Abstract
The risk of severe disease caused by co-infection with SARS-CoV-2 and influenza virus (IAV) raises an annual concern for global public health. Extracellular vesicles (EV) derived from mesenchymal stem cells (MSC) possess anti-inflammatory properties that can attenuate the inflammatory cytokine levels induced by viral infection. However, the effects of MSC-EV treatment on SARS-CoV-2 and IAV co-infection have not been elucidated. In the present study, we co-induced lung epithelial cells (EpiC) with SARS-CoV-2 Spike protein (S) and H1N1 influenza viral HA protein (HA) and found robust upregulation of inflammatory cytokines in comparison to those induced by either S or HA protein. Consequently, treatment of lung endothelial cells (EC) with conditioned medium from EpiC co-induced by both S and HA proteins resulted in increased apoptosis and impaired angiogenic ability, suggesting the effects of co-induction on epithelial-endothelial crosstalk. In addition, lung EpiC co-induced by both S and HA proteins showed paracrine effects on the recruitment of immune cells, including monocytes, macrophages and neutrophils. Of Note, EV derived from Wharton Jelly’s MSC (WJ-EV) transferred miR-146a to recipient lung EpiC, which impaired TRAF6 and IRAK1, resulting in the downregulation of NF-κB pathway and secretion of inflammatory cytokines, rescuing the epithelial-endothelial crosstalk, and reducing the elevation of immune cell recruitment. Moreover, the anti-inflammatory properties of WJ-EV are affected by type 2 Diabetes Mellitus. WJ-EV derived from donors with type 2 Diabetes Mellitus contained less miR-146a and showed impaired ability to downregulate the NF-κB pathway and inflammatory cytokines in recipient cells. Taken together, our findings demonstrate the role of miR-146a in targeting the NF-κB pathway in the anti-inflammatory abilities of WJ-EV, which is a promising strategy to rescue the epithelial-endothelial crosstalk altered by co-infection with SARS-CoV-2 and IAV.
Similar content being viewed by others
Introduction
Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been controlled; however, the incidence of persistent long-term post-COVID-19 syndrome, even in patients with mild symptoms, increases the risk for human healthcare1,2,3. Several studies have shown that SARS-CoV-2 is co-infected with influenza virus (IAV) and is associated with disease severity and mortality4,5. Both SARS-CoV-2 and IAV have similar mechanisms of infection, boost each other’s pathogenesis, and elevate inflammatory cytokines, resulting in increased lung damage and disease severity6,7,8. After recovery from COVID-19, SARS-CoV-2 viral proteins are still detected in the sera of these subjects over the long term, raising concerns about the co-effects of SARS-CoV-2 and IAV proteins if these subjects are infected by IAV. Therefore, it is necessary to investigate the co-impact of SARS-CoV-2 and IAV on the lung function and develop effective therapeutic strategies for the treatment of influenza patients with post-COVID-19 syndrome.
Mesenchymal stem cell (MSC)-derived EV offers a promising option for cell-free therapy for various diseases. EV contain biological signals, including proteins, mRNAs, and miRNAs, which can be incorporated into target cells and modulate the genotypic and phenotypic characteristics of recipient cells9,10,11. Numerous studies have reported the role of EV-derived MSC in improving severe lung injury induced by bacteria or viruses9,12,13,14,15. In addition, our previous study showed that Wharton’s jelly MSC-derived EV (WJ-EV) reduced inflammatory cytokines in SARS-CoV-2 protein-induced lung EpiC under normal conditions and in the presence of high levels of glucose and uremic toxins16. However, the mechanisms through which WJ-EV downregulate the inflammatory cytokines in cells induced by viral proteins remain unclear; in addition, whether WJ-EV have the potential to regulate inflammatory cytokines and repair lung cell dysfunction co-induced by both SARS-CoV-2 and IAV is still unknown.
Type 2 diabetes mellitus (T2DM) is one of the comorbidities associated with the severity and progression of COVID-1917,18,19. Furthermore, T2DM alters the characteristics and impairs the functions of MSC from numerous sources, such as adipose tissues and umbilical cords20. Notably, Wharton’s jelly MSC (WJ-MSC) derived from diabetic mothers exhibited decreased plasticity, higher efficiency of differentiation into adipocytes, and increased proliferative capacity in comparison to WJ-MSC from healthy mothers21,22. However, to date, no research has examined the effects of T2DM on the function of EV derived from MSC as immunomodulatory agents has been reported.
In the present study, we examined the effects of co-induction of SARS-CoV-2 and H1N1 viral proteins on lung epithelial-endothelial cell crosstalk and the ability of WJ-EV to rescue these alterations. Furthermore, the anti-inflammatory abilities of WJ-EV derived from healthy donors were compared with those derived from T2DM patients to clarify the impact of T2DM on the functions of WJ-EV.
Results
Co-induction of SARS-CoV-2 and IAV viral proteins resulted in the upregulation of inflammatory cytokines in lung EpiC and the altered paracrine effects on EC and immune cells
Co-infection between SARS-CoV-2 and other pulmonary viruses is associated with high severity and hospitalization rates in acute or long-term COVID-19 patients23,24,25. Among pulmonary viruses, IAV has the highest prevalence of co-infection with SARS-CoV-226. Therefore, we examined the effects of the co-induction of SARS-CoV-2 S protein, persisting in the human body up to 1-year post-COVID-19 infection, and HA protein, a pathogenic protein of IAV, on lung EpiC. Lung EpiC, which express ACE2 and TMPRSS216, receptors that serve as entry points for SARS-CoV-2 into cells and are widely used as an in vitro model for SARS-CoV-2 infection experiments27,28, were induced with both S and HA proteins, and the cell morphology, proliferation, and expression of inflammatory cytokines were assessed.
Lung EpiC showed no alterations in morphology (Fig. 1A) or proliferation (Fig. 1B) when induced with a single viral protein (S or HA) or a combination of both proteins (S and HA). Meanwhile, both single viral proteins, S and HA, induced the expression and secretion of inflammatory cytokines in lung EpiC, including IL1β, IL6, TNFα, and IL8, compared to non-induced cells (Fig. 1C, D). Interestingly, co-induction with both S and HA proteins resulted in dramatic upregulation of IL1β, IL6, IL8 and TNFα relative to non-induced cells (Fig. 1C, D). These data suggest that co-infection with SARS-CoV-2 and IAV exacerbates inflammatory cytokine production in the lung EpiC.
Co-induction of S and HA proteins resulted in the severely altered characteristics of lung EpiC. (A) Morphology of lung EpiC. (B) Proliferation of lung EpiC. (C) Inflammatory cytokine gene expression and (D) protein secretion were highly upregulated in lung EpiC co-induced by S and HA proteins. (E) Experimental design to examine the paracrine effects of lung EpiC on lung EC. (F) Conditioned medium from lung EpiC co-induced by S and HA proteins significantly induced the apoptosis of lung EC. (G) Conditioned medium from lung EpiC co-induced by S and HA proteins significantly impaired the angiogenic ability of lung EC. (H) Lung EpiC co-induced by S and HA proteins showed increased monocyte recruitment. (I) Lung EpiC co-induced by S and HA proteins showed increased macrophage recruitment. Scale bars indicate 500 μm. Values represent the mean ± SD. (n = 5, *p ≤ 0.05; **p ≤ 0.01, ***p ≤ 0.001).
Previous studies have reported coagulation abnormalities and cardiovascular complications in patients with COVID-193,29, suggesting damage to the pulmonary blood vessels during viral infection. In addition, since EC dysregulation has been widely reported in COVID-19 patients, we hypothesized that there was an interaction between the infected lung EpiC and the neighboring lung EC, which resulted in the impaired function of lung EC. Therefore, we next collected CM from lung EpiC and used CM to treat lung EC (Fig. 1E). Firstly, the effects of CM from the control lung EpiC, without viral protein induction, on lung EC survival was compared with EC-specific culture medium (Lonza) and lung EpiC-culture medium. The results showed no significant differences on the cell viability of lung EC cultured with either EC-specific culture medium, lung EpiC-culture medium, or CM from the control lung EpiC for 24 h and 48 h (Supplementary Fig. 1), suggesting that CM derived from the control lung EpiC showed no toxicity on the survival of lung EpiC.
Next, the effects of CM from lung EpiC induced by viral protein on the apoptosis of lung EC were examined. The results showed that lung EC treated with CM derived from S protein-induced lung EpiC or CM derived from HA protein-induced lung EpiC exhibited a significant increase in apoptosis compared to cells treated with CM from the control lung EpiC without induction (Fig. 1F). In addition, lung EC treated with CM derived from lung EpiC co-induced with both S and HA proteins showed a higher apoptotic rate in comparison to those treated with CM derived from lung EpiC induced by either S or HA protein (Fig. 1F). Moreover, a tube formation assay of lung EC treated with CM derived from lung EpiC was performed. The results showed that treatment with CM derived from viral protein-induced lung EpiC significantly impaired the tube formation ability of lung EC, and this impairment was highest in the CM of lung EpiC with co-induction of both S and HA proteins (Fig. 1G).
Infection of lung EpiC with respiratory viruses results in the production of antiviral substances, including inflammatory cytokines, which recruit cells to activate adaptive immunity. Therefore, we examined the paracrine effects of viral protein-induced lung EpiC on the recruitment of monocytes and macrophages using a transwell insert. As a result, compared to lung EpiC without viral protein induction, lung EpiC induced by either S or HA protein showed stimulated paracrine effects on recruiting monocytes and macrophages, and these effects were significantly enhanced in lung EpiC co-induced by both S and HA proteins (Fig. 1H, I).
Taken together, these data suggested that SARS-CoV-2 and IAV co-infection promoted the exacerbation of inflammatory cytokines in lung EpiC, which consequently resulted in the altered paracrine effects on the lung EC functions and the recruitment of immune cells.
WJ-EV downregulated inflammatory cytokines and the NF-κB pathway in lung EpiC co-induced by S and HA proteins resulting in the rescued paracrine effects on lung endothelial and immune cells
We previously reported that WJ-EV has the ability to reduce inflammatory cytokines in lung EpiC induced by the S protein16. However, it remains unclear whether WJ-EV can regulate the inflammatory cytokines in cells co-induced by both SARS-CoV-2 virus and IAV. In addition to screening previous reports regarding the effects of EV derived from MSC treatment on inflammatory cytokines induced by SARS-CoV-2 and influenza viruses, we performed a systematic review using the following keywords: (covid-19 OR sars-cov-2 OR influenza) AND (extracellular vesicles OR exosomes) AND (mesenchymal stem cells OR MSC) AND cytokines.
A total of 296 articles were identified through an electronic database search. Of these, following the inclusion and exclusion criteria (Supplementary Table 1), three studies were found involving EV derived from MSC treatment for SARS-CoV-2 or influenza infection, (Supplementary Fig. 2). Among these three studies, 1 study used human nasal epithelial cells induced by IAV A or HCoV-OC430 and 2 studies used the lung epithelial cells, Calu-3 cell line induced by S1 SARS-CoV-2 S protein15 and SARS-CoV-2 S-protein16. All studies showed robust cytokine levels after SARS-CoV-2 or IAV induction and were improved after MSC-EV treatment. Even though they used different EV origins, Oh et al. used EV from umbilical cord-derived MSC, Cloer et al. used EV from bone marrow-derived MSC, and Khanh et al. used EV from either adipose tissue or Wharton’s jelly derived MSC, the same tendency that MSC derived EV reduced the cytokine level on infected cells were reported (Supplementary Table 2).
As a systematic review revealed no previous studies on the effects of WJ-EV on the regulation of inflammatory cytokines in cells co-induced by both SARS-CoV-2 and IAV, highlighting the novelty of our study, we proceeded to investigate the response of lung EpiC co-induced by both S and HA proteins to WJ-EV treatment. A gene expression analysis showed that treatment with WJ-EV significantly reduced the upregulation of inflammatory genes and the secretion of inflammatory cytokines, including IL1β, IL6, IL8 and TNFα, in lung EpiC co-induced by both S and HA proteins (Fig. 2A, B). Numerous studies have reported the important roles of the NF-κB signaling pathway in modulating the immune system and regulating the inflammatory response to viral infection. In addition, we previously reported the effects of WJ-EV treatment on the downregulation of inflammatory cytokines in Calu-3 cells induced by S protein by the impairment of p65 NF-κB pathway16. Therefore, we next examined the protein expression of p65 in lung EpiC. In accordance with the mRNA expression levels and secretion of the inflammatory cytokines (Fig. 2A, B), the protein expression of p65 was upregulated in lung EpiC co-induced by both S and HA proteins (Fig. 2C). Of note, treatment of lung EpiC cells co-induced by both S and HA proteins with WJ-EV significantly reduced the expression of p65 protein, similar to the non-viral protein-induced state (Fig. 2C).
WJ-EV rescued the altered characteristics of lung EpiC co-induced by S and HA proteins. (A) WJ-EV treatment reduced the upregulation of inflammatory cytokine gene expression and (B) protein secretion in lung EpiC co-induced by S and HA proteins, (C) WJ-EV treatment reduced the upregulation of p65 in lung EpiC co-induced by S and HA proteins; Full length blot was shown in Supplementary Fig. 5, (D) WJ-EV treatment rescued the paracrine effects of lung EpiC co-induced by S and HA proteins inducing apoptosis of lung EC, (E) WJ-EV treatment rescued the paracrine effects of lung EpiC co-induced by S and HA proteins impairing the tube formation ability of lung EC. (F) WJ-EV treatment rescued the paracrine effects of lung EpiC co-induced by S and HA proteins altering the angiogenic gene expression in lung EC. (G) WJ-EV treatment reduced the recruitment of monocytes, (H) macrophages and (I) neutrophils by lung EpiC co-induced by S and HA proteins. Scale bars indicate 200 μm. Each value represents the mean ± SD. (n = 5, *p ≤ 0.05; **p ≤ 0.01, ***p ≤ 0.001).
Next, we investigated the ability of WJ-EV to rescue the impaired crosstalk between lung EpiC and EC induced by S and HA proteins. CM from lung EpiC co-induced by S and HA proteins followed by treatment with WJ-EV was collected and used to treat lung EC. The results showed that lung EC treated with CM derived from lung EpiC co-induced by S and HA proteins, followed by treatment with WJ-EV, showed a lower apoptotic rate than those treated with CM derived from lung EpiC co-induced by S and HA proteins (Fig. 2D). In addition, lung EC treated with CM derived from lung EpiC co-induced by S and HA proteins followed by treatment with WJ-EV restored tube formation ability, similar to lung EC with no viral protein induction (Fig. 2E).
Next, we examined angiogenic genes in lung EC treated with CM derived from lung EpiC. The results showed that lung EC treated with CM derived from lung EpiC co-induced by S and HA proteins exhibited a significant downregulation of VEGF, ANG1 and bFGF in comparison to the control lung EC without viral protein induction (Fig. 2F). Notably, lung EC treated with CM derived from lung EpiC co-induced by S and HA proteins followed by treatment with WJ-EV showed the rescue of angiogenic genes, including VEGF, ANG1 and bFGF to a similar expression level to control lung EC without viral protein induction (Fig. 2F).
Furthermore, we examined the paracrine effects of lung EpiC co-induced by both S and HA proteins following WJ-EV treatment on the recruitment of monocytes and macrophages. Lung EpiC co-induced by both S and HA proteins after treatment with WJ-EV showed reduced paracrine effects on recruiting monocytes (Fig. 2G) and macrophages (Fig. 2H). In addition to monocytes and macrophages, during viral infection, neutrophils are the predominant immune cells recruited31; therefore, the paracrine effects of lung EpiC on the recruitment of neutrophils was further examined. As a result, while lung EpiC co-induced by both S and HA proteins showed the induced recruitment of neutrophils, those treated with WJ-EV exhibited the decreased paracrine effect (Fig. 2I).
Taken together, these data suggest that WJ-EV can downregulate inflammatory cytokines in lung EpiC co-induced by both S and HA proteins and rescue the altered paracrine effects of these cells on lung EC and immune cells.
miR-146a-5p derived from WJ-EV played a critical role in the impaired activation of NF-κB in lung EpiC co-induced by S and HA proteins
EV contain a variety of biomolecules such as proteins, RNAs, and miRNAs, among which miRNAs play a critical role as the regulators of gene expression. miRNAs derived from EV can be delivered to recipient cells where they bind to target genes, leading to a reduction in their expression by increasing mRNA degradation or decreasing mRNA translation, thus altering the phenotypes of recipient cells30. Therefore, we next performed small RNA sequencing of WJ-EV to identify the miRNAs that contribute to the anti-inflammatory effects (Fig. 3A). The top 20 miRNAs with the highest expression were mapped to pathways involved in inflammatory cytokine regulation using the mirparthDB database (mpd.bioinf.uni-sb.de). The results showed that among the top 20 miRNAs with the highest expression in WJ-EV, miR-21, miR-143, and miR-146a were involved in the regulation of the NF-κB signaling pathway and inflammatory cytokines (Fig. 3B).
miRNA sequencing analysis of WJ-EV. (A) Top 50 miRNAs highly expressed in WJ-EV, (B) Prediction of involved pathways using the mirparthDB database (mpd.bioinf.uni-sb.de). The results showed that miR-21, miR-143 and miR-146a are involved in the regulation of NF-κB signaling pathway and inflammatory cytokines. (C) The enrichment analysis of miRNA target genes using mirNET database (www.mirnet.ca). The results showed that miR-21 and miR-146a target the genes involved in the NF-κB signaling pathway and inflammatory cytokines.
Next, an enrichment analysis of miRNA target genes was performed to identify the interaction between candidate miRNAs and genes involved in the NF-κB activation pathway using the mirNET database (www.mirnet.ca). Enrichment was defined using the Kyoto Encyclopedia of Genes and Genomes (KEGG), Reactome, and Gene Ontology: Biological Process (GO: BP). The results showed the interactions of miR-21 and miR-146a with genes involved in NF-κB activation, including Myeloid differentiation primary response 88 (Myd88), Interleukin-1 receptor-associated kinase 1 (IRAK1), IRAK4, TNF receptor associated factor 6 (TRAF6), NFKB1, as well as with inflammatory cytokines such as IL6 and IL1β (Fig. 3C). Therefore, we hypothesized that miR-21 and miR-146a may contribute to the impairment of the NF-κB signaling pathways and inflammatory cytokines.
A further analysis was performed using miR-21 and miR-146a inhibitors to determine the miRNAs that play a crucial role in the impairment of the NF-κB signaling pathway. Modified WJ-EV were collected from WJ-MSC treated with inhibitors of miR-21 (anti21-EV), miR-146a (anti146-EV), or a combination of both miR-21 and miR-146a (anti21-146EV). Next, the effects of these modified WJ-EV on activation of the NF-κB pathway in lung EpiC co-induced by both S and HA proteins were examined. The results showed that the incorporation of lung EpiC co-induced by S and HA proteins with WJ-EV or anti21-EV resulted in the decreased expression of p65 (Fig. 4A). In contrast, the incorporation of lung EpiC co-induced by S and HA proteins with anti146-EV or anti21-146EV did not alter the expression of p65 (Fig. 4A).
miR-146a- derived from WJ-EV contributed a critical role to impair the activation of NF-κB in lung EpiC co-induced by S and HA proteins. (A) The expression of p65 in lung EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV, Anti21-EV, Anti146-EV, or Anti21-146EV; Full length blot was shown in Supplementary Fig. 6, (B) The gene expression and (C) protein secretion of inflammatory cytokines in lung EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV, Anti21-EV, Anti146-EV, or Anti21-146EV. (D) A tube formation assay of lung EC treated with CM derived from lung EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV, Anti21-EV, Anti146-EV, or Anti21-146EV. (E) Migration of immune cells to lung EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV, Anti21-EV, Anti146-EV, or Anti21-146EV. Each value represents the mean ± SD. (n = 5, *p ≤ 0.05; **p ≤ 0.01, ***p ≤ 0.001).
Next, we examined the expression and secretion of inflammatory cytokines in response to NF-κB activation in lung EpiC. Consistent with the NF-κB expression, the incorporation of either WJ-EV or anti21-EV into lung EpiC co-induced by S and HA proteins downregulated inflammatory cytokine gene expression and protein secretion (TNFα, IL1β, IL6, and IL8) in comparison to lung EpiC co-induced by S and HA proteins without EV treatment (Fig. 4B, C). Meanwhile, anti146-EV and anti21-146EV lost their effects on the regulation of inflammatory cytokines in lung EpiC co-induced by S and HA proteins (Fig. 4B, C), suggesting that miR-146a plays a critical role in the ability of WJ-EV to downregulate inflammatory cytokines.
Moreover, the paracrine effects of lung EpiC co-induced by S and HA proteins incorporated with modified WJ-EV on lung EC and immune cells were examined. Relative to the incorporation of WJ-EV, the incorporation of anti21-EV into lung EpiC co-induced by S and HA proteins showed no alteration in the rescued paracrine effects on lung EC tube formation ability (Fig. 4D) or the recruitment of immune cells, including monocytes, macrophages and neutrophils (Fig. 4E). Meanwhile, anti146-EV and anti21-146EV lost their ability to rescue the paracrine effects of lung EpiC co-induced by S and HA proteins (Fig. 4D-E).
Furthermore, to clarify the mechanism of how miR-146a regulates NF-κB pathway, we performed the enrichment analysis of miRNA target genes involved in the NF-κB activation pathway using the the mirDB database (https://mirdb.org/). The result suggested TRAF6 and IRAK1 as two promising candidates with the highest score for prediction (score 100). In addition, the interaction of miR-146a with TRAF6 and IRAK1 was confirmed using the Targetscan database (https://www.targetscan.org/vert_80/) to predict the binding sites. As shown in Fig. 5A, the binding sites of miR-146a to TRAF6 were predicted, including positions 473–480, 538–545 and 1272–1279 of TRAF6 3’ UTR. Meanwhile, the binding sites of miR-146a to IRAK1 included positions 40–47 and 56–63 of IRAK1 3’ UTR.
miR-146a downregulates NF-κB p65 via the impairment of TRAF6 and IRAK1 in lung EpiC co-induced by S and HA proteins. (A) Predicted binding sites of miR-146a with traf6 or irak1 by Targetscan database (https://www.targetscan.org/vert_80/). (B) The expression of TRAF6 and (C) IRAK1 in lung EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV, Anti21-EV, Anti146-EV, or Anti21-146EV; Full length blot was shown in Supplementary Fig. 7. (D) The expression of TRAF6 and IRAK1 in lung EpiC co-induced by S and HA proteins and treated with miR-146a mimic; Full length blot was shown in Supplementary Fig. 8.
Next, the protein expression of TRAF6 and IRAK1 were examined in lung EpiC co-induced by S and HA proteins followed by treatment with WJ-EV. The results showed that in consistent with the expression of p65, both TRAF6 and IRAK1 showed the upregulation in lung EpiC co-induced by S and HA proteins, in comparison to the control lung EpiC (Fig. 5B, C). Of note, lung EpiC co-induced by S and HA proteins followed by incorporated with WJ-EV or anti21-EV showed the reduced expression of both TRAF6 and IRAK1; meanwhile, those incorporated with anti146-EV or anti21-146EV still remained the induced expression of TRAF6 and IRAK1 (Fig. 5B, C), suggesting the involvement of TRAF6 and IRAK1 in the regulation of miR-146a on NF-κB p65 expression. In order to confirm the direct role of miR-146a on the regulation of NF-κB p65 expression induced by S and HA proteins, lung EpiC co-induced by S and HA proteins were treated with miR-146a mimic. The results showed that treatment with miR-146a mimic significantly reduced the expression of TRAF6 and IRAK1, consistent with the downregulation of p65 protein in the cellular nucleus (Fig. 5D).
Taken together, these data suggested that miR-146a plays a key role in the ability of WJ-EV to downregulate the NF-κB signaling pathway and inflammatory cytokines induced by S and HA viral proteins in lung EpiC via the impairment of TRAF6 and/IRAK1.
T2DM impaired the abilities of WJ-EV to rescue lung EpiC from co-induction of S and HA proteins
T2DM is strongly linked to greater cytokine storm severity during viral infection, leading to more unfavorable outcomes for patients17,18,19. In addition, several studies have reported the impaired functions of MSC derived from T2DM patients20,32,33. We previously reported the upregulation of inflammatory cytokines in MSC derived from adipose tissue of donors with T2DM which altered the functions of these cells20; therefore, in the present study, we speculated that MSC derived from Wharton’s Jelly tissues of T2DM donors might also exhibit an inflammatory activation state. To confirm this speculation, we compared the upregulation of NF-κB p65 protein and secretion of inflammatory cytokines in MSC derived from Wharton’s Jelly tissues of healthy donors (WJ-MSC) and those from T2DM donors (T2WJ-MSC). As shown in Supplementary Fig. 3A, T2WJ-MSC exhibited the higher expression of p65 and inflammatory cytokine expression, especially IL6, TNFα and IL1β, in comparison to WJ-MSC (Supplementary Fig. 3A, B).
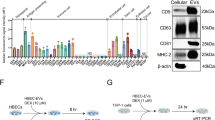
These data raised concerns about the effects of T2DM on the immunomodulatory ability of EV derived from MSC. Therefore, we next compared the abilities of WJ-EV derived from T2DM donors (T2WJ-EV) to regulate the responses of lung cells to S and HA proteins relative to WJ-EV derived from healthy donors (WJ-EV). Firstly, T2WJ-EV were characterized, which were nanosized (40–500 nm) (Supplementary Fig. 4A), expressed EV markers, such as CD63 and TSG101 and negative with APOA1 (Supplementary Fig. 4B), and showed the ability to be internalized by lung EpiC (Supplementary Fig. 4C, D), similar to WJ-EV. Of note, T2WJ-EV showed a lower amount of miR-146a-5p than WJ-EV (Fig. 6A).
WJ-EV derived from T2DM donors showed the impaired ability to downregulate inflammatory cytokines and rescued the alteration of lung EpiC co-induced by S and HA proteins. (A) The expression of miR-146a in WJ-EV and T2WJ-EV. (B) The gene expression and (C) protein secretion of inflammatory cytokines in lung EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV or T2WJ-EV. (D) The expression of p65 in lung EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV or T2WJ-EV; Full length blot was shown in Supplementary Fig. 9. (E) Apoptotic assay of lung EC treated with CM derived from EpiC co-induced by S and HA proteins, followed by the incorporation of WJ-EV or T2WJ-EV. (F) Tube formation ability of lung EC treated with CM derived from lung EpiC co-induced by S and HA proteins followed by incorporation with WJ-EV or T2WJ-EV. (G) The angiogenic gene expression of lung EC treated with CM derived from lung EpiC co-induced by S and HA proteins followed by incorporation with WJ-EV or T2WJ-EV. G. Migration of monocytes, (H) macrophages, and (I) neutrophils to lung EpiC co-induced by S and HA proteins followed by incorporation with WJ-EV or T2WJ-EV. Each value represents the mean ± SD. (n = 5, *p ≤ 0.05; **p ≤ 0.01, ***p ≤ 0.001).
Next, the abilities of T2WJ-EV to regulate the expression of inflammatory cytokines in lung EpiC co-induced by S and HA proteins was compared with that of WJ-EV. Lung EpiC were co-induced with S and HA proteins for 24 h and then treated with WJ-EV or T2WJ-EV for a further 24 h. The results showed that while the incorporation of lung EpiC with WJ-EV downregulated inflammatory cytokines gene expression and protein secretion in these cells, the incorporation of lung EpiC with T2WJ-EV instead amplified the expression and secretion levels of these inflammatory cytokines (Fig. 6B, C). Consistently, in contrast with WJ-EV, T2WJ-EV lost the ability to impair the expression of p65 in NF-κB pathways in lung EpiC co-induced by S and HA proteins (Fig. 6D).
Moreover, to examine the effects of T2WJ-EV on the crosstalk between EpiC and EC, CM was collected from lung EpiC co-induced with S and HA proteins, followed by incorporation with WJ-EV or T2WJ-EV and used to treat lung EC. The results showed that while WJ-EV reduced the apoptotic rate of lung EC treated with CM derived from lung EpiC co-induced by both S and HA proteins, T2WJ-EV CM lost these effects (Fig. 6E). In addition, in contrast to lung EC treated with CM derived from lung EpiC incorporated with WJ-EV, lung EC treated with CM derived from lung EpiC incorporated with T2WJ-EV could not maintain the ability to form capillary-like structures (Fig. 6F). Consistent with the tube formation ability, the expression levels of VEGF, ANG-1 and bFGF in lung EC treated with CM derived from lung EpiC incorporated with T2WJ-EV were not rescued and were even lower than those in lung EC treated with CM derived from lung EpiC co-induced by both S and HA proteins without EV incorporation (Fig. 6G).
Furthermore, we examined the ability of T2WJ-EV treatment to rescue the altered paracrine effects of lung EpiC, which recruit immune cells co-induced by both S and HA proteins. The results showed that lung EpiC co-induced by both S and HA proteins incorporated with T2WJ-EV showed similarly increased immune cell recruitment, including monocytes, macrophages, and neutrophils (Fig. 6H–J), in comparison to those without EV treatment. These data suggested that, in contrast to WJ-EV, T2WJ-EV lost the ability to rescue lung EpiC from the induction of both S and HA proteins.
Taken together, these data suggest that T2DM impairs the ability of WJ-EV to rescue the epithelial-endothelial cellular dysfunction induced by S and HA proteins. T2WJ-EV amplified the production of inflammatory cytokines in lung EpiC co-induced by S and HA proteins, resulting in altered paracrine effects on lung EC and immune cells.
Discussion
Studies have reported that co-infection with SARS-CoV-2 and IAV is frequent, especially during winter, when pulmonary infections caused by IAV increase. Co-infection with SARS-CoV-2 and IAV leads to increased patient hospitalization, illness severity, and higher mortality rates5,24,26,34,35. A previous study of SARS-CoV-2 and IAV H1N1 coinfection in the K18-hACE2 transgenic mouse model demonstrated that co-infection led to higher inflammatory cytokine levels in Bronchoalveolar-lavage fluid (BALF) in comparison to a single infection6. In addition, pre-infection with IAV enhanced SARS-CoV-2 infectivity and boosted the viral load7. However, the severity of this co-infection on lung epithelial-endothelial cell interactions is not yet fully understood. In the current study, our data showed that the co-induction of S protein of SARS-CoV-2 and HA protein of IAV robust the expression level of inflammatory cytokines, including IL1β, IL6, IL8, and TNFα, in lung EpiC, in comparison, to single induction of either S protein or HA protein. Our results are consistent with those of a previous study showed that inflammatory cytokines (TNF-α, IL-1α, IL-6, and IFN-β) increased rapidly in mice induced with SARS-CoV-2 and IAV and the levels were higher than those in mice induced with a single virus (SARS-CoV-2 or IAV)6.
In virus-infected lungs, dysfunction of the alveolar barrier formed by EpiC and EC is reported to be the main reason for the cytokine storm, which increases the severity and mortality. Upon viral infection, lung EpiC produces inflammatory cytokines to activate EC, and signals from EpiC-EC recruit immune cells to the infection site for viral clearance. In severe viral infectious conditions, a defective immune response occurs, in which the dysfunction of EpiC-EC leads to the leakage of immune cells into the lung, inducing a cytokine storm, a main cause of Acute respiratory distress syndrome (ARDS), coagulation, and mortality. In COVID-19, while the inflammatory responses of lung EpiC are induced directly by SARS-CoV-2, EC are less sensitive to the induction of SARS-CoV-2. A previous study found that the expression of the SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 was low in human lung EC collected from autopsy samples of COVID-19 patients, and 8 out of 10 samples were negative for SARS-CoV-2 S protein36, suggesting an indirect response to viral induction via paracrine effects of lung EpiC37. Excessive production of inflammatory cytokines in viral-induced lung EpiC leads to the dysfunction of other cells in the surrounding area, including EC38. For instance, IL6 signaling plays an important role in vascular EC dysfunction6. This is evidenced by our finding that the damage to lung EC, including increased apoptosis and impaired tube formation ability, when induced with CM containing secreted molecules from lung EpiC co-induced with both S and HA proteins, was more severe than that induced by CM from lung EpiC induced with a single protein. Our findings suggest the role of dysregulation of EC functions in the disease severity of SARS-CoV-2 and IAV co-infection due to the alteration of lung epithelial-endothelial crosstalk.
It has been widely reported that MSC-EV has immunosuppressive and immunomodulatory effects that can enhance the repair of lung damage39,40,41. Therefore, we sought to determine whether the impairment of the epithelial-endothelial function could be restored by treatment with WJ-EV derived from healthy donors. Our findings showed that treatment with WJ-EV significantly suppressed the cytokine expression by inhibiting the activation of the NF-κB pathway in lung EpiC co-induced by S and HA proteins, thus rescuing EC angiogenic functions. NF-κB is a central transcription factor that regulates a broad range of genes involved in various immune and inflammatory responses, including the induction of pro-inflammatory genes. Previous studies have reported that SARS-CoV-2 S-protein and IAV activate NF-κB via the canonical pathway, facilitated by different Toll-like receptors (TLR), TLR2, and TLR7. Upon sensing, the two proteins activate downstream cascades by activating MYD88 and retinoic acid-inducible gene 1 (RIG-1), respectively, leading to activation of the NF-κB transcription factor, resulting in robust pro-inflammatory production28,42. This might be involved in the dramatically higher cytokine production levels observed in lung EpiC induced by the combination of both S and HA proteins in comparison to the levels induced by either of the proteins individually.
The role of IRAK1 and TRAF6 in TLR signaling and NF-κB activation has been reported43. Upon activation by pathogens or antigen-antibody complexes, TLRs associate with MyD88. MyD88 subsequently recruits IRAK1 and IRAK4, which activate TRAF6. TRAF6 facilitates the synthesis of Lys-63-linked polyubiquitination on both TRAF6 and IκB kinase γ (NEMO). Thereafter, a complex including transforming growth factor-β-activated kinase 1 (TAK1), TAK1 binding protein 2 (TAB2), and TAB3 is recruited to TRAF6. TAK1 stimulates the IKK complex, resulting in NF-κB activation and the production and release of substantial amounts of inflammatory cytokines, additionally enhanced by TRAF6- and MyD88-activated IRF5.
In accordance with our study, the role of miR-146a regulating the NF-κB signaling pathway via IRAK1 and TRAF6 has been reported in several previous studies. In the rat model of spinal cord injury, miR-146a induces the recovery by inhibiting proinflammatory cytokine secretion through direct binding to IRAK1 and TRAF6 which suppressed these protein expressions44. In addition, IRAK1 and TRAF6 were downregulated in human corneal epithelial cells with miR-146a overexpression, resulting in the inhibition of NF-κB p65 translocation from cytoplasm to nucleus and reduced TNF-α-induced expression of IL6, IL8, COX2, and ICAM145. Moreover, miR-146a showed the anti-inflammatory effects in microglial cells which reduced the expression of pro-inflammatory cytokines, such as TNF-α and IL-1β, and phenotype-related genes (iNOS and CD86) through the inhibition of TRAF6/IRAK146.
In addition, we evaluated the effects of T2DM on the function of WJ-EV as immunomodulators. Our findings revealed that WJ-EV derived from donors with T2DM failed to suppress the elevation of inflammatory cytokines induced by the co-induction of both S and HA proteins in lung EpiC, resulting in an impaired ability to rescue the EC function. Of note, the incorporation of T2WJ-EV into lung EpiC amplified the production of inflammatory cytokines induced by S and HA proteins. Previous studies have reported reduced levels of miR-146a-5p in the serum of T2DM patients47. Consistent with these studies, our data also showed a lower amount of miR-146a-5p in T2WJ-EV than in WJ-EV, suggesting the involvement of miR-146a-5p in the impaired immunomodulation ability of WJ-EV by T2DM.
Conclusion
In the present study, we reported the co-effects of SARS-CoV-2 S proteins and H1N1 viral HA proteins on the upregulation of inflammatory cytokines in lung EpiC, which resulted in the impairment of lung EC via epithelial-endothelial crosstalk and the elevation of immune cell recruitment. The treatment of lung EpiC with WJ-EV derived from healthy donors reduced the viral upregulation of inflammatory cytokines and immune cell recruitment in EpiC via the miR-146a/NF-κB signaling pathways and rescued the functions of lung EC. However, in contrast to WJ-EV, T2WJ-EV derived from patients with T2DM contributed to the upregulation of inflammatory cytokines in lung EpiC and dysfunction of lung EC induced by both SARS-CoV-2 and H1N1 viral proteins (Fig. 7).
Proposed model. WJ-EV derived from healthy donors (but not T2DM donors) downregulated inflammatory cytokines and rescued the altered paracrine effects of lung EpiC co-induced by S and HA proteins on lung EC and immune cells via miR-146a targeting TRAF6 and IRAK1 resulted in the downregulation of the NF-κB pathway.
Materials and methods
Ethics statement
All procedures in this study were conducted in accordance with the amended Declaration of Helsinki, and approval for the study was obtained from the Ethics Committee of the University of Tsukuba. Human umbilical cords were collected after obtaining consent from a parent for both study participation and publication of identifying information/images in an online open-access journal. Umbilical cords were obtained from full-term neonates without infection and were born to healthy or diabetic pregnant women.
Wharton’s Jelly-MSC isolation
Wharton’s jelly was obtained from the umbilical cords of newborns delivered by healthy mothers (n = 5, average age of 33, HbAc1 < 5.0) and mothers with T2DM (n = 5, average age of 33, HbAc1 > 6.0) who underwent cesarean section at the Department of Obstetrics and Gynecology, University of Tsukuba Hospital after receiving consent for both study participation and publication. The human umbilical cord was washed with cold phosphate-buffered saline (PBS), followed by removal of blood clots and blood vessels. Wharton’s jelly was then obtained and cut into 1–2 mm pieces. Pieces of Wharton’s jelly derived from healthy donors (WJ) and donors with T2DM (T2WJ) were exposed to 0.1% collagenase solution and incubated at 37 °C for 30 min. After incubation, the solution was centrifuged at 1600 rpm for 7 min and washed with PBS. The pellet was gently placed in a culture dish containing MSC culture medium, Ischove’s modified Dulbecco’s medium (IMDM) with 10% heat-inactivated fetal bovine serum (FBS), 2 mg/mL l-glutamine, 5 ng/mL human basic-FGF, and 0.1% penicillin-streptomycin (100 U/mL penicillin, 0.1 mg/mL streptomycin; Life Technologies, USA). The dish was incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 5 days. Then, MSCs were sorted using MSC markers, and the sorted cells were cultured and expanded. For future experiments, the cells were cryopreserved using Cell Banker solution (ZENOAQ, Koiyama, Japan) and stored in liquid nitrogen.
Cell lines
Human lung adenocarcinoma (Calu-3) cells were obtained from Riken Cell Bank (Japan) and used as lung EpiC in this study. Calu-3 cells were cultured in Iscove’s Modified Dulbecco’s medium (IMDM; Gibco) containing 10% FBS and 1% penicillin/streptomycin at 37 °C under 5% CO2 in a humidified atmosphere. The medium was changed every 3 days.
Human Lung Microvascular Endothelial Cells (HMVEC-L, Lonza, Walkersville, MD, USA) were used as lung EC in this study. HMVEC-L cells were cultured in endothelial basal medium (EBM, Lonza, Walkersville, MD, USA) supplemented with 2% FBS, 0.04% hydrocortisone, 0.4% hFGF-B, 0.1% VEGF, 0.1% R3-IGF-1, 0.1% ascorbic acid, 0.1% hEGF, 0.1% GA-1000, and 0.1% heparin.
Human monocytes THP-1 were obtained from Riken Cell Bank and cultured using IMDM (Gibco) containing 10% FBS and 1% penicillin/streptomycin at 37 °C under 5% CO2 in a humidified atmosphere. To differentiate monocytes THP-1 to macrophages, cells were seeded with seeding density of 2 × 105 on a 6-cm dish in IMDM (Gibco) supplemented with 10% FBS, 1% penicillin/streptomycin (Life Technologies, USA) and 0.05mM 2-mercapthoethanol with 20nM phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) for differentiation into macrophages. After two days of incubation, macrophage-like cells were confirmed, then the macrophages were collected for a transwell migration assay.
Human pro-myeloblasts HL-60 cells were obtained from the Japanese Collection of Research Bioresources Cell Bank and cultured using IMDM containing 20% FBS and 1% penicillin/streptomycin at 37 °C under 5% CO2 in a humidified atmosphere. HL-60 cells were induced to differentiate to neutrophils by being cultured in the differentiation IMDM-based medium containing 1.3% v/v DMSO for 6 days, as previous reports48. The differentiation of HL-60 to neutrophils was examined by a neutrophil marker analysis, including APC-Mouse Anti-human-CD15 (551376, BDBiosciences, Franklin Lakes, NJ, USA), FITC-Mouse Anti-human-CD11b (562793, BDBiosciences) and FITC-Mouse Anti-human-CD16 (555406, BDBiosciences) using a flow cytometer (LSRFortessa X-20, BD BioSciences).
Viral protein induction of lung EpiC
Calu-3 cells were seeded in 24-well plates with a number of 2 × 105 cells/well for 24 h and then induced with SARS-CoV-2 pepTivator Peptide Pools Prot_S (6pmol, Miltenyi Biotec, Bergisch Gladbach, Germany), or Influenza Virus HA protein (6pmol, Miltenyi Biotec), or the combination of both proteins, SARS-CoV-2 pepTivator Peptide Pools Prot_S and Influenza Virus HA protein (at a concentration of 3pmol for each protein) for 24 h. Cells and conditioned medium (CM) were collected for further experiments. To collect CM, supernatant from Calu-3 cell culture was centrifuged at 1500 rpm for 5min and then at 2100 rpm for 20 min to remove cell debris.
Treatment of lung EC with EpiC-derived CM
To examine the paracrine effects of EpiC on EC, HMVEC-L cells were seeded in 24-well plates with 105 cells for 24 h. Then, the medium was changed with CM from induced lung EpiC and EV-treated induced lung EpiC separately and incubated for 24 h before collection for a further analysis.
Systematic review
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines49. The search strategy was performed using three electronic databases (PubMed, Embase, and Web of Science) from inception to April 11, 2024, using the following keywords: (covid-19 OR sars-cov-2 OR influenza) AND (extracellular vesicles OR exosomes) AND (mesenchymal stem cells OR MSC) AND cytokines. After removing duplicate papers, at least 2 independent reviewers (NA and PS) screened the titles, abstracts, and full texts to meet the eligibility criteria.
Cytokine level data from the first and third articles were extracted using PlotDigitizer (https://plotdigitizer.com). Subsequently, percentages were calculated and compared with the control treatment. The second article presented cytokine levels using a heatmap, which hindered data extraction.
EV isolation
Conditioned medium from WJ-MSC and T2WJ-MSC was collected and centrifuged at 1500 rpm for 5 min and then at 2100 rpm for 20 min to remove cell debris. The pellet was then ultracentrifugged at 100,000×g for 70 min at 4 °C (Optima L-100 K, Beckman Coulter, Brea, CA, US). The pellet was stained with PKH67 (PKH67 linker; Sigma-Aldrich, St. Louis, MO) for 5 min at room temperature. The pellet, considered to be composed of EV, was washed twice with PBS and ultracentrifuged under the same conditions. WJ-MSC-derived EV (WJ-EV) and T2WJ-MSC-derived EV (T2WJ-EV) were collected after washing. The protein level of EV was measured by Bradford assay and sizes were examined by dynamic light scattering (Zetasizer NanoZS, Melvern Instruments, United Kingdom).
Internalization of Induced lung EpiC with EV
Lung EpiC (2 × 105) were seeded in 24-well plates and incubated at 37 °C under 5% CO2 for 24 h. The cells were then induced by 3pmol of SARS-CoV-2 Peptide Pools Prot_S and 3pmol of Influenza Virus HA protein for 24 h, followed by internalization with PKH-67-labeled WJ-EV or T2WJ-EV at a concentration of 50ng for an additional 24 h. The internalization of PKH-67-labeled EV into lung EpiC was assessed by flow cytometry (Attune NxT, Thermo Fisher Scientific, Waltham, MA, USA), and the morphology was observed under a microscope (Keyence, BZ-X710). The cells and CM from co-induced lung EpiC, co-induced lung EpiC treated with WJ-EV, or co-induced lung EpiC treated with T2WJ-EV were then collected for subsequent experiments.
Fluorescence activated cell sorting analysis of lung EC apoptosis
Annexin V apoptosis detection kit (BioLegend) was used to analyze the apoptotic rate of lung EC. EC were collected in binding buffer 1x and incubated with 2µL of PE-Annexin V and 2µL 7AAD in the dark for 15 min at room temperature. Apoptotic cells were analyzed using a flow cytometer (LSRFortessa X-20, BD BioSciences).
Gene expression analysis
Total RNA was isolated from lung EpiC and lung EC using Sepasol-RNA I Super G (Nacalai Tesque) according to the manufacturer’s protocols. Next, 1 µg of total RNA was used to synthesize cDNA using an RT-PCR kit (Toyobo Co., Ltd., Osaka, Japan). SYBR Green Real-time PCR Master Mix (Toyobo, Japan) was used to run the quantitative PCR and analyzed using a GeneAmp 7,500 Fast Real-time PCR System (Applied Biosystems, Waltham, MA) to determine the target gene expression. The primer sequences used in this study are listed in Table 1. The expression levels of the target genes were analyzed by the ΔΔCT method using β-actin as a control gene.
ELISA assay of inflammatory cytokines
CM from lung EpiC, WJ-MSC and T2WJ-MSC were collected and centrifuged at 1500 rpm for 5 min and then at 2100 rpm for 20 min before being used to measure the inflammatory cytokine concentration. The concentrations of TNFα, IL1β, IL6, and IL8 were measured respectively using TNFα (KE00154, Proteintech, USA), IL1β (KE00021, Proteintech, USA), IL6 (KE00139, Proteintech, USA), and IL8 (KE00006, Proteintech, USA) human ELISA kits following the manufacturer’s instructions.
Briefly, 100 µl of standard or CM was added to each well of a 96-well plate which has been pre-coated with a specific antibody and incubated for 2 h at 37 °C. After being washed with wash buffer for four times, a detection antibody solution was added into each well and incubated for one hour at 37 °C followed by a 4-time washing step. Next, horseradish peroxidase (HRP)-conjugated antibody (TNFα and IL8) or Sterptravidin-HRP (IL-1β and IL6) was added into each well and incubated for 40 min at 37 °C, followed by adding Tetramethyl-benzidine (TMB) reagent and incubated for 20 min for signal development. Finally, sulfuric acid solution was added to stop color development. The absorbance at 450 nm with the correction wavelength set at 630 nm was measured by a microplate reader (Varioskan LUX, Thermo Fisher Scientific). The inflammatory cytokine concentration was calculated according to the standard curve and substrated to the inflammatory cytokine concentration in EpiC culture medium.
Western blotting
Total proteins were extracted from lung EpiC using RIPA buffer (Nacalai, Kyoto, Japan). For p65 expression, nuclear protein was extracted from lung EpiC as described previously16. Thirty micrograms of protein were electrophoresed on a 7.5% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and then transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore). The membranes were blocked with 2% bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) for 1 h at room temperature and incubated with the primary antibody at 1:1000 dilution rate, including rabbit NF-κB anti-p65 antibody (GTX107678, GeneTex, Irvine, CA, USA), rabbit anti-TRAF6 antibody (67591, Cell Signaling Technology, Danvers, MA, USA), or rabbit anti-IRAK1 antibody (10478-2-AP, Proteintech, Rosemont, IL, USA) at 4 °C overnight. For internal control, mouse anti-β-actin (bsm-33036 M, Bioss Antibody, Woburn, MA, USA) or rabbit anti-Lamin B1 antibody (GTX103292, GeneTex) was used. HRP-conjugated goat anti-rabbit IgG or HRP-conjugated goat anti-mouse IgG (Thermo Fisher Scientific) was used as the secondary antibody at 1:10000 dilution rate. Quantification of the bands was performed using ImageJ (NIH, Bethesda, MD, USA).
For EV markers, total protein was extracted from EV or MSC using RIPA buffer (Nacalai). Total protein (20 µg) was electrophoresed on 7.5% SDS-PAGE gels and transferred onto PVDF membranes (Immobilon-P; Millipore). Then, the membrane was blocked using 2% BSA and incubated with primary antibodies, including rabbit anti-CD63 (CSB-PA006039Cusabio Technology LLC, Houston, TX, US), rabbit anti-TSG101 (CSB-PA060017, Cusabio Technology LLC), or rabbit anti-APOA1 (GTX112692, GeneTex) at 1:1000 dilution at 4 °C overnight. The membranes were then washed with TBS-T followed by incubation with a secondary antibody, HRP-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific) at 1:10000 dilution. The protein bands were detected by and chemiluminescence reagents (Merck Millipore) then analyzed using a luminescence imager (Image Quant LAS4000; GE HealthCare, Little Chalfont, United Kingdom). Quantification was performed using ImageJ (NIH, Bethesda, MD, USA).
Tube formation assay
Four-well culture plates with a volume of 300µL of growth factor-reduced Matrigel (Corning) were added to each well and incubated at 37 °C for 30 min. Then, 105 EC seeded with 500µL of culture medium or conditioned medium in Matrigel-coated 4-well culture plates and incubated at 37 °C. Images of the tubes were captured every 3 h using a microscope (Keyence, BZ-X710). The average tube length was analyzed using ImageJ (NIH, Bethesda, MD, USA).
Transwell migration assay
Monocytes THP-1 (105 cells/200 µL), THP-1-derived macrophages (5 × 104cells/200µL) or HL-60-derived neutrophils (105cells/200µL) were placed into BD Falcon 8.0-µm pore cell Transwell culture inserts, which were then placed into a 24-well plate that had already been seeded with the viral-protein-induced lung EpiC. Following a 24 h incubation period, transwell inserts were collected and cells were fixed with 4% paraformaldehyde and any non-migrated cells were gently removed from the apical side of the transwell inserts membrane using a cotton-bud swab. Transwell membrane images were obtained under a dissecting microscope after 10 min of methanol permeabilization, 5min of staining with 2% crystal violet dye solution and washing with distilled deionized water. The estimated number of cells on the membrane was calculated by counting the number of cells in 39 random regions of a constructed grid.
Small RNA sequencing
Small RNA sequencing was performed according to previously described methods10. Briefly, cDNA was synthesized using 500ng of total RNA extracted from EV samples and sequenced using an Illumina NextSeq500 (Illumina, San Diego, CA, USA). The NEBNext Small RNA Library Prep Kit (E7330, New England Biolabs, Ipswich, MA, USA) was used to build a small RNA sequencing library, which was selected according to its size using AMPure beads (NC9933872, Thermo Fisher Scientific, Inc.) and verified using a Bioanalyzer DNA High-sensitivity Kit (5067 − 4626, Agilent). Reads were grouped by sequence, analyzed using a small RNA analysis tool and CLC Genomics Workbench (Ver.9.5.3, Qiagen, Germantown, MD, USA), and then matched to miR-base-annotated microRNAs (miRbase v21). A total count of one million or z-score normalization was used to normalize the raw counts of each sample.
miRNA gene expression analysis
To examine miRNA expression levels, RNA was isolated from EV samples using ISOGEN-LS (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. One microgram of total RNA was transcribed into cDNA using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems). 500ng cDNA samples were analyzed using the TaqMan 2 × Universal PCR Master Mix, which included AmpErase UNG (Applied Biosystems). RNU48 (Thermo Fisher Scientific) was used as an internal control. PCR cycles were performed using a GeneAmp 7500Fast Real-Time PCR System (Applied Biosystems). The expression levels of the target genes were calculated using the ΔΔCt method. The primer sequences used are listed in Table 1.
Isolation of modified WJ-EV with the inhibition of target miRNAs
WJ-MSC was seeded in a number of 1.2 × 105cells/well in a 6 well-plate in culture medium for 48 h followed by the replacement with fresh medium. After that, Lipofectamine RNAiMAX (Invitrogen) was diluted in Opti-MEM I Reduced Serum Media (Thermo Fisher Scientific), then mixed with miR-21 inhibitor or miR-146a inhibitor, or a combination of both miR-21 and miR-146a inhibitors (Thermo Fisher Scientific). Then, miRNAs mixtures were added to each well of WJ-MSC and incubated for 48 h and the inhibition of target miRNAs was confirmed by miRNA expression analysis. For EV isolation, after 48 h-incubation with miRNA mixtures, the medium was changed with EV-depleted cultured medium for 48 h and the conditioned medium was collected to isolate EV.
Treatment of lung EpiC co-induced by S and HA viral proteins with miR-146a mimic
Lung EpiC co-induced by S and HA viral protein was seeded in a number of 5 × 105cells/well in a 6 well-plate in culture medium for 48 h followed by the replacement with fresh medium. After that, Lipofectamine RNAiMAX (Invitrogen) was diluted in Opti-MEM I Reduced Serum Media (Thermo Fisher Scientific) before being mixed with miR-146a mimic (Thermo Fisher Scientific) then added to each well of lung EpiC and incubated for a further 48 h. The expression of miR-146a was confirmed by a miRNA expression analysis.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistics were analyzed using Mann Whitney U test by GraphPad Prism 5 software program (GraphPad Software, San Diego, CA, US). P values of < 0.05 were considered to indicate statistical significance.
Data availability
The data in the present study are available from the corresponding author upon reasonable request. miRNA sequencing raw data can be accessed from Gene Expression Omnibus (GEO) database, National Center for Biotechnology Information (NCBI), with an accession number GSE273241 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?%20acc=GSE273241) and token number: mlsjssoulzqfhgv.
References
Salamanna, F., Veronesi, F., Martini, L., Landini, M. P. & Fini, M. Post-COVID-19 syndrome: The persistent symptoms at the post-viral stage of the Disease. A systematic review of the current data. Front. Med. 8, 1–30 (2021).
Haque, A. & Pant, A. B. Long covid: Untangling the complex syndrome and the search for therapeutics. Viruses 15, 42 (2023).
Desai, A. D., Lavelle, M., Boursiquot, B. C. & Wan, E. Y. Long-term complications of COVID-19. Am. J. Physiol. Cell. Physiol. 322, C1–C11 (2022).
Dao, T. L., Hoang, V. T., Colson, P., Million, M. & Gautret, P. Co-infection of SARS-CoV-2 and influenza viruses: A systematic review and meta-analysis. J. Clin. Virol. Plus 1, 100036 (2021).
Yue, H. et al. The epidemiology and clinical characteristics of co-infection of SARS‐CoV‐2 and influenza viruses in patients during COVID‐19 outbreak. J. Med. Virol. 92, 2870–2873 (2020).
Kim, E. H. et al. Coinfection with SARS-CoV-2 and influenza A virus increases disease severity and impairs neutralizing antibody and CD4+ T cell responses. J. Virol. 96, e01873–e01821 (2022).
Bai, L. et al. Coinfection with influenza a virus enhances SARS-CoV-2 infectivity. Cell Res. 31, 395–403 (2021).
Oishi, K., Horiuchi, S., Minkoff, J. M. & tenOever, B. R. The host response to influenza A Virus interferes with SARS- CoV-2 replication during coinfection. J. Virol. 96, 1–12 (2022).
Monsel, A. et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 192, 324–336 (2015).
Chang, Y. H. et al. Extracellular vesicles derived from Wharton’s Jelly mesenchymal stem cells inhibit the tumor environment via the miR-125b/HIF1α signaling pathway. Sci. Rep. 12, 13550 (2022).
Ngo, N. H. et al. Transformed extracellular vesicles with high angiogenic ability as therapeutics of distal ischemic tissues. Front. Cell. Dev. Biol. 10, 1–15 (2022).
Zhu, Y. et al. Human mesenchymal stem cell microvesicles for treatment of E. coli endotoxin-induced acute lung injury in mice. Stem Cells 32, 116–125 (2014).
Hao, Q. et al. Mesenchymal stem cell–derived extracellular vesicles decrease lung injury in mice. J. Immunol. 203, 1961–1972 (2019).
Khatri, M., Richardson, L. A. & Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell. Res. Ther. 9, 17 (2018).
Cloer, C. et al. Mesenchymal stromal cell-derived extracellular vesicles reduce lung inflammation and damage in nonclinical acute lung injury: Implications for COVID-19. PLoS One 16, e0259732 (2021).
Khanh, V. C. et al. Wharton’s Jelly mesenchymal stem cell-derived extracellular vesicles reduce SARS-CoV2-induced inflammatory cytokines under high glucose and uremic toxin conditions. Stem Cells Dev. 30, 758–772 (2021).
Bode, B. et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 14, 813–821 (2020).
Barron, E. et al. Associations of type 1 and type 2 diabetes with COVID-19- related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 8, 813–822 (2020).
Huang, I., Lim, M. A. & Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia–a systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. Clin. Res. Rev. 14, 395–403 (2020).
Trinh, N. T. et al. Increased expression of EGR-1 in diabetic human adipose tissue-derived mesenchymal stem cells reduces their Wound Healing Capacity. Stem Cells Dev. 25, 760–773 (2016).
Pierdomenico, L. et al. Diabetes mellitus during pregnancy interferes with the biological characteristics of Wharton’s jelly mesenchymal stem cells. Open. Tissue Eng. Regen. Med. J. 4, 103–111 (2011).
Kong, C. M. et al. Changes in stemness properties, differentiation potential, oxidative stress, senescence and mitochondrial function in Wharton’s Jelly stem cells of umbilical cords of mothers with gestational diabetes mellitus. Stem Cell Rev. Rep. 15, 415–426 (2019).
Zheng, J. et al. Clinical and virological impact of single and dual infections with influenza a (H1n1) and sars-cov-2 in adult inpatients. PLoS Negl. Trop. Dis. 15, 1–15 (2021).
Baala, L. et al. Case Report: Co-infection with SARS-CoV-2 and influenza H1N1 in a patient with acute respiratory distress syndrome and a pulmonary sarcoidosis. F1000Research 9, 1482 (2022).
Liang, X. et al. Coinfection of SARS-CoV-2 and influenza A (H3N2) detected in bronchoalveolar lavage fluid of a patient with long COVID using metagenomic next-generation sequencing: A case report. Front. Cell. Infect. Microbiol. 13 (2023).
Maltezou, H. C. et al. COVID-19 and respiratory virus co-infections: A systematic review of the literature. Viruses 15, 865 (2023).
Chu, H. et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 1, e14–e23 (2020).
Khan, S. et al. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife 10 (2021).
Kasho, A. K. A. et al. PBMC MicroRNAs: Promising biomarkers for the differential diagnosis of COVID-19 patients with abnormal coagulation indices. Curr. Microbiol. 80, 248 (2023).
Oh, S. J. et al. Anti-viral activities of umbilical cord mesenchymal stem cell-derived small extracellular vesicles against human respiratory viruses. Front. Cell Infect. Microbiol. 12, 850744 (2022).
Johansson, C. & Kirsebom, F. C. M. Neutrophils in respiratory viral infections. Mucosal Immunol. 14, 815–827 (2021).
Cassidy, F. C. et al. Impact of type 2 diabetes mellitus on human bone marrow stromal cell number and phenotypic characteristics. Int. J. Mol. Sci. 21, 1–20 (2020).
Kornicka, K., Houston, J. & Marycz, K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 Diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell. Rev. Rep. 14, 337–345 (2018).
Krammer, F. & Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 14, 167–182 (2015).
Wu, D. et al. Coinfection of influenza virus and severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). Pediatr. Infect. Dis. J. 39, E79 (2020).
Schimmel, L. et al. Endothelial cells are not productively infected by SARS-CoV-2. Clin. Transl. Immunol. 10, e1350 (2021).
Mulay, A. et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. Cell. Rep. 35, 109055 (2021).
Harrison, A. G., Lin, T. & Wang, P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 41, 1100–1115 (2020).
Wang, L. T., Liu, K. J., Sytwu, H. K., Yen, M. L. & Yen, B. L. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl. Med. 10, 1288–1303 (2021).
Farkhad, N. et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: A successful phase 1, control-placebo group, clinical trial. Stem Cell Res. Ther. 13, 283 (2022).
Bian, D., Wu, Y., Song, G., Azizi, R. & Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell. Res. Ther. 13, 24 (2022).
Hao, W., Wang, L. & Li, S. FKBP5 regulates RIG-I-mediated NF-κB activation and influenza a virus infection. Viruses 12, 672 (2020).
Konno, H. et al. TRAF6 establishes innate immune responses by activating NF-κB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One 4 (2009).
Wei, J. et al. MicroRNA-146a contributes to SCI recovery via regulating TRAF6 and IRAK1 expression. Biomed. Res. Int. (2016).
Han, R. et al. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci. Rep. 13, 1–12 (2023).
Liu, G. J. et al. MiR-146a ameliorates hemoglobin-induced microglial inflammatory response via TLR4/IRAK1/TRAF6 associated pathways. Front. Neurosci. 14, 1–12 (2020).
Baldeón, L. R. et al. Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PLoS One 9, e115209 (2014).
Babatunde, K. A. et al. Chemotaxis and swarming in differentiated HL-60 neutrophil-like cells. Sci. Rep. 11, 1–13 (2021).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Acknowledgements
We would like to acknowledge the support from the Japanese Ministry of Education, Culture, Sports, Science & Technology (MEXT).
Author information
Authors and Affiliations
Contributions
N.A. conducted contributed to the experiments, systematic review, data analysis, and the original draft of manuscript. C.-K.V. raised the study concept and experimental design, contributed to experiments and editing of the manuscript. P.S. contributed to systematic review and editing of the manuscript. M.F. contributed to the systematical review. M.O. and H.H. provided human samples. T.Y. contributed to the experimental support. O.O. raised the study concept and experimental design, the editing of the manuscript and final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Anggraeni, N., Vuong, CK., Silvia, P. et al. Mesenchymal stem cell-derived extracellular vesicles reduce inflammatory responses to SARS-CoV-2 and Influenza viral proteins via miR-146a/NF-κB pathway. Sci Rep 14, 26649 (2024). https://doi.org/10.1038/s41598-024-77258-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77258-0