Abstract
Pilocytic astrocytoma (PA) is classified as a Grade I benign neuroglial tumor. The extent of surgical resection is a critical factor influencing the prognosis for patients with PA. In prior researches of PA, the extent of surgical resection is generally categorized into GTR, STR and biopsy. In some researches on brain tumor surgeries, the extent of resection also includes GTL. There is no existing research specifically comparing the efficacy of GTR versus GTL in PA treatment. In this study, the data we used are from the SEER database. We categorized the extent of resection into GTL, GTR, STL, STR, biopsy, and no surgery based on SEER classification of surgical procedures, to investigate the impact of extent of resection on PA patient survival. A multivariate logistic regression model was utilized to acquire odds ratios (OR) for different extent of resection. Survival outcomes across different extent of resection (GTL, GTR, STL, STR, biopsy, no surgery) were assessed using Kaplan–Meier survival curve analysis, with curve comparisons conducted via log-rank tests. The impact of various risk factors on survival was assessed using the Cox proportional hazards model. The hazard ratio (HR) was employed to quantify the influence of one or more factors on overall survival throughout the follow-up period. Multivariate Cox analysis revealed that age, tumor location, extent of resection, as well as the application of radiotherapy and chemotherapy, all significantly impacted prognosis. Compared to GTL, GTR did not significantly increase the risk of mortality (HR 1.17; 95% CI 0.73–1.86, p = 0.5). Furthermore, there was no statistically significant difference between the Kaplan–Meier survival curves of the two groups (p = 0.18). We employed propensity score matching (PSM) to balance the differences in baseline characteristics of patients receiving chemotherapy or radiotherapy. A total of 4429 patients were included in this study. Age, diagnosis period, race, tumor size, and tumor location as influential on the extent of resection. Age, tumor location, extent of resection, and application of radiotherapy and chemotherapy influenced the survival of PA patients. The Kaplan–Meier survival curves revealed that the long-term survival rate for GTR is slightly higher than that for GTL. The PSM analysis revealed that the application of radiotherapy and chemotherapy was associated with the reduction of overall survival in PA patients. In conclusion, there was no significant difference in survival between GTR and GTL, so GTR with less damage was preferred. The application of radiotherapy and chemotherapy can reduce overall survival of patients with PA.
Similar content being viewed by others
Introduction
Pilocytic astrocytoma (PA) is classified as a Grade I benign neuroglial tumor1,2. It typically manifests with a range of clinical symptoms that adversely impact patients’ quality of life, including headaches, motor impairments, and speech difficulties3. According to the Central Brain Tumor Registry of the United States (CBTRUS), the incidence of PA in the U.S. population is 2.9 per 100,0004, with a predominance among adolescents5. The overall prognosis for PA is generally favorable, with a 10-year postoperative survival rate reaching up to 95.8%6. The extent of resection is a critical factor influencing the prognosis for patients with PA7.
In prior researches of PA, the extent of resection is generally categorized into gross total resection (GTR), subtotal resection (STR), and biopsy. Compared to patients undergoing subtotal resection, biopsy, or no surgery, those who undergo GTR typically exhibit a more favorable prognosis8,9. In some researches on brain tumor surgeries, GTR is classified as a form of supramarginal resection. Besides GTR, supramarginal resection includes gross total resection with lobectomy (GTL)10,11, which is recognized as a more extensive form of resection, known as supramaximal resection12. Although there is no existing research specifically comparing the efficacy of GTR versus GTL in PA treatment, detailed comparisons between the GTL and GTR have been explored in researches of glioblastoma (GBM). Theoretically, GTL, with its clearer resection margins and broader extent, allow for more comprehensive removal of malignant and pre-malignant cells, potentially extending the time to tumor recurrence and overall survival13. Extensive researches in glioblastoma have indicated that GTL is often associated with improved survival outcomes compared to GTR, extending patient survival and enhancing overall survival rates10,14,15,16,17. However, some early researches suggest that there is no significant difference in survival times between GTL and GTR18.
Although both PA and GBM are classified as gliomas, they exhibit substantial differences. PA is a WHO Grade I benign tumor, predominantly observed in children and adolescents, characterized by slow growth and non-invasive behavior19. In contrast, GBM is a WHO Grade IV malignant tumor, typically occurring in adult and elderly individuals, with rapid growth and highly invasive characteristics20. The molecular genetics of these two tumors also differ significantly. PA is commonly associated with alterations in the Ras/RAF/mitogen-activated protein kinase/extracellular signal-regulated kinase pathway19, whereas GBM is characterized by mutations in genes regulating receptor tyrosine kinase /rat sarcoma /phosphoinositide 3-kinase, p53, and retinoblastoma protein signaling20. These distinctions contribute to differences in treatment strategies and survival time. PA is predominantly treated through surgical intervention, typically without the need for radiotherapy or chemotherapy, and has a favorable prognosis19. GBM is managed through a combination of surgical resection, radiotherapy, and chemotherapy. However, the prognosis remains unfavorable, with limited survival time despite these aggressive treatment modalities20. Therefore, the treatment protocols and clinical practices for GBM cannot be directly applied to PA, indicating that findings from studies comparing GTL and GTR in GBM should not be extrapolated to PA.
Given the low incidence of PA, conducting large-scale clinical studies poses significant challenges. Consequently, it becomes crucial to identify databases with rigorous data recording standards, precise parameter classification, large sample sizes, and long follow-up durations for research on PA. In this study, the Surveillance, Epidemiology, and End Results (SEER) program is particularly well-suited for our study. We categorized the extent of resection based on the SEER surgery codes into GTL, GTR, STL, STR, biopsy, and no surgery, to investigate the impact of extent of resection on PA patient survival. We hope that our findings will assist neurosurgeons in making informed clinical decisions and contribute to further improving the prognosis of PA patients.
Materials and methods
Data source and selection
Data for this study were obtained from the Surveillance, Epidemiology, and End Results (SEER). The SEER provides cancer statistics through population-based cancer registries, covering 35% of the U.S. population, and includes data on incidence, survival rates, and clinical interventions21.
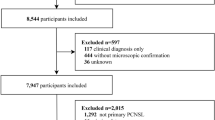
To ensure statistical robustness and stability, two SEER datasets released in April 2021 were utilized to maximize sample size. The International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), was employed to identify the code for PA as 9421. All PA patients from the 1992–2018 (13 Registries) and 2000–2018 (18 Registries) datasets were included. Tumors located in the brain were screened, and duplicate entries across the two datasets were removed, resulting in a cohort of 5,283 patients. Exclusions were applied to 13 cases with unknown survival time, 282 cases that did not meet primary tumor criteria or presented with multiple tumors, and 562 patients lacking documented information on lobectomy, tumor resection, biopsy, unspecified surgery types, or with unknown surgery. After applying these criteria, a total of 4429 patients was selected for analysis (see Fig. 1).
Study variables and significance
This study included variables such as the extent of resection, age, sex, diagnosis period, race, tumor size, tumor location, radiotherapy, chemotherapy, and follow-up status. The extent of resection was categorized as GTL (code 55), GTR (code 30), STL (code 40), STR (code 21), biopsy (code 20), and no surgery (code 00). Age was stratified into four groups: 0–19 years, 20–39 years, 40–59 years, and > 60 years. The diagnosis periods were divided into three intervals: 1998–2004, 2005–2011, and 2012–2018. Race was delineated as White, Black, American Indian/Alaska Native, Asian or Pacific Islander, and unknown. Tumor locations included the cerebellum, brainstem, cerebrum, frontal lobe, occipital lobe, overlapping lesions in the brain, parietal lobe, temporal lobe, ventricle, and brain NOS (not otherwise specified). Tumor size was classified into the following categories: < 20 mm, 20–40 mm, > 40 mm, and unknown size. The primary endpoint of this study was overall survival, defined as the time from diagnosis to death from any cause, measured in months.
Data analysis methods
Pearson’s chi-square test was used to compare clinical characteristics among different extent of resection. The extent of resection was treated as the dependent variable, with GTL as the reference group. Multivariate logistic regression analysis was performed to obtain odds ratios (ORs) for different extent of resection. Kaplan–Meier survival analysis was conducted for patients with different extent of resection, and log-rank tests were used to compare differences between Kaplan–Meier curves. The Cox proportional hazards model was used to assess the impact of various risk factors on survival, with hazard ratios (HRs) indicating the effect of one or more factors on overall survival throughout the follow-up period. Propensity score matching (PSM, 1:1 matching) was employed to balance baseline characteristics between patients who received chemotherapy or radiotherapy and those who did not. Kaplan–Meier survival analysis was then performed on the matched results to further investigate whether other factors influenced the prognosis of patients who received chemotherapy or radiotherapy. A significance level of p < 0.05 was set for all statistical analyses in this study.
Results
Overall findings and baseline characteristics
This study included a total of 4429 patients. Patients were categorized into groups based on the extent of resection, including GTL, GTR, STL, STR, biopsy, and no surgery. The analyzed factors included age, sex, diagnosis period, race, tumor size, tumor location, radiotherapy, and chemotherapy. Significant differences were observed among the groups in terms of age, diagnosis period, tumor size, tumor location, radiotherapy, chemotherapy, and the number of deaths (p < 0.001). However, no statistically significant differences were observed for sex (p = 0.827) and race (p = 0.176) (Table 1).
In the entire cohort of 4429 patients, a substantial portion underwent gross total resection (GTR and GTL), accounting for 47.1% of the cases. Subtotal resection (STR and STL) and biopsy were performed in 23.5% and 20.1% of patients, respectively. Tumors were predominantly observed in the 0–19 years age group, comprising 73.3% of cases. The most common tumor location was the cerebellum (39.6%), followed by the brainstem (12.2%), with other locations less frequently involved. A significant proportion of patients did not receive radiotherapy (92.1%) or chemotherapy (88.7%). During the follow-up period, 316 patients (7.1% of the total cohort) died (Table 1).
Different tumor locations and diagnosis periods affect extent of resection
Univariate and multivariate logistic regression analyses were conducted to evaluate the predictive significance of various extent of resection. Factors that reached statistical significance (p < 0.05) in the univariate logistic regression analysis were subsequently included in the multivariate logistic regression (Table 2), with GTL as the reference group. Univariate logistic analysis revealed that sex did not significantly influence the selection of extent of resection (p > 0.05). In the multivariate logistic analysis, we observed a gradual increase in the likelihood of selecting GTR and STR over time. Figure 2 also demonstrates the same trend. Compared to patients with cerebellum tumors who underwent GTL, those with tumors located in the brainstem, cerebrum, and ventricles were more likely to undergo STR, biopsy, STL, or no surgery.
Different extent of resection were popular in different periods
We identified temporal trends in the selection of extent of resection across different periods. As depicted in Fig. 2, the distribution of extent of resection varied significantly across distinct time intervals. During the 1998–2004 period, lobectomy was the predominant surgical approach, with 46.0% of patients undergoing GTL and 21.0% undergoing STL, while fewer than 2.0% of patients underwent GTR or STR. In the 2005–2011 period, there was a noticeable shift, characterized by a gradual increase in the proportion of tumor excisions (including GTR and STR), though lobectomy (encompassing both GTL and STL) remained the most commonly selected procedure. By the 2012–2018 period, this trend had evolved significantly, with a marked rise in tumor excisions, evidenced by 47.0% of patients undergoing GTR and 25.0% undergoing STR. Conversely, the proportion of patients receiving lobectomy procedures (GTL at 3.0% and STL at 1.0%) had sharply declined.
The significant impact of age, tumor location, surgical extent, radiotherapy, and chemotherapy on patient survival rates
The results of the univariate Cox regression analysis demonstrated that age, tumor location, extent of resection, radiotherapy, and chemotherapy significantly impacted patient survival (Table 3). Subsequently, these significant variables identified in the univariate analysis—age, tumor location, extent of resection, radiotherapy, and chemotherapy—were incorporated into a multivariate Cox regression analysis to further elucidate their independent effects on survival. The multivariate analysis confirmed the strong influence of these factors on survival. When compared to the 0–19 years age group, patients aged 20–39 years (HR: 2.69, 95% CI 1.97–3.68, p < 0.001), 40–59 years (HR: 6.61, 95% CI 4.32–8.52, p < 0.001), and over 60 years (HR: 18.3, 95% CI 12.63–26.43, p < 0.001) demonstrated a progressively higher risk of mortality. Tumor location also significantly influenced survival outcomes, with cerebellum tumors associated with the most favorable prognosis.
In contrast, tumors in the cerebrum (HR: 2.37, 95% CI 1.55–3.62, p < 0.001), ventricles (HR: 2.15, 95% CI 1.40–3.32, p < 0.001), brainstem (HR: 2.07, 95% CI 1.41–3.03, p < 0.001), overlapping lesions in the brain (HR: 2.06, 95% CI 1.15–3.66, p = 0.014), and frontal lobe (HR: 2.05, 95% CI 1.26–3.34, p = 0.004) were associated with a significantly elevated risk of mortality compared to cerebellum tumors. In terms of extent of resection, patients who did not undergo surgery (HR: 2.19, 95% CI 1.46–3.28, p < 0.001) and those who had STR (HR: 1.60, 95% CI 1.02–2.52, p = 0.042) faced a significantly increased mortality risk relative to those who underwent GTL. However, no statistically significant differences in survival were observed for patients who underwent GTR (HR: 1.17, 95% CI 0.73–1.86, p = 0.500), STL (HR: 1.46, 95% CI 0.99–2.15, p = 0.056), or biopsy (HR: 1.36, 95% CI 0.94–1.99, p = 0.100). Furthermore, patients who received radiotherapy exhibited a significantly higher mortality risk (HR: 3.10, 95% CI 2.35–4.09, p < 0.001), as did those who received chemotherapy (HR: 2.49, 95% CI 1.84–3.37, p < 0.001) (Table 3).
Patients with GTL and GTR have the highest survival rates
We conducted a comprehensive analysis using Kaplan–Meier survival curves to assess the influence of various extent of resection on patient survival, as illustrated in Fig. 3. We subsequently calculated the 5-year, 10-year, and 15-year survival rates corresponding to each extent of resection, which are detailed in Table 4. To rigorously evaluate the differences among the Kaplan–Meier curves, we employed log-rank tests, with the results summarized in Table 5. Notably, the analysis revealed no statistically significant differences in survival curves between GTR and GTL (p = 0.18), nor between STR and STL (p = 0.97). Overall, our findings indicate that both GTL and GTR are associated with superior survival outcomes compared to STL and STR.
Why do patients who receive chemotherapy and radiotherapy have worse survival rates
Cox regression analysis revealed that patients who received chemotherapy and radiotherapy exhibited poorer prognoses, a finding that piqued our interest due to its apparent contradiction to established expectations. Consequently, we intend to delve deeper into the underlying reasons for this anomalous observation. To begin our investigation, we classified patients based on their receipt of chemotherapy and radiotherapy and conducted a comparative analysis of their baseline characteristics. As illustrated in Table 6, a significant disparity was observed between patients who received chemotherapy or radiotherapy and those who did not, with notable differences in several key characteristics, including age, extent of resection, and tumor size (p < 0.001).
Kaplan–Meier survival analysis of patients after PSM. (a) Kaplan–Meier survival analysis of patients after PSM who received chemotherapy compared to those who did not receive chemotherapy; (b) Kaplan–Meier survival analysis of patients after PSM who received radiotherapy compared to those who did not receive radiotherapy.
Given that patient age, tumor size, and surgical intervention (or extent of resection) are recognized as potential factors influencing patient survival, we undertook a further investigation to ascertain whether chemotherapy itself might adversely impact survival. Patients were stratified into two groups based on their receipt of chemotherapy. After addressing the discrepancies in baseline characteristics between those who received chemotherapy and those who did not, we proceeded to analyze the survival disparities between the two cohorts. To achieve a meaningful comparison, we employed propensity score matching (PSM) at 1:1 ratio. The matching variables included age, sex, diagnosis period, race, tumor size, tumor location, extent of resection, and the application of radiotherapy. The results, as summarized in Table 7, demonstrated that post-matching, there were no significant differences in any of the matching variables between the chemotherapy and non-chemotherapy groups. Subsequent Kaplan–Meier survival analysis following PSM, presented in Fig. 4a, revealed that patients who underwent chemotherapy experienced significantly poorer survival compared to their counterparts who did not receive chemotherapy (p < 0.001), thereby corroborating the initial findings observed prior to PSM.
In a similar vein, we sought to investigate whether the application of radiotherapy could be associated with a reduction in patient survival. Patients were stratified into two groups based on their receipt of radiotherapy. Following the adjustment for discrepancies in baseline characteristics between individuals who received radiotherapy and those who did not, we proceeded to analyze the survival rate variations between the two cohorts. To ensure a reliable comparison, the groups were matched using the propensity score matching (PSM) methodology. The matching parameters encompassed age, sex, diagnosis period, race, tumor size, tumor location, extent of resection, and receipt of chemotherapy. The findings, detailed in Table 8, indicated that post-matching, there were no significant differences between the radiotherapy group and the non-radiotherapy group across all matching variables. Subsequent Kaplan–Meier survival analysis following PSM, illustrated in Fig. 4b, revealed that patients who underwent radiotherapy exhibited significantly poorer survival outcomes compared to those who did not receive radiotherapy (p < 0.0001), thus reaffirming the trends observed prior to the application of PSM.
Discussion
Our findings indicate a significant correlation between advanced age and reduced survival, suggesting an increased risk of mortality as age progresses. This observation aligns with the research conducted by Yusuke Tomita et al., who reported consistent trends in age-adjusted incidence and mortality rates among elderly individuals, particularly those over 60 years of age diagnosed with PA22. Similarly, the study by Yang et al. reached comparable conclusions, utilizing competing risk analysis to demonstrate that, after adjusting for factors beyond age, the cumulative incidence of cancer-specific mortality was notably higher in the adult population9. As a predominantly low-grade tumor that is most commonly observed in pediatric patients, PA typically presents with symptoms during childhood; however, a minority of cases may manifest in adulthood, often leading to a first-time diagnosis19. This phenomenon may help explain the lower incidence of PA in adult patients relative to pediatric cohorts. Despite its rarity in adults, PA is characterized by a relatively high recurrence rate. Case series and systematic reviews conducted by Kamila M. Bond et al. support this assertion, indicating that while PA is uncommon in adults, its occurrence is associated with a poorer prognosis, as adult patients demonstrate a higher likelihood of recurrence following subtotal resection compared to their pediatric counterparts. This finding underscores the critical importance of striving for gross total resection whenever feasible23.
Further investigations indicate that PA in adult patients exhibits characteristics indicative of a non-benign tumor, marked by a notable propensity for recurrence24,25. Although data from the Surveillance, Epidemiology, and End Results (SEER) program does not incorporate details regarding recurrence, it is well-established that cancer recurrence often correlates with a poorer prognosis, thereby potentially exacerbating the unfavorable prognostic outlook noted in adult patients. Research by Jason A. Ellis et al. reinforces this perspective, shedding light on the clinical trajectory of adult patients, in which tumor recurrence and malignant transformation may manifest following the initial surgical intervention in a subset of individuals. Consequently, rigorous clinical surveillance and imaging follow-up are essential for adults diagnosed with PA, particularly during the first four years post-resection26. In contrast, studies focusing on pediatric PA patients suggest that those undergoing gross total resection may require less frequent imaging follow-up. Recommendations indicate that a regimen of six postoperative MRI scans may suffice, given that pediatric patients who achieve gross total resection generally demonstrate excellent event-free survival (EFS) and overall survival. Additionally, these patients typically exhibit slow disease progression following recurrence, often remaining asymptomatic27. This finding advocates for a considered reduction in unnecessary imaging follow-up for pediatric patients, thereby alleviating financial burdens on families while optimizing the allocation and utilization of medical resources.
Based on the findings reported by Joo Whan Kim et al., achieving gross total resection of PA located in the cerebrum and cerebellum appears to be relatively attainable28. However, the decision-making process surrounding surgical intervention, the extent of resection, and subsequent postoperative prognosis is influenced by various critical factors. Our study outcomes reveal that tumors situated in the cerebrum exhibit the most dismal prognosis, followed by those in the ventricles and brainstem, with progressively favorable prognoses observed in overlapping brain lesions, as well as lesions in the frontal, occipital, parietal, temporal lobes, and cerebellum. Importantly, multivariate logistic regression analyses indicate that patients with tumors located in the brainstem, cerebrum, and ventricles are more likely to pursue STR, biopsy, STL, or refraining from surgery altogether, in contrast to their counterparts with cerebellum tumors. This inclination may be attributed to the increased risk of neurological deficits and potentially life-threatening complications that accompany extensive resection of brainstem tumors29. In particular, extensive resection within the cerebrum may precipitate functional impairments, memory deficits, and enduring language expression disorders30, while analogous procedures targeting tumors in the ventricles are often associated with substantial intraoperative blood loss, significantly compromising postoperative quality of life31. As a consequence, individuals harboring tumors in these high-risk regions (brainstem, cerebrum, and ventricles) tend to favor less invasive and more conservative treatment strategies.
Standard treatment protocols for gliomas typically incorporate radiotherapy and chemotherapy due to their established efficacy in eradicating malignant cells and inhibiting tumor progression. However, it is imperative to recognize that each therapeutic intervention for PA, a generally benign neoplasm, carries inherent risks of late complications that may substantially compromise patients’ quality of life32. Our analysis reveals that both radiotherapy and chemotherapy may adversely affect survival rates in PA patients, irrespective of whether surgical intervention is undertaken (data not shown). This observation aligns with findings reported by Matthew W. Parsons et al.33. The observed decline in survival among patients undergoing radiotherapy may be attributed to the severe side effects associated with radiation exposure, which include endocrine dysfunctions, hearing loss, vasculopathy34, and the potential for radiotherapy to catalyze the malignant transformation of PA. Notably, certain studies have delineated radiotherapy as a critical contributor to the malignant transformation of PA35, particularly within the pediatric population36. Similarly, the poorer prognostic outcomes in patients undergoing chemotherapy may be linked to the inherent sensitivity of PA to chemotherapeutic agents, which can precipitate the emergence of multidrug-resistant tumor cell populations37,38.While the SEER database does not provide granular details regarding the specific chemotherapeutic and radiotherapeutic regimens employed—merely categorizing patients as having received or not received such treatments—single-center retrospective cohort studies have indicated that a significant proportion of PA patients treated with Bevacizumab demonstrate favorable clinical responses39. Consequently, the observed decline in prognosis among patients receiving chemotherapy may be associated with the administration of suboptimal pharmacological agents during treatment. Although our findings, in conjunction with previous research, suggest that both radiotherapy and chemotherapy may culminate in unfavorable outcomes, the underlying mechanisms remain inadequately understood and necessitate further investigation to elucidate the complex interplay between these treatment modalities and their impact on patient prognosis.
Kaplan–Meier survival analysis and Log-rank tests revealed significant differences in survival among patients with varying extent of resection. Specifically, patients undergoing GTR and GTL demonstrated markedly better survival compared to those who received biopsy, STR, or STL, with the no surgery cohort exhibiting the poorest survival rates. Notably, there was no statistically significant difference in survival between the GTL and GTR groups, nor between the STL and STR cohorts. In the realm of glioma research, the pursuit of optimal complete tumor resection is a widely adopted paradigm. However, there is a paucity of detailed investigations specifically examining the distinctions between STL and STR, which accounts for our inability to identify pertinent literature on these procedures. Consequently, our analysis predominantly focuses on a more comprehensive exploration of GTL and GTR. Prior studies have consistently demonstrated that gross total resection is superior to subtotal resection8, corroborating our own findings. Nonetheless, these previous investigations did not directly compare survival curves between GTR and GTL, a gap that our study addresses by illustrating the absence of a significant survival difference between GTL and GTR. Both GTR and GTL are classified as supramaximal resections, designed to maximize the excision of the FLAIR signal, indicative of tumor infiltration, in order to mitigate the risk of tumor recurrence10. While GTL may signify a more extensive supramaximal resection, its application in glioma patients carries potential risks, including seizures, personality changes40. Furthermore, certain scholars contend that for tumors that are amenable to complete resection, additional lobectomy may be unnecessary. They suggest that such an intervention could potentially lead to a reduction in overall survival duration for patients18.
Our analysis suggests that GTL does not exhibit higher early postoperative mortality rates, likely attributable to advancements in microsurgical techniques; however, it does not demonstrate improved long-term prognosis compared to GTR. While previous studies have indicated similar survival rates between GTL and GTR in the management of glioblastoma18, there has yet to be any investigation distinguishing between GTL and GTR specifically in the treatment of PA. Existing population-based studies related to PA, such as the research conducted by Yang et al., have classified resection extents into STR, GTR/ TR, biopsy, and unknown categories9. In contrast, our study further refines this classification by delineating the resection extents into GTL, GTR, STL, STR, biopsy, and no surgery. To the best of our knowledge, this is the first report demonstrating that in the context of surgical treatment for PA, GTL and GTR yield essentially equivalent survival rates. Consequently, we advocate for prioritizing GTR over GTL, given its marginally superior long-term survival, reduced postoperative complications, and enhanced quality of life for patients. Therefore, we recommend that patients should prioritize GTR whenever clinically feasible. Moreover, we observed a noticeable decline in the prevalence of lobectomy procedures alongside a corresponding increase in tumor resection practices over time. This trend suggests that complete tumor resection without the necessity of lobectomy is becoming increasingly achievable owing to advancements in medical technology. The integration of a growing array of innovative techniques in brain tumor surgery, such as preoperative imaging assessments, intraoperative ultrasound modalities, and 5-ALA imaging agents, provides neurosurgeons with diverse options to optimize the extent of gross total resection of tumors41,42. Furthermore, emerging research indicates that the presence of eosinophilic Rosenthal fibers (RFs) and eosinophilic granular bodies can facilitate the delineation of PA margins, thereby enhancing the potential for complete neurosurgical resection and ultimately improving patient prognosis43.
In addition to the potential biases and inaccuracies inherent in the data, this study is subject to several notable limitations. Given that the focus is on a benign tumor characterized by generally high long-term survival rates, a significant number of patients were lost to follow-up, which may compromise the robustness of our analysis. Furthermore, the SEER database lacks specific clinical and molecular outcome data, thereby constraining our ability to conduct more intricate investigations into the molecular aspects of PA. It is important to note that optic nerve PA were excluded from this study due to the substantial differences in surgical treatment approaches between optic and intracranial PA; thus, our analysis is confined exclusively to brain PA. Additionally, a considerable portion of patients (15.5%) had tumors located in unspecified regions of the brain, and a significant subset of patients (25.6%) presented with undetermined tumor sizes. Furthermore, the availability of detailed information regarding adjuvant therapies, such as radiotherapy and chemotherapy, was limited. These factors collectively contribute to the potential influence on the analytical outcomes.
Radiosurgery represents a viable therapeutic alternative for the management of residual or recurrent volumetric disease in PA, providing long-term local tumor control32. While the primary approach to PA treatment remains complete surgical resection, certain tumor locations may present significant challenges that hinder total resection. In such cases, the application of radiosurgery, particularly Gamma Knife procedures, becomes a pertinent consideration. Initial studies have demonstrated the effectiveness of Gamma Knife radiosurgery for tumors that are inaccessible via conventional surgical approaches44.Regrettably, the SEER database utilized in this study does not provide a distinct categorization for radiosurgery; rather, it encompasses this treatment modality under the broader umbrella of radiation therapy. This limitation significantly restricts our capacity to perform a comprehensive analysis of the specific impact of radiosurgery on treatment outcomes for patients with PA.
Conclusion
In conclusion, there was no significant difference in PA patients’ survival between GTR and GTL, so GTR with less damage was preferred. The application of radiotherapy and chemotherapy can reduce overall survival of patients with PA.
Gliomas can be classified into infiltrative and non-infiltrative types based on their growth patterns. Infiltrative gliomas exhibit a growth pattern akin to the distribution of tree roots within soil, making it exceedingly challenging to delineate tumor boundaries during surgical intervention. Common imaging modalities used to define tumor margins often underestimate the extent of tumor infiltration, which can consequently lead to recurrence after surgery. Therefore, it is recommended that such tumors be subjected to supra total/supramaximal resection. In contrast, the focus of this study is on PA, a non-infiltrative tumor characterized by well-defined edges. Given this clarity in delineation, gross total resection based on imaging-defined boundaries theoretically enables the achievement of gross total resection of tumor. This may elucidate why, in our study centered on PA, there were no statistically significant differences in survival between the GTR and GTL. Furthermore, GTR likely minimizes damage to the surrounding normal brain tissue, a factor contribute to the slightly superior long-term survival observed in patients undergoing GTR compared to those undergoing GTL.
Data availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request. SEER database data can be directly accessed and obtained from seer.cancer.gov.
References
Louis, D. N. et al. The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neuro Oncol. 23, 1231–1251. https://doi.org/10.1093/neuonc/noab106 (2021).
Ostrom, Q. T. et al. CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 22, iv1–iv96. https://doi.org/10.1093/neuonc/noaa200 (2020).
Nabors, L. B. et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice guidelines in Oncology. J. Natl. Compr. Canc Netw. 18, 1537–1570. https://doi.org/10.6004/jnccn.2020.0052 (2020).
Tabash, M. A. Characteristics, survival and incidence rates and trends of pilocytic astrocytoma in children in the United States; SEER-based analysis. J. Neurol. Sci. 400, 148–152. https://doi.org/10.1016/j.jns.2019.03.028 (2019).
Ostrom, Q. T. et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 19, v1–v88. https://doi.org/10.1093/neuonc/nox158 (2017).
Burkhard, C. et al. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J. Neurosurg. 98, 1170–1174. https://doi.org/10.3171/jns.2003.98.6.1170 (2003).
Cler, S. J. et al. Genetic and histopathological associations with outcome in pediatric pilocytic astrocytoma. J. Neurosurg. Pediatr. 29, 504–512. https://doi.org/10.3171/2021.9.Peds21405 (2022).
Johnson, D. R., Brown, P. D., Galanis, E. & Hammack, J. E. Pilocytic astrocytoma survival in adults: analysis of the Surveillance, Epidemiology, and end results program of the National Cancer Institute. J. Neurooncol. 108, 187–193. https://doi.org/10.1007/s11060-012-0829-0 (2012).
Yang, W. et al. Comparison of adult and pediatric pilocytic astrocytomas using competing risk analysis: a population-based study. Clin. Neurol. Neurosurg. 212, 107084. https://doi.org/10.1016/j.clineuro.2021.107084 (2022).
Shah, A. H. et al. Survival benefit of lobectomy for glioblastoma: moving towards radical supramaximal resection. J. Neurooncol. 148, 501–508. https://doi.org/10.1007/s11060-020-03541-5 (2020).
Ndirangu, B., Bryan, K. & Nduom, E. Extent of resection and outcomes of patients with primary malignant brain tumors. Curr. Treat. Options Oncol. 24, 1948–1961. https://doi.org/10.1007/s11864-023-01158-0 (2023).
Nichols, N. M., Hadjipanayis, C. G. & Editorial Supramaximal resection of eloquent glioblastoma: a continued paradigm shift in neurosurgical oncology. J. Neurosurg. 138, 58–60. https://doi.org/10.3171/2022.3.Jns22564 (2023).
Zheng, Y. et al. Lobectomy versus gross total resection for glioblastoma multiforme: a systematic review and individual-participant data meta-analysis. J. Clin. Neurosci. 115, 60–65. https://doi.org/10.1016/j.jocn.2023.07.016 (2023).
Incekara, F., Koene, S., Vincent, A., van den Bent, M. J. & Smits, M. Association between Supratotal Glioblastoma Resection and Patient Survival: a systematic review and Meta-analysis. World Neurosurg. 127, 617–624e612. https://doi.org/10.1016/j.wneu.2019.04.092 (2019).
Dimou, J., Beland, B. & Kelly, J. Supramaximal resection: a systematic review of its safety, efficacy and feasibility in glioblastoma. J. Clin. Neurosci. 72, 328–334. https://doi.org/10.1016/j.jocn.2019.12.021 (2020).
Schneider, M. et al. Surgery for temporal glioblastoma: lobectomy outranks oncosurgical-based gross-total resection. J. Neurooncol. 145, 143–150. https://doi.org/10.1007/s11060-019-03281-1 (2019).
Schneider, M. et al. Safety metric profiling in surgery for temporal glioblastoma: lobectomy as a supra-total resection regime preserves perioperative standard quality rates. J. Neurooncol. 149, 455–461. https://doi.org/10.1007/s11060-020-03629-y (2020).
Höllerhage, H. G., Zumkeller, M., Becker, M. & Dietz, H. Influence of type and extent of surgery on early results and survival time in glioblastoma multiforme. Acta Neurochir. (Wien). 113, 31–37. https://doi.org/10.1007/bf01402111 (1991).
Bornhorst, M., Frappaz, D. & Packer, R. J. Pilocytic astrocytomas. Handb. Clin. Neurol. 134, 329–344. https://doi.org/10.1016/b978-0-12-802997-8.00020-7 (2016).
Wirsching, H. G., Galanis, E., Weller, M. & Glioblastoma Handb. Clin. Neurol. 134, 381–397 https://doi.org/10.1016/b978-0-12-802997-8.00023-2 (2016).
Enewold, L. et al. Updated Overview of the SEER-Medicare Data: Enhanced Content and Applications. J. Natl. Cancer Inst. Monogr. 3–13. https://doi.org/10.1093/jncimonographs/lgz029 (2020).
Tomita, Y. et al. Age is a major determinant for poor prognosis in patients with pilocytic astrocytoma: a SEER population study. Clin. Exp. Med. 23, 2301–2309. https://doi.org/10.1007/s10238-022-00882-5 (2023).
Bond, K. M. et al. Adult pilocytic astrocytoma: an Institutional Series and Systematic Literature Review for extent of resection and recurrence. World Neurosurg. 110, 276–283. https://doi.org/10.1016/j.wneu.2017.11.102 (2018).
Stüer, C. et al. Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer. 110, 2799–2808. https://doi.org/10.1002/cncr.23148 (2007).
Newton, H. B. Do pilocytic astrocytomas have a benign course in adult patients? Nat. Clin. Pract. Neurol. 4, 296–297. https://doi.org/10.1038/ncpneuro0787 (2008).
Ellis, J. A. et al. Rapid recurrence and malignant transformation of pilocytic astrocytoma in adult patients. J. Neurooncol. 95, 377–382. https://doi.org/10.1007/s11060-009-9935-z (2009).
Dodgshun, A. J., Maixner, W. J., Hansford, J. R. & Sullivan, M. J. Low rates of recurrence and slow progression of pediatric pilocytic astrocytoma after gross-total resection: justification for reducing surveillance imaging. J. Neurosurg. Pediatr. 17, 569–572. https://doi.org/10.3171/2015.9.Peds15449 (2016).
Kim, J. W. et al. Comparison of the clinical features and treatment outcomes of pilocytic astrocytoma in pediatric and adult patients. Childs Nerv. Syst. 39, 583–591. https://doi.org/10.1007/s00381-023-05839-x (2023).
Xu, Q. W., Bao, W. M., Mao, R. L., Jiang, D. J. & Yang, G. Y. Surgical treatment of solid brain stem tumors in adults: a report of 22 cases. Surg. Neurol. 48, 30–36. https://doi.org/10.1016/s0090-3019(97)00125-0 (1997).
Fang, S., Wang, Y. & Jiang, T. The influence of Frontal Lobe tumors and Surgical Treatment on Advanced Cognitive functions. World Neurosurg. 91, 340–346. https://doi.org/10.1016/j.wneu.2016.04.006 (2016).
Dash, C. et al. Intraventricular Pilocytic Astrocytoma: a single centre experience. Neurol. India. 70, 1468–1474. https://doi.org/10.4103/0028-3886.355185 (2022).
Simonova, G., Kozubikova, P., Liscak, R. & Novotny, J. Jr. Leksell Gamma Knife treatment for pilocytic astrocytomas: long-term results. J. Neurosurg. Pediatr. 18, 58–64. https://doi.org/10.3171/2015.10.Peds14443 (2016).
Parsons, M. W. et al. The use and efficacy of chemotherapy and radiotherapy in children and adults with pilocytic astrocytoma. J. Neurooncol. 151, 93–101. https://doi.org/10.1007/s11060-020-03653-y (2021).
Merchant, T. E., Conklin, H. M., Wu, S., Lustig, R. H. & Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 27, 3691–3697. https://doi.org/10.1200/jco.2008.21.2738 (2009).
Shibahara, I. et al. Pilocytic astrocytoma with histological malignant features without previous radiation therapy–case report. Neurol. Med. Chir. (Tokyo). 51, 144–147. https://doi.org/10.2176/nmc.51.144 (2011).
Jager, B., Schuhmann, M. U., Schober, R., Kortmann, R. D. & Meixensberger, J. Induction of gliosarcoma and atypical meningioma 13 years after radiotherapy of residual pilocytic astrocytoma in childhood. Pediatr. Neurosurg. 44, 153–158. https://doi.org/10.1159/000113120 (2008).
Brown, M. T. et al. Chemotherapy for pilocytic astrocytomas. Cancer. 71, 3165–3172 (1993).
Bredel, M. & Zentner, J. Brain-tumour drug resistance: the bare essentials. Lancet Oncol. 3, 397–406. https://doi.org/10.1016/s1470-2045(02)00786-6 (2002).
Carabenciov, I. D., Bhargav, A. G., Uhm, J. H. & Ruff, M. W. Bevacizumab Use in Refractory Adult Pilocytic Astrocytoma: a single-Center Case Series. Neurologist. 24, 87–89. https://doi.org/10.1097/nrl.0000000000000227 (2019).
Miller, A. The lobotomy patient–a decade later: a follow-up study of a research project started in 1948. Can. Med. Assoc. J. 96, 1095–1103 (1967).
Sastry, R. et al. Applications of Ultrasound in the resection of brain tumors. J. Neuroimaging. 27, 5–15. https://doi.org/10.1111/jon.12382 (2017).
Hadjipanayis, C. G., Widhalm, G. & Stummer, W. What is the Surgical Benefit of utilizing 5-Aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 77, 663–673. https://doi.org/10.1227/neu.0000000000000929 (2015).
Ganz, J. C. Low grade gliomas. Prog Brain Res. 268, 271–277. https://doi.org/10.1016/bs.pbr.2021.10.036 (2022).
Ganz, J. C., Smievoll, A. I. & Thorsen, F. Radiosurgical treatment of gliomas of the diencephalon. Acta Neurochir. Suppl. 62, 62–66. https://doi.org/10.1007/978-3-7091-9371-6_13 (1994).
Acknowledgements
We thank SEER database for its openness and accessibility to data and thank the corresponding author for his help in the process of writing and revising the paper.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82204332), Xinxiang Medical University (Grant No. XYBSKYZZ201810) and Starting Research Funding from Xinxiang Medical University (No. 505492).
Author information
Authors and Affiliations
Contributions
JS, SG, ZC and PY was responsible for conception, design, quality control of this study, reviewed, and edited the manuscript. YH, JY, QT, YM, HZ and GH performed the study selection, data extraction, statistical analyses, and were major contributors in writing the manuscript and contributed in classification criteria discussion. CG, GD and PY participated in studies selection and statistical analyses. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Data for this study were obtained from the Surveillance, Epidemiology, and End Results (SEER) public user database. As a public database, SEER database can be accessed by anyone. The patient information utilized in this study contains de-identifiable information. Therefore, this study has received approval exemption from the ethics committee of Xinxiang Medical University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Su, J., Guo, S., Chen, Z. et al. Efficacy of various extent of resection on survival rates of patients with pilocytic astrocytoma: based on a large population. Sci Rep 14, 24646 (2024). https://doi.org/10.1038/s41598-024-75751-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75751-0