneal ultrafiltration27,28. Studies have demonstrated that Genistein possesses anti-angiogenic properties. Notably, Genistein flavonoids exert inhibitory effects on angiogenesis in rheumatoid arthritis by targeting IL-6/JAK2/STAT3/VEGF signaling pathway29. Moreover, Genistein inhibited angiogenesis and inflammation in a mouse model of peritoneal endometriosis20 and suppressed the expression of CXCL16 and VEGF-A in breast cancer cells30. In our animal experiments, the administration of Genistein demonstrated a significant decrease in peritoneal vessel density. Moreover, it effectively suppressed glucose uptake while simultaneously mitigating peritoneal thickening and reducing fibrillar collagen deposition in the peritoneum. These comprehensive observational results indicate that in the peritoneal environment, Genistein exhibits significant anti-angiogenic properties, improves PF, and enhances peritoneal function.
In conclusion, our study showed Genistein inhibited MMT and improved PF by regulating the O-GlcNAcylation status of HIF-1α via the mTOR/OGT pathway (Fig. 7), which provides novel insights for the treatment of PF.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Balzer, M. S. Molecular pathways in Peritoneal Fibrosis. Cell. Signal. 75, 109778 (2020).
Krediet, R. T. Acquired decline in Ultrafiltration in Peritoneal Dialysis: the role of glucose. J. Am. Soc. Nephrol. 32, 2408–2415 (2021).
Krediet, R. T. & Parikova, A. Relative contributions of Pseudohypoxia and inflammation to peritoneal alterations with long-term peritoneal Dialysis patients. Clin. J. Am. Soc. Nephrol. 17, 1259–1266 (2022).
Elzakra, N. & Kim, Y. Hif-1Alpha metabolic pathways in Human Cancer. Adv. Exp. Med. Biol. 1280, 243–260 (2021).
Ke, Q. & Costa, M. Hypoxia-inducible Factor-1 (Hif-1). Mol. Pharmacol. 70, 1469–1480 (2006).
Morishita, Y., Ookawara, S., Hirahara, I., Muto, S. & Nagata, D. Hif-1Alpha mediates Hypoxia-Induced epithelial-mesenchymal transition in peritoneal mesothelial cells. Ren. Fail. 38, 282–289 (2016).
Manuprasert, W. et al. Intermittent hypoxia in rat enhancing peritoneal membrane thickening through Hif-1Alpha-Induced cytokines in Peritoneum. Asian Pac. J. Allergy Immunol. 40, 177–185 (2022).
Wang, Q., Xu, L., Zhang, X., Liu, D. & Wang, R. Gsk343, an inhibitor of Ezh2, mitigates fibrosis and inflammation mediated by Hif-1Alpha in Human Peritoneal Mesothelial Cells Treated with high glucose. Eur. J. Pharmacol. 880, 173076 (2020).
Sekiguchi, Y. et al. Rapamycin inhibits transforming growth factor Beta-Induced Peritoneal Angiogenesis by blocking the secondary hypoxic response. J. Cell. Mol. Med. 16, 1934–1945 (2012).
Wang, J. et al. Canagliflozin alleviates high glucose-Induced Peritoneal Fibrosis Via Hif-1Alpha Inhibition. Front. Pharmacol. 14, 1152611 (2023).
Yang, X. & Qian, K. Protein O-Glcnacylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell. Biol. 18, 452–465 (2017).
Martinez, M. R., Dias, T. B., Natov, P. S. & Zachara, N. E. Stress-Induced O-Glcnacylation: an adaptive process of injured cells. Biochem. Soc. Trans. 45, 237–249 (2017).
Herzog, R. et al. Dynamic O-Linked N-Acetylglucosamine modification of proteins affects stress responses and survival of Mesothelial cells exposed to peritoneal Dialysis fluids. J. Am. Soc. Nephrol. 25, 2778–2788 (2014).
Wang, J. et al. O-Glcnacylation regulates Hif-1Alpha and induces mesothelial-mesenchymal transition and fibrosis of human peritoneal mesothelial cells. Heliyon. 9, e22916 (2023).
Mukund, V. et al. Its role in metabolic diseases and Cancer. Crit. Rev. Oncol. Hematol. 119, 13–22 (2017).
Ning, Y. et al. Genistein ameliorates renal fibrosis through Regulation Snail Via M6a Rna Demethylase Alkbh5. Front. Pharmacol. 11, 579265 (2020).
Qin, W. et al. Genistein alleviates pressure overload-Induced Cardiac Dysfunction and interstitial fibrosis in mice. Br. J. Pharmacol. 172, 5559–5572 (2015).
Tong, M. et al. Genistein attenuates Advanced Glycation End Product-Induced expression of fibronectin and connective tissue growth factor. Am. J. Nephrol. 36, 34–40 (2012).
Li, S. et al. Genistein suppresses aerobic glycolysis and induces Hepatocellular Carcinoma Cell Death. Br. J. Cancer. 117, 1518–1528 (2017).
Sutrisno, S. et al. Genistein modulates the Estrogen Receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J. Tradit Complement. Med. 8, 278–281 (2018).
Sodi, V. L. et al. mTOR/Myc Axis Regulates O-Glcnac Transferase expression and O-Glcnacylation in breast Cancer. Mol. Cancer Res. 13, 923–933 (2015).
Very, N. et al. Cross Regulation between mTOR Signaling and O-Glcnacylation. J. Bioenerg Biomembr. 50, 213–222 (2018).
Park, S., Pak, J., Jang, I. & Cho, J. W. Inhibition of mTOR affects Protein Stability of Ogt. Biochem. Biophys. Res. Commun. 453, 208–212 (2014).
Qin, H., Song, Z., Shaukat, H. & Zheng, W. Genistein regulates lipid metabolism via Estrogen Receptor beta and its downstream signal Akt/mTOR in HepG2 cells. Nutrients.13, 4015 (2021).
Jia, Q., Yang, R., Liu, X. F., Ma, S. F. & Wang, L. Genistein attenuates renal fibrosis in Streptozotocininduced Diabetic rats. Mol. Med. Rep. 19, 423–431 (2019).
Stavenuiter, A. W., Schilte, M. N., Wee, T., Beelen, R. H. & P. M. & Angiogenesis in Peritoneal Dialysis. Kidney Blood Press. Res. 34, 245–252 (2011).
Devuyst, O., Margetts, P. J. & Topley, N. The pathophysiology of the peritoneal membrane. J. Am. Soc. Nephrol. 21, 1077–1085 (2010).
Kariya, T. et al. Tgf-Beta1-Vegf-a pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal Dialysis. Am. J. Physiol. -Renal Physiol. 314, F167–F180 (2018).
Cheng, W. X. et al. Genistein inhibits Angiogenesis developed during rheumatoid arthritis through the IL-6/Jak2/Stat3/Vegf signalling pathway. J. Orthop. Transl. 22, 92–100 (2020).
Uifalean, A. et al. Influence of Soy isoflavones in breast Cancer angiogenesis: a Multiplex Glass Elisa Approach. J. BUON. 23, 53–59 (2018).
Acknowledgements
This study was supported by “Tianjin Medical Talents” project, the second batch of high-level talents selection project in health industry in Tianjin (no.TJSJMYXYC-D2-014), Key Project of Natural Science Foundation of Tianjin (no.22JCZDJC00590), Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital (no.ZXY-ZDSYSZD-1), Tianjin Science and Technology Major Special Project and Engineering Public Health Science and Technology Major Special Project (no.21ZXGWSY00100).
Author information
Authors and Affiliations
Contributions
JW, XL, and YL performed the experiments and wrote the manuscript. AA and HL managed and analyzed data. SZ and PY designed the project, edited the manuscript. All authors reviewed the manuscript and approved of this submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Lv, X., Lin, Y. et al. Genistein inhibits HIF-1α and attenuates high glucose-induced peritoneal mesothelial-mesenchymal transition and fibrosis via the mTOR/OGT pathway. Sci Rep 14, 24369 (2024). https://doi.org/10.1038/s41598-024-74879-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74879-3