Abstract
Depression frequently comorbidities with cancer, adversely affecting survivors’ quality of life. Liver dysfunction is also prevalent among cancer survivors. However, the association between these two conditions remains unclear. This study aimed to explore the relationship between depression and liver function biomarkers in US cancer survivors. A cross-sectional study was conducted utilizing data from the National Health and Nutrition Examination Survey (NHANES) 2005–2020. Cancer survivors were screened and depression was assessed using the Patient Health Questionnaire-9 (PHQ-9), and 18 liver function biomarkers were included. Survey-weighted generalized linear models with multiple covariables adjusted were employed to examine the associations between depression and liver function biomarkers. A total of 4118 cancer survivors were included, representing a weighted population of 21 501 237. After adjusted with age, gender, race, marital status, education level, family income-to-poverty ratio, and number of cancer types, 8 biomarkers demonstrated positive correlations with depression in cancer survivors, included alanine aminotransferase (ALT, OR = 1.007, 95% CI: 1.000 to 1.013), alkaline phosphatase (ALP, 1.006 [1.002, 1.010]), gamma glutamyl transferase (GGT, 1.004 [1.001, 1.007]), lactate dehydrogenase (LDH, 1.004 [1.000, 1.009]), total protein (TP, 1.040 [1.009, 1.072]), globulin (GLB, 1.060 [1.030, 1.091]), total cholesterol (TC) to high-density lipoprotein cholesterol (HDL-C) ratio (1.162 [1.050, 1.286]), and low-density lipoprotein cholesterol (LDL-C) to HDL-C ratio (1.243 [1.012, 1.526]); while 4 other biomarkers exhibited negative correlations, included HDL-C (0.988 [0.980, 0.997]), total bilirubin (TBi, 0.501 [0.284, 0.883]), aspartate aminotransferase (AST) to ALT ratio (0.588 [0.351, 0.986]), albumin (ALB) to GLB ratio (0.384 [0.229, 0.642]). Following sensitivity analysis, 5 biomarkers included LDH, HDL-C, TBi, AST/ALT and LDL-C/HDL-C lost their statistical significance for the association. This study identified certain associations between 7 liver function biomarkers and depression in US cancer survivors. Further research, particularly prospective longitudinal studies, is warranted to elucidate the causal relationships and explore the potential of improving liver function for the management of depression in cancer patients.
Similar content being viewed by others
Introduction
Depression occurs in up to 20% of cancer patients and major depression strikes in almost 13%1,2. Among cancer survivors, depression significantly impairs their quality of life3. Antidepressant treatment has limited efficacy in cancer patients as almost one-third of patients with clinically significant depression do not respond to either psychotherapy or pharmacotherapy4. Identifying therapeutic targets for managing oncological depression is of crucial importance. Previous studies have mainly focused on exploring the associations with inflammation-related biomarkers such as serum cortisol5, C-reactive protein (CRP)6, interleukin-6 (IL-6)7, interferon gamma (IFN-γ)8, tumor necrosis factor alpha (TNF-α)9, transforming growth factor beta (TGF-β)10, etc. There has been no research shed the light on the potential correlation between liver function biomarkers and depression in oncological patients.
Many previous studies have suggested correlations between depression and impaired liver function including metabolic-associated fatty liver diseases11,12,13, alcoholic liver diseases14, chronic hepatitis15, etc. Two studies have investigated the association of depression with liver function biomarkers included alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), albumin (ALB), globulin (GLB) and bilirubin in post-stroke patients16 and healthy older adults17, respectively. However, the association among cancer patients hasn’t been illuminated, despite that liver function abnormalities are common in cancer patients, either caused by cancer itself or induced from anti-cancer therapies18.
In light of the above literature review, improve patients’ liver performance may alleviate their depression, or conversely, alleviating patients’ depression may improve their liver function performance. But the potential association between liver function biomarkers and depression in oncological patients hasn’t been illuminated. Therefore, to provide fundamental data support for further investigate on the association of them, the current cross-sectional study was conducted to explore the relationships between depression and liver function biomarkers in cancer survivors, utilizing data from the National Health and Nutrition Examination Survey (NHANES) 2005–2020.
Methods
The current cross-sectional study utilized a nationally representative sample from the US NHANES database. The NHANES is an epidemiological program conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of the noninstitutionalized civilian resident population in the United States. Since 1999, the sample design has employed a multi-year, stratified, clustered four-stage sampling approach, with data release in 2-year cycles.
All the NHANES protocols were approved by the NCHS ethics review board, and written informed consents were obtained from all participants19. We confirm that all methods were performed in accordance with the relevant guidelines and regulations for current research type.
Cancer survivors
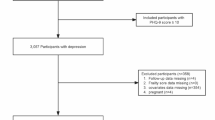
In the current study, the NHANES surveys conducted from 2005 to 2020 were included. The cancer survivors were defined as the individuals who responded “Yes” to the question “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?”20. A total of 6172 cancer survivors remained after excluding those without cancer or responses. We then excluded the individuals who did not complete all the nine Patient Health Questionnaire (PHQ-9) questions, leaving 4118 participants for the association analysis. Furthermore, to perform a sensitivity analysis with complete cases, 392 participants with incomplete data in any kinds of covariables were eliminated.
Depression
A well-validated nine-item depression screening instrument called PHQ-9 was administered to determine the frequency of depression symptoms. Each item on depression symptom was scored from 0 to 3 according to participants’ responses. The depression was defined as a score of 10 or above on the PHQ-9 according to references21.
Liver function biomarkers
The NHANES collected biological specimens for laboratory analysis to provide detailed information about participants’ health and nutritional status. The biospecimen collection took place in the mobile examination center (MEC), the controlled environment of which allowed laboratory test to be performed under standardized conditions at each survey location. The 18 liver function biomarkers of alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), AST to ALT ratio (AST/ALT), alkaline phosphatase (ALP, U/L), gamma glutamyl transferase (GGT, U/L), lactate dehydrogenase (LDH, U/L), total bilirubin (TBi, mg/dL), total protein (TP, g/L), albumin (ALB, g/L), globulin (GLB, g/L), ALB to GLB ratio (ALB/GLB), total cholesterol (TC, mg/dL), triglycerides (TG, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL), TC to HDL-C ratio (TC/HDL-C), TG to HDL-C ratio (TG/HDL-C), LDL-C to HDL-C ratio (LDL-C/HDL-C) were collected. NHANES provides a series of standardized procedures for specimen collection and biomarker measurement as researchers can find these instructions on the NHANES official website (https://www.cdc.gov/nchs/nhanes/index.htm)22.
Covariables
The number of cancer types per participant was categorized according to the series of questions: “1st cancer—what kind was it?” “2nd cancer—what kind was it?” “3rd cancer—what kind was it?” “More than 3 kinds of cancer?”. Data on the course of cancer from the first cancer diagnosis to the interview were excessively deficient in the NHANES dataset hence it was not collected23. Sociodemographic characteristics included age, gender (male, female), race (Hispanic, non-Hispanic white, non-Hispanic black, other), marital status (never married, widowed/divorced/separated, married/living with partner), education level (less than high school, high school or equivalent, college or above) and family income-to-poverty ratio (PIR, < 1.3, 1.3–3.5 < , ≥ 3.5) were collected. Anthropometric indexes included body mass index (BMI, < 18.5, 18.5–25, 25–30, ≥ 30) and waist circumference (WC; for male: < 94, 94–102, ≥ 102; for female: < 80, 80–88, ≥ 88) were collected. Health behaviors included smoking status (never, former, present) and alcohol consumption (never, former, mild, moderate, heavy) were collected. BMI24,25, WC26,27, smoking status28,29, and alcohol consumption30,31 were adjusted in sensitivity analysis, in consideration of the fact that they were not only correlated with depression, but also directly associated with liver function biomarkers32.
Statistical analyses
Dummy variables were used to indicate missing covariate values. Continuous variables were presented as mean ± standard error and categorical variables were demonstrated as frequencies and percentages. Weighted χ2 test (categorical variable), t-test (normal distribution), or Mann-Whittney U test (skewed distribution) were employed to detect the differences among depression and non-depression groups. Survey-weighted generalized linear models were introduced to elucidate the association between depression and liver function biomarkers. A total of 3 statistical models were constructed in each analysis. Crude model was the non-adjusted model with no covariables adjusted. Model I adjusted for age and gender. Model II adjusted for age, gender, race, marital status, education level, PIR, and number of cancer types. Sensitivity analysis was conducted in two ways: by excluding the participants with missing data in covariables and by adding BMI, WC, smoking status and alcohol consumption into adjusted variables. Stratified analysis was presented to examine the potential subgroup variations in the association between depression and liver function biomarkers across genders and races. Multicollinearity among variables were examined with generalized variance inflation factor (GVIF). Data analysis was performed with the statistical software R (http://www.R-project.org). Survey-weighted statistical models were constructed with the “survey” package of R. Multicollinearity test was conducted with the “car” package of R. A P value < 0.05 was considered as statistically significant33,34.
Results
Study sample characteristics
A total of 4118 cancer survivors were included in this study. Table 1 presented the baseline characteristics of the study sample. According to the cut-off point of 10 for PHQ-9, 444 cancer survivors were divided into the depression group and the other 3674 were assigned into the non-depression group. After weighting with the recommended weights from the NHANES, the depression group and the non-depression group could represent 1 975 315 and 19 525 922 individuals from the US cancer survivors, respectively. Compared with non-depressive cancer survivors, the depressive ones exhibited the following characteristics: lower AST/ALT, TBi, ALB, ALB/GLB, and HDL-C; higher ALP, GGT, TP, GLB, TG, TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C; lower age, lower proportion of non-Hispanic White; higher proportion of female, widowed/divorced/separated for marital, less than high school for education, PIR less than 1.3, obese for BMI, and present smoker.
Association of depression and liver function biomarkers
The relationships between the depression and liver function biomarkers were accessed across 3 survey-weighted models. The detailed odds ratios (ORs) with their correspondent 95% confidence intervals (CIs) were presented in Table 2. In the crude model with no covariable adjusted, 10 liver function biomarkers showed associations with depression in cancer survivors: ALP, GGT, TBi, TP, ALB, GLB, ALB/GLB, HDL-C, TC/HDL-C, LDL-C/HDL-C. In the model I with age and gender adjusted, ALT and LDH also exhibited associations in addition to the first 10 biomarkers. In the model II fully adjusted with age, gender, race, marital status, education level, PIR, and number of cancer types, 8 biomarkers demonstrated positive correlations with depression in cancer survivors: ALT (OR = 1.007, 95% CI: 1.000 to 1.013, P = 0.035), ALP (OR = 1.006, 95% CI: 1.002 to 1.010, P = 0.003), GGT (OR = 1.004, 95% CI: 1.001 to 1.007, P = 0.012), LDH (OR = 1.004, 95% CI: 1.000 to 1.009, P = 0.038), TP (OR = 1.040, 95% CI: 1.009 to 1.072, P = 0.011), GLB (OR = 1.060, 95% CI: 1.030 to 1.091, P < 0.001), TC/HDL-C (OR = 1.162, 95% CI: 1.050 to 1.286, P = 0.004), LDL-C/HDL-C (OR = 1.243, 95% CI: 1.012 to 1.526, P = 0.038). And 4 other biomarkers demonstrated negative correlations: AST/ALT (OR = 0.588, 95% CI: 0.351 to 0.986, P = 0.044), TBi (OR = 0.501, 95% CI: 0.284 to 0.883, P = 0.017), ALB/GLB (OR = 0.384, 95% CI: 0.229 to 0.642, P < 0.001), HDL-C (OR = 0.988, 95% CI: 0.980 to 0.997, P = 0.007). Multicollinearity among variables were not detected between the 7 covariables and each liver function biomarker. Detailed multicollinearity test results in Supplementary material.
Sensitivity analysis and stratified analysis for the association
To validate the above associations between depression and liver function biomarkers, and assess the robustness of these results, a sensitivity analysis was conducted by excluding a subset of missing covariables. The results of the sensitivity analysis indicated stable associations between depression and 9 liver function biomarkers: ALT (P = 0.024), AST/ALT (P = 0.040), ALP (P = 0.009), GGT (P = 0.011), TBi (P = 0.029), TP (P = 0.009), GLB (P < 0.0001), ALB/GLB (P < 0.001), TC/HDL-C (P = 0.012), whereas the other 3 biomarkers fell apart: LDH (P = 0.075), HDL-C (P = 0.065), LDL-C/HDL-C (P = 0.127). Detailed statistics for above sensitivity analysis in Table 3. Following another sensitivity analysis by adding body mass index, waist circumference, smoking status and alcohol consumption into adjusted variables, AST/ALT (P = 0.059), TBi (P = 0.103), HDL-C (P = 0.142), LDL-C/HDL-C (P = 0.092) fell apart on association with depression. Details in Supplementary material.
To examine the potential subgroup variations in the association between depression and liver function biomarkers, stratified analysis was presented across genders and races. The results indicated the gender have interaction on the association between ALP (Pinteraction = 0.013) and depression in cancer survivors, whereas the race have interaction on GGT (Pinteraction = 0.008), GLB (Pinteraction = 0.010), TC (Pinteraction = 0.012), HDL (Pinteraction = 0.023), LDL (Pinteraction < 0.0001) with the depression. Detailed statistics for stratified analysis in Supplementary material.
Discussion
The current study explored the association of depression and 18 liver function biomarkers in US cancer survivors. Following the sensitivity analysis, 6 biomarkers included ALT, ALP, GGT, TP, GLB, and TC/HDL-C demonstrated stable positive correlations with depression in cancer survivors; and ALB/GLB demonstrated consistent negative correlations. Given the particularities of oncological patients, our results are consistent with some studies and inconsistent with others, as discussed below.
Enzymes
ALT is an enzyme which universally participates in amino acid metabolism and serves as a crucial biomarker of liver health35. It facilitates the conversion of alanine to pyruvate and glutamate to α-ketoglutarate, essential intermediates in various metabolic pathways35. The result from the St. Jude Lifetime Cohort Study indicated 41.3% adult childhood cancer survivors had elevated ALT36, and another cohort study in Israel suggested elevated ALT independently predicts new onset of depression in employees undergoing health screening examinations37. In reversal from ALT, AST/ALT exhibited the opposite, namely a negative, association with depression as results among post-stroke patients17. The results were reasonable and consistent with current study as AST presented no significant association and ALT acted as the denominator in the ratio.
ALP is a multifaceted enzyme with essential roles in various biological processes, including bone mineralization, intestinal absorption, and bile production38. A cross-sectional study, utilizing NHANES 2007–2014 surveys, suggested that higher ALP levels, even within the normal range, were significantly associated with a higher risk of depression in US adults39, which is consistent with the current study. Another cross-sectional study in China also indicated that serum ALP was independently associated with vascular depression in patients with cerebrovascular disease40.
GGT is an enzyme which primarily responsible for the metabolism of glutathione, an antioxidant that protects cells from damage41. Ziqiang Lin et al. found GGT was the one of five most important features for depression prediction with data from NHANES 2007–2016 surveys42. Jun Gong found evaluated GGT in post-stroke depression patients compared with non-depressed ones16. Wataru Tomeno et al. reported, even in patients both suffered from the non-alcoholic fatty liver disease, those with major depressive disorder have higher GGT than those without, which further validated the GGT feature43.
Bilirubin
Bilirubin is a yellow pigment produced by the liver when it breaks down old red blood cells, and growing evidence suggests that bilirubin is a potent antioxidant and cytoprotective agent against multiple metabolic diseases44. Bilirubin have two forms in the blood: the unconjugated bilirubin bound to albumin and conjugated bilirubin bound to glucuronic acid45. In the current study, only the total bilirubin was analyzed because there was no further data on the separate components of total bilirubin. Previous studies have been consistent in the direction of the relationship between bilirubin and the cancer risk46. Numerous studies have suggested a negative association between bilirubin and the cancer risk including lung cancer47,48, colorectal cancer49,50, and breast cancer51. However, here is still a lack of agreement on the direction of the relationship between bilirubin and depression. In postpartum female, Yi Liu et al. reported decreased total bilirubin was associated with the incidence of postpartum depression52. In post-stroke patients, the outcomes were more variable. Wai Kwong Tang et al. reported high bilirubin level is associated with post-stroke depression in China Hong Kong population53, while the study by Jun Gong et al. indicated a negative association between total bilirubin and post-stroke depression in China Chongqing population16 and Yanyan Wang et al. reported a higher level of indirect bilirubin can lower the risk of post-stroke depression in China Wuhan population54. Furthermore, another study from China Shenyang discovered more detailed relationships: higher level of total bilirubin on admission was related to the major depression occurrence within 3 months after post-ischemic stroke; but lower level on admission was characteristic for the occurrence between 3 and 6 months post-stroke; while 6 months after stroke, there was no relationship from them55. Ultimately, here was a study utilizing the similar NHANES 2009 to 2018 sample and it indicated depressed men had lower total bilirubin level than control men in general US population, which is to some extent in accordance with our results56.
Proteins
As for the total protein, one recent study with UK Biobank data revealed a significant association between depression and low levels of total protein in females57. Likewise, the study from China Chongqing also indicated a negative association of depression and total protein among post-stroke population16. However, in the analysis used NHANES data, no statistically significant association was produced between total protein and depression56.
Globulins play crucial roles in the immune system, nutrient transport, and blood clotting58. Studies mainly focus on some specific fractions of globulins including alpha-1-globulin, alpha-2-globulin, and sex hormone-binding globulins59,60,61. An earlier study indicated major depressed subjects showed significantly lower total protein and a higher percentage of alpha-1-globulin fraction than normal controls and minor depressed subjects62. In the Chongqing study, the globulin was negatively associated with post-stroke depression16. In the previous NHANES study, the globulin was positively associated with depression in models of no-adjusted and adjusted with age and race, however fell apart after adjusted with education, PIR, marital status, alcohol consumption, body mass index, waist circumference56.
In reversal from GLB, ALB/GLB exhibited the opposite, namely a positive, association with depression as results among post-stroke patients, which is reverse to what the current study found17. However, the reversion exactly validated our results as ALB presented no significant association and GLB acted as the denominator in the ratio.
Lipids
Relationships between cholesterols and depression varies widely across studies. Some reported low cholesterol is associated with depression using NHANES 2005–2008 surveys63, while the others reported low cholesterol is not associated with depression using NHANES 2005–2018 surveys64. Furthermore, Yang Wang et al. reported that the remnant cholesterol (i.e. TC-(HDL-C + LDL-C)) positively correlated with depression with NHANES 2005–2016 data65.
As for HDL-C, O P Almeida et al. reported 5-year probability of depression in older men increased as the HDL-C decreased66, Soili M Lehto et al. reported those with long-term depression may have lower HDL-C compared with healthy controls67, and a large sample pooled analysis indicated lower levels of HDL-C was associated with increased odds of depression68. As for non-HDL-C, Xianlin Zhu et el. reported there was a positive association between it and depression with NHANES 2005–2018 data69. Consistently, Xiaoyi Qi et al. reported the non-HDL-C to HDL-C ratio was significantly associated with a higher risk of depression in U.S. adults with NHANES 2005–2016 data70.
As for TC/HDL-C, A Lum Han reported it was not associated with depression with data from Korea NHANES71. However, in a large sample population-based cohort study covering 211 200 participants across 10 years, low levels of HDL were associated with future risk of depression and depression patients had higher levels of TC72.
Clinical implications
The current study has provided preliminary evidence for the association between depression and liver function biomarkers in cancer survivors from US population. Our findings suggest that enhancing patients’ liver performance may mitigate their depression, or conversely, alleviating patients’ depression could potentially improve their liver function performance. The interplay between these two conditions in cancer patients warrants further exploration in clinical trials to determine their therapeutic implications. Unlike previous studies focused on the general population or the post-stroke patients, we restricted our target population to cancer survivors this time. And in the current study, except the specific fractions of bilirubin, the most complete liver function indices have been included. Furthermore, we have utilized a large representative US population sample, three survey-weighted generalized linear models and multiple covariable adjustments for the association analysis, which have enabled our results more consistent with how the real-world cancer survivors experience. Nevertheless, there were also some limitations in current study. A cross-sectional study design is incapable of determining the causality, therefore prospective longitudinal studies are expected in the future. Moreover, a cross-sectional study design is also unable to remove the insidious residual confusing results from unmeasured or unidentified confounding factors.
Conclusion
ALT, ALP, GGT, TP, GLB, and TC/HDL-C were positively associated with depression in US cancer survivors, while ALB/GLB demonstrated negative correlations. The therapeutic role of liver function improvement for depression in cancer patients deserves further investigation, and large sample prospective longitudinal studies are anticipated.
Data availability
The datasets used and/or analyzed in the current study are available in the article or supplementary material.
References
Pitman, A. et al. Depression and anxiety in patients with cancer. BMJ 361, k1415 (2018).
Bortolato, B. et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 52, 58–70 (2017).
Yi, J. C. & Syrjala, K. L. Anxiety and depression in cancer survivors. Med. Clin. North Am. 101(6), 1099–1113 (2017).
Panjwani, A. A. et al. Biomarker opportunities in the treatment of cancer-related depression. Trends Mol. Med. 28(12), 1050–1069 (2022).
Qin, N. et al. Associations of serum cortisol and inflammatory marker features with depression and anxiety in young women with gynecologic cancer. Support Care Cancer 31(12), 674 (2023).
Renna, M. E. et al. Depression and anxiety in colorectal cancer patients: Ties to pain, fatigue, and inflammation. Psychooncology 31(9), 1536–1544 (2022).
Cohen, M. et al. Low physical activity, fatigue and depression in breast cancer survivors: Moderation by levels of IL-6 and IL-8. Int. J. Psychophysiol. 158, 96–102 (2020).
Ho, H. Y. et al. Circulating cytokines as predictors of depression in patients with breast cancer. J. Psychiatr. Res. 136, 306–311 (2021).
Tzang, B. S. et al. Differential associations of proinflammatory and anti-inflammatory cytokines with depression severity from noncancer status to breast cancer course and subsequent chemotherapy. BMC Cancer 20(1), 686 (2020).
Miranda, D. O. et al. Elevated serum levels of proinflammatory cytokines potentially correlate with depression and anxiety in colorectal cancer patients in different stages of the antitumor therapy. Cytokine 104, 72–77 (2018).
Gu, Y. et al. Association between nonalcoholic fatty liver disease and depression: A systematic review and meta-analysis of observational studies. J. Affect. Disord. 301, 8–13 (2022).
Xiao, J. et al. Is fatty liver associated with depression? A meta-analysis and systematic review on the prevalence, risk factors, and outcomes of depression and non-alcoholic fatty liver disease. Front. Med. (Lausanne) 8, 691696 (2021).
Shea, S. et al. Non-alcoholic fatty liver disease and coexisting depression, anxiety and/or stress in adults: A systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 15, 1357664 (2024).
Huang, X., Liu, X. & Yu, Y. Depression and chronic liver diseases: Are there shared underlying mechanisms?. Front. Mol. Neurosci. 10, 134 (2017).
Khan, A. N. et al. Frequency of depression among patients with chronic liver disease. J. Ayub. Med. Coll. Abbottabad 32(4), 535–539 (2020).
Gong, J. et al. Liver function test indices-based prediction model for post-stroke depression: A multicenter, retrospective study. BMC Med. Inform. Decis. Mak. 23(1), 127 (2023).
Savage, K. et al. Liver and inflammatory biomarker relationships to depression symptoms in healthy older adults. Exp. Gerontol. 177, 112186 (2023).
De Martin, E. et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 68(6), 1181–1190 (2018).
Chen, T. C. et al. National health and nutrition examination survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat. 2(184), 1–35 (2020).
Cao, C., Friedenreich, C. M. & Yang, L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol. 8(3), 395–403 (2022).
Levis, B., Benedetti, A. & Thombs, B. D. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: Individual participant data meta-analysis. Bmj 365, l1476 (2019).
NHANES 2017–2018 Laboratory Methods. Available from: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2017.
Cao, C. et al. Association of N-terminal pro-brain natriuretic peptide with survival among US cancer survivors. J. Natl. Cancer Inst. 116, 938–947 (2024).
Khurana, T. et al. Clinically meaningful body mass index change impacts pediatric nonalcoholic fatty liver disease. J. Pediatr. 250, 61-66.e1 (2022).
Fan, R., Wang, J. & Du, J. Association between body mass index and fatty liver risk: A dose-response analysis. Sci. Rep. 8(1), 15273 (2018).
Lee, J. H. et al. Cutoff points of waist circumference for predicting incident non-alcoholic fatty liver disease in middle-aged and older Korean adults. Nutrients 14(14), 2994 (2022).
Rahmani, J. et al. Waist circumference and risk of liver cancer: A systematic review and meta-analysis of over 2 million cohort study participants. Liver Cancer 9(1), 6–14 (2020).
Marti-Aguado, D., Clemente-Sanchez, A. & Bataller, R. Cigarette smoking and liver diseases. J. Hepatol. 77(1), 191–205 (2022).
Lai, S. W. Smoking and nonalcoholic fatty liver disease. Am. J. Gastroenterol. 114(6), 998 (2019).
Testino, G. & Caputo, F. Alcohol-related liver disease. Hepatobiliary Surg. Nutr. 13(2), 347–351 (2024).
Mackowiak, B. et al. Alcohol-associated liver disease. J. Clin. Invest. 134(3), 1. https://doi.org/10.1172/JCI176345 (2024).
Johnson, C. L. et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2(161), 1–24 (2013).
Qi, X. et al. Vitamin E intake is inversely associated with NAFLD measured by liver ultrasound transient elastography. Sci. Rep. 14(1), 2592 (2024).
Qi, X. et al. The associations between dietary fibers intake and systemic immune and inflammatory biomarkers, a multi-cycle study of NHANES 2015–2020. Front. Nutr. 10, 1216445 (2023).
Ruhl, C. E. & Everhart, J. E. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology 55(2), 447–454 (2012).
Green, D. M. et al. Serum alanine aminotransferase elevations in survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Hepatology 69(1), 94–106 (2019).
Zelber-Sagi, S. et al. Elevated alanine aminotransferase independently predicts new onset of depression in employees undergoing health screening examinations. Psychol. Med. 43(12), 2603–2613 (2013).
Siller, A. F. & Whyte, M. P. Alkaline phosphatase: Discovery and naming of our Favorite enzyme. J. Bone Miner. Res. 33(2), 362–364 (2018).
Liang, Y. et al. Association of serum alkaline phosphatase and depression in US adults: A population-based cross-sectional study. Front. Psychiatry 14, 1131105 (2023).
Tao, X. et al. Serum alkaline phosphatase was independently associated with depression in patients with cerebrovascular disease. Front. Psychiatry 14, 1184673 (2023).
Brennan, P. N., Dillon, J. F. & Tapper, E. B. Gamma-glutamyl transferase (γ-GT)—An old dog with new tricks?. Liver Int. 42(1), 9–15 (2022).
Lin, Z. et al. Classifying depression using blood biomarkers: A large population study. J. Psychiatr Res. 140, 364–372 (2021).
Tomeno, W. et al. Non-alcoholic fatty liver disease comorbid with major depressive disorder: The pathological features and poor therapeutic efficacy. J. Gastroenterol. Hepatol. 30(6), 1009–1014 (2015).
Vítek, L. & Tiribelli, C. Bilirubin: The yellow hormone?. J. Hepatol. 75(6), 1485–1490 (2021).
Hinds, T. D. Jr. & Stec, D. E. Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension 72(4), 788–795 (2018).
Freisling, H. et al. Gilbert’s syndrome, circulating bilirubin and lung cancer: A genetic advantage?. Thorax 75(11), 916–917 (2020).
Wen, C. P. et al. The ability of bilirubin in identifying smokers with higher risk of lung cancer: A large cohort study in conjunction with global metabolomic profiling. Clin. Cancer Res. 21(1), 193–200 (2015).
Horsfall, L. J. et al. Genetically raised serum bilirubin levels and lung cancer: A cohort study and Mendelian randomisation using UK Biobank. Thorax 75(11), 955–964 (2020).
Seyed Khoei, N. et al. Circulating bilirubin levels and risk of colorectal cancer: serological and Mendelian randomization analyses. BMC Med. 18(1), 229 (2020).
Zucker, S. D., Horn, P. S. & Sherman, K. E. Serum bilirubin levels in the U.S. population: Gender effect and inverse correlation with colorectal cancer. Hepatology 40(4), 827–835 (2004).
Kühn, T. et al. Albumin, bilirubin, uric acid and cancer risk: Results from a prospective population-based study. Br. J. Cancer 117(10), 1572–1579 (2017).
Liu, Y. et al. Bilirubin and postpartum depression: An observational and Mendelian randomization study. Front. Psychiatry 15, 1277415 (2024).
Tang, W. K. et al. Association between high serum total bilirubin and post-stroke depression. Psychiatry Clin. Neurosci. 67(4), 259–264 (2013).
Wang, Y. et al. Nomogram including indirect bilirubin for the prediction of post-stroke depression at 3 months after mild acute ischemic stroke onset. Front. Neurol. 14, 1093146 (2023).
Gao, J. et al. Changes of serum uric acid and total bilirubin in elderly patients with major postischemic stroke depression. Neuropsychiatr. Dis. Treat. 14, 83–93 (2018).
Li, X. et al. Relationship between depressive disorders and biochemical indicators in adult men and women. BMC Psychiatry 23(1), 49 (2023).
Kiewa, J. et al. Comprehensive sex-stratified genetic analysis of 28 blood biomarkers and depression reveals a significant association between depression and low levels of total protein in females. Complex Psychiatry 10(1–4), 19–34 (2024).
Suh, B. et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann. Oncol. 25(11), 2260–2266 (2014).
Zhu, H. et al. Causal relationship between sex hormone-binding globulin and major depression: A Mendelian randomization study. Acta Psychiatr Scand. 148(5), 426–436 (2023).
Bahrini, L., Ouanes, S. & Ghachem, R. Inflammatory profile in depression and associated clinical and sociodemographic features in a Middle-Eastern North-African population. J. Affect. Disord. 198, 122–126 (2016).
Van Hunsel, F. et al. Lower total serum protein, albumin, and beta- and gamma-globulin in major and treatment-resistant depression: Effects of antidepressant treatments. Psychiatry Res. 65(3), 159–169 (1996).
Maes, M. et al. Total serum protein and serum protein fractions in depression: Relationships to depressive symptoms and glucocorticoid activity. J. Affect. Disord. 34(1), 61–69 (1995).
Tedders, S. H. et al. Low cholesterol is associated with depression among US household population. J. Affect. Disord. 135(1–3), 115–121 (2011).
Zhang, Q. et al. Low cholesterol is not associated with depression: Data from the 2005–2018 national health and nutrition examination survey. Lipids Health Dis. 21(1), 35 (2022).
Wang, Y. & Shen, R. Association of remnant cholesterol with depression among US adults. BMC Psychiatry 23(1), 259 (2023).
Almeida, O. P. et al. HDL cholesterol and the risk of depression over 5 years. Mol. Psychiatry 19(6), 637–638 (2014).
Lehto, S. M. et al. Low HDL cholesterol associates with major depression in a sample with a 7-year history of depressive symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 32(6), 1557–1561 (2008).
Bot, M. et al. metabolomics profile in depression: A pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol. Psychiatry 87(5), 409–418 (2020).
Zhu, X. et al. Association of non-HDL-C and depression: a cross-sectional analysis of the NHANES data. Front. Psychiatry 14, 1274648 (2023).
Qi, X. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: A cross-sectional NHANES study. J. Affect. Disord. 344, 451–457 (2024).
Han, A. L. Association between lipid ratio and depression: a cross-sectional study. Sci. Rep. 12(1), 6190 (2022).
Chourpiliadis, C. et al. Metabolic profile and long-term risk of depression, anxiety, and stress-related disorders. JAMA Netw. Open 7(4), e244525 (2024).
Acknowledgements
The authors would like to express their sincere gratitude to all participants and investigators who have been part of the NHANES study.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515110718), the Guangdong Provincial Medical Science and Technology Research Foundation (Grant No. A2022179), and the National Natural Science Foundation of China (Grant No. 82305385).
Author information
Authors and Affiliations
Contributions
YLL designed the study. ZKY, XYR and JTL collected the data. YLL, ZKY, XYR, JTL, HL, PZ, and SS analyzed the data and drafted the manuscript. JL and YLL revised the final version of the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All the NHANES protocols were approved by the Ethics Review Board of U.S. National Center for Health Statistics, and written informed consents were obtained from all participants of NAHNES surveys.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Ye, Z., Ran, X. et al. Association between depression and liver function biomarkers among US cancer survivors in NHANES 2005–2020. Sci Rep 14, 27501 (2024). https://doi.org/10.1038/s41598-024-78890-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78890-6


