Abstract
Radiofrequency ablation (RFA) is effective treatment for Barrett’s esophagus (BE). Product of successful RFA is neosquamous epithelium (NSE), which resembles native squamous epithelium and has lower risk for neoplastic transformation. Dilated intercellular spaces (IS) are common microscopic feature of reflux induced injury of esophagus. The aim of this study was to analyze the ultrastructure of NSE by transmission electron microscopy (TEM), depending on the post RFA treatment modalities and to asses impact of these findings on BE recurrence. Prospective observational clinical study based on TEM analysis of biopsy of specimens obtained from patients in whom CE of BE was achieved minimum 6 months after the last RFA session. In each patient biopsies were taken from NSE and proximal esophagus. Two groups of patients were defined according to the post RFA treatment: proton pump inhibitors (PPI’s) or laparoscopic Nissen fundoplication (LNF). Comparative analysis of IS length was made between two groups. Endoscopic surveillance with biopsies was conducted for 5 years. Overall 22 patients with CE of BE after RFA underwent complete study protocol, out of whom in 10 LNF was performed, while 12 were treated with PPI’s. The mean values of IS length in the proximal esophagus and NSE in LNF group were 0.378 ± 0.116 µm and 0.878 ± 0.354, while in PPI’s group 0.724 ± 0.325 µm and 1.228 ± 0.226 µm, respectively. Mean lenghts of IS were statistically significantly higher in PPI’s group both in NSE (p = 0.032) and proximal esophagus (p = 0.009). There were 5 BE recurrences after 5 years surveillance, 4 in PPI group and 1 in LNF group, without statistical significance (p = 0.084). Dilated IS are commonly presented in NSE of patients with CE of BE with RFA who are treated with PPI’s. LNF provides may offer better reflux protection of NSE than PPI’s and may reduce the rates of recurrence after successful RFA treatment.
Similar content being viewed by others
Introduction
Barrett’s esophagus (BE) is defined as the presence of metaplastic columnar epithelium on the esophagus, which contains goblet cells1,2. Through the course of low and high- grade dysplasia, BE may be transformed into esophageal adenocarcinoma (EAC)1,2.
Endoscopic radiofrequency ablation (RFA) today is the most accepted treatment modality for dysplastic BE, with long-term proven safety and efficacy profile. The end goal of the procedure is to achieve complete eradication (CE) of BE related metaplasia and dysplasia3,4,5. The epithelium, that covers esophagus after ablation, is being called neosquamous epithelium (NSE)6. This type of epithelium resembles normal squamous, and what seems to be most important, genetic malformations which are present in dysplastic BE are not observed inside the NSE, indicating significantly reduced risk of neoplastic transformation7,8,9. However, a study which employed electrone microscopy evaluation of NSE, revealed presence of dilated intercellular spaces (DIS) in all of the examined biopsy specimens10. By examining claudin 4 expression, the major contributor to tight junctions inside squamocellular epithelium, same study also indicated possible higher permeability of NSE10. This higher NSE permeability might be due to the week nature of newly formed epithelium itself, or a result of the ongoing gastroesophageal reflux11. Further investigation of NSE properties may be of great interest when critically evaluating recurrence of BE after RFA.
Recurrence rates after successful RFA and CE of BE, are up to 10% annually12. Recurrence of BE has a greater probability to occur in the first year following the ablation, and is far less common after that period13,14. The majority of recurrences occur at the level of neo squamocolumnar junction and distal ablating segment of the BE, the area most exposed to reflux13,14. It has been shown that patients with uncontrolled acid and weakly acid reflux have higher probability for residual or recurrent BE after RFA, making assessment of NSE microstructure even more important15.
The aim of our study was to evaluate the NSE through the electron microscopy examination of intercellular spaces (IS) length, and to seek out how different post RFA treatment modalities can affect the properties of the NSE in the term of IS diameter. Also, this study addressed the impact of post RFA treatment and IS length in NSE on BE recurrence.
Material and methods
This was a prospective clinical observational study conducted at the Department of Esophagogastric Surgery, Hospital for Digestive Surgery, University Clinical Center of Serbia, School of Medicine, University of Belgrade. It was initiated at 2010, the biopsy material was collected until 2015, and the follow-up conducted until 2020. The study was conducted in collaboration with the Institute for Histology, School of Medicine, University of Belgrade. The Hospital Board and School of Medicine Ethics Committee approved the study, and the informed consent was obtained from all patients. All methods were performed in accordance with the relevant guidelines and regulations.
The study was supported by Serbian Academy of Sciences and Arts (SASA, grants No. F-35, F-77).
As a part of regular prospective follow-up of patients in whom Barrx RFA was performed due to dysplastic/metaplasic BE, separate analysis of biopsy specimens was obtained from patients in whom complete eradication (CE) of BE was achieved with one, or several RFA sessions. The criteria for CE of BE were routine four quadrant biopsies negative for presence of IM, and macroscopically absent columnar epithelium in distal esophagus. The basic inclusion criteria were CE of metaplasia/dysplasia and minimal period from the last RFA session of 6 months.
Two groups of patients were created. The first group consisted of patients who were treated with proton pump inhibitors (PPI) therapy (daily dose of 40 mg esomeprazole) after the CE of BE was achieved. Patients who underwent laparoscopic Nissen fundoplication (NF) after the CE of BE were classified into the second group.
Patients who had signs of erosive esophagitis, disrupted fundoplication or reherniation on endoscopy were excluded from the study.
Biopsy and electrone microscopy evaluation were scheduled at least 6 months after NF, or at least 6 months after CE of metaplasia/dysplasia was obtained in PPI treatment group.
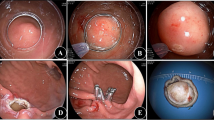
Endoscopic biopsy specimens were taken with “jumbo” forceps. In each patient two specimens were obtained from NSE (3 cm above the newly formed squamocolumnar junction) and from proximal esophagus (5 cm below the level of upper esophageal sphincter). Biopsy specimens were analyzed under transmission electron microscopy (TEM).
Comparative analysis was made between the groups with regard to mean diameter of IS in NSE, both in distal and proximal esophagus.
Follow-up consisted of regular upper GI endoscopy and four quadrant biopsies of NSE which were performed yearly up to 5 years.
Transmission electron microscopy analysis of intercellular spaces in NSE
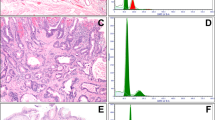
Biopsy samples were fixed with 3% glutaraldehyde in cacodylate buffer and postfixed in 1% OsO4. After dehydration in graded alcohols, cells were embedded in Epoxy medium (Sigma-Aldrich, 45345). Thin sections were mounted on copper grids (Sigma-Aldrich, G4901), and stained with uranyl acetate and lead citrate for examination on an electron microscope (Morgagni 268D, FEI, Hillsboro, OR). The sections and micrographs for the analysis were selected by using Systematic Uniform Random Sampling16. The intercellular spaces were measured as distances in between 80 cells, on 10 micrographs per sample at 2800 × magnification by the use of TEM. The same micrographs were used for fractional volume analysis as previously described using the following formula: volume fraction \(\, = \,\sum {\text{PAP}}/\sum {\text{Pcyt }} \times \, \rho\) , where ∑PAP is the number of points of a dense grid counted on intercellular spaces, ∑Pcyt the number of points of a second grid counted within the cytoplasm, and ρ is the number of points on the dense grid that represent each point of the grid used for the cytoplasm (in this case 25) Fig. 1.
Statistical analysis
The normality assumption for continuous variables was evaluated by the Kolmogorov–Smirnov test. Continuous variables are presented as means and standard deviations for normally distributed variables or as medians and interquartile ranges (IQR) for non-normally distributed ones. Differences in continuous variables were assessed by the two-sided Student’s t test for normal distributed, or Mann–Whitney U test for non-normal distributed variables. Differences in categorical variables were tested by χ2 test or Fisher test, as appropriate. Our sample size provided 80% statistical power, ensuring adequate sensitivity to detect significant differences and the expected effect size, thereby validating the study’s findings. Statistical power for main outcomes (Table 2) of this study was 78% for NSE and 88% for proximal esophagus17.
Differences in survival without recurrence due to therapy modality were compared using Kaplan–Meier survival curves and statistical significance was determined by the log rank test. Cox proportional-hazards analyses was used to identify predictors of mortality in the follow-up period.
Statistical analyses were performed using the statistical package for social sciences, version 18 (SPSS, Chicago, Ill). Statistical significance was defined as p < 0.05.
Results
Overall, 22 patients in which CE-IM/D was achieved with RFA underwent biopsy protocol for transmission electron microscopy evaluation. 12 patients were treated with PPI, while 10 of them underwent LNF.
The data of basic demographics, upper GI endoscopy and RFA procedure are shown in Table 1. There were no differences in the term of age, gender, nor in the term of the mean length of BE segment. The IM/D ratio did not differ between two groups of patients. There were no statistical differences regarding the mean number of RFA sessions between the two groups.
The mean value of IS length in NSE within the group of patients under the PPI regimen was 1.228 ± 0.226 µm. The mean value of NSE IS length in the group of patients with CE-IM who underwent antireflux surgery was 0.878 ± 0.354 µm, which was statistically significantly lower than in the PPI group (p = 0.032).
The mean value of proximal esophagus IS length in group of patients taking PPI and the group in whom NF was performed was 0.724 ± 0.325 µm and 0.378 ± 0.116 µm accordingly, demonstrating statistically significant difference (p = 0.009).
The mean values of IS length inside NSE and proximal esophagus are presented in Table 2.
Fractional volume analysis showed that percentage of IS with the regard to whole thickness of NSE in the group of patients treated with PPI’s was 27.82% (range from 20.1 to 38.9%). The percentage of IS inside the squamous epithelium of proximal esophagus was 19.93% (range from 11.58 to 24.48%). In the group of patients undergoing antireflux surgery fractional volume analysis showed that percentage of IS with the regard to whole thickness was 22.23% (range from 15.52 to 42.57%) in NSE, and 15.35% (range from 12.86 to 18.03%) in proximal esophagus. No statistical differences were found when NSE IS volume percentages were compared between the groups of patients with different post RFA treatment (p = 0.052).
Mean values of IS diameter were analyzed with regard to post RFA treatment, BE histology and length prior to RFA and number of RFA sessions. Of all analyzed parameters only post RFA treatment showed to be significant as a predictor of NSE IS length (p = 0.032) Table 3.
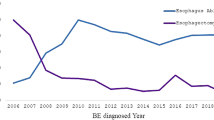
In all patients follow-up was obtained for 60 months through regular yearly endoscopic check- ups. Overall, recurrence occurred in 5 patients (27%), in 4 of them (40%) in PPI group, with median interval of recurrence detection 33 months, and in one (8.3%) in NF group, detected 36 months after CE BE. All of the recurrences were IM, located within 10 mm from neo-squamocolumnar junction.
There was no statistical difference regarding the impact of post RFA treatment modality on recurrence of BE (p = 0.084) (Fig. 2).
None of the analyzed factors had predictive value regarding the BE when evaluated through the regression analysis (Table 4).
Discussion
Barrett’s esophagus represents the end-stage of GERD, regarding both structural damage and functional deterioration of the esophagus18. Successful treatment of BE with RFA only means that the consequence has been eradicated for some time. The issue that remains is the treatment of the cause, which in case of BE is long lasting gastroesophageal reflux, usually in environment of hiatal hernia, duodenogastroesophageal reflux and esophageal dismotility2,12.
The neosquamous epithelium is a product of re-epithelization after successfully performed ablative endoscopic procedure. The basic histology of NSE resembles the one of native squamous epithelium with one to two basal cell layers, followed by multiple layers of more mature cells7. In the early post-ablation phase, hyperplasia of the basal layer and papillar elongation of lamina propria may be present, indicating the regenerative reflux induced changes. Most importantly, the in-depth evaluation of the NSE in patients that had preneoplastic and neoplastic lesions, showed normalization of the genetic profile, that hopefully carries less risk for recurrent dysplasia and carcinoma8,9.
Adopted treatment after RFA consists of PPI’s, usually for lifetime, with the aim of preventing reflux damages of healed esophageal mucosa and achieving effective symptom control. There is evidence that despite PPI’s treatment, there are patients that have persistent or recurrent BE after RFA, which is related to ongoing reflux, in form of the increased overall number of reflux episodes and prevailing weakly acidic reflux15,19,20. Further, patients with larger hiatal hernia are more likely to have failure of RFA treatment, which cannot be impacted by PPI treatment21. Basic intent of our study was to assess the impact of ongoing reflux on NSE. The patients included were treated with PPI’s and antireflux surgery after CE of BE achieved with RFA. The rationale for this methodology was under the premise that fundoplication will mechanically prevent gastroesophageal reflux, regardless of its acidity.
Our study pointed out that DIS are more frequently present in NSE of patients that are submitted to PPI therapy, than in those who underwent Nissen fundoplication. Also, mean values of IS length were statistically significantly higher in those receiving PPI’s as a selected post RFA treatment. Yet, this statistical difference was not confirmed when fractional volume analysis of intercellular spaces was performed, probably due to limited number of patients. The presence of DIS in patients with ongoing PPI treatment may be explained by ongoing weakly acidic and alkaline reflux. Calabrese et al., proved that DIS is initiated not only by acid but also with the bile reflux as well. In their study, there were no differences regarding the width of intercellular spaces in GERD patients in whom predominantly acidic or bile reflux was recorded (2.27 ± 0.47 μm in patients with pure acidic reflux and 2.11 ± 0.23 μm in those with mixed bile/acid reflux). Authors indicated that ultrastructural damage of esophageal epithelium may occur even with adequate acid suppression therapy, due to the noxious effect of bile salts22. In rabbit experimental model it has been shown that bile acids may contribute to DIS formation both in acidic and weakly acidic environment. In this study, bile acids in the acid (pH 2), and weakly acid solutions (pH 5) lead to induction of increased permeability of esophageal mucosa and decreased transepithelial electrical resistance23. Same study group investigated 14 healthy volunteers, who were treated with infusion of acidic, weakly acidic, acid-bile and neutral infusions above esophagogastric junction24. The study showed that both acid and weakly acid infusions provoked DIS without any differences in the effect. Ghatak et al., studied the possible mechanism of bile salts acting as main pathologic determinant in induction of DIS by treating cell cultures with bile salts cocktails at pH 7.4, pH 5 and control media. It has been shown that exposure to bile salts at pH 5 decreases the epithelial barrier function, causing the loss of stratification of squamocolumnar epithelium and disruption of major epithelial junctions25,26. The pH 5 media on which bile salts proved these pathogenic effects resemble the one in GERD patients treated with PPI’s.
Intercellular spaces length in proximal esophagus differ significantly between two groups of our patients. Caviglia et al.27 found DIS equally in distal and proximal esophagus, in subgroup of patients with non-erosive reflux disease. We are usually referring to the IS length in the proximal healthy esophagus to be normal. But, we also have to be aware that reflux may have high extent, which may lead to DIS in proximal esophageal segments. It is our opinion that DIS analysis must be made with the reference to proposed cut-off values, and certainly not within the presumption that length of IS in proximal esophagus is normal.
Antireflux surgery after RFA may have a beneficial role in preventing recurrent BE, especially in patients with long segments and significant hiatal hernias. Patients with long segment BE are those with highest incidence of hiatal hernia, esophageal dysmotility, lowest values of mean lower esophageal sphincter pressure, as well as highest incidence of pathologic reflux measured on pH/impedance28. Antireflux surgery was beneficial as an adjustment treatment in those who have not achieved CE of BE after several RFA sessions, providing excellent reflux control and high rate of complete BE remission29. Also, Johnson et al.30 showed that Nisssen fundoplication after RFA treatment had protective effect on NSE, with none of the patients progressing towards dysplasia, and majority of them having CE of BE in the follow-up of 48 months.
Five year follow-up in our study indicated higher recurrence rates in the group of patients treated with PPI’s, however due to sample size, this did not reach statistical significance. We find 5 years follow-up to be sufficient in objective evaluation of recurrence of RFA, as it has been shown that almost all of the BE recurrences happen in first 2 years after RFA14.
There are several limitations of our study. The first is the limited number of patients, which is directly influenced by the very demanding study methodology. This limitation was at least partially overcome with the high number of data obtained from each biopsy sample. The internal data checking for every biopsy sample was performed with presentation of three-dimensional model, which provided the valuable informations in the term of overall IS occupying surface regarding the whole thickness of epithelium. The second limitation is that patients were not stratified according to the baseline characteristics, prior to RFA treatment, or randomized regarding the post RFA treatment.
The conclusion may be as follows. Dilated intercellular spaces are present in NSE and there is statistical evidence that DIS are present to less extent in those who underwent antireflux surgery than in patients receiving PPI’s regimen after RFA. Also, we lack the esophageal pH-impedance metry data, which could add more to objectivity of IS length value.
When these data are combined with those obtained from endoscopic follow-up and histology in terms of BE recurrence, we might get better insight into RFA perspective in the term of future treatment protocols. Antireflux surgery may be proposed after RFA and CR of BE, with the intent to prevent reflux damages of NSE, and potential recurrence of BE.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
Sharma, P. Barrett esophagus: a review. JAMA 328(7), 663–671 (2022).
Spechler, S. J. & Souza, R. F. Barrett’s esophagus. N. Engl. J. Med. 371(9), 836–845 (2014).
Shaheen, N. J. et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 141(2), 460–468 (2011).
Haidry, R. & Lovat, L. Long-term durability of radiofrequency ablation for Barrett’s-related neoplasia. Curr. Opin. Gastroenterol. 31(4), 316–320 (2015).
Visrodia, K., Zakko, L. & Wang, K. K. Radiofrequency ablation of Barrett’s esophagus: efficacy, complications, and durability. Gastrointest. Endosc. Clin. N. Am. 27(3), 491–550 (2017).
Berenson, M. M. et al. (1993) Restoration of squamous mucosa after ablation of Barrett’s esophageal epithelium. Gastroenterology 104, 1686–1691 (1993).
Odze, R. D. & Lauwers, G. Y. Histopathology of Barrett’s esophagus after ablation and endoscopic mucosal resection therapy. Endoscopy 40(12), 1008–1015 (2008).
Pouw, R. E. et al. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am. J. Gastroenterol. 104(6), 1366–1373 (2009).
Levert-Mignon, A. et al. Changes in gene expression of neo-squamous mucosa after endoscopic treatment for dysplastic Barrett’s esophagus and intramucosal adenocarcinoma. United Eur. Gastroenterol. J. 5(1), 13–20 (2017).
Jovov, B., Shaheen, N. J., Orlando, G. S., Djukic, Z. & Orlando, R. C. Defective barrier function in neosquamous epithelium. Am. J. Gastroenterol. 108(3), 386–391 (2013).
Orlando, R. C. How good is the neosquamous epithelium?. Dig. Dis. 32, 164–170 (2012).
Mittal, S. K., Baboli, K. M. & Bremner, R. Reflux control after Barrett’s esophagus ablation. Foregut 1(1), 78–85 (2021).
Sami, S. S. et al. Timeline and location of recurrence following successful ablation in Barrett’s oesophagus: an international multicentre study. Gut 68(8), 1379–1385 (2019).
Cotton, C. C. et al. Late recurrence of barrett’s esophagus after complete eradication of intestinal metaplasia is rare: final report from ablation in intestinal metaplasia containing dysplasia trial. Gastroenterology 153(3), 681-688.e2 (2017).
Krishnan, K. et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 143(3), 576–581 (2012).
Lucocq, J. M. & Hacker, C. Cutting a fine figure: on the use of thin sections in electron microscopy to quantify autophagy. Autophagy 9, 1443–1448 (2013).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39(2), 175–191 (2007).
Savarino, E. et al. Characteristics of gastro-esophageal reflux episodes in Barrett’s esophagus, erosive esophagitis and healthy volunteers. Neurogastroenterol. Mot. 22(10), 1061-e280 (2010).
van Vilsteren, F. G. et al. Predictive factors for initial treatment response after circumferential radiofrequency ablation for Barrett’s esophagus with early neoplasia: a prospective multicenter study. Endoscopy 45(7), 516–25 (2013).
Korst, R. J., Santana-Joseph, S., Rutledge, J. R. et al. Patterns of recurrent and persistent intestinal metaplasia after successful radiofrequency ablation of Barrett’s esophagus. J. Thorac. Cardiovasc. Surg. 145, 1529–1534 (2013).
Korst, R. J. et al. Effect of hiatal hernia size and columnar segment length on the success of radiofrequency ablation for Barrett’s esophagus: a single-center, phase II clinical trial. J. Thorac. Cardiovasc. Surg. 142(5), 1168–73 (2011).
Calabrese, C. et al. Intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol. Ther. 18(5), 525–32 (2003).
Farré, R. et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut 57(10), 1366–74 (2008).
Farré, R. et al. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut 59(2), 164–9 (2010).
Ghatak, S. et al. Bile salts at low pH cause dilation of intercellular spaces in in vitro stratified primary esophageal cells, possibly by modulating Wnt signaling. J. Gastrointest. Surg. 20(3), 500–9 (2016).
Ghatak, S. et al. Bile acid at low ph reduces squamous differentiation and activates EGFR signaling in esophageal squamous cells in 3-D culture. J. Gastrointest. Surg. 17(10), 1723–1731 (2013).
Caviglia, R. et al. Dilated intercellular spaces and acid reflux at the distal and proximal oesophagus in patients with non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol. Ther. 25(5), 629–36 (2007).
Lord, R. V. et al. Hiatal hernia, lower esophageal sphincter incompetence, and effectiveness of Nissen fundoplication in the spectrum of gastroesophageal reflux disease. J. Gastrointest. Surg. 13(4), 602–10 (2009).
Komanduri, S. et al. Recurrence of Barrett’s esophagus is rare following endoscopic eradication therapy coupled with effective reflux control. Am. J. Gastroenterol. 112(4), 556–566 (2017).
Johnson, C. S. et al. The durability of endoscopic therapy for treatment of barrett’s metaplasia, dysplasia, and mucosal cancer after Nissen fundoplication. J. Gastrointest. Surg. 19(5), 799–805 (2015).
Funding
The study was supported by Serbian Academy of Sciences and Arts (SASA, grants No. F-35, F-77).
Author information
Authors and Affiliations
Contributions
OS, PP, VB contributed in manuscript design. OS, AS, TKS, TM collected the data, performed the data analysis. OS and AS wrote the manuscript. OS, TKS and TM prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Skrobic, O., Simic, A., Pesko, P. et al. Impact of post RFA treatment on neosquamous epithelium microstructure. Sci Rep 14, 28895 (2024). https://doi.org/10.1038/s41598-024-80081-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80081-2