Abstract
This study aims to mine and analyze adverse events (AEs) of Vedolizumab based on the FAERS database to better understand its safety and potential risks in the real world. Data from the second quarter of 2014 to the third quarter of 2023 were collected, employing various signal mining methods such as Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayesian Geometric Mean (EBGM). The study gathered 14,753,012 reports of AEs, of which 46,726 were related to Vedolizumab. Signal mining identified 401 Preferred Terms (PTs) involving 27 System Organ Classes (SOCs). There was an increasing trend in the number of reports, with a slightly higher proportion of reports from women compared to men, and the primary reporting group was adults, especially those aged between 18 and 65 years. New potential AE signals were identified, such as a higher incidence of Pregnancy, Haematochezia, and Clostridium difficile infection. Although less frequent, strong signals were noted for Incisional hernia, Intestinal fistula infection, Anastomotic complication, Drug metabolising enzyme increased, Gingival graft, Intestinal anastomosis complication, Anorectal infection, Perineal rash, and Abdominal hernia obstructive. Despite the positive prospects of Vedolizumab in the treatment of inflammatory bowel diseases, the AEs related to its use identified in this study, particularly the newly identified potential risks, suggest that even targeted therapies can have systemic effects beyond expectations.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) represents a group of complex chronic intestinal disorders that significantly impact patients’ quality of life. Traditional treatments such as corticosteroids, immunosuppressants, and anti-tumor necrosis factor agents may be ineffective, fail, or cause adverse reactions in some patients1,2. Previous studies have shown that patients receiving these treatments often experience a range of adverse events (AEs), including severe infections, liver toxicity, and increased risks of malignancy1. Additionally, biologic therapies such as infliximab and adalimumab have been associated with higher incidences of systemic immune suppression, leading to complications like opportunistic infections and lymphoma2.
Given the limitations of traditional therapies, Vedolizumab, a biologic targeting the α4β7 integrin with selective action on the gut, has emerged as a promising alternative for the treatment of IBD, including ulcerative colitis and Crohn’s disease. Its mechanism of action involves blocking the interaction between α4β7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1), thereby reducing intestinal inflammation3. In 2014, Vedolizumab was approved by the FDA and subsequently gained widespread use in over 60 countries worldwide, improving the quality of life for many patients with IBD.
Although Vedolizumab is regarded as a member of the gut-selective biologics and is highly esteemed for its unique safety profile, the increase in its use has been accompanied by a growing number of reports of adverse reactions. In August 2021, the FDA revised the prescribing information for Vedolizumab, further highlighting concerns about its safety. Our research is among the first to systematically mine and analyze Vedolizumab-related adverse events from the FDA Adverse Event Reporting System (FAERS) database4,5,6,7,8. The novelty lies in identifying both known and new potential AEs through advanced signal mining techniques.
Methods
Data source
The data were obtained from the FAERS, one of the largest publicly available databases of AEs in the world. It contains information on AEs reported spontaneously by healthcare professionals, patients, and pharmaceutical companies. FAERS is updated quarterly, providing a rich data resource for this study. This study collected reports of AEs related to Vedolizumab from the second quarter of 2014 to the third quarter of 2023 in the FAERS database.
Data standardization
To ensure the accuracy and consistency of the data, the study utilized the Preferred Terms (PTs) from the Medical Dictionary for Regulatory Activities (MedDRA) to standardize the Vedolizumab-related AE data from FAERS9. The standardization process involved mapping each AE report to MedDRA PTs, followed by categorization into the corresponding System Organ Classes (SOCs). This mapping ensured uniformity in the description of AEs across different reports, facilitating a more accurate comparison and analysis of the data.
Signal mining methods
This study employed various methods of disproportionality analysis, including the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayesian Geometric Mean (EBGM) for data mining10,11,12,13. These methods are based on the disproportionality contingency table (Table 1) to calculate the values of ROR, PRR, BCPNN, and EBGM (Table 2), which are used to assess the correlation between Vedolizumab and AEs. Higher values indicate a stronger association between Vedolizumab and the AEs, thus providing important information about the frequency of occurrence and signal strength of AEs in this study.
Results
Basic situation of AEs
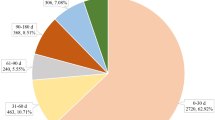
After data cleaning, from the second quarter of 2014 to 2023, a total of 14,753,012 reports of AEs were obtained, of which 46,726 were related to Vedolizumab. As shown in Table 3, from 2014 to 2023, there was an increasing trend in the number of reports, with a slightly higher proportion of reports from women (51.64%) compared to men (41.44%). The primary reporting group was concentrated among adults, particularly those aged 18 to 65 years. The reports mainly came from consumers and physicians, accounting for 54.04% and 30.13% respectively. Geographically, Canada and the United States had the highest number of reports, accounting for 43.17% and 31.87% respectively. Regarding the severity of AEs, the reports indicated that 3.45% of cases resulted in death, 0.69% in disability, and 30.47% required hospitalization - initial or prolonged (Table 3; Fig. 1). The occurrence of AEs varied widely in terms of time after medication, with 7.96% occurring within 0 to 30 days of medication and 11.97% as long-term events over 360 days. However, there were high rates of missing or anomalous values, accounting for 60.02%.
Results of AE signal mining
Signal mining for AE signals where Vedolizumab was the primary suspected drug identified 401 PTs involving 27 SOCs (Fig. 2). In Table 4, sorted by frequency of occurrence, the top ten AEs had already been mentioned in the drug’s label, including gastrointestinal disorders, general disorders and administration site conditions, injury, poisoning, and procedural complications, among others. Vascular disorders, neoplasms benign, malignant and unspecified (including cysts and polyps), metabolism and nutrition disorders, cardiac disorders, pregnancy, puerperium and perinatal conditions were relatively high in incidence and were potential new AEs.
In Table 5, sorted by the EBGM value, the top 30 are listed. Therapeutic reaction time decreased and loss of therapeutic response were ranked first and second in signal strength and were frequent, consistent with the drug’s label. Incisional hernia, intestinal fistula infection, anastomotic complication, drug metabolising enzyme increased, gingival graft, intestinal anastomosis complication, anorectal infection, perineal rash, abdominal hernia obstructive, although less frequent, had strong signal strengths, possibly indicating new AEs. Additionally, pregnancy had both a high incidence rate and signal strength, further confirming its potential importance as a new AE signal.
Discussion
Vedolizumab, as a novel biologic, specifically targets the intestine and blocks the interaction between α4β7 integrin and MAdCAM-1, thereby reducing intestinal inflammation. Due to its unique mechanism of action, Vedolizumab shows an advantage in reducing systemic immunosuppressive responses and is considered to have higher safety compared to other biologics. This study provides an in-depth analysis of AE reports related to Vedolizumab, revealing the safety characteristics of this drug in actual clinical use. From 2014 to 2023, with the increasing use of Vedolizumab and improvements in the reporting system, the number of related AE reports showed an upward trend14. The number of AE reports was slightly higher in women than in men, mainly concentrated in adults aged 18 to 65 years. This could be related to the higher prevalence of certain IBDs in middle-aged populations, and may also align with the tendency of women to be more proactive in seeking medical help for health issues. Most AE reports came from consumers and physicians, highlighting the importance of patient self-monitoring and the key role of healthcare professionals in the assessment of drug safety. Notably, the reporting of serious AEs, such as those leading to death, disability, and prolonged hospitalization, underscores the necessity of careful monitoring of patients when using Vedolizumab clinically. The analysis of the timing of AEs showed that some occurred shortly after medication, while others were long-term events. This distribution might relate to the drug’s mechanism of action and the long-term treatment nature of inflammatory bowel diseases. It is important to note that a significant amount of missing or anomalous data exists, which could impact the interpretation of the analysis results.
In this study, most identified AE signals were consistent with those listed in the Vedolizumab label, including gastrointestinal disorders, general disorders and administration site conditions, injury, poisoning, and procedural complications15,16,17. The continued reporting of these known AEs not only validates the results of previous clinical trials but also reflects their prevalence in actual clinical practice. For example, reports on digestive diseases like colitis ulcerative and Crohn’s disease may indicate the complexity and challenge of these conditions themselves, as well as the difficulty in maintaining a therapeutic response. Additionally, signals identified from the EBGM value analysis, such as decreased therapeutic reaction time and loss of therapeutic response, although consistent with the label, their increased intensity and frequency warrant attention. This may reflect a weakening response to Vedolizumab over time in some patients. These results suggest the need for continuous monitoring of these known AEs when treating IBD with Vedolizumab and adjusting treatment strategies as necessary.
More notably, new potential AE signals were identified, including vascular disorders, neoplasms benign, malignant and unspecified (including cysts and polyps), metabolism and nutrition disorders, cardiac disorders, and pregnancy, puerperium and perinatal conditions. Particularly noteworthy are the high incidence rates of pregnancy, haematochezia, and Clostridium difficile infection, which could be new AE signals. Newly identified signals like incisional hernia, intestinal fistula infection, and anastomotic complication, although less frequent, have strong signal strengths and should be noted in clinical practice.
Vedolizumab reduces the activity of specific immune cells (like T cells) in the gut by inhibiting α4β7 integrin, thus alleviating inflammation. However, this inhibition might indirectly affect the integrity of the intestinal mucosal barrier, crucial for preventing microbial and foreign substance invasion18. Vedolizumab might cause a decrease in intestinal mucosal defense, making it more susceptible to damage and infection, leading to haematochezia and intestinal fistula infection. Normal healing of the intestine is critical in IBD patients, especially those requiring intestinal surgery. Postoperative anastomotic healing relies on a healthy inflammatory response, adequate blood supply, and appropriate immune-cell-mediated tissue rebuilding. The immunomodulatory action of Vedolizumab could interfere with these processes, especially during inflammation and repair stages19, possibly leading to poor anastomotic healing and increased risk of leakage, infection, or fistula formation. A complex immune network in the gut maintains local immune balance and protects intestinal mucosa. Vedolizumab might disrupt this balance by altering the distribution and function of immune cells in the gut20. This disturbance could lead to excessive or insufficient intestinal reactions, triggering haematochezia, intestinal fistula infection, or anastomotic complication.
Vedolizumab reduces specific immune cell activity in mucosal tissues by inhibiting α4β7 integrin21. While this effect is primarily significant in the intestinal mucosa, it might also affect other mucosal tissues like oral and anal regions. This change could reduce the immune defense against bacteria and other pathogens, increasing infection risk. The oral and anal areas are frequently in contact with the external environment and normally under effective immune surveillance to prevent infection and inflammation. Vedolizumab might disrupt this normal immune surveillance mechanism at these sites, leading to overgrowth of bacteria or fungi and triggering infections and inflammatory responses like gingival graft and anorectal infection. In the perineal area, Vedolizumab may affect the production and regulation of inflammatory mediators and cytokines22. This impact could lead to an excessive or insufficient response to skin irritants, resulting in perineal rash. Additionally, reduced immune defense in the skin could make this area more susceptible to external irritants and infectious agents.
The potential mechanism behind “Drug metabolising enzyme increased” may involve the impact of Vedolizumab on liver metabolic functions23. Vedolizumab inhibits α4β7 integrin, affecting immune cell activity in the gut, which could indirectly influence liver immunoregulation. The liver has a complex immune network involved in regulating both local and systemic immune responses. Vedolizumab might disrupt liver immune balance, thereby affecting the expression of drug-metabolizing enzymes. Additionally, the use of Vedolizumab could lead to systemic or local inflammatory responses. These inflammatory responses might affect liver function through various mechanisms. Inflammatory cytokines and signaling molecules, such as tumor necrosis factor-alpha (TNF-α) and interleukins (ILs), could alter the activity of drug-metabolizing enzymes, impacting drug metabolism24.
Pregnancy as a potential AE of Vedolizumab use involves complex mechanisms, including the drug’s impact on the immune system and possible indirect effects on hormonal balance. During pregnancy, the immune environment of the placenta is crucial for the normal development of the embryo. Vedolizumab, by inhibiting α4β7 integrin, might affect placental immune regulation, thereby impacting the pregnancy process. Specifically, it could alter the migration and function of immune cells in the placenta, affecting fetal immune protection25. Additionally, it might indirectly influence hormonal balance, particularly hormones related to immune function, such as cortisol and sex hormones.
The occurrence of incisional hernia and abdominal hernia obstructive might be related to poor tissue integration during the postoperative healing process. Especially in IBD patients undergoing abdominal surgery, Vedolizumab might affect the healing of the surgical area, leading to the development of hernias. Given the potential for Vedolizumab to impact postoperative healing and increase the risk of complications such as incisional hernias and anastomotic issues, clinicians should be vigilant in monitoring patients undergoing abdominal surgery. It may be beneficial to implement targeted follow-up protocols and adjust treatment strategies to mitigate these risks and improve patient outcomes.
However, it is important to acknowledge the limitations inherent to this study design. As with any large registry-based study, there is a significant risk of bias due to the high volume of missing data. The significant amount of missing or anomalous data (60.02%) may affect the reliability of the results and could potentially lead to skewed interpretations of the adverse event frequencies and severities. This is a common limitation of spontaneous reporting systems like the FAERS, where AEs, especially those occurring in outpatient settings, may be underreported. As a result, some events may not have been included in the database, leading to potential underestimation of certain AEs. In contrast, real-world observational studies with fewer missing data provide a more comprehensive view of Vedolizumab’s safety profile. For example, a recent observational study26 reported an increased risk of gut infections in patients treated with Vedolizumab compared to other biologics. This finding aligns with some of the new AE signals identified in our study, such as the higher incidence of intestinal fistula infections and Clostridium difficile infections. These real-world results further emphasize the importance of careful monitoring for infections in patients treated with Vedolizumab, particularly considering its gut-selective immunosuppressive effects, which may compromise the intestinal mucosal barrier.
Besides, this study analyzing AEs of Vedolizumab in the treatment of IBD has certain limitations. Firstly, the study relies on spontaneous reporting in the FAERS database, which may lead to biases in the reporting of AEs, particularly in terms of reporting frequency and severity. The spontaneous reporting system might be influenced by reporting willingness and recognition ability, potentially leading to overestimation or underestimation of certain AEs. Secondly, the data in the database lack the rigor of randomized controlled trials, so causal relationships cannot be established, and only associative evidence is provided. Additionally, due to the lack of detailed individual patient information in the database, such as baseline health conditions, comorbidities, and concurrent medications, our understanding of the mechanisms behind AEs is limited. Future studies should aim to address these data gaps to provide a more accurate assessment of Vedolizumab’s safety profile.
Conclusion
This study provides an in-depth exploration of the use of Vedolizumab in treating IBD and its associated AEs. Through detailed analysis of the FAERS database, this study has confirmed several known AEs, such as gastrointestinal disorders and general disorders and administration site conditions, and also revealed several new potential AEs, including haematochezia, intestinal fistula infection, pregnancy, transurethral prostatectomy, etc. These findings offer important guidance for clinicians using Vedolizumab in the treatment of IBD. They not only provide valuable information on the safety of the drug but also highlight areas that require special attention during treatment, especially for patient groups with specific risk factors. Future research should focus on exploring the specific mechanisms of these potential AEs and finding ways to minimize adverse reactions while ensuring therapeutic efficacy, thus providing safer and more effective treatment options for IBD patients. Through research, we can anticipate greater progress in the field of IBD treatment, improving patients’ quality of life and long-term health outcomes.
Data availability
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Jairath, V. & Feagan, B. G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 5(1), 2–3 (2020).
Cai, Z., Wang, S. & Li, J. Treatment of inflammatory bowel disease: a comprehensive review. Front. Med. 8, 765474 (2021).
Luzentales-Simpson, M. et al. Vedolizumab: potential mechanisms of action for reducing pathological inflammation in inflammatory bowel diseases. Front. Cell. Dev. Biol. 9, 612830 (2021).
Zhu, H. et al. Mining and analysis of adverse event signals of cariprazine based on the real-world data of FAERS database. J. Affect. Disord. 347, 45–50 (2024).
Zhou, Q. et al. Adverse events of epidiolex: a real-world drug safety surveillance study based on the FDA adverse event reporting system (FAERS) database. Asian J. Psychiatry. 90, 103828 (2023).
Jiang, Y. et al. The correlation of Esketamine with specific adverse events: a deep dive into the FAERS database. Eur. Arch. Psychiatry Clin. NeuroSci. 1–9. (2023).
Jiang, Y. et al. Safety assessment of brexpiprazole: real-world adverse event analysis from the FAERS database. J. Affect. Disord. 346, 223–229 (2024).
Ou, M. et al. Analysis of the adverse events of Aristada: a real-world study based on FAERS database. Asian J. Psychiatry. 94, 103968 (2024).
Brown, E. G. Using MedDRA: implications for risk management. Drug Saf. 27(8), 591–602 (2004).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13(8), 519–523 (2004).
Evans, S. J. W., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10(6), 483–486 (2001).
Bate, A. et al. A bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321 (1998).
DuMouchel, W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 53(3), 177–190 (1999).
Goodman, W. A., Erkkila, I. P. & Pizarro, T. T. Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat. Revi Gastroenterol. Hepatol. 17(12), 740–754 (2020).
Loftus, E. V. Jr et al. Long-term safety of Vedolizumab for inflammatory bowel disease. Aliment. Pharmacol. Therap. 52(8), 1353–1365 (2020).
Sandborn, W. J. et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 158(3), 562–572.e12 (2020).
Peyrin-Biroulet, L. et al. Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. 22(1), 1–16 (2022).
Osterman, M. T. et al. Mucosal biomarker of innate immune activation predicts response to vedolizumab in Crohn’s disease. Inflamm. Bowel Dis. 26(10), 1554–1561 (2020).
Ungar, B. et al. Dose optimisation for loss of response to Vedolizumab—pharmacokinetics and immune mechanisms. J. Crohn’s Colitis. 15(10), 1707–1719 (2021).
Guo, X. Y., Liu, X. J. & Hao, J. Y. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J. Dig. Dis. 21(3), 147–159 (2020).
Hsu, P. et al. Responsiveness to Vedolizumab therapy in ulcerative colitis is associated with alterations in immune cell-cell communications. Inflamm. Bowel Dis. 29(10), 1602-1612 (2023).
Bertani, L. et al. Assessment of serum cytokines predicts clinical and endoscopic outcomes to vedolizumab in ulcerative colitis patients. Br. J. Clin. Pharmacol. 86(7), 1296–1305 (2020).
Sun, W. et al. Assessment of vedolizumab disease-drug‐drug interaction potential in patients with inflammatory bowel diseases. Clin. Pharmacol. Drug Dev. 10(7), 734–747 (2021).
Stanke-Labesque, F. et al. Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol. Therap. 215, 107627 ( 2020).
Mitrova, K. et al. Differences in the placental pharmacokinetics of vedolizumab and ustekinumab during pregnancy in women with inflammatory bowel disease: a prospective multicentre study. Therap. Adv. Gastroenterol. 14, 17562848211032790 (2021).
Innocenti, T. et al. Infectious risk of vedolizumab compared with other biological agents in the treatment of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 33(1S), e574–e579 (2021).
Acknowledgements
This study was performed using the FAERS source that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA.
Funding
The work is supported by the Wuxi Traditional Chinese Medicine Hospital Innovation Development Fund Project (ZYYZD24001).
Author information
Authors and Affiliations
Contributions
Qinyun Xu, Pu Yuan conceived the study; Qinyun Xu, Jing Zhang, Weihong Tang, Minghong Zhou, Xiaoling Zhang and Pu Yuan collected the report; Qinyun Xu and Pu Yuan wrote the manuscript and edited the manuscript. All authors have approved publishment of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Q., Zhang, J., Tang, W. et al. Data mining and analysis of adverse events of Vedolizumab based on the FAERS database. Sci Rep 15, 278 (2025). https://doi.org/10.1038/s41598-024-75421-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75421-1