Abstract
Scorpion venom may include pharmacological substances that have the potential to provide benefits. Multiple scientific investigations have shown that particular scorpion venoms induce apoptosis and inhibit the development of cancerous cells. The present study investigated the potential anticancer properties of the crude venom derived from Hottentotta saulcyi (H. saulcyi) on both in vivo mice models and in vitro breast carcinoma cells. The venom of scorpions belonging to the species H. saulcyi was obtained with the application of electrical stimulation at voltages of 8 and 10 V. The determination of the Average Lethal Dose 50 (LD50) was conducted. The present work assessed the in vitro cytotoxicity and morphological characteristics of H. saulcyi venom using fluorescence microscopy, MTT assay, and flow cytometry assessment. Additionally, research was performed to assess the cytotoxic effects in vivo on a mouse model with breast cancer. The examination of MCF-7 cells treated with scorpion venom at a microscopic level revealed the existence of cells undergoing apoptosis. The venom of H. saulcyi has anticancer properties, as shown by the observation that MCF-7 cells had a 62.12% apoptotic rate when exposed to a dose of 1.47 mg/L. Based on the results obtained, it can be shown that the viability of MCF-7 cells has exhibited a substantial reduction (P < 0.01). Furthermore, the findings indicated that the venom of H. saulcyi resulted in a significant increase in the synthesis of TNF-α, IL-6, IL-10, TGF-β, and caspase (P < 0.05). The treatment groups administered with H. saulcyi venom exhibited a significant augmentation in the expression of proapoptotic genes compared to the control group of healthy individuals. The transcription of the BCL2 gene exhibited a statistically significant increase in the healthy control group compared to both the healthy venom-treated group (P < 0.05) and the malignant venom-treated group (P < 0.01). The crude venom of H. saulcyi has considerable promise in demonstrating anticancer properties. Further investigation may be warranted to explore the potential of using H. saulcyi crude venom as a medicinal platform for the prevention of breast cancer.
Similar content being viewed by others
Background
Breast cancer is the most often occurring and lethal kind of cancer among women. According to a study conducted in 2008, there has been a notable 20% rise in the global prevalence of breast cancer1. Annually, about 1.5 million novel occurrences of breast cancer are documented, constituting a quarter of all cancer cases2. Historically, breast cancer has been widely prevalent among women, constituting around 15% of the total female cancer population. Recent research indicates that, after lung cancer, breast cancer has become the second greatest cause of mortality globally, which was responsible for about 40,000 deaths in 20183. Approximately 42,250 women and 530 men in the United States are projected to succumb to breast cancer by the year 20244. Therefore, identifying innovative, effective, and secure therapies for this life-threatening and prevalent neoplasm is of utmost importance. Despite the existence of several cancer treatment modalities, such as surgery, chemotherapy, and radiation, their efficacy in achieving a complete cure for cancer patients is typically limited, and they may also be associated with undesirable side effects5,6.
Differentiation therapy, a novel methodology, can potentially induce lasting modifications in the characteristics of malignant cells. This approach can reactivate the differentiation process, minimizing the detrimental effects on adjacent healthy cells while eliminating the tumor7. In contrast, previous studies have shown that animal venoms involve diverse bioactive substances, spanning from minuscule phenolic compounds to substantial polypeptide chains with high molecular weights, hence exhibiting enduring biological impacts8. The venom of every scorpion species comprises around 100 different peptides, each with specific biological features9. According to reports, the number of peptides in scorpion venoms exceeds 100,000. However, it should be noted that a limited number of peptides, namely 200, have been subjected to rigorous examination of their structural and functional characteristics10. The Hottentotta genus, which belongs to the Buthidae family, has a wide distribution over the continents of Africa and Asia. The Buthidae family includes most of the lethal scorpion species found globally, accounting for about 90% of the total. One example of a somewhat big scorpion species is Hottentotta saulcyi (H. saulcyi), which is characterized as a burrowing arachnid11. This particular species of scorpions has a substantial physical size, often measuring between 75 and 120 mm, with some individuals reaching lengths of up to 13 cm. According to a study12, it has been observed that male scorpions possess a range of 28 to 30 shoulder teeth, whereas female scorpions typically exhibit a range of 24 to 29 shoulder teeth. H. saulcyiis characterized by sturdy, sizable claws12. The scorpion species known as Hottentotta saulcyi has been documented in Syria, Iraq, and Iran. The venom of H. saulcyi contains proteins at relatively low concentrations (16.26%), characterized by short chains and small-molecular-weight neurotoxins. The venom of H. saulcyihas an albumin content of 11.7%. The venom’s characteristics are influenced by several factors, including genetic variations, geographical regions, and environmental conditions in which the scorpions are confined13,14,15.
Different peptides, chemicals, and proteins gathered during the milking process are found in scorpion venom. The milking method entails securely confining the snake and inducing it to strike against a specific receptacle, often a glass or plastic vial, to collect the venom. After extraction, the venom is processed and kept under optimal conditions for further examination or used in antivenom manufacturing. Currently, this toxic substance is referred to as Curd Venom. Scorpions possess venom comprising several low molecular-weight peptides, exhibiting a wide range of pharmacological characteristics such as anti-epileptic, antimicrobial, and channel-blocking capabilities16,17. A study conducted by Karsch showed that the venom of Buthus martensii (B. martensii)exhibited a significant reduction in the growth of glioma tumors. This effect was attributed to the induction of cell death in glioma cells within an in vivo setting18. Furthermore, it was shown that the venom derived from Leirus quinquertiumshad inhibitory effects on primary brain tumors19. Iran harbors a substantial population of H. saulcyi, a venomous scorpion species. The venom derived from scorpions native to Iran has been shown to include peptides that can impede the development of cancer cells and induce their demise via mechanisms involving cell death and apoptosis20. Based on a study conducted by Zargan et al21., it has been shown that the unrefined venom derived from Odontobuthus doriae and Androctonus crassicauda has anticancer capabilities and induces apoptosis in MCF-7 (a cell line associated with breast cancer) and SH-SY5Y (a cell line associated with neuroblastoma) cells within an in vitro setting. While many studies have been conducted on the pharmacological effects of H. saulcyivenom, its cytotoxic pattern remains undetermined22.
Anticancer peptides (ACPs) include a collection of compact peptides composed of 10 to 60 amino acids, which can impede the proliferation and metastasis of malignant cells and inhibit the formation of neoplastic vasculature. Adenocarcinoma of the prostate has a reduced inclination towards the development of treatment resistance. In general, it is typical for antimicrobial peptides (AMPs) to induce cell destruction via the formation of transmembrane pores or ion channels23. The cellular penetration is facilitated by a connection between the anionic cell membranes and the AMPs present in the venom of H. saulcyi. The principal targets of ACPs are mostly the lipids present in cellular membranes24. The peptides exhibit affinity towards phosphate groups and anionic phospholipids. The transmembrane pore generated by the venom of H. saulcyi may be characterized by the toroidal model. The formation of a toroidal shape occurs when venom molecules from H. saulcyipenetrate the bilayer membrane perpendicularly, resulting in the continuous bending of the membrane monolayer from its upper to lower surfaces25,26. Incorporating peptides into the phospholipid bilayer of the cytoplasm membrane may be facilitated by the peptides’ capacity to bind to the membrane and induce changes in its structure. The anticancer properties of H. saulcyivenom peptides may be attributed to the interaction with intracellular targets, including DNA, RNA, and proteins, in addition to their ability to disrupt cellular membranes. Consequently, the internal systems experience disruption26,27,28,29.
This study aimed to analyses the anticancer capabilities and cellular mechanisms of H. saulcyi venom in breast cancer. The physicochemical characteristics and anticancer efficacy of H. saulcyi venom were evaluated. Finally, an investigation was conducted on the attributes of H. saulcyi venom, specifically concerning its effects on the mouse model.
Methods
Chemicals
The fetal bovine serum (FBS), DMEM, and RPMI 1640 used in this study were acquired from Gibco, a reputable supplier in the United States (Gibco, USA). The following reagents were obtained from Sigma-Aldrich, USA: agarose, RNase A, proteinase K, ethidium bromide, polysorbate (Tween 80: TW80), ethylene diamine tetra acetic acid (EDTA), cholesterol (CHOL), polyvinyl alcohol, MTT kit, annexin V FITC, propidium iodide, and Trypan blue (Sigma-Aldrich, USA). All other chemicals used in the study were purchased from local suppliers and were of analytical grade.
Cell cultures and ethics
The MCF-7 and fibroblast cell lines were obtained from the Iranian Biological Resource Centre in Tehran, Iran (IBRC, Iran). The cell culture media was supplemented with penicillin (100 µg/mL), streptomycin (100 µg/mL), gentamycin (100 µg/mL), 10% heat-inactivated fetal bovine serum (FBS), and RPMI 1640 or DMEM. Fibroblast cells were cultivated in DMEM culture medium, whereas MCF-7 cells were cultivated in RPMI 1640 culture media. Subsequently, the cells were placed in a CO2 incubator set at 37 °C, creating a humidified atmosphere with 5% CO218,19,20. The research was carried out in adherence to the principles of the Biotechnology Research Centre, Falavarjan Branch, Islamic Azad University, located in Isfahan, Iran. The study received approval from the Medical Research Ethics Committee of the Biotechnology Research Centre, Falavarjan Branch, Islamic Azad University, Isfahan, Iran, under the reference number IR.IAU.FALA.REC.1400.025. The research has been conducted in conformity with the ARRIVE guidelines, as outlined on the official website (https://arriveguidelines.org).
Collection of the scorpions and venom
The scorpion species H. saulcyi, which has medicinal significance, was obtained from the Esfahan Farm Scorpion Company and maintained inside laboratory conditions using containers designed to mimic its natural habitat. In a manner that ensured the animal’s well-being, individuals of the species H. saulcyi were carefully captured by gently grasping the distal end of their tails with elongated forceps. Subsequently, scorpions were placed in containers containing soil with a depth of roughly two inches and a layer of small stones on the surface. The venom was discharged using electrical stimulation of H. saulcyiat a voltage range of 8–10 V. Then, the venom was subjected to lyophilization and then stored at a temperature of -50 °C. The lyophilized powders were stored at -20 °C for future use. Following the dissolution of the powder in phosphate-buffered saline (PBS), protein concentration was quantified using the Bradford method12.
Minimize animal suffering
The ARRIVE guidelines were developed to minimize the distress experienced by laboratory mice in group settings. These guidelines suggest several processes, such as strain selection, grouping, cleaning, and environmental enrichment. Due to the lower level of aggression shown by the BALB/c mouse compared to other mouse species (source: www.mice.jax.org), we opted for the BALB/c mouse. Before puberty, cohesive social groups were formed with classmates and instances of social disturbances were minimal. When allocating mice to experimental groups, unfamiliar animals were not mixed. This study used female BALB/c mice to ensure consistency in the group with regards to the gender variable. The cages underwent daily cleaning, with efforts to minimize the frequency of thorough cage cleaning. The clean and dry nest material, utilized but not soiled, was moved during cage changes. The animals were kept under regulated temperature conditions, with a 12-hour light/dark cycle, and provided unlimited access to water and nourishment. Every possible effort was made to ad libitum minimize the anguish experienced by animals (https://www.ncbi.nlm.nih.gov/books/NBK4039/)52.
Determination of lethality of scorpion venom
The LD50 values of H. saulcyi venom were determined by in vivo experimentation to assess its lethality. To assess each venom dosage’s toxicity, four groups of 2-month-old BALB/c mouse models weighing 18 and 20 g were used. Among these groups, one group of mice was designated as the control, as shown in Table 1. During the experiment, the mice were accommodated in an environment maintained at room temperature, where they were provided with unrestricted access to rodent feed and surface water. Four distinct dosages were assessed, exhibiting death rates that varied from 0 to 100%. The experimental groups were subjected to subcutaneous administration of four concentrations. The venoms extracted from H. saulcyiscorpions at concentrations of 1.2, 1.5, 1.8, and 2.1 mg/kg body weight were diluted in 0.2 mL of phosphate-buffered saline (PBS). An equivalent quantity of PBS was administered just to the control group. To ascertain the LD50, the animals were subjected to a 24-hour observation period12,30.
Determination of amino acids using high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS)
The identification of amino acids in the venom of H. saulcyi, is accomplished via the use of high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) technology. The HPLC-MS/MS system comprises the Agilent 1200 high-performance liquid chromatography and the Agilent 6430 class triple quadrupole mass spectrophotometer with an electrospray ionization source. The separation process included a CORTECS C18 column with dimensions of 4.6 mm × 150 mm and a particle size of 2.7 m. The column temperature was carefully controlled at 30 °C. The gradient elution method used in this study consisted of three distinct phases: the first phase lasted from 0 to 10 min, during which the solvent composition ranged from 10 to 85% B; the second phase spanned from 10 to 13 min, with a solvent composition transition from 85 to 95% B; and finally, the third phase included the time interval from 13 to 19 min, maintaining a constant solvent composition of 95% B. The mobile phases in the experiment consisted of acetonitrile (B) and a solution of 0.1% formic acid in water (A). The injection volume was 10 µl, while the flow rate was 0.3 mL/min. The data was analyzed via the Mass Hunter workstation program developed by Agilent Technologies, based in the United States. The mass spectrometer was used in positive and negative ionization multiple reaction monitoring modes. The source parameters used in this study were as follows:
-
The temperature of the drying gas was set to 320 °C.
-
The flow rate was 11 L/min.
-
The pressure of the nebulizing gas was 30 psi.
The capillary voltage was set at 300 V for positive and − 300 V for negative ionization modes.
LC-MS/MS analysis to identify the venom components and protein concentration
The solutions used were of chromatographic innocence, and the water was filtered using deionization. The majority of the criteria were provided by Fluka–Sigma–Aldrich. Using the Biuret technique, protein concentrations were calculated30. In this method, proteins’ peptide bonds emit a violet color when alkaline copper is present. The intensity of the color is measured using a spectrometer at a wavelength of 540 nm.
Confocal microscopy
The examination of nuclear integrity in breast carcinoma cells was conducted employing a confocal laser scanning microscope, namely the Leica TCS-SP2 equipment manufactured in Germany. The cells were first cultivated on 6 well glass bottom plates using a complete medium. In this study, the cells (MCF-7) were rinsed using ice-cold phosphate buffer saline (PBS). The control and cells treated with scorpion venom formulations underwent this rinsing step. Following the rinsing, the cells were labelled with ethidium bromide at 100 µg/mL concentration in PBS. Subsequently, the cells were placed onto slides. The Leica confocal microscope was employed for observation during the final experiment. Images were acquired using a 590 nm filter from a laser that emitted argon and krypton.
Fluorescence microscopy
The cellular permeability of the membrane and nuclear integrity were assessed utilizing a fluorescence microscopy instrument (Motic, Germany). The 2 × 105 cells were subjected to scorpion venom at a concentration that resulted in a 50% inhibition of cell growth after 48 h. Cells that had not been treated were collected and then centrifuged at 1000 revolutions per minute for 5 minutes. Following two washes, the pellet was reconstituted in phosphate-buffered saline (PBS). Subsequently, the sample was incubated with a solution containing ethidium bromide and acridine orange liquid, specifically at 100 µg/mL concentration in phosphate-buffered saline (PBS). The identification of apoptotic cells was then performed by qualitative analysis using a fluorescence microscope.
Cytotoxicity assay
According to the MTT kit protocol (Sigma-Aldrich, USA), the MCF-7 and control (fibroblast) cells were planted in 96-well plates at a concentration of 2 × 105 cells (200000 cells). The plates were then placed in a CO2 incubator and cultivated overnight. Afterwards, the medium was replaced with fresh media containing scorpion venom at dosages of 5, 2.5, 1.25, 0.625, 0.312, and 0.156 µg/mL. The samples were then kept at a constant temperature of 37 °C, with a 5% (v/v) concentration of CO2 and 80% (v/v) humidity throughout the experiment. One well was designated as the control group and was not subjected to the introduction of venom. The plate was placed in an incubator and kept at a temperature of 37 °C for a duration of 3 h. Following the incubation period, a volume of 150µL of MTT solvent was introduced into each well. The plate was covered in aluminum foil and placed on an orbital shaker for a duration of 15 min. The optical absorbance was measured at a wavelength of 590 nm. Three-time intervals of 24, 48, and 72 h were used for the MTT test.
Analysis of apoptosis via flow cytometry
A flow cytometric study assessed the extent of apoptotic activity induced by the scorpion venom. Briefly, 2 × 105MCF-7 cells and control cells (fibroblasts) were cultured under treatment with scorpion venom. The cells were then incubated for 48 h before being subjected to centrifugation at 4 °C. The cells underwent two rounds of centrifugation after their suspension in an annexin-HEPES solution. The pellets were reconstituted in the same buffer solution (100 µl), including propidium iodide and annexin V FITC (Sigma-Aldrich, USA). The analysis was conducted employing a flow cytometer (Becton Dickinson, LSRFortessa™) after a 15-minute incubation period at ambient temperature and under conditions of low light. The flow cytometric observations were acquired using a 488 nm excitation laser and 530/30 nm and 585/42 nm bandpass filters to detect PI. The program Cell Quest, which is designed for the Macintosh platform, was used for data analysis53.
Comet assay
The present study used comet experiments or single-cell electrophoresis analyses to examine the extent of DNA damage inflicted upon MCF-7 cells due to exposure to scorpion venom. MCF-7 cells were subjected to a 48-hour treatment with scorpion venom at the half-maximal inhibitory concentration (IC50). Following two washes with cold PBS (pH 7.2), the cells were centrifugated at 4 °C. The slides were first coated with a layer of agarose with a typical melting point (0.75% in PBS). After the agarose solution had solidified, a volume of 85 µl containing 104 cells was added. After solidification, an additional layer of low melting point agarose (100 µl) was introduced. The slides were immersed in a cold lysis solution containing 10% DMSO, 100 mM EDTA, 2.5 M NaCl, 10 mM Tris, 1% Triton X-100, and pH 10 for one hour in the absence of light at a temperature of 4 °C. Subsequently, the slides were transferred to an electrophoresis apparatus and exposed to new buffer consisting of 1 mM EDTA, pH 13.5, and 300 mM NaOH for 20 min. Electrophoresis was conducted for 20 min at an applied voltage of 18 V. The slides were immersed in a neutralizing buffer solution of 0.4 M Tris-HCl at a pH of 7.5 for five minutes. Next, the slides were treated with ethidium bromide at a concentration of 10 µg/mL. A fluorescence microscopy device manufactured by Motic in Germany was used to achieve a 100-fold magnification of the slides with a green filter. A total of 100 cells were enumerated on each slide in order to obtain the comet score. The width and length of the comet tail were measured using the Motic Image Plus 2.0 software30.
The effects of venom on murine biomodels induced with cancer
(A) Development of a breast cancer animal model and mouse treatment
The animal model of breast cancer was generated using 7,12-Dimethylbenz[a]anthracene (DMBA, C20H16) obtained from Thermo Scientific Chemicals, USA, with a purity of 98% (Fig S1). The molecular weight of this chemical is 256.34 g/mol. Upon administration to animals, this chemical can elicit the development of breast cancer54,55. The BALB/c mice were allocated into four distinct groups, as shown in Table 1. Subsequently, injections of the drug were administered close to the mammary gland. The experiment included the administration of scorpion venom to mice, as described in the section titled “Determination of lethality of scorpion venom” and documented in Table 1. In this research, the control group is defined as the group that was not administered any venom. On the other hand, the treated group specifically denotes the group that was administered with the LD50 dosage (1.47 mg/kg) of scorpion venom.
The mortality, body weight fluctuations, and medical evaluations of mice were selected randomly and documented daily for seven days. The criteria for assigning clinical scores to the bio-models used in this study were as follows: 0 (normal, active, healthy), -1 (slightly sick, slightly ruffled fur, otherwise normal), -2 (ill, ruffled fur, sluggish movement, hunching), -3 (extremely sick, ruffled hair, very slow movement, stooped, eyes shut), -4 (moribund), and − 5 (dead).
(B) Development of venom mixtures for vaccination and pathology analysis
The Venom formulations were suspended in a buffer solution containing 0.2 M sodium bicarbonate, 5% casein hydrolysate, and 0.5% weight/volume glucose. These suspensions are then delivered to mice, as shown in Table 1. The mice are euthanized, and the tumors are excised 48 h after administering the last dosage. The tumors are immersed in a solution of 10% formalin, followed by the preparation of paraffin slices. These slices are then subjected to staining using hematoxylin and eosin (E&H), after which a histopathological study is conducted. Briefly, slides containing paraffin sections were placed in a slide holder. Then deparaffinization and rehydration of the sections were done. While the sections were in water, the hematoxylin surface was washed with a Kimwipe to remove oxidized particles. Then the excess water in the slide was removed and stained with hematoxylin, and the slide was washed with deionized water. The slide was dried at room temperature for 1 h and then eosin staining was performed. After that, the slide was dehydrated. Slides were cover-slipped using a drop of Permount (xylene based) to cover the entire tissue. The slide was dried overnight in the hood.
(C) Cytokine secretion
Blood serum samples were collected from mice 48 h after administering the last dosage. A 96-well plate was filled with 200 µL of the blood serum samples. Following a 48-hour incubation period, the concentrations of Tumor Necrosis Factor (TNF-α), Interleukin-10 (IL-10), Interleukin-6 (IL-6), Transforming Growth Factor-beta (TGF-β), and caspase were assessed in the blood serum samples. This was achieved using mouse Enzyme-Linked Immunosorbent Assay (ELISA) kits from Karmania Pars Gene in Iran. In instances when the mice did not experience mortality during the experimental procedures, they were subsequently euthanized after the injection of ketamine/xylazine.
(D) Quantitative real-time PCR analysis of apoptosis-related transcription
This study employed a quantitative real-time PCR technique with SYBR green amplification to assess the transcription levels of apoptosis-inducing genes, including P53, STAT3, and CDK7, together with the anti-apoptotic gene BCL2. The quantitative real-time PCR was conducted following the guidelines provided by the manufacturer. The SYBR® Premix Ex TaqTM II kit from TaKaRa, Japan, and specific primers listed in Table 2 were used for this purpose. Relative gene transcription rates were obtained by normalizing the corresponding GAPDH level. The trials were conducted on two separate occasions.
Statistical analysis
The data were analyzed with the GraphPad Prism software, and the results were reported as the mean ± standard deviation. The study included One-way ANOVA, T-Test, and Tukey analysis to investigate the differences between the treated and control cells. The statistical significance of the differences was assessed using the following notation: P < 0.05(*), P < 0.01(**), P < 0.001(***), and P < 0.0001(****). Each experiment was done at least three times.
Results
The LD50 potency of scorpion venom was established
Table 3 displays the recorded mortality rates of mice resulting from the venom of H. saulcyiaccording to our group study12. The experiment used four doses (1.2, 1.5, 1.8, and 2.1 mg/kg b.w.) according to our previous study12. The mortality rates for mice were 100% and 75% at doses of 2.1 and 1.8 mg/kg, respectively. Furthermore, it was observed that there was a mortality rate of 25% while administering a dose of 1.2 mg/kg. Nevertheless, when administered at 1.5 mg/kg, the survival rate exhibited a mere 50% efficacy. The conclusive outcome of the research yielded an LD50 value of 1.47 mg/kg of body weight. Therefore, the determined LD50 value for the venoms under investigation in H. saulcyi was 1.47 mg/kg.
Amino acid and protein determination
Thirty amino acids are found in H. saulcyi venom, of which eleven are found in small concentrations (Table 4). Argininosuccinic acid and selenocysteine are not present in the examined H. saulcyi venom. Cystathionine and homocysteine are absent from H. saulcyi. Hydroxylysine is present in H. saulcyi. The largest concentrations of amino acids were found in H. saulcyi venom, except serine, arginine, aspartic acid, glutamine, and threonine.
The amount of protein was also measured. The venom’s albumin concentration was 11.7% (H. saulcyi). The albumin concentration in the H. saulcyi venom was less than that of the control group (61.1%). For H. saulcyi venom, the total protein ratio was 16.2 (Table 5). Chromatogram curves of total protein and albumin protein of scorpion venom with concentrations of 16.2% and 11.7% were shown in Fig. 1A and B. Table 5 reveals that the venom of this scorpion contains cytochrome c oxidase subunit I as the active component. The study also revealed that the concentration of cytochrome c oxidase subunit I in scorpion venom is 1.1 µM.
Results of in vitro morphological studies
Confocal microscopy examined the cytotoxic and apoptotic effects induced by the scorpion venom. The administration of scorpion venom therapy at an IC50 dosage over 48 h resulted in notable differences compared to the confocal images of untreated control cells. In the untreated control cells, the nuclei seemed intact. However, in the MCF-7 cells, there was a distinct occurrence of nuclear disintegration, chromatin condensation, and margination. In contrast to the control cells, fluorescence microscopic examinations of the MCF-7 cells treated with scorpion venom at the IC50 dosage for 48 h revealed the existence of apoptotic cells, both in the early and late stages. The observation of apoptotic cell formation and chromatin condensation, indicative of an apoptotic process including both early and late apoptotic stages, constituted several nuclear alterations.
Histological alterations and proinflammatory cytokines
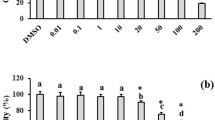
The experimental groups were compared for any variations in apoptosis using a technique using Annexin V-PI staining. Figure 1C and D are shown as percentages of the distribution of early, late, necrotic, and survival cells in control fibroblast cells. In the healthy control group and the group treated with venom, the rates of early, late, and necrotic cell death were all below 20%. A significant proportion, over 80%, of the fibroblast cells in both the control and venom-treated groups exhibited viability. The apoptosis rates observed in fibroblast cells for the control group (Fig. 1C) and the venom-treated group (Fig. 1D) were 3.77% and 18.38%, respectively. The treatment of MCF-7 cells with control and venom resulted in apoptotic rates of 2.5% (Fig. 1E) and 62.12% (Fig. 1F), respectively.
The accelerated cell proliferation phenomenon was closely associated with the advancement of the cell cycle. The cells from both the control and the venom-treated groups were subjected to flow cytometry analysis to assess cell cycle regulation. Compared to fibroblast cells, the MCF-7 cells subjected to venom treatment exhibited an elevation in the G0/G1 phase ratio and a reduction in the S phase to G2/M phase ratio. 64% (64%) of fibroblast cells treated with scorpion venom were in the S phase, and there was no discernible decrease in cell cycle complete. A significant reduction in cell cycle completion was seen in MCF7 cells treated with scorpion venom; 58% of the cells failed to move into the S phase and stayed in the G0/G1 phase. (Figure 2A and B). The findings of our study demonstrated that the venom of H. saulcyi had inhibitory effects on the cell cycle progression of MCF-7 cancer cells. Furthermore, Fig. 2C illustrates the findings obtained from examining apoptotic and antiapoptotic gene expression levels at IC50 concentrations and concentrations above and below IC50 in MCF-7 and fibroblast cell lines.
Mass spectrometry of crude venom from H. saulcyi analysis by LC-MS/MS. (A) Chromatogram of total protein (16.2%) of scorpion venom. (B) Albumin protein chromatogram of scorpion venom with the highest percentage (11.7%). Necrotic and apoptotic cells are analyzed using a flow cytometer and Annexin V-FITC in fibroblast healthy control (C), venom treated fibroblast (D), MCF-7 cancer control (E) and venom treated MCF-7 (F) cells. Q1: Necrotic %, Q2: late apoptotic %, Q3: early apoptotic %, and Q4: Live %.
Cell cycle analysis in cancer cells and fibroblast cells. (A) In fibroblast cells treated with scorpion venom, there was no significant reduction in cell cycle completion and 64% of cells were in S phase. (B) In MCF7 cells treated with scorpion venom, there was a significant decrease in cell cycle completion and 58% of cells remained in G0/G1 phase and failed to enter S phase. (C) The results of investigating the expression of apoptotic and antiapoptotic genes in concentrations of IC50, higher than IC50 and lower than IC50 in MCF-7 and fibroblast cell lines.
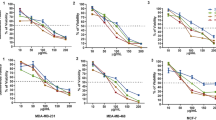
Research was conducted to assess the cytotoxicity of MCF-7 and control (fibroblast) cell lines. This study used the MTT test to investigate the possible anticancer properties of H. saulcyi venom on MCF-7 cell lines. No harmful effects of the H. saulcyi venom on control (fibroblast) cell lines were seen within the tested dilution range. Based on the present discovery, it was shown that the venom of H. saulcyi did not induce any harmful effects within the studied dilution ranges when applied to control cell lines, namely fibroblasts (Fig. 3). The study’s findings indicate that the venom of H. saulcyi has anticancer properties, as shown by the observation that MCF-7 cells exhibited a survival rate of 32% when exposed to a dose of 5 µg/L. Based on the results obtained, it can be shown that the viability of MCF-7 cells has exhibited a substantial reduction (P < 0.01) (Fig. 3A). A significant proportion of MCF-7 and fibroblast cells, when exposed to scorpion venom at a concentration of 5 µg/L for 48 h, exhibited comet formation indicative of DNA fragmentation, as seen under a fluorescence microscope.
In contrast, control cells did not display any obvious comet formations, as shown in Fig. 3B. Table 6 presents the comparative analysis of the length-to-breadth ratio and the prevalence of comet formations in both untreated and treated cells. The mean length-to-breadth ratio of treated fibroblast and MCF-7 cells was significantly increased compared to untreated cells.
(A) Using the MTT test, the anticancer potential of the H. saulcyi venom was examined in this work using MCF-7 and fibroblast cell lines. A dilution range did reveal cytotoxicity of the H. saulcyi venom on MCF-7 more than control (fibroblast) cell lines. *P < 0.05, ** P < 0.01. (B) Comet testing of untreated and venom-treated cells. The venom-treated cells clearly displayed comet formations indicative of DNA breakage, in contrast to the control cells that lacked any comet-shaped structures. Both intact and apoptotic cells are shown.
Results of in vivo study
Survival rate, body weight changes and clinical scores of mice
The mortality, body weight fluctuations, and medical evaluations of mice were selected randomly and documented daily for seven days. Table 7 displays the data on the weight and mortality rate of all animals in the following groups: healthy control, Venom-treated healthy, cancer control, and Venom-treated Cancer. The mice in the healthy control, Venom-treated healthy, cancer control, and Venom-treated Cancer groups all had a 7-day survival rate of 100%. The body weight of mice in the Venom Treated Cancer, Cancer Control, Venom Treated Healthy, and Healthy Control groups after 7 days were 16.75 ± 0.14, 15.67 ± 0.2, 18.87 ± 0.3, and 19.93 ± 0.4, respectively. The results indicated that mice in the Cancer Control group had symptoms such as illness, disheveled hair, slow movement, and hunching. On the other hand, mice in the Venom Treated Cancer, Venom Treated Healthy, and Healthy Control groups showed mild sickness and somewhat disheveled fur but were otherwise normal.
Cytotoxicity study
The provided visual representation in Fig. 4A showcases representative photomicrographs of breast tumor slices stained with H&E, representing each treatment group. The healthy control group consisted of densely populated and highly functioning cells. Even though the densities of the venom-treated healthy group were lower compared to the healthy control group, it should be noted that breast control tumors exhibited viable tumor cells. In contrast, the breast tumor density in the group treated with H. saulcyi venom was lower than in the control tumor groups. The breast tumor stroma subjected to H. saulcyi venom treatment exhibited necrotic areas and degenerative scars, indicating the efficacy of the combined treatment in suppressing tumor growth. Additionally, these tumors had the lowest density of malignant cells.
(A) Tumor anatomy. Breast tumors treated with H. saulcyi venom were stained with hematoxylin and eosin (H&E) in comparison to the cancer control; (×200) high power field. Scale bar in black: 100 μm. (B) We are analyzing immunological reactions and suppressive effects on splenic T-cells. The relative ratios and levels of TNF-α, IL-6, IL-10, TGF-β, and caspase in different mouse groups as determined by ELISA. H. saulcyi venom cause a significant increase in the transcription of the (C) pro-apoptotic genes P53, STAT3, and CDK7 and a reduction in the transcription of the (D) anti-apoptotic BCL2 gene at a significant value ** P < 0.001. By using GAPDH as a reference gene, data were normalized. In the healthy control groups, there was no discernible change in the transcription of pro- or anti-apoptotic genes. E) image of tumor tissue taken while sectioning a mouse body.
Following the administration of the last dosage, blood serum samples were collected from mice belonging to each experimental group. The blood serum samples were analyzed using the enzyme-linked immunosorbent assay (ELISA) technique to detect the presence of TNF-α, IL-6, IL-10, TGF-β, and caspase secretions. TNF-α, IL-6, IL-10, TGF-β, and caspase production levels exhibited differences between the group treated with venom from H. saulcyi and the healthy control group. Notably, the blood serum samples obtained from the animals treated with H. saulcyi venom showed significantly higher levels of these substances compared to the healthy control group (P < 0.05) (Fig. 4B). Furthermore, the results indicated that the venom of H. saulcyi led to a significant increase in the production of TNF-α, IL-6, IL-10, TGF-β, and caspase at a statistically significant level of P < 0.05, as seen in Fig. 4B.
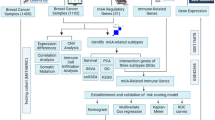
In the current study, real-time polymerase chain reaction (PCR) was used to evaluate the transcriptional activity of the proapoptotic genes P53, STAT3, and CDK7 and the antiapoptotic gene BCL2 and compared to the healthy control group, the groups treated with H. saulcyi venom exhibited a significant increase in the transcription of proapoptotic genes (Fig. 4C, P < 0.01). The investigation of the transcription of the antiapoptotic gene BCL2 was subsequently conducted across many groups. The transcription of the BCL2 gene was found to be significantly more significant in the healthy control group compared to both the healthy venom treatment group (P < 0.05) and the malignant venom treated group (P < 0.01) (Fig. 4D). Furthermore, Fig. 4E depicts the visual representation of the tumor tissue. At the same time, it is being sectioned from the mouse’s body. The general diagram of the working protocol of this study was drawn by the Biorender online website (https://app.biorender.com/gallery) (Fig. 5).
Discussion
The therapeutic potential of scorpion venom in illness therapy has been attributed to many pharmacologically significant compounds, including amino acids, bases, inorganic minerals, biogenic amines, proteins, and poisonous peptides30. The biological effects of the venom are likely significantly influenced by the amino acid and peptide of the substance. Numerous investigations have demonstrated that venom elements are capable of breaking down the protein collagen and the intercellular matrix among cells, break down the membranes of cells, produce oxidizing substances, disrupt blood’s procoagulant and anticoagulant properties, and damage nerve tissue31,32. Thus, it appears that dissecting the venom’s constituent parts can offer us a thorough understanding of its biological effects. This study revealed many protein groups found in scorpion venom, including lipolysis, metallopeptidase, phospholipase, albumin, Cyt-C, and ScoTox-alphaI. Previous research has demonstrated that the majority of venom metalloproteinases are hemorrhagic, meaning they catalyze the breakdown of a wide range of structural components and physiologically significant enzymes. Its hydrolytic targets, which have significant hemorrhagic effects, include collagen, fibrinogen, and coagulation-related factors33,34. Therefore, the metallopeptidase present in scorpion venom in this study probably plays an important role in the destruction or reduction of vital enzymes in the cell cycle of cancer cells. Enzymes called phospholipases facilitate the breakdown of the sn-2 ester bond in phospholipids found in cell membranes35. The phospholipases discovered in this study’s scorpion venom have the ability to cause resistant cancer cells’ cell membranes to break down. Albomin Cyt-C and ScoTox-alphaI from Scorpion venom enhance the susceptibility of the cell membrane to sodium by blocking calcium-activated potassium channels, which causes relative hyperkalemia and triggers the dissolution of catecholamines, and releasing voltage-sensitive sodium channels that are accompanied by calcium entry36. These results are consistent with the current study’s findings on cancer cells, and more research may be needed to fully comprehend the inhibitory effect of scorpion venom on cancer cells.
Despite extensive efforts, the efficacy of existing cancer therapies, including radiation and chemotherapy, remains unsatisfactory, and they often result in adverse consequences on the healthy tissue around tumors37. In addition, it has been shown that tumor cells exhibit resistance against various therapeutic interventions38. In recent studies, evidence has emerged indicating that scorpion venoms can impede the proliferation of various cancer cell lines using distinct mechanisms. These mechanisms include the obstruction of specific ion channels or the binding to specific targets located within the membranes of cancer cells. Additionally, scorpion venoms have been found to inhibit the metastasis and invasion of cancer cells and activate intracellular pathways that ultimately result in apoptosis and cell cycle arrest36,39. The venom derived from scorpions has been seen to impede the entry of chloride ions into glioma cells while exhibiting little or no impact on normal cells originating from the same region29,39. Additionally, the matrix metalloproteinase 2 (MMP-2) plays a crucial role in tumor invasion and has heightened expression in glioma. The binding of scorpion venom to MMP-2 significantly inhibits the gelatinase function of MMP-2, hence impeding the process via which Cl − internalizing channels alter currents29,39. According to research, there are a variety of proteins and peptides in scorpion venom that have the capacity to block neurotransmitter molecules40. Our findings concurred with earlier research demonstrating the anticancer properties of scorpion venom.
Scorpions possess diverse bioactive compounds inside their venom39,40. Venoms possess a diverse array of pharmacologically active chemicals that exhibit a broad spectrum of biological effects, including the ability to block channels and AMPs and ACPs41.
Researchers42investigated the pharmacological characteristics of the venom derived from H. bengalensis, the Indian black scorpion. Their research findings revealed the presence of phospholipases, a kinin-releasing component, and a component responsible for smooth muscle contraction43. A comprehensive examination of the existing scholarly works revealed that the venom derived from B. martensii karsch and L. quinquestriatusshowed inhibitory effects on the formation of primary brain tumors and glioma tumors, respectively15,44. Consistent with earlier research, our investigation revealed the existence of bioactive substances in scorpion venom, all of which possess the capacity to interfere with the cell cycle and ultimately cause cell death.
The term LD50 denotes an approximation of the quantity of toxic substance that, under controlled circumstances, would result in the death of 50% of a significant population of test animals belonging to a particular species. The evaluation of the LD50 test indicates that the accuracy of the process relies on the quantity of animals used. However, even when a significant number of animals are used, there are significant variations in the test results due to various factors that influence the numerical value of the LD50. These factors include the species and strain of the animal, their age and sex, their diet, the period of food deprivation before dosing, the temperature, the caging conditions, the season, and the experimental procedures employed. Therefore, the LD50 value should not be considered an unchanging biological parameter44,45. In this work, we examined the LD50 value of H. saulcyivenom. Our investigation aimed to examine the potential alterations in the LD50 of this research compared to our prior work12. Based on this investigation’s findings, we noted no disparity in the LD50 value of H. saulcyivenom compared to the previous study12. In contrast to prior research12,45, this study’s findings indicate that the LD50 value may be a consistent biological factor for a particular animal species. These findings suggest a persistent biological impact of a hazardous chemical on the genetic and biological aspects of a model organism. Hence, the findings of this investigation, when contrasted with our prior work12, demonstrate that environmental influences have no impact on the LD50 threshold.
Many investigations have shown that scorpion venom has several effects, such as apoptotic induction, anticancer activity, cytotoxicity, and immunosuppression44,45. Apoptosis is a regulated cellular mechanism that destroys superfluous and unwanted cells, contributing to immune responses and certain pathological conditions44,45. According to a study, some scorpion species’ venoms can inhibit DNA synthesis and induce apoptosis in malignant cells46. This study investigated the apoptotic, antimutagenic, cytotoxic, and oxidative stress effects of the crude venom from H. saulcyi on MCF-7 and fibroblast cell lines. The MTT studies demonstrated that the exposure of MCF-7 cells lines to crude venom from H. saulcyiresulted in increased cytotoxicity compared to fibroblast cell lines, although to a lower extent. The tests mentioned above functioned as a dual-purpose assessment, both as an indication of cytotoxicity and as a means to evaluate the integrity of the cell membrane47. Using a confocal microscope in the experiment unveiled that the genetic material inside the cell is subject to degradation upon exposure to the unrefined venom produced by H. saulcyi. The study’s results revealed that the initiation of apoptosis positively correlated with the dosage administered, as shown by microscopic analysis, particularly in MCF-7 cell lines. This study’s protein analysis and confocal microscopy results were in line with other research demonstrating the potency of scorpion venom in eliminating resistant cancer cells48,49.
In comparison, fibroblast cell lines exhibited a comparatively lower level of apoptosis induction. In a study conducted in 2011, it was shown by researchers that the unrefined venom of Odontobuthus doriaeinduced apoptosis in MCF-7 cells44,49. The morphological analysis of the MCF-7 cell line exposed to raw venom from H. saulcyi demonstrated the presence of apoptotic bodies and oedema. The fibroblast cells exhibited no injury due to the small quantity of crude venom, in contrast to the MCF-7 cells. When exposed to crude venom concentrations above five µg/L, an inverted microscope revealed cellular injury in both cells.
Apoptosis, also known as programmed cell death, confers advantages to cancer therapy50. Apoptosis is characterized by many morphological changes, such as transmembrane blebbing, nuclear condensation, chromatin condensation, nuclear fragmentation, and the generation of apoptotic bodies51. Observing morphological alterations by confocal microscopy provided evidence for the induction of apoptosis by venom.
The venom administration at the IC50 concentration for 48 h altered the phospholipid distribution across the plasma membrane. Both cell lines increased in early and late apoptotic/necrotic cells, indicating venom-induced apoptosis in the MCF-7 cell lines. The results of the cell cycle study revealed that the venom induced a significant increase in the proportion of cells in the sub-G1 phase while simultaneously reducing the DNA content in the G0/G1, S, and G2/M phases. Therefore, it was shown that the venom-induced apoptotic cell death in both MCF-7 cells (P < 0.01) and fibroblast cells (P< 0.05), in addition to causing cell cycle arrest. The researchers also conducted experiments to assess tumor cell growth inhibition in mice afflicted with tumors52,53,54,55.
Our findings supported the work of earlier studies and shown that, by causing apoptosis and preventing the advancement of the cell cycle, scorpion venoms are highly useful weapons for the treatment of cancer56. One important mechanism that can stop the unchecked cell proliferation that is a defining feature of cancer progression is the production of cytotoxic action. In the context of a research study, the rAGAP peptide has been shown to induce apoptosis and suppress the growth of SW480 cells, a type of human colon cancer54,55,56. The toxin can increase the expression of p27, resulting in a halt in the G1 phase of the cell cycle. Additionally, it has been shown that the compound inhibits the activation of Bcl-2, phosphatidylinositol 3-kinase (PI3K), and phospho-Akt (p-Akt) while simultaneously increasing the expression of Bax and PTEN (Phosphatase and Tensin Homolog) in SW480 cells46,47,48. The migration and invasion of HepG2 cells, a type of human hepatoma, are inhibited by the introduction of recombinant AGAP. This inhibition is mediated via a voltage-gated sodium channel (VGSC) β1 subunit and a cell adhesion molecule (CAM). However, it is essential to note that the presence of the β1 subunit is necessary for the observed effects since human liver HL7702 cells lacking this subunit do not exhibit any changes in response to recombinant AGAP. A study conducted by54,55showed that overexpression of the VGSC β1 subunit in HL7702 normal cells resulted in the inhibition of their migration and invasion by rAGAP. The present investigation and other research demonstrated that the venom of the Iranian scorpion species can enhance apoptosis via the activation of pro-apoptotic genes55,57.
The use of rAGAP in the therapy of breast cancer cells MCF-7 and MDA-MB-231 has been shown to effectively suppress cancer cell stemness epithelial-mesenchymal transition (EMT) and hinder migration and invasion processes. The peptide in question facilitates the reduction of pentraxin 3 (PTX3), a mediator of inflammatory processes that plays a role in complement activation and inflammation regulation, utilizing the NF-κB and Wnt/β-catenin signaling pathway. A potential association has been proposed between the reduced expression of the Nav 1.5 channel and a decreased level of PTX3 in breast tumors after therapy with rAGAP. The present investigation, consistent with other research, demonstrated that the venom of the Iranian scorpion can enhance apoptosis via the activation of pro-apoptotic genes58,59. The injection of scorpion venom into the body of an individual may lead to significant medical issues and perhaps result in premature mortality. Neurotoxins are the primary constituents of scorpion venom and are recognized as the causative agents behind the clinical signs seen in cases of envenomation. In addition to neurotoxins, scorpion venoms include a diverse array of bioactive compounds. The progress in separation, characterization, and biotechnological methodologies has facilitated the advancement of more efficacious interventions for scorpion envenoming’s45,60. Additionally, these advancements have resulted in the identification of multiple scorpion venom peptides that possess intriguing therapeutic attributes. Hence, it is plausible that scorpion venom has the potential to not only pose a medical risk to human well-being but also to provide a significant reservoir of bioactive compounds that might serve as promising starting points for the discovery of novel treatments targeting both existing and upcoming illnesses61,62. The present review analyses scorpion venom toxins’ deleterious and advantageous characteristics while proposing recommendations for the latest advancements in formulating novel therapies63. This underscores the imperative for further investigations into scorpion elimination and the medicinal potential of scorpion venoms in pharmaceutical exploration.
Conclusion
Scorpion venom’s many constituents have stimulated investigations into its toxicity, development of antivenom, and potential medical uses. These chemicals possess a multitude of pharmacological activities, such as antibacterial, immune-suppressive, and anticancer actions. Nevertheless, before venom-derived biotherapeutics can be introduced into the market, there are many technical obstacles that need to be addressed. These problems include obtaining access to venomous materials, accurately characterizing isolated components, establishing effective production procedures, and finding ways to minimize any negative consequences. Recent research has shown that scorpion venom has the ability to hinder the growth of tumor cells and prevent the activation of genes responsible for programmed cell death. The venom of H. saulcyi has shown antiproliferative and apoptogenic properties on MCF-7 cell lines and in mouse models of breast cancer. Additional investigation is required to determine the precise constituents accountable for this action and the cytotoxicity mechanism.
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- (H. saulcyi):
-
Hottentotta saulcyi
- LD 50 :
-
Lethal Dose
- ACPs:
-
Anticancer peptides
- FBS:
-
Fetal bovine serum
- EDTA:
-
Ethylene diamine tetraacetic acid
- CHOL:
-
Cholesterol
- DMBA:
-
7,12-Dimethylbenz[a]anthracene
- TNF-α:
-
Tumor necrosis factor
References
Tao, Z. et al. Breast cancer: epidemiology and etiology. Cell Biochem. Biophys. 72, 333–338. https://doi.org/10.1007/s12013-014-0459-6 (2015).
Bevilacqua, G. The viral origin of human breast Cancer: from the mouse mammary tumor virus (MMTV) to the human Betaretrovirus (HBRV). Viruses. 14 (8), 1704. https://doi.org/10.3390/v14081704 (2022).
Roy, P., Sur, S., Das, S. & Wui, W. T. Phytochemical-conjugated bio-safe gold nanoparticles in breast cancer: a comprehensive update. Breast Cancer. 29 (5), 761–777. https://doi.org/10.1007/s12282-022-01368-8 (2022).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74 (1), 12–49 (2024).
Ebrahim, K., Vatanpour, H., Zare, A., Shirazi, F. H. & Nakhjavani, M. Anticancer activity a of caspian cobra (Naja naja Oxiana) snake venom in human cancer cell lines via induction of apoptosis. Iran. J. Pharm. Research: IJPR. 15 (Suppl), 101 (2016). PMID: 28228809.
Dezianian, S. et al. In vitro study of hottentotta schach crude venom anticancer effects on mcf-7 and vero cell lines. Iran. J. Pharm. Research: IJPR. 19 (1), 192. https://doi.org/10.22037/ijpr.2020.1100957 (2020).
Johari, B. & Zargan, J. Simultaneous targeted inhibition of Sox2-Oct4 transcription factors using decoy oligodeoxynucleotides to repress stemness properties in mouse embryonic stem cells. Cell. Biol. Int. 41 (12), 1335–1344. https://doi.org/10.1002/cbin.10847 (2017).
Roy, A. & Bharadvaja, N. Venom-derived bioactive compounds as potential anticancer agents: a review. Int. J. Pept. Res. Ther. 27, 129–147. https://doi.org/10.1007/s10989-020-10073-z (2021).
Almaaytah, A. et al. Antimicrobial/cytolytic peptides from the venom of the north African scorpion, Androctonus Amoreuxi: biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides. 35 (2), 291–299. https://doi.org/10.1016/j.peptides.2012.03.016 (2012).
Wulff, H., Christophersen, P., Colussi, P., Chandy, K. G. & Yarov-Yarovoy, V. Antibodies and venom peptides: new modalities for ion channels. Nat. Rev. Drug Discovery. 18 (5), 339–357. https://doi.org/10.1038/s41573-019-0013-8 (2019).
Sousa, P., Froufe, E., Harris, D. J., Alves, P. C. & Van der Meijden, A. Genetic diversity of Maghrebian Hottentotta (Scorpiones: Buthidae) scorpions based on CO1: new insights on the genus phylogeny and distribution. Afr. Invertebrates. 52 (1), 135–143 (2011). https://hdl.handle.net/10520/EJC84689
Boghozian, A., Nazem, H., Fazilati, M., Hejazi, S. H. & Sheikh Sajjadieh, M. Toxicity and protein composition of venoms of Hottentotta saulcyi, Hottentotta Schach and Androctonus crassicauda, three scorpion species collected in Iran. Veterinary Med. Sci. 7 (6), 2418–2426. https://doi.org/10.1002/vms3.593 (2021).
Nakhjavani, M. et al. In Vivo effect of Lidocaine on mouse exposed to Odontobuthos Doriae Scorpion venom.2016. 10536/DRO/DU:30160693.
Hussen, F. S. & Ahmed, S. T. New data of scorpion fauna, include two new records with identification key of scorpion species (Arachnida: Scorpiones) in Iraq. Plant. Archives. 20 (2), 6711–6725 (2020). http://plantarchives.org/20-2/6711-6725%20(6632).pdf
Gupta, S. D. et al. Indian black scorpion (Heterometrus Bengalensis Koch) venom induced antiproliferative and apoptogenic activity against human leukemic cell lines U937 and K562. Leuk. Res. 31 (6), 817–825. https://doi.org/10.1016/j.leukres.2006.06.004 (2007).
Wang, C. G. et al. Molecular characterization of an anti-epilepsy peptide from the scorpion Buthus Martensi Karsch. Eur. J. Biochem. 268 (8), 2480–2485. https://doi.org/10.1046/j.1432-1327.2001.02132.x (2001).
Sangboonruang, S. et al. Potentiality of melittin-loaded niosomal vesicles against Vancomycin-intermediate Staphylococcus aureus and staphylococcal skin infection. Int. J. Nanomed. 16, 7639. https://doi.org/10.2147/IJN.S325901 (2021).
Wang, W. X. & Ji, Y. H. Scorpion venom induces glioma cell apoptosis in vivo and inhibits glioma tumor growth in vitro. J. Neurooncol. 73, 1–7 (2005).
Dardevet, L. et al. Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins. 7 (4), 1079–1101. https://doi.org/10.3390/toxins7041079 (2015).
Keshavarz Alikhani, H., Bidmeshkipour, A. & Zargan, J. Cytotoxic and apoptotic induction effects of the venom of Iranian scorpion (Odontobuthus Bidentatus) in the Hepatocellular carcinoma cell line (HepG2). Int. J. Pept. Res. Ther. 26, 2475–2484. https://doi.org/10.1007/s10989-020-10029-3 (2020).
Zargan, J. et al. Scorpion venom (Odontobuthus Doriae) induces apoptosis by depolarization of mitochondria and reduces S-phase population in human breast cancer cells (MCF-7). Toxicol. In Vitro. 25 (8), 1748–1756. https://doi.org/10.1016/j.tiv.2011.09.002 (2011).
Kazemi, S. M. & Sabatier, J. M. Venoms of Iranian scorpions (Arachnida, Scorpiones) and their potential for drug discovery. Molecules. 24 (14), 2670. https://doi.org/10.3390/molecules24142670 (2019).
Xie, M., Liu, D. & Yang, Y. Anti-cancer peptides: classification, mechanism of action, reconstruction and modification. Open. Biology. 10 (7), 200004. https://doi.org/10.1098/rsob.200004 (2020).
Guo, R. et al. Scorpion peptide Smp24 exhibits a potent Antitumor Effect on Human Lung Cancer cells by damaging the membrane and cytoskeleton in vivo and in Vitro. Toxins. 14 (7), 438. https://doi.org/10.3390/toxins14070438 (2022).
Mihajlovic, M. & Lazaridis, T. Antimicrobial peptides in toroidal and cylindrical pores. Biochim. et Biophys. Acta (BBA)-Biomembranes. 1798 (8), 1485–1493. https://doi.org/10.1016/j.bbamem.2010.04.004 (2010).
Hong, J. et al. How melittin inserts into cell membrane: conformational changes, inter-peptide cooperation, and disturbance on the membrane. Molecules. 24 (9), 1775. https://doi.org/10.3390/molecules24091775 (2019).
Piri-Gharaghie, T. et al. Molecular detection of fungal APR1 gene in serum of multiple sclerosis patients: a personalized medicine research. Personalized Medicine Journal. 7 (25), 15–24 (2022).
Ghourchian, H. et al. Novel niosome-encapsulated 2, 5-Diketopiperazine (BHPPD): synthesis, formulation, and anti-breast cancer activity. Applied Biochemistry and Biotechnology. 196 (6), 3126–3147 (2024).
Dabbagh Moghaddam, F. et al. Delivery of melittin-loaded niosomes for breast cancer treatment: an in vitro and in vivo evaluation of anti-cancer effect. Cancer Nanotechnol. 12 (1), 14. https://doi.org/10.1186/s12645-021-00085-9 (2021).
Azzam, N. N. The protective effects and ameliorative potency of the haemolymph from the Saudi scorpion Androctonus crassicauda against the oxidative stress induced by its crude venom: a pharmacological study. J. Bioscience Appl. Res. 4 (3), 218–259 (2018).
Yong, Y., Hiu, J. J. & Yap, M. K. The secretory phenotypes of envenomed cells: insights into venom cytotoxicity. Adv. Protein Chem. Struct. Biology. 133, 193–230 (2023).
Piri-Gharaghie, T. et al. (S)-3-(3, 4-Dihydroxybenzyl) piperazine-2, 5-dione (cyclo-Gly-L-DOPA or CG-Nio-CGLD) peptide loaded in Chitosan Glutamate-Coated Niosomes as anti-Colorectal cancer activity. BMC Pharmacology and Toxicology. 25 (1), 44 (2024).
Gutiérrez, J. M., Escalante, T., Rucavado, A. & Herrera, C. Hemorrhage caused by snake venom metalloproteinases: a journey of discovery and understanding. Toxins. 8 (4), 93 (2016).
Markland, F. S. Jr & Swenson, S. Snake venom metalloproteinases. Toxicon. 62, 3–18 (2013).
Dennis, E. A., Cao, J., Hsu, Y. H., Magrioti, V. & Kokotos, G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111 (10), 6130–6185 (2011).
Petricevich, V. L. Scorpion venom and the inflammatory response. Mediators of inflammation. ;2010. (2010).
Wang, S. et al. A review on curability of cancers: more efforts for novel therapeutic options are needed. Cancers. 11 (11), 1782 (2019).
Rebucci, M. & Michiels, C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 85 (9), 1219–1226 (2013).
Dueñas-Cuellar, R. A. et al. Scorpion toxins and ion channels: potential applications in cancer therapy. Toxins. 12 (5), 326 (2020).
AlAsmari, A. K., Islam, M. & AlZahrani, A. M. In vitro analysis of the anticancer properties of scorpion venom in colorectal and breast cancer cell lines. Oncol. Lett. 11 (2), 1256–1262 (2016).
Luo, L., Kamau, P. M. & Lai, R. Bioactive peptides and proteins from wasp venoms. Biomolecules. 12 (4), 527. https://doi.org/10.3390/biom12040527 (2022).
Lahiri, S. C. & Chaudhuri, A. K. Action of the scorpion (Heterometrus bengalensis CL Koch) venom on smooth muscle. Indian J. Exp. Biol. 20 (7), 545–548 (1982).
Kar, P. K., Sarangi, B., Datta, A., Gomes, A. & Lahiri, S. C. Occurrence of phospholipase in the venom of the scorpion (Heterometrus bengalensis). Indian J. Med. Res. 83, 332–337 (1986). https://pubmed.ncbi.nlm.nih.gov/3733194/
Zargan, J. et al. Scorpion (Odontobuthus Doriae) venom induces apoptosis and inhibits DNA synthesis in human neuroblastoma cells. Mol. Cell. Biochem. 348, 173–181. https://doi.org/10.1007/s11010-010-0652-x (2011).
Zbinden, G. & Flury-Roversi, M. Significance of the LD 50-test for the toxicological evaluation of chemical substances. Arch. Toxicol. 47, 77–99 (1981).
Einstein, A., Podolsky, B. & Rosen, N. Can quantum-mechanical description of physical reality be considered complete? Phys. Rev. 47, 777–780 (1935).
Hemmati, S. & Rasekhi Kazerooni, H. Polypharmacological cell-penetrating peptides from venomous Marine animals based on Immunomodulating, Antimicrobial, and Anticancer properties. Mar. Drugs. 20 (12), 763. https://doi.org/10.3390/md20120763 (2022).
Nakhjavani, M. et al. In Vivo effect of Lidocaine on mouse exposed to Odontobuthos Doriae Scorpion venom.2016.
Rapôso, C. Scorpion and spider venoms in cancer treatment: state of the art, challenges, and perspectives. J. Clin. Translational Res. 3 (2), 233 (2017).
Diepstraten, S. T. et al. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer. 22 (1), 45–64. https://doi.org/10.1038/s41568-021-00407-4 (2022).
Kahalian, S., Koopaie, M., Hakimiha, N. & Kolahdooz, S. Assessment of the methylene blue mediated photodynamic therapy on BCL2 and BAX genes expression at mRNA level and apoptosis of head and neck squamous cell carcinoma cell line. Folia Med. 64 (2), 221–228 (2022).
Arunima, S. & Verulkar, S. Comparative analysis of different protein estimation methods. Pharma Innov. J. 11 (4), 2091–2095 (2022).
Piri Gharaghie, T., Doosti, A. & Mirzaei, S. A. Prevalence and antibiotic resistance pattern of Acinetobacter spp. infections in Shahrekord medical centers. Dev. Biol. 13 (4), 35–46 (2021).
Kumaravel, T. S., Vilhar, B., Faux, S. P. & Jha, A. N. Comet assay measurements: a perspective. Cell Biol. Toxicol. 25, 53–64 (2009).
Yağmur, E. A., Özkan, Ö. & Karaer, K. Z. Determination of the median lethal dose and electrophoretic pattern of Hottentotta saulcyi (Scorpiones, Buthidae) scorpion venom. J. arthropod-borne Dis. 9 (2), 238 (2015).
Al-Asmari, A. K., Riyasdeen, A. & Islam, M. Scorpion venom causes apoptosis by increasing reactive oxygen species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell lines. J. evidence-based Integr. Med. 23, 2156587217751796 (2018).
Gu, Y., Liu, S. L., Ju, W. Z., Li, C. Y. & Cao, P. Analgesic-antitumor peptide induces apoptosis and inhibits the proliferation of SW480 human colon cancer cells. Oncol. Lett. 5 (2), 483–488 (2013).
Guo, G. et al. Analgesic-antitumor peptide inhibits the migration and invasion of HepG2 cells by an upregulated VGSC β1 subunit. Tumor Biology. 37, 3033–3041 (2016).
Piri-Gharaghie, T. et al. A review of the epidemiology and clinical signs of SARS-COV-2. NCMB J. 11 (41), 103–20 (2020).
Wendler, D. Suffering in animal research: the need for limits and the possibility of compensation. Kennedy Inst. Ethics J. 32 (3), 297 (2022).
Chong, H. P., Tan, K. Y. & Tan, C. H. Cytotoxicity of snake venoms and cytotoxins from two southeast Asian cobras (Naja sumatrana, Naja kaouthia): exploration of anticancer potential, selectivity, and cell death mechanism. Front. Mol. Biosci. 7, 583587 (2020).
Plante, I. Dimethylbenz (a) anthracene-induced mammary tumorigenesis in mice. InMethods in Cell Biology 2021 Jan 1 (Vol. 163, pp. 21–44). Academic Press.
Angeline Kirubha, S. P. et al. Evaluation of mammary cancer in 7, 12-dimethylbenz (a) anthracene-induced wister rats by asymmetrical temperature distribution analysis using thermography: A comparison with serum Cea levels and histopathology. BioMed Research International. ;2012. (2012).
Acknowledgements
The authors would like to thank the staff members of Biotechnology Research Center of Islamic Azad University, Falavarjan Branch, Iran for their help and support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Formal analysis, investigation, methodology and writing-original draft: Mahshid Nosouhian. Methodology, resources, supervision, validation, visualization, writing-review and editing: Ali Asghar Rastegari. Conceptualization, data curation, supervision, validation, visualization, writing-review and editing: Kahin Shahanipur. Formal analysis, investigation, methodology, project administration, writing-review and editing: Ali Mohammad Ahadi. Methodology, validation, visualization, writing-review and editing: Mohammadreza Sheikh Sajjadieh.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was conducted in accordance with the Biotechnology Research Center, Falavarjan Branch, Islamic Azad University, Isfahan, Iran principles. It was approved by the Medical Research Ethics Committee of Biotechnology Research Center, Falavarjan Branch, Islamic Azad University, Isfahan, Iran. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Consent to publish
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nosouhian, M., Rastegari, A.A., Shahanipour , K. et al. Anticancer potentiality of Hottentotta saulcyi scorpion curd venom against breast cancer: an in vitro and in vivo study. Sci Rep 14, 24607 (2024). https://doi.org/10.1038/s41598-024-75183-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75183-w
Keywords
This article is cited by
-
Modeling and Characterization of Hottentotta saulcyi Scorpion Venom and Investigation of Its Anticancer Properties
International Journal of Peptide Research and Therapeutics (2025)
-
Invertebrate venoms: A treasure trove of bioactive compounds with anticancer potential
Archives of Toxicology (2025)