Abstract
Myrmecophilus acervorum, previously considered a parthenogenetic species widely-distributed in Europe, has been observed to have both sexes in populations inhabiting the central part of the distribution range. Specimens from those heterosexual populations have been found being infected with Wolbachia. New mitochondrial data (COI and 16S markers) revealed the well-supported differentiation of M. acervorum populations inhabiting western Polesie (Poland) and southern Europe. In turn, analyses of EF1α marker support the hypothesis on the unfinished lineage sorting at the nuclear DNA level. Interestingly, we found that parthenogenetic populations inhabiting western Polesie are infected with Wolbachia belonging to supergroup A, while endosymbionts occurring in sexual populations of M. acervorum observed in Romania belong to supergroup B. Furthermore, new and potentially diagnostic characteristics in the external structures of the eyes of M. acervorum were identified. The surface of ommatidia in specimens occurring in southern Europe was smooth. In contrast, the ommatidia surface of individuals collected in Poland was visibly sculptured. To sum up, the significant genetic variability found in the present case, and the differentiating morphological character, are almost certainly effects of cryptic species being present within M. acervorum. This is indicative of ongoing speciation within the populations of this insect, and of simultaneous unfinished lineage sorting at the nuclear DNA level.
Similar content being viewed by others
Introduction
The advent of the DNA barcoding concept initiated the widespread application of mitochondrial DNA (mtDNA) in taxonomic research1. Mitochondrial markers swiftly became integral components in diverse research areas, including the systematics, phylogeography, population genetics, ecology, and evolutionary biology of animals2. The versatility of these markers is closely linked to the characteristics of the mitochondrial genome, i.e. its general uniparental inheritance (Ref.3 but see4), high rate of mutagenesis5 due to the lack of protective histones and exposure to the impact of free radicals6, as well as fourfold smaller effective population size compared to the nuclear genome7,8. Consequently, intraspecific variation of mitochondrial DNA may surpass that observed in nuclear DNA, making analyses based on mitochondrial markers valuable for resolving population divergences that remain elusive using coding nuclear markers9.
Divergent mtDNA lineages detected within species may be considered as distinct species according to the value of the genetic distance between them10. However, not every divergent mitochondrial DNA lineage should be regarded as a separate species11,12,13,14. The divergence detected at the mitochondrial level is not always supported by nuclear DNA data15,16,17. Discrepancies between mitochondrial and nuclear DNA patterns of diversity may arise from various factors. Analyses based on mitochondrial markers may be more susceptible to false results owing to the effective amplification of pseudogenes (e.g. NUMTs) instead of the functional target genes18. Even after this factor has been eliminated in laboratory and bioinformatic analyses, mito-nuclear discordance may persist as a result of biological processes like hybridization and the subsequent introgression of the mitochondrial genome15,16,19, incomplete lineage sorting of ancestral polymorphisms19,20 or selection acting directly or indirectly on mitochondrial genes21. The presence of bacterial endosymbionts can also indirectly influence genetic diversity at the mitochondrial level, with correlations observed between infection patterns and distinct phylogenetic clades or specific haplotypes of hosts22,23,24,25. Furthermore, endosymbiotic bacteria can be associated with both greater and smaller genetic variation of hosts at the mitochondrial level26,27,28 or deep divergences in mitochondrial phylogenies29,30. Therefore, analyses based on mitochondrial markers should incorporate tests for the presence of endosymbiotic bacteria, e.g. Wolbachia31.
Wolbachia is a well-known endosymbiotic bacterium, infecting a broad range of insects, crustaceans and nematode species32,33,34. It is estimated that approximately 66% of insect species are infected by Wolbachia35. The bacterium has been identified in species belonging to several major insect orders, including Coleoptera, Diptera, Hymenoptera, Lepidoptera and Orthoptera36,37.
Orthopterans, one of the largest hemimetabolic insect orders, inhabit diverse terrestrial habitats worldwide, even the nests of social insects38. Ant-loving crickets within the genus Myrmecophilus (Orthoptera: Myrmecophilidae) are commonly associated with various ant species39,40,41. The genus currently comprises 63 valid species, with 10 described in Europe. Most of the latter species occur regionally in southern and south-eastern parts of the continent42. All Myrmecophilus species are morphologically similar, with subtle diagnostic characters based mainly on body colour and the number of spurs on the hind leg, and also the male genitalia and the variability of the female ovipositor’s shape43,44,45,46. However, species identification based on these diagnostic characteristics may be challenging, particularly as nymphs lack the full set of characters of adult specimens. Thus, molecular tools serve as effective means for individual identification, the discovery of potential new species, and the determination of phylogenetic relationships47,48,49.
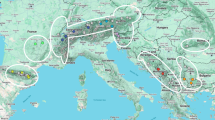
Myrmecophilus acervorum (Panzer, 1799) (Fig. 1) is distributed across Europe and inhabits the nests of approximately 20 host ant species from at least eight genera41,50. Although it is thought to be thelytokous parthenogenetic species, recent surveys in the central part of its distribution range have revealed the presence of both sexes42. Nonetheless, the phylogenetic and phylogeographic relationships among M. acervorum populations remain enigmatic. Initial attempts to elucidate these relationships using molecular markers identified several haplotypes of M. acervorum in Europe, with six forming a parthenogenetic clade in populations distributed west of the Carpathians42. Additionally, testing all collected samples for Wolbachia infection revealed its presence only in populations where both sexes were observed.
In the present study, we employ morphological and molecular approaches to assess the diversity of parthenogenetic M. acervorum occurring in the western Polesie region of Poland and compare the observed patterns with previous data. All the specimens collected were examined morphologically to identify potentially diagnostic traits for routine identification. Subtle differences in the external structure of cricket eyes were noted, appearing to be congruent with interspecific diversity in M. acervorum. At the same time, similar patterns were revealed for mitochondrial markers (COI and 16S), though not fully detectable for the nuclear marker (EF1α). Additionally, all specimens were tested for the presence of endosymbiotic Wolbachia. Analyses based on the Wolbachia 16S gene fragment confirmed infection in all but one of the tested samples, representing the first report of the presence of Wolbachia in parthenogenetic populations of M. acervorum.
Results
Morphological characteristics
The external structure of the M. acervorum eyes was tested for 20 individuals collected in Poland and 3 specimens from Romanian populations (Fig. 2). The surface of ommatidia in members of the Romanian population of ant crickets was smooth (Fig. 3a, b). In contrast, the surface of the ommatidia in the specimens collected in Poland was visibly sculptured (Fig. 3c, d). Two types of appendages can be distinguished: larger ones, ranging from a dozen to over twenty on one ommatidium, and numerous small protuberances (Fig. 3d).
Mitochondrial markers—COI and 16S
Mitochondrial markers were amplified for a subgroup of collected M. acervorum specimens (up to 5 individuals per sample). As a result, high-quality sequences of the cox1 gene fragment were obtained for 83 specimens (Table 1). In the case of 16S mitochondrial marker, only 50 sequences could be generated. Alignment of COI sequences (619 bp) included three variable sites and all of them were parsimony informative. In turn, alignment of 16S sequences (415 bp) included only one variable site, which was not parsimony informative. COI marker sequences were more variable and four unique sequences were identified, while in the case of 16S only two haplotypes were noted (Table 1). Overall haplotype diversity (Hd) was 0.2421 for COI and 0.0400 for 16S. In turn, nucleotide diversity (π) was 0.0004 for COI and 0.0001 for 16 S.
Phylogenetic analyses were performed on a combined alignment (1034 bp) in which we identified five haplotypes (H20-H24; Table 1). Haplotype H20 was common and occurred in all but two of the samples tested (not observed in samples WW-1 and P-1). Subsequent analyses included sequences determined by Iorgu et al.42 for M. acervorum and further 11 congeneric individuals. The genetic distance among selected Myrmecophilus species varied between 0.028 and 0.246 (the lowest value of K2P distance was between M. fuscus and M. gallicus and the highest between M. fuscus and M. manni) (Table 2). In turn, the average intraspecific variation ranged from 0.006 ± 0.002 for M. nonveilleri to 0.012 ± 0.002 for M. acervorum. Thus, we additionally calculated the values of the K2P distance separately for the M. acervorum samples studied by Iorgu et al.42 and those collected in this study (Table 3). In this analysis, the distance between the two M. acervorum subgroups was estimated at 0.020. Distances within the subgroups were smaller (0.005 ± 0.001 for the samples studied by Iorgu et al.42 and 0.002 ± 0.001 for the samples collected in this study). We also calculated the K2P distances among all the concatenated haplotypes (Supplementary Table S1). The highest value (0.028) was recorded between haplotype H7 and haplotypes H21-H24. In addition, we calculated the K2P genetic distances based only on the barcode fragment of the cox1 mitochondrial gene. The overall K2P distance within M. acervorum was 0.035 ± 0.004, but after separation into two groups according to their geographic origin, these values were 0.002 ± 0.001 for the samples collected in the present study and 0.041 ± 0.005 for the samples collected by Iorgu et al.42. The genetic distance between these two groups was 0.035 ± 0.005.
Additionally, we used concatenated haplotypes as an input data for a sequences-based species delineation attempt according to the protocol described by Iorgu et al.42 (Supplementary Table S2). At the 3% value of the barcode gap, we determined six initial and seven recursive partitions. The obtained pattern of separation was similar to previously determined, i.e. the distinctiveness of M. balcanicus, M. gallicus, and M. nonveilleri was supported, as well as internal split within M. nonveilleri. Interestingly, we also observed the clear separation within M. acervorum and as a result we determined three separate inner groups, including: (1) haplotypes H1-H6, H8, and H10 determined by Iorgu et al.42 for M. acervorum samples collected in Romania, Hungary, Bulgaria, and Germany; (2) haplotype H7 determined for M. acervorum sample collected in Pobit Kamak (Bulgaria); and (3) haplotypes H20-H24 determined for samples collected in western Polesie (Poland).
The phylogenetic trees constructed for the concatenated data according to the BI and ML approaches had similar topologies with high branch support. The topology was congruent with that previously determined by Iorgu et al.42 and revealed well-supported groups (Fig. 4). Additionally, two well-separated clades were observed within M. acervorum. These clades grouped haplotypes determined for the previously studied samples and those identified for the samples collected in this study. The populations of M. acervorum from Poland are most probably parthenogenetic41. Males were only historically recorded in Poland, but in a different region (the Pomeranian Lakeland in northern Poland)51. Samples collected from parthenogenetic populations of M. acervorum occurring in Germany (with haplotypes H1-H3 and H8-H10) were also included in the previous study. However, this feature was not reflected in the pattern of clade separation (Fig. 4).
Median-joining network (Fig. 5) was determined for dataset of concatenated mitochondrial sequences. Thus, two haplotypes determined previously for samples collected from parthenogenetic populations occurring in Germany (i.e. haplotypes H8 and H9) were not included in this analysis. In turn, haplotypes H1 and H10 had the same sequence (marked with an asterisk in Fig. 5). The topology of determined network revealed the presence of two well-separated haplogroups (Fig. 5). The pattern of this separation was congruent with that previously observed in the ML tree topology, and the M. acervorum reproductive system (sexual vs. parthenogenetic) was not the grouping factor.
Median-joining network of Myrmecophilus acervorum, based on concatenated sequences of COI and 16S mitochondrial markers. The circle size reflects haplotype frequency (for the haplotypes determined in this study). The black circles represent intermediate haplotypes not encountered in the sampling. The small perpendicular lines represent mutational steps. Haplotypes H1 and H10 had the same sequence. Thus, haplotype H1 is marked with an asterisk.
Nuclear marker—EF1α
This nuclear marker was successfully amplified for 15 individuals of M. acervorum collected from 11 sampling sites (Table 1). Analysis of sequence variation (483 bp) revealed 3 unique variants marked as HE1-HE3. Sequence HE3 was common and occurred in all but two of the samples (was not found in Brus 2 and Lake Czarne 4; Table 1). Further analyses included sequences determined by Hsu et al.45 for another 7 congeners, as well as sequences determined for two specimens collected in Romania and previously analysed by Iorgu et al.42 (specimen MA4.1 from Lepşa and specimen MA59 from Magura Baita).
The genetic distance among the Myrmecophilus species varied between 0.024 and 0.417 (Table 4), and the average intraspecific variation ranged from 0.001 ± 0.001 (M. antilucanus) to 0.009 ± 0.004 (M. mayaealberti). The intraspecific distance calculated for M. acervorum was 0.004 ± 0.002 and the pairwise K2P distance calculated for this species was from 0.002 to 0.006. The highest value was noted for HE1-HE2, HE1-HE4, and HE2-HE5 haplotype pairs (Supplementary Table S3). The value calculated for two M. acervorum subgroups (this study vs. previous study) was 0.005 ± 0.003.
The maximum-likelihood phylogeny reconstructed on the nuclear marker showed well-supported groups but a weak structure within M. acervorum (Fig. 6). The pattern of separation revealed at the mitochondrial level was not fully supported by nuclear data.
Wolbachia prevalence in the samples of Myrmecophilus acervorum
A positive sign of Wolbachia infection was present in all but one of the M. acervorum samples tested (in 101 out of 141 individuals tested collected in 22 out of 23 samples). Only in the sample collected at Łowiszów (LW), the endosymbiont was not detected (Table 5).
Four high-quality PCR products were sequenced and 561 bp long reads were obtained. Among them, two haplotypes were identified (HW1 was observed at Zienki 3, Brus 3, and Lake Czarne 1; haplotype HW2 was observed only at Lake Czarne 1). These haplotypes were analysed with sequences obtained from the GenBank, revealing a high percentage of identity with the previously determined 16 S haplotypes of known Wolbachia endosymbionts infecting other insects (Supplementary Table S4).
The identified 16S haplotypes differed from a single haplotype determined previously for Wolbachia infecting sexual populations of M. acervorum. The values of the p-distance determined for HW1-HW2 and HW1-HW3 pairs were 0.016 and 0.018, respectively (Supplementary Table S5). Similar values of the genetic distance were also found e.g. between haplotypes belonging to supergroups A and E (e.g. 0.011 for KT319093-AJ575104 pair) or between haplotypes representing supergroups C and J (0.014 for AJ010276- AJ548802 pair) (Supplementary Table S5). The ML tree topology revealed that Wolbachia haplotypes HW2 and HW3 determined for bacteria infecting parthenogenetic populations of M. acervorum occurring in western Polesie grouped together with sequences identified for Wolbachia belonging to supergroup A (Fig. 7). In turn, haplotype HW1 determined previously for endosymbionts identified in sexual populations of M. acervorum was found within clade including sequences of Wolbachia belonging to supergroup B (Fig. 7).
Maximum likelihood tree constructed with the 16S molecular marker of Wolbachia infecting Myrmecophilus acervorum and other host species. The references of both M. acervorum reproductive system and corresponding Wolbachia supergroups were added. The numbers at the nodes indicate bootstrap values based on 1000 replicates. The 16S sequences obtained for Ehrlichia ruminantium and E. chaffeensis were used as the outgroup.
Discussion
Knowledge of the biology, ecology and also the morphological and genetic diversity of the myrmecophilous cricket M. acervorum remains limited. Our analyses indicated three unusual phenomena observed in populations inhabiting Lasius nests located in western Polesie (Poland) compared to previously tested samples collected in southern Europe: (i) the presence of potentially diagnostic characteristics in the external structures of M. acervorum eyes differentiating these two groups of samples, (ii) genetic distinctiveness identified between given groups of samples determined at the mitochondrial level with subtle support by data determined at the nuclear level, and (iii) the presence of endosymbiotic Wolbachia bacteria infecting the parthenogenetic populations of M. acervorum in western Polesie.
Morphological characteristics
The taxonomic diagnostics of insects is based on their external morphology. For this reason, ever since the early development of the systematics of these arthropods, the classic ways to identify their systematic position were constructed on the basis of selected morphological characters52,53,54. Nowadays, morphological analysis remains a relevant approach in studies on integrative taxonomy and systematics of insects, including Orthoptera55,56,57. Important diagnostic characters in members of the genus Myrmecophilus include the coloration, the number, size and shape of spines on the tibia and the hindleg pretarsus, the body chaetotaxy and the appearance of the female subgenital plate42,46,58.
Comparative analysis of the morphological structure of 23 specimens of M. acervorum from Poland (20) and Romania (3) generally revealed subtle differences in a few key structures, e.g. the structure of the components of the female’s ovipositor, the structure of the spines on the tibia and the hindleg pretarsus, and the chaetotaxy of the insect’s body. However, since only a small number of specimens from Romania were studied, the data obtained to date cannot be binding, which suggests the need for further analyses based on a much larger sample of research material. In contrast, distinct differences were found in the eye structure. The appearance of the eyes in the Romanian specimens corresponds with a character described by Panzer (1799) in the original description of the species M. acervorum (then Blatta acervorum): oculi parvi subprominuli nitidi, which should be translated as “inconspicuous, shining eyes”. In the Polish specimens, the eyes are matt and covered with a distinctive microstructure with characteristic tubercules. The contemporary literature makes no mention of this character, and what references there are to the structure of the eyes concern solely their presence in a partially reduced form53 and sometimes their coloration59.
Patterns of the genetic diversity at the mitochondrial and nuclear levels
Molecular markers (e.g. mitochondrial fragments) have been frequently applied in studies on orthopteran taxonomy to resolve problems arising from observed phenotypic plasticity, the lack of descriptions of immature stages, and cryptic species complexes60. Some molecular markers are considered to be neutral, i.e. their variants confer no fitness advantage (e.g. microsatellites). In the past, also mtDNA was considered as a source of neutral markers. However, performed studies showed that mtDNA is not always neutrally evolving61. More recently, genome-wide analyses depicted that most nucleotide sites experience fluctuating selection with mean selection coefficients near zero62. However, combining mtDNA and nuclear markers, as well as a better understanding their properties, can improve the power of molecular data.
DNA barcoding is one of the most emblematic contemporary techniques for molecular taxonomy. This approach involves analyses of the genetic distance among sequences of the 5’ end-region of the mitochondrial gene cytochrome c oxidase subunit I with diagnosing and delimiting species63. According to this concept, distinct species can be identified based on the difference between the maximum intraspecific and the minimum interspecific genetic distance, i.e. the barcode gap64. The majority of genera within the order Orthoptera exhibit interspecific distances from 7 to 15% and intraspecific distances of less than 5%60. In the case of the genus Myrmecophilus, the interspecific distance based on COI sequences exceed 13%, and intraspecific distances are less than 1%42. In the present study, the COI-based genetic distance within M. acervorum was 3.5%. After data separation based on a tree topology, the genetic distance between the two groups took the same value (0.035 ± 0.005), while the values within the groups were 0.24% for samples tested by Iorgu et al.42 and 0.2% for samples tested in the present study. The genetic distance between groups raises further questions about the reasons for the observed divergence within M. acervorum. Firstly, the value obtained is higher than the interspecific genetic distance determined for M. fuscus and M. gallicus (2.66%), two morphologically well-supported species. Thus, taking into account the morphological distinctiveness of the two groups of M. acervorum samples based on external eye structures, it is possible that the two identified lineages belong to different species. On the other hand, an alternative explanation should also be considered. Although morphological characteristics of orthopteran eyes are included in species descriptions65,66,67, the references to microstructures present or absent on their surface are often unavailable. Thus, we cannot state unequivocally that this trait is diagnostic within the order Orthoptera or within its genera. However, even if these eye characteristics are not diagnostic, the level of genetic difference may suggest the existence of cryptic species within M. acervorum. A similar case has been described for M. nonveilleri. There, two separate lineages were identified, and the distance between them was estimated at 5.03% on the COI sequences and 3.38% on the concatenated COI and 16S markers. In the present study, we also analysed concatenated mitochondrial data (COI + 16S), and the distance between the two M. acervorum groups was 2%. However, it is worth noting that the genetic distances among haplotypes belonging to these two groups were even higher (up to 2.8%).
Observed genetic differences within and among Myrmecophilus species were also confronted with the result of the preliminary sequence-based delineation test based on concatenated haplotypes. Interestingly, the test confirmed the clear separation within M. acervorum and the presence of three separate groups including: (1) haplotypes determined by Iorgu et al.42 for M. acervorum samples collected in Romania, Hungary, Bulgaria, and Germany; (2) haplotype H7 determined for M. acervorum sample collected in Pobit Kamak (Bulgaria); and (3) haplotypes determined for samples collected in the present study in western Polesie (Poland). We suspect that M. acervorum could be recognized as a complex of cryptic species. Evidences for such phenomena were identified e.g. for Myrmecophilus species associated with Anoplolepis gracilipes45. Nevertheless, in the case of diversity observed in M. acervorum, more samples and specimens collected across its distribution range are needed to perform detailed analyses.
In turn, the nuclear marker (EF1α) is a conserved protein-coding gene that has previously been reported to be useful not only in phylogenetic analyses at the intra- and interordinal levels, but also for resolving relationships among congeneric insect species68,69,70. Moreover, EF1α was analysed together with the mitochondrial marker cytb in order to resolve phylogenetic relationships among Myrmecophilus species associated with Anoplolepis gracilipes ants in the Indo-Pacific region45. The intraspecific genetic distance calculated for the nuclear marker was comparable to values determined for other Myrmecophilus species. Although the haplotypes determined for specimens collected in Romania and Poland did not form distinct clades, as was earlier the case for mitochondrial data, the haplotypes identified for specimens collected in western Polesie seem to have originated from those identified for samples from southern Europe. However, taking into account the low bootstrap values within the identified clade, our explanation should be treated with caution, pending more detailed analysis. Nevertheless, we suggest that the observed phylogenetic pattern may be interpreted as a result of the incomplete sorting of nuclear lineages, potentially indicating an ongoing speciation process. On the other hand, it could also be an effect of introgression resulting from secondary contact of formerly peripatric or allopatric mitochondrial lineages. A similar complex pattern of the genetic diversity at both mitochondrial and nuclear levels has been described for the water louse Asellus aquaticus, widely distributed across Europe, suggesting its possible cryptic speciation71. However, in the case of M. acervorum, more samples collected at a wider spatial scale should be included in any future study to test the proposed hypotheses.
Wolbachia prevalence and its genetic diversity
All 141 individuals of M. acervorum collected in western Polesie (Poland) were tested for Wolbachia infections: the endosymbiont was found in 101 of them (72%). Wolbachia were identified in all but one of the samples obtained from parthenogenetic populations of ant crickets (22 out of 23). This result contradicts the recently published observations described by Iorgu et al.42, who found Wolbachia only in biparental populations of M. acervorum. In that group, the endosymbiont was present at a lower frequency (5 out of 30 specimens tested; 17% of the endosymbiont’s prevalence) than determined here for parthenogenetic populations. A lower frequency in sexual populations of M. acervorum could be associated with Wolbachia-mediated mechanisms induced in its host (for instance, endosymbionts causing male-killing are expected to occur at lower frequencies)72. In turn, a high Wolbachia prevalence in the parthenogenetic population of M. acervorum tested in the present study may be strictly related to its vertical transmission via egg cytoplasm73.
Although our study was focused primarily on testing M. acervorum samples for the presence of Wolbachia, the results shed light on the genetic diversity of this endosymbiont. A comparative analysis with data deposited in GenBank confirmed the > 99% similarity of Wolbachia found in M. acervorum to endosymbionts infecting other insects (e.g. the flies Stomoxys calcitrans and Melieria omissa, the moth Schoenobius gigantella or the hoverfly Microdon myrmicae). Moreover, Wolbachia infecting parthenogenetic populations of M. acervorum differed significantly from endosymbionts previously identified in sexual populations (1.6% difference between the sequences HW1 (found in sexual populations) and HW2 (found in parthenogenetic populations), and 1.8% between the sequences HW1 and HW3 (also found in parthenogenetic populations)). In turn, both Wolbachia haplotypes identified in the parthenogenetic populations tested in the present study differ from each other to a lesser extent (0.2%).
The phylogeny reconstruction including also sequences representing Wolbachia from various supergroups clearly confirmed a genetic differentiation between endosymbionts infecting populations of M. acervorum occurring in western Polesie and Romania. Haplotype HW1 determined previously clustered with sequences identified for Wolbachia belonging to supergroup B, while newly found haplotypes HW2 and HW3 grouped with sequences representing supergroup A. These two Wolbachia supergroups are frequently observed in insects37, also among species representing order Orthoptera74. For example, species belonging to genus Hemiandrus (Family Anostostomatidae) are infected with supergroup A or B but none of the tested species was identified to simultaneously harbour bacteria from both supergroups. Thus, we suggest that both groups of M. acervorum populations (i.e. parthenogenetic from western Polesie and sexual from Pașcani, Coșereni and Lepșa sampling sites in Romania) were independently infected with genetically divergent endosymbionts. Moreover, we cannot exclude that Wolbachia infecting M. acervorum and belonging to separate supergroups differ in rates of transfer or induced mechanisms manipulating host’s reproduction. Those characteristics might explain differences in endosymbiont frequency described above. Interestingly, the parthenogenetic populations belonging to the western clade determined by Iorgu et al.42 remain uninfected with Wolbachia. The background of this phenomenon remains unsolved. Thus, more comprehensive analyses are needed to investigate both the genetic diversity of Wolbachia infecting M. acervorum within its distribution range, as well as endosymbiont transmission among host’s parthenogenetic and sexual populations (e.g. based on MLST system or phylogenomic approach)75,76.
Proposed scenarios inducing observed diversity within Myrmecophilus acervorum
Myrmecophilus acervorum is an Orthopteran highly adapted to co-existence with ants. Inhabiting the distinctive habitat of an ant’s nest, it possesses a series of adaptive morphological characters, e.g. partially reduced eyes, the lack of wings and small body size53,59, which account for its poor dispersal abilities. Another important feature of this species is its reproductive strategy based on thelytokous parthenogenesis, which occurs in populations over a wide area of its distribution range42. Parthenogenesis enables insects to survive in unpropitious conditions and facilitates the faster colonization of new terrain77. Speciation after the transition to parthenogenesis is considered to be very rare, and so are reversals to sexuality78.
Parthenogenesis is rare among Orthoptera79,80, and known cases relate not only to species living in highly distinctive habitats, such as cave crickets of the genus Hadenoecus Scudder, 186381, but also to those inhabiting less specialized niches, such as the flightless bush cricket Poecilimon intermedius Fieber, 1853, which is associated with grassy habitats82 or grasshopper Saga pedo Pallas, 1771 inhabiting various habitats (preferably areas with dry summers and mild winters, e.g. grasslands, meadows, pastures, or gorges)83. According to Kirkendall and Normark84, its inability to fly and its consequent poor dispersal abilities might be recompensed by parthenogenesis.
It is known that following the last glaciation, parthenogenesis enabled many species to colonize available terrain faster, which is probably why they are now more widely distributed than their heterosexual counterparts82,85,86. Myrmecophilus acervorum is by a large margin the most widely distributed member of the genus in Europe87, far more than other, heterosexual Myrmecophilus spp. that inhabit only the Balkans and other regions in southern Europe. This ant cricket is therefore a perfect example of the crucial role of parthenogenetic reproduction in the effective colonization of new terrain by living organisms. Moreover, there are further examples of this phenomenon among other orthopterans. The distribution of most members of the genus Poecilimon Fischer, 1853, with populations in which males are present, is restricted to the southern Balkans, Turkey and the Caucasus, whereas the parthenogenetic P. intermedius occurs from central Europe all the way to western China82,88. Likewise, the parthenogenetic Saga pedo Pallas, 1771, is widely distributed in southern Europe, and also in Russia and China, whereas the ranges of the other heterosexual species from this genus are limited to the Balkans83.
Analysis of the present-day distribution of M. acervorum in the context of the postglacial migration history of the European fauna suggests that the Balkan region was probably the centre of occurrence and the refugial area from which Europe became colonized by M. acervorum. This hypothesis is also supported by data given by Varga89, who regards the areas to the south and east of the Carpathians as key to the survival and evolution of autochthonous lineages of poorly mobile species. In this part of Europe, the Carpathians was the only region during the last glaciation that was not covered by the ice sheet, which enabled the survival of many species of flora and fauna90,91.
On the basis of literature data describing the directions of post-glacial migrations of species of flora and fauna from their glacial refugia in southern Europe92, one can set up a probable hypothesis regarding the directions of northwards dispersal of M. acervorum during the post-glacial period and assume that recolonization routes were generally congruent with the well-known migration corridors. Nevertheless, the exact locations of glacial refugia of M. acervorum remain unknown and more comprehensive, phylogeographic approach is needed to determine them. On the one hand, we may suggest that the glacial refugium was likely located in Carpathian region, where sexual populations of M. acervorum have been identified42. In turn, parthenogenetic populations have been broadly observed across species distribution range (i.e. in Bulgaria, western Romania, Hungary, Germany, and Poland). Parthenogenetic forms are better colonizers and thus, could efficiently colonize new areas available during interglacial cycles93,94. Postglacial history could support the divergence among populations of M. acervorum, giving rise to the separate phylogenetic lineages recorded in this study, and which, moreover, differ in the external structure of the eye. The division at the level of mitochondrial DNA, not explicitly supported by the results of nuclear marker analysis, could be associated with the dynamics of lineage sorting, which is quicker in the case of the mitochondrial genome. Furthermore, the possibility cannot be ruled out that the phylogenetic lineages of M. acervorum identified here are in fact hitherto undescribed cryptic species defined as genetically distinct units, which on the basis of their evident morphological similarity, are classified as a single species95. This hypothesis is supported by the result of the sequences-based species delineation but needs more data to be confirmed.
On the other hand, the observed intraspecific divergence (observed primarily at the mitochondrial level) could be linked with the presence of Wolbachia infecting M. acervorum. Endosymbionts detected in sexual populations group with Wolbachia belonging to supergroup B, while those found in parthenogenetic populations cluster with Wolbachia from supergroup A. The identified diversity could explain the difference in Wolbachia frequency within sexual and parthenogenetic populations of M. acervorum. We detected a high percentage of endosymbionts in parthenogenetic populations inhabiting western Polesie. That may suggest that in these populations Wolbachia is predominantly vertically transmitted, while in sexual populations could induce one of the well-known mechanisms of manipulation of host’s reproduction. However, Wolbachia has not been found in parthenogenetic populations from Romania and thus, further studies are needed to explain this phenomenon (especially in the context of induced parthenogenesis).
Nevertheless, the presence of endosymbionts infecting M. acervorum and belonging to distinct supergroups is in line with the pattern of host’s genetic divergence. Like mtDNA, transmission of Wolbachia is effected through the maternal lineage and can be associated with the correlated transmission of the symbiont together with its associated mitogenome haplotype96. Nonetheless, in the case of M. acervorum, we also discovered a distinctive morphological character, the state of which differentiates ant cricket populations in accordance with the separation pattern at the mitochondrial DNA level. To date, no influence on the part of Wolbachia has been reported on the variability of diagnostic morphological characters, so the alternative hypothesis outlined above appears to be less probable.
Conclusions
A range of factors are known, e.g. the isolation of particular populations, climatic conditions, phenotypic plasticity associated with the type of habitat, and genetic drift, which can affect the genetic and morphological diversity of constantly evolving organisms. But where intraspecific diversity is concerned, particular individuals usually differ only in the morphometrics of specific body parts97,98 or in their coloration99. Hence, the significant genetic variability at the mitochondrial DNA level (COI and 16S) found in the present case, and the differentiating morphological character (i.e. eye structure), are almost certainly effects of cryptic species being present within M. acervorum. This, in turn, is indicative of ongoing speciation within the populations of this insect, and of simultaneous unfinished lineage sorting at the nuclear DNA level. However, the absence of males evidently limits the effectiveness of a morphological analysis performed solely on the basis of available female specimens as regards establishing their actual taxonomic status. It is therefore essential to continue this research and to establish genetic variability in populations of the ant cricket from all parts of its distribution range, based on both mitochondrial and nuclear markers. In addition, further studies need to focus on detecting and analysing the genetic variability in Wolbachia bacteria in both heterosexual and parthenogenetic ant cricket populations. In this way, the hypothesis regarding the independent infection of identified lineages of M. acervorum by endosymbionts from separate groups can be tested.
Methods
Sample collection
The 141 specimens of M. acervorum from Poland used in this research were collected from May to October 2021 in western Polesie (Table 6). The main habitats of the ant crickets in this area were dry pine forests with decaying birch trunks lying on the forest floor, colonized by Lasius platythorax or Formica fusca ants (Fig. 2). Some specimens were found in different habitats (Table 6). Sampling included preliminary screening of selected nests for the presence of M. acervorum individuals. Once ant-loving crickets were found in a particular nest, a sample of nest material (about 1 dm3) was collected and transported to the laboratory. Then, all samples were separately searched and each M. acervorum specimen found was preserved in the appropriate dilution of ethanol (i.e. 75% EtOH for analyses of morphology and 99% EtOH for further genetic analyses).
Microscopic methods
Material selection and a general analysis of specimens (20 from Poland and 3 from Romania) were carried out using an OLYMPUS SZ51 stereoscopic microscope. For the SEM techniques, samples were dried at the critical point of CO2 using an Emitech K850 Critical Point Dryer. The 23 dried specimens were then coated with a gold layer using an Emitech K550X Sputter Coater, after which the samples were placed directly in the VEGA3 TESCAN SEM chamber for examination and visualization. The SEM parameters were as follows: HV 30.00 kV, magnification 3.00 kx (Fig. 3a, c) and 9.00 kx (Fig. 3b, d). WD 18.68–18.86 mm.
DNA extraction and amplification of selected molecular markers of Myrmecophilus acervorum
Genomic DNA was extracted from 141 collected specimens using the Sherlock AX kit (A&A Biotechnology, Gdańsk, Poland) according to the manufacturer’s protocol. Genetic material was extracted from whole body of all the specimens collected.
Both mitochondrial (COI and 16S) and nuclear (EF1α) markers were chosen to examine the genetic diversity of M. acervorum (for up to 5 specimens per sample). Selected mitochondrial markers were previously examined in M. acervorum samples collected in south-eastern and western Europe42. Thus, for further comparative analyses of sequence data, we applied the PCR protocols in our study. The COI fragment was amplified using the LCO-1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’) and HCO-2189 (5’-TAAACTTCAGGGTGACCAAAAAATCA-3’) primer pair100. In turn, the 16S fragment was amplified using 16Sar-Dr (5’-CGCCTGTTTAACAAAAACAT-3’) and 16Sbr (5’- CCGGTCTGAACTCAAGATCACGT-3’) primers101,102. The nuclear marker (EF1α) was amplified using the EF-alpha-1 F (5’-ATCGAGAGGTTCGAGAARGARGC-3’) and EF-alpha-1R (5’-CCAYCCCTTRAACCANGGCAT-3’) primer pair45. PCR reactions were performed in a 20 µL volume containing 0.8x JumpStart REDTaq ReadyMix (1 U of JumpStart REDTaq DNA polymerase, 4 mM Tris-HCl, 20 mM KCl, 0.6 mM MgCl2, 0.08 mM of dNTP; Sigma-Aldrich, Germany), 0.4 µM of forward and reverse primers and ~ 100 ng of DNA. The volume was made up to 20 µL with autoclaved water. The amplification of these molecular markers involved the same conditions – 5 min at 94 °C; 35 cycles of 1 min at 94 °C, 1 min at 46.9 °C (COI and 16S) or 52.9 °C (EF1α), 1 min at 72 °C; a final extension at 72 °C for 5 min. All molecular markers were amplified for the same individuals.
The amplified fragments were separated by 1% agarose gel electrophoresis in a 1x SB buffer and visualized with SimplySafe™ (ethidium bromide replacement; EURx, Poland) in UV light. All PCR products were purified by Eppic Fast (A&A Biotechnology, Poland) treatment according to the relevant protocols and sequenced using the BigDye™ terminator cycle sequencing method. All the newly obtained sequences were deposited in the GenBank database (accession nos.: PP896881-PP896884 for COI, PP886506-PP886507 for 16 S, PP915641-PP915645 for EF1α).
Testing for Wolbachia infections
The M. acervorum samples were tested for Wolbachia infections. All 141 specimens were screened using primers specific for the Wolbachia 16S fragment (WF: 5’-CGGGGGAAAATTTATTGCT-3’ and WR: 5’- AGCTGTAATACAGAAAGGAAA-3’)103. Amplifications were performed with a total volume of 25 µl using DreamTaq Hot Start Green PCR Master Mix (Thermo Scientific, UK), in accordance with the manufacturer’s instructions. Amplifications were performed with positive and negative controls. For the positive control, the DNA template isolated from D. melanogaster individuals infected with the wMel Wolbachia strain was used, while the negative control was undertaken without the DNA template.
The amplified fragments were separated by 1% agarose gel electrophoresis in a 1x SB buffer and visualized with SimplySafe™ (ethidium bromide replacement; EURx, Poland) in UV light. Selected good-quality products were purified by Eppic Fast (A&A Biotechnology, Poland) treatment according to the relevant protocols and sequenced using the BigDye™ terminator cycle sequencing method. All the newly obtained sequences were deposited in the GenBank database (accession nos.: PP897406-PP897407).
Bioinformatic analyses
The BLAST application (Altschul et al. 1990) was used to compare the sequences with those deposited in the NCBI database. Alignments were prepared using Geneious Prime 2023.2.1 (https://www.geneious.com) with Clustal Omega algorithm104. Mitochondrial sequences were analysed separately and as a concatenated alignment. Haplotypes were determined using DnaSP v.6.12.03105.
In Mega X106, we estimated pairwise genetic distances using the Kimura 2-parameter (K2P) nucleotide substitution model with 1,000 bootstrap replicates. Reconstructions of phylogenetic relationships among the identified haplotypes and genotypes with a median-joining algorithm (MJ107) were performed in PopART 1.7 software108. The best-fit models for nucleotide substitution (for mitochondrial and nuclear data) were estimated using jModeltest 2.1.10109 with both the Akaike and Bayesian information criterions (AIC and BIC, respectively).
Phylogenetic analyses were generated using the maximum likelihood (ML) and Bayesian inference (BI) methods. ML analyses were performed in RAxML-v.8 software110 implemented online on the CIPRES Science Gateway111, using 1,000 bootstrap resampling and other parameters as default. In turn, MrBayes v.3.2.7112 was used for BI analyses. The Markov Chain Monte Carlo (MCMC) search was run with four chains for 10 million generations with a sampling frequency of 1/1000 trees, with a burn-in of 10%. The DNA sequence-based species delineation approach was applied on concatenated sequences of COI and 16S, as described by Iorgu et al.42. Mentioned analysis was performed using ABGD web platform113.
In turn, maximum likelihood reconstruction of Wolbachia phylogeny based on 16S rRNA gene sequences were performed in Mega X software106 with 1,000 bootstrap replicates. Additional 16S sequences determined for Wolbachia belonging to various supergroups, as well as sequences identified for Ehrlichia ruminantium and E. chaffeensis (served as the outgroup), were added to the analysis114. Final phylogenetic trees were edited in FigTree v.1.4.2. (http://tree.bio.ed.ac.uk/software/figtree/).
Data availability
The data underlying this article are available in the article and in its online supplementary material, as well as in the GenBank Nucleotide Database and can be accessed with accession numbers PP896881-PP896884 for COI, PP886506-PP886507 for 16S, PP915641-PP915645 for EF1α of Myrmecophilus acervorum, and PP897406-PP897407 for 16S of Wolbachia infecting selected ant cricket species.
References
DeSalle, R. & Goldstein, P. Review and interpretation of trends in DNA barcoding. Front. Ecol. Evol. 7, 302 (2019).
Harrison, R. G. Animal mitochondrial DNA as a genetic marker in population and evolutionary biology. Trends Ecol. Evol. 4, 6–11 (1989).
Birky, C. W. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu. Rev. Genet. 35, 125–148 (2001).
Breton, S. & Stewart, D. T. Atypical mitochondrial inheritance patterns in eukaryotes. Genome 58, 423–431 (2015).
Brown, W. M., George, M. & Wilson, A. C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. U.S.A. 76, 1967–1971 (1979).
Shigenaga, M. K., Hagen, T. M. & Ames, B. N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. 91, 10771–10778 (1994).
Birky, C. W., Fuerst, P. & Maruyama, T. Organelle gene diversity under migration, mutation, and drift: equilibrium expectations, approach to equilibrium, effects of heteroplasmic cells, and comparison to nuclear genes. Genetics 121, 613–627 (1989).
Hudson, R. R. & Turelli, M. Stochasticity overrules the three-times rule: genetic drift, genetic draft, and coalescence times for nuclear loci versus mitochondrial dna. Evolution 57, 182–190 (2003).
Lin, C. P. & Danforth, B. N. How do insect nuclear and mitochondrial gene substitution patterns differ? Insights from bayesian analyses of combined datasets. Mol. Phylogenet. Evol. 30, 686–702 (2004).
Petrusek, A., Thielsch, A. & Schwenk, K. Mitochondrial sequence variation suggests extensive cryptic diversity within the western palearctic Daphnia longispina complex. Limnol. Oceanogr. 57, 1838–1845 (2012).
Dasmahapatra, K. K., Elias, M., Hill, R. I., Hoffman, J. I. & Mallet, J. Mitochondrial DNA barcoding detects some species that are real, and some that are not. Mol. Ecol. Resour. 10, 264–273 (2010).
Hernández-López, A. et al. Host tracking or cryptic adaptation? Phylogeography of Pediobius saulius (Hymenoptera, Eulophidae), a parasitoid of the highly invasive horse-chestnut leafminer. Evol. Appl. 5, 256–269 (2012).
Mardulyn, P., Othmezouri, N., Mikhailov, Y. E. & Pasteels, J. M. Conflicting mitochondrial and nuclear phylogeographic signals and evolution of host-plant shifts in the boreo-montane leaf beetle Chrysomela lapponica. Mol. Phylogenet. Evol. 61, 686–696 (2011).
Thielsch, A., Knell, A., Mohammadyari, A., Petrusek, A. & Schwenk, K. Divergent clades or cryptic species? Mito-nuclear discordance in a Daphnia species complex. BMC Evol. Biol. 17, 227 (2017).
Gompert, Z., Forister, M. L., Fordyce, J. A. & Nice, C. C. Widespread mito-nuclear discordance with evidence for introgressive hybridization and selective sweeps in Lycaeides. Mol. Ecol. 17, 5231–5244 (2008).
Linnen, C. R. & Farrell, B. D. Mitonuclear discordance is caused by rampant mitochondrial introgression in Neodiprion (Hymenoptera: Diprionidae) sawflies. Evolution 61, 1417–1438 (2007).
Shu, X. X. et al. Rapid genetic divergence and mitonuclear discordance in the Taliang knobby newt (Liangshantriton taliangensis, Salamandridae, Caudata) and their driving forces. Zool. Res. 43, 129–146 (2022).
Song, H., Buhay, J. E., Whiting, M. F. & Crandall, K. A. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc. Natl. Acad. Sci. U.S.A. 105, 13486–13491 (2008).
Funk, D. J. & Omland, K. E. Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 34, 397–423 (2003).
Franco, F., Lavagnini, T., Sene, F. & Manfrin, M. Mito-nuclear discordance with evidence of shared ancestral polymorphism and selection in cactophilic species of Drosophila. Biol. J. Linn. Soc. 116, 1 (2015).
Pavlova, A. et al. Perched at the mito-nuclear crossroads: divergent mitochondrial lineages correlate with environment in the face of ongoing nuclear gene flow in an Australian bird. Evol. Int. J. Org. Evol. 67, 3412–3428 (2013).
D. D Hurst, G. & M Jiggins, F. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc. R. Soc. B Biol. Sci. 272, 1525–1534 (2005).
Kambhampati, S., Rai, K. S. & Verleye, D. M. Frequencies of mitochondrial DNA haplotypes in laboratory cage populations of the mosquito, Aedes albopictus. Genetics 132, 205–209 (1992).
Sun, X. J., Xiao, J. H., Cook, J. M., Feng, G. & Huang, D. W. Comparisons of host mitochondrial, nuclear and endosymbiont bacterial genes reveal cryptic fig wasp species and the effects of Wolbachia on host mtDNA evolution and diversity. BMC Evol. Biol. 11, 86 (2011).
Wendt, M., Kulanek, D., Varga, Z., Rákosy, L. & Schmitt, T. Pronounced mito-nuclear discordance and various Wolbachia infections in the water ringlet Erebia pronoe have resulted in a complex phylogeographic structure. Sci. Rep. 12, 5175 (2022).
Cariou, M., Duret, L. & Charlat, S. The global impact of Wolbachia on mitochondrial diversity and evolution. J. Evol. Biol. 30, 2204–2210 (2017).
Jäckel, R., Mora, D. & Dobler, S. Evidence for selective sweeps by Wolbachia infections: phylogeny of Altica leaf beetles and their reproductive parasites. Mol. Ecol. 22, 4241–4255 (2013).
Zhu, X. et al. Diversity of Wolbachia infection and its influence on mitochondrial DNA variation in the diamondback moth, Plutella xylostella. Mol. Phylogenet. Evol. 182, 107751 (2023).
Kodandaramaiah, U., Simonsen, T. J., Bromilow, S., Wahlberg, N. & Sperling, F. Deceptive single-locus taxonomy and phylogeography: Wolbachia-associated divergence in mitochondrial DNA is not reflected in morphology and nuclear markers in a butterfly species. Ecol. Evol. 3, 5167–5176 (2013).
Ritter, S. et al. Wolbachia infections mimic cryptic speciation in two parasitic butterfly species, Phengaris teleius and P. nausithous (Lepidoptera: Lycaenidae). PLoS ONE 8, e78107 (2013).
Sucháčková Bartoňová, A. et al. Wolbachia affects mitochondrial population structure in two systems of closely related Palaearctic blue butterflies. Sci. Rep. 11, 3019 (2021).
Sazama, E. J., Ouellette, S. P. & Wesner, J. S. Bacterial endosymbionts are common among, but not necessarily within, insect species. Environ. Entomol. 48, 127–133 (2019).
Sazama, E. J., Bosch, M. J., Shouldis, C. S., Ouellette, S. P. & Wesner, J. S. Incidence of Wolbachia in aquatic insects. Ecol. Evol. 7, 1165–1169 (2017).
Zug, R. & Hammerstein, P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7, e38544 (2012).
Hilgenboecker, K., Hammerstein, P., Schlattmann, P., Telschow, A. & Werren, J. H. How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220 (2008).
De Oliveira, C. D. et al. Broader prevalence of Wolbachia in insects including potential human disease vectors. Bull. Entomol. Res. 105, 305–315 (2015).
Werren, J. H. & Windsor, D. M. Wolbachia infection frequencies in insects: evidence of a global equilibrium?. Proc. R. Soc. B Biol. Sci. 267, 1277–1285 (2000).
Naskrecki, P. Grasshoppers and their relatives. In Encyclopedia of Biodiversity 722–736. https://doi.org/10.1016/B978-0-12-384719-5.00168-4 (2013).
Komatsu, T., Maruyama, M. & Itino, T. Nonintegrated host association of Myrmecophilus tetramorii, a specialist myrmecophilous ant cricket (Orthoptera: Myrmecophilidae). Psyche J. Entomol. 2013, e568536 (2013).
Komatsu, T. & Maruyama, M. Taxonomic recovery of the ant cricket Myrmecophilus albicinctus from M. americanus (Orthoptera, Myrmecophilidae). ZooKeys 589, 97–106 (2016).
Żurawlew, P. et al. Distribution of Myrmecophilus acervorum (Panzer, 1799) (Orthoptera: Myrmecophilidae) in Poland. Fragm. Fauna 65, 55–68 (2022).
Iorgu, I. Ş et al. Ant crickets and their secrets: Myrmecophilus acervorum is not always parthenogenetic (Insecta: Orthoptera: Myrmecophilidae). Zool. J. Linn. Soc. 197, 211–228 (2023).
Desutter-Grandcolas, L. First record of ant-loving crickets (Orthoptera: Myrmecophilidae: Myrmecophilinae) in New Caledonia. Aust J. Entomol. 36, 159–163 (1997).
Hebard, M. Revision of the north American species of the genus Myrmecophila (Orthoptera; Gryllidae; Myrmecophilinae). Trans. Am. Entomol. Soc. 46, 91–111 (1920).
Hsu, P. W. et al. Ant crickets (Orthoptera: Myrmecophilidae) associated with the invasive yellow crazy ant Anoplolepis gracilipes (Hymenoptera: Formicidae): evidence for cryptic species and potential co-introduction with hosts. https://doi.org/10.25849/MYRMECOL.NEWS_030:103 (2020).
Stalling, T. & Birrer, S. Identification of the ant-loving crickets, Myrmecophilus Berthold, 1827 (Orthoptera: Myrmecophilidae), in Central Europe and the northern Mediterranean Basin. Articulata 28, 1–11 (2013).
Komatsu, T., Maruyama, M. & Itino, T. Differences in host specificity and behavior of two ant cricket species (Orthoptera: Myrmecophilidae) in Honshu, Japan. J. Entomol. Sci. 45, 227–238 (2010).
Komatsu, T., Maruyama, M., Ueda, S. & Itino, T. mtDNA phylogeny of Japanese ant crickets (Orthoptera: Myrmecophilidae): diversification in host specificity and habitat use. 52 (2008).
Ortega-Morales, A. I. et al. First record of the ant cricket Myrmecophilus (Myrmecophilina) americanus (Orthoptera: Myrmecophilidae) in Mexico. Zootaxa 4258, 195–200 (2017).
Franc, V., Majzlan, O., Krištín, A. & Wiezik, M. On the distribution and ecology of the ant cricket (Myrmecophilus Acervorum) (Orthoptera: Myrmecophilidae) in Slovakia. Matthias Belivs Univ. Proc. 5, 40–50 (2015).
Bazyluk, W. Nowe dla Polski lub rzadsze gatunki z rzędów Blattodea, Mantodea, Orthoptera i Dermaptera. Fragm. Fauna 7, 263–282 (1957).
Dominik, J. Klucze Od Oznaczania Owadów Polski. Cz. XIX Chrząszcze—Coleoptera. Zeszyt 41 Kołatki—Anobiidae (Państwowe Wydawnictwo Naukowe, 1955).
Bazyluk, W. Prostoskrzydłe—Orthoptera (Saltatoria) (Warszawa, 1956).
Nunberg, M. Obumierki—Rhizophagidae Vol. 64 (Warszawa, 1967).
Bellman, H. Szarańczaki. Łatwe Oznaczanie Gatunków Europy Środkowej (MULTICO Oficyna Wydawnicza, 2009).
Louveaux, A., Amédégnato, C., Poulain, S. & Desutter-Grandcolas, L. Catalogue and keys of the Acridomorpha (Insecta, Orthoptera) from north West Africa. Zoosystema 35, 175–181 (2013).
Smith, T., Froeba, J. & Capinera, J. Key to the grasshoppers (Orthoptera: Acrididae) of Florida. Fla. Entomol. 87, 537–550 (2009).
Stalling, T. A new species of ant-loving cricket from Mallorca, Balearic Islands, Spain (Orthoptera, Myrmecophilidae). Graellsia 69, 153–156 (2013).
Stalling, T. MyrmecopGallicusllicus, a new species of ant cricket from France (Orthoptera: Myrmecophilidae). Articulata 32, 1–7 (2017).
Timm, V. F., Gonçalves, L. T., Valente, V. L. D. S. & Deprá, M. The efficiency of the COI gene as a DNA barcode and an overview of Orthoptera (Caelifera and Ensifera) sequences in the BOLD system. Can. J. Zool. 100, 710–718 (2022).
Dong, Z., Wang, Y., Li, C., Li, L. & Men, X. Mitochondrial DNA as a molecular marker in insect ecology: current status and future prospects. Ann. Entomol. Soc. Am. 114, 470–476 (2021).
Lynch, M., Wei, W., Ye, Z. & Pfrender, M. The genome-wide signature of short-term temporal selection. Proc. Natl. Acad. Sci. 121, e2307107121 (2024).
Hebert, P. D. N., Cywinska, A., Ball, S. L. & deWaard, J. R. Biological identifications through DNA barcodes. Proc. R Soc. B Biol. Sci. 270, 313–321 (2003).
Meyer, C. P. & Paulay, G. DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 3, e422 (2005).
K Tan, M. & Robillard, T. Rugabinthus, a new genus of Lebinthina (Orthoptera, Gryllidae, Eneopterinae) from New Guinea. J. Orthoptera Res. 31, 9–40 (2022).
Cao, C., Rong, H. & Naveed, H. Two new species of the genus Xya Latreille, 1809 (Orthoptera, Tridactyloidea, Tridactylidae) from Yunnan with a key to all Xya species in China. ZooKeys 947, 103–112 (2020).
Gu, J. J., Zhou, Y. & Yuan, W. New Genera and species of trigonidiidae (Orthoptera: Grylloidea) from the mid-cretaceous of Myanmar with a redescription of Birmaninemobius hirsutus. Insects 15, 442 (2024).
Kim, S., Lee, W. & Lee, S. Estimation of a new molecular marker of the genus Stathmopoda (Lepidoptera: Stathmopodidae): comparing EF1a and COI sequences. J. Asia-Pac. Entomol. 20, 269–280 (2017).
Marques, J. F., Winde, I., Jönsson, A. M. & Anderbrant, O. Taxonomic relationship among four European physokermes species (Hemiptera: Coccomorpha) based on nuclear and mitochondrial DNA. Front. Glob. Change 6, 1 (2023).
Simon, S., Schierwater, B. & Hadrys, H. On the value of elongation factor-1α for reconstructing pterygote insect phylogeny. Mol. Phylogenet. Evol. 54, 651–656 (2010).
Sworobowicz, L. et al. Revisiting the phylogeography of Asellus aquaticus in Europe: insights into cryptic diversity and spatiotemporal diversification. Freshw. Biol. 60, 1824–1840 (2015).
Hurst, G. & Jiggins, F. M. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6, 329–336 (2000).
Huigens, M. E., de Almeida, R. P., Boons, P. A. H., Luck, R. F. & Stouthamer, R. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. B Biol. Sci. 271, 509–515 (2004).
Bridgeman, B., Morgan-Richards, M., Wheeler, D. & Trewick, S. A. First detection of Wolbachia in the New Zealand biota. PLoS ONE 13, e0195517 (2018).
Wang, X. et al. Phylogenomic analysis of Wolbachia strains reveals patterns of genome evolution and recombination. Genome Biol. Evol. 12, 2508–2520 (2020).
Baldo, L. et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72, 7098–7110 (2006).
O’Woma, O. O., Chigozirim, U. P., Emmanuel, O. & Chukwuebuka, E. M. Reproductive and survival strategies utilized by insect. A review. Am. J. Zool. Res. 4, 1–6 (2016).
Bell, G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality (Routledge, 2019).
Normark, B. Modes of reproduction. In The Evolution of Insect Mating Systems Vol. 352 (eds Shuker, D. M. & Simmons, L. W.) (Oxford University Press, 2014).
Fernandes, M. L., Zacaro, A. A. & Serrão, J. E. First report of a parthenogenetic Grylloidea and new genus of Neoaclini (Insecta: Orthoptera: Grylloidea: Phalangopsidae: Phalangopsinae). Zootaxa 4032, 407–416 (2015).
Lamb, R. Y. & Willey, R. B. The First parthenogenetic populations of orthoptera saltatoria to be reported from North America. Ann. Entomol. Soc. Am. 68, 721–722 (1975).
Borissov, S. B., Hristov, G. H. & Chobanov, D. P. Phylogeography of the Poecilimon ampliatus species group (Orthoptera: Tettigoniidae) in the context of the Pleistocene glacial cycles and the origin of the only thelytokous parthenogenetic phaneropterine bush-cricket. Arthropod. Syst. Phylogeny 79, 401–418 (2021).
Dutrillaux, A. M. et al. Origin of the complex karyotype of the polyploid parthenogenetic grasshopper Saga pedo (Orthoptera: Tettigoniidae). Eur. J. Entomol. 106, 477–483 (2009).
Kirkendall, L. & Normark, B. Parthenogenesis. In Encyclopedia of Insects (Resh, V. H. & Cardé, R.) 851–856 (Academic Press, 2003).
Vandel, A. P. La parthénogenèse géographique: contribution á l’étude biologique et cytologique de la parthénogenèse naturelle. Bull. Biol. Fr. Belg. 62, 164–281 (1928).
Cosendai, A. C., Wagner, J., Ladinig, U., Rosche, C. & Hörandl, E. Geographical parthenogenesis and population genetic structure in the alpine species Ranunculus kuepferi (Ranunculaceae). Heredity 110, 560–569 (2013).
de Jong, Y., Fauna Europaea Consortium. Checklist dataset. Fauna Europaea. https://doi.org/10.15468/ymk1bx (2016).
Wu, C. & Liu, C. New record of a Poecilimon species (Orthoptera: Tettigoniidae: Phaneropterinae; Barbitistini) from China. Zootaxa 4612, 296–300 (2019).
Varga, Z. Post-glacial Dispersal Strategies of Orthoptera and Lepidoptera in Europe and in the Carpathian Basin 93–105 (2003).
Hewitt, G. M. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 68, 87–112 (1999).
Zasadni, J. & Kłapyta, P. The tatra mountains during the last glacial maximum. J. Maps 10, 440–456 (2014).
Taberlet, P., Fumagalli, L., Wust-Saucy, A. & Cosson, J. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 7, 453–464 (1998).
Morgan-Richards, M., Trewick, S. A. & Stringer, I. A. N. Geographic parthenogenesis and the common tea-tree stick insect of New Zealand. Mol. Ecol. 19, 1227–1238 (2010).
Hoffmann, A. A., Reynolds, T., Nash, K. & Weeks, A. M. A. R. A high incidence of parthenogenesis in agricultural pests. Proc. R. Soc. B Biol. Sci. 275, 2473–2481 (2008).
Pfenninger, M. & Schwenk, K. Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evol. Biol. 7, 121 (2007).
Oliveira, D. C. S. G., Raychoudhury, R., Lavrov, D. V. & Werren, J. H. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera: Pteromalidae). Mol. Biol. Evol. 25, 2167–2180 (2008).
Agrawal, A. A. Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326 (2001).
Pan, Z., Hong, F. & Jiang, G. Morphometrics reveal correlation between morphology and bioclimatic factors and population mixture in Tetrix japonica (Orthoptera: Tetrigidae). Acta Zool. 99, 199–210 (2018).
Yadav, S., Stow, A. J., Harris, R. M. B. & Dudaniec, R. Y. Morphological variation tracks environmental gradients in an agricultural pest, Phaulacridium vittatum (Orthoptera: Acrididae). J. Insect Sci. Online 18, 13 (2018).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299 (1994).
Palumbi, S. et al. The Simple Fool’s Guide to PCR, Ver. 2 (Department of Zoology and Kewalo Marine Laboratory, University of Hawaii, 1991).
Palumbi, S. R. Molecular Systematics 2nd edn, 205–247 (Sinauer, 1996).
Singh, S. T., Kumar, J., Thomas, A., Ramamurthy, V. V. & Rajagopal, R. Detection and localization of Rickettsia sp. in Mealybug. Environ. Entomol. 42, 711–716 (2013).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Rozas, J. et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 34, 3299–3302 (2017).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Bandelt, H. J., Forster, P. & Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16, 37–48 (1999).
Leigh, J. W. & Bryant, D. Popart: full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (2012).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Miller, M. A., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE) 1–8. https://doi.org/10.1109/GCE.2010.5676129 (IEEE, 2010)
Ronquist, F. et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877 (2012).
Konecka, E. & Olszanowski, Z. Phylogenetic analysis based on the 16S rDNA, gltA, gatB, and hcpA gene sequences of Wolbachia from the novel host Ceratozetes thienemanni (Acari: Oribatida). Infect. Genet. Evol. 70, 175–181 (2019).
Acknowledgements
We would like to thank Jarosław Pawelec for support in production of scanning electron microscope imagery.
Author information
Authors and Affiliations
Contributions
A.K.Z.—conceptualization; data curation; resources; methodology; formal analysis; writing—original draft preparation; writing—review and editing; visualization; G.K.W.—conceptualization; methodology; resources; writing—original draft preparation; visualization; B.S—resources; supervision; project administration; funding acquisition; writing—review and editing; M.Z.—resources; writing—review and editing; E.P.T.—resources; writing – review and editing; E.I.I.—resources; writing—review and editing; I.S.I.—resources; writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kaczmarczyk-Ziemba, A., Wagner, G.K., Staniec, B. et al. Intraspecific diversity of Myrmecophilus acervorum (Orthoptera: Myrmecophilidae) indicating an ongoing cryptic speciation. Sci Rep 14, 23984 (2024). https://doi.org/10.1038/s41598-024-75335-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75335-y