Abstract
Cholinergic treatments with an emphasis on M1 muscarinic acetylcholine receptor (mAChR) agonists as potential modulating agents are a new approach in Alzheimer’s disease (AD) therapy. In previous research, we designed and characterized novel thiazolidine-2,4-dione (TZD)-derived compounds that possess anti-AD properties and enhance the expression of mAChRM1 in rats. This study evaluated a novel orthosteric agonist of mAChRM1 from related pathways that has shown promising anti-Alzheimer’s disease activity. PC12 cells were exposed to various concentrations of TZ4M before they were exposed to scopolamine (3 µM). Immunocytochemistry and western blot analyses revealed that TZ4M increased the expression of mAChRM1 in differentiated cells induced by scopolamine-treated PC12 cells. The results showed that TZ4M (3 and 5 µM) markedly upregulated PKC and ChAT protein expression, and the cells were significantly protected against increased ROS levels followed by neuronal cell loss, as evidenced by the MTT assay. TUNEL staining indicated that TZ4M impeded the shaping of apoptotic bodies. Analysis of the amino acid sequences of the ligand–protein binding site indicated that TZ4M is bound to the orthosteric site (acetylcholine site). This study revealed that TZ4M, a derivative of TZD, effectively protects against scopolamine-induced damage. TZ4M, a novel mACRM1 orthosteric agonist, is promising for treating AD.
Similar content being viewed by others
Introduction
Neurodegenerative disorders are frequently correlated with cholinergic circuit dysfunction1. The cholinergic pathway plays a critical role in learning and memory functions to regulate memory processes, which are impaired in neurodegenerative disorders2. Defects in the cholinergic signaling pathway, characterized by decreased ChAT activity (EC 2.3. 1.6), reduced acetylcholine (ACh) levels, and apoptosis of cholinergic neurons, cause Alzheimer’s disease (AD) and its symptoms3. Muscarinic receptor activation can alter the processing of amyloid precursor proteins toward the nonamyloidogenic pathway, affecting major features of AD, such as cholinergic deficit, cognitive dysfunction, tau, and Aβ diseases4. The loss of cholinergic neurons and M1 muscarinic acetylcholine receptor (mAChR) in the cerebral cortex and hippocampus plays a significant role in AD5. mAChRs are prototypical allosterically-modulated G-protein-coupled receptors6. The M1 subtype is strongly expressed in the cerebral cortex and hippocampus in the cholinergic-muscarinic system7.
In AD therapy, targeting mostly involves cholinesterase inhibitors, muscarinic agonists, and antagonists8,9. The deterrence of mAChR neurotransmission by the prescription of nonselective mAChR antagonists, such as scopolamine, results in consequential cognitive deterioration in humans and animals. Scopolamine regenerates a model of AD and leads to cognitive dysfunction, synaptic dysfunction (blocked information transmission such as acetylcholine), oxidative stress injury, central cholinergic dysfunction, and other pathological changes5,10. mAChRs, especially mAChRM1, are potential therapeutic targets for treating neurological diseases and psychiatric disorders due to their extensive physiological functions in the central and peripheral nervous systems11. Several orthosteric mAChRM1 agonists that bind to acetylcholine binding sites, such as GSK103470212, xanomeline13, and HTL993614, have shown good efficacy and have progressed to early clinical development15.
In a previous study, thiazolidine-2,4-dione (TZD)-based derivatives were designed for CNS targeting and synthesized after investigating their permeability, absorption, distribution, metabolism, excretion (ADME), and toxicity16. These findings demonstrated that TZD derivatives exhibit neuroprotective effects in a scopolamine-induced AD model in adult Wistar rats. An investigation of (E)-5-(3-hydroxybenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (TZ3O) and (E)-5-(4-methoxybenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (TZ4M) derivatives revealed a decrease in acetylcholinesterase (AChE) activity and an increase in mAChRM1 incidence density and protein expression in the rat hippocampus (DG, CA1) and cortex16. Additionally, these animals had increased BDNF and NRF2 levels and reduced p-Tau protein expression in the hippocampus. Furthermore, the findings demonstrated decreased cell death and improved memory function while demonstrating no considerable hepatotoxic or cell-damaging effects16.
In the search for drugs from bioactive molecules among extensive heterocyclic scaffolds, TZD rings have been recognized as a powerful platform in medicinal chemistry and several clinically used TZDs have been shown to have anti-HDAC, anti-inflammatory, anticonvulsant, antidepressant, muscarinic agonist, and antidiabetic effects17,18,19. According to PubMed, 9512 articles have reported on the effectiveness of TZD-based agents through 2023, with 1459 clinical trial articles (https://pubmed.ncbi.nlm.nih.gov/). Because of their expansive pharmacological specificity, TZDs are currently being investigated as better, safer, and potential pharmacological agents18. TZDs have been studied extensively for their potential to treat AD20. New thiazolidinone derivatives show strong binding affinity for mAChRM1, reverse learning impairment in male Wistar rats with AD, and have no apparent cholinergic toxicity21,22.
This study aimed to evaluate the potential of TZ4M treatment as an mAChRM1 agonist in scopolamine-induced differentiated PC12 cells. Scopolamine, an mAChR antagonist, was used to disrupt cholinergic activity to study the potential of TZ4M to improve mAChRM1 incidence density and protein expression via cholinergic pathway factors in in vitro models of AD. We report that TZ4M effectively activates the M1 receptor an orthosteric agonist of ChAT and PKC-related pathways. This knowledge can help with the design of agonists and aid in identifying new therapeutic targets.
Results
Cytotoxic effect of scopolamine and TZ4M on PC12 cells
In neuroscience-related research, scopolamine is applied to an induced model of cognitive impairment or a neurotoxicology model23. To evaluate the neurotoxicity caused by scopolamine and TZ4M, PC12 cells were exposed to different concentrations of scopolamine (0.5, 1, 3, 5, and 10 µM) and TZ4M (0.1, 1, 3, 5, 10, 20, 50, 100, 500 and 1000 µM) for 48 h. Scopolamine-induced toxicity was observed to occur in a dose-dependent manner by decreasing cell viability. The cell viability rate was determined to be 50% after treatment with 3 µM scopolamine, and this concentration was chosen for further tests (F(1.254, 2.509) = 66.68, P = 0.007, ANOVA) (Fig. 1A). TZ4M exhibited no significant cytotoxicity up to 50 µM (81%); however, at higher concentrations (1000 µM), the cell viability decreased to 64% (F(10, 20) = 42.52, P < 0.0001, ANOVA) (Fig. 1B).
Cytotoxic effects of scopolamine and TZ4M on PC12 cells. (A) Dose-dependent effects of scopolamine and (B) cytotoxic effects of TZ4M on the viability of PC12 cells are shown. The data are expressed as the mean ± standard deviation (SEM) (n = 3). The significance of P values < 0.05 was determined, #p < 0.05, ##p < 0.01, ###p < 0.001 vs the control.
Protective effect of TZ4M on scopolamine-induced cytotoxicity in PC12 cells
Numerous studies have documented the pharmacological effects of TZD, which has neuroprotective effects and prevents neurotoxicity16,24. To investigate the protective effects of TZ4M against scopolamine-induced cytotoxicity, the cells were pretreated with varying concentrations of TZ4M (0.1, 1, 3, 5, 10, 20, 50, and 100 µM) for 1 h. The cells were then exposed to scopolamine (3 µM) for 48 h. Figure 2A (1–4) depicts the growth and progression of PC12 cells in response to various conditions after 24 h. In the scopolamine (3 µM) treatment group (Fig. 2A-2), the presence and density of PC12 cells were decreased compared with those in the nontreated group (control) (Fig. 2A-1). However, compared with the scopolamine group, the groups pretreated with 3 µM (Fig. 2A-3) and 5 µM (Fig. 2A-4) TZ4M and exposed to scopolamine, exhibited a noticeable increase in the density of PC12 cells.
Protective effects of TZ4M on scopolamine-induced cytotoxicity in scopolamine-induced PC12 cells and the growth of PC12 cells in response to various treatments. (A) The progression of PC12 cells is shown under multiple conditions. PC12 cell growth in (1) the control group, after exposure to (2) 3 µM scopolamine, (3) 3 µM scopolamine + 3 µM TZ4M, and (4) 3 µM scopolamine + 5 µM TZ4M after 24 h. (B) The dose-dependent protective effect of TZ4M on scopolamine-induced cytotoxicity on the viability of PC12 cells for 48 h has been shown. The data are expressed as the mean ± standard deviation (SEM) (n = 3). The significance of P values < 0.05 was determined, #p < 0.05, ##p < 0.01, ###p < 0.001 vs the control, *p < 0.05, **p < 0.01, ***p < 0.001 vs the scopolamine group.
The results showed that scopolamine-induced cell death (10.59%) significantly decreased with increasing concentrations of TZ4M (F(10, 22) = 57.11, P < 0.0001, ANOVA) (Fig. 2B). At a concentration of 100 µM, the viability of the cells reached 85.89% of that of the control. This study demonstrated that TZ4M had dose-dependent protective effects against scopolamine-induced cytotoxicity in PC12 cells. TZ4M at concentrations of 3 µM and 5 µM with ~ 60% viability was selected for further experiments. Using 10 µM rivastigmine resulted in 64.58% cell viability, while TZ4M, at the same concentration, exhibited 61.25% viability.
Effect of TZ4M on ROS levels in differentiated scopolamine-treated PC12 cells
The ROS level plays a crucial role in the ability of cells to withstand cell death. Disorders of oxidative stress regulation are correlated with AD25. Oxidative stress caused by intracellular ROS levels was detected by measuring the fluorescence intensity of DCF (F(4, 10) = 107.5, P < 0.0001, ANOVA) (Fig. 3). After exposure to 3 µM scopolamine for 48 h, ROS production increased 2.46-fold. PC12 cells were pretreated with 3 and 5 µM TZ4M for 1 h. The ROS levels (1.38- and 1.19-fold, respectively) were significantly reduced in a concentration-dependent manner compared with those in the scopolamine group. Additionally, rivastigmine (as a positive control) reduced ROS levels by 1.94-fold. The ROS levels are shown as the fold change compared with those in the control group. A comparison between the levels of ROS in scopolamine-induced cells in the TZ4M (3 and 5 µM) and rivastigmine (10 µM) treated groups revealed that TZ4M remarkably decreased the ROS levels in scopolamine-treated differentiated PC12 cells.
Effect of TZ4M on oxidative stress in scopolamine-induced PC12 cells. The figure shows fluorescence intensity values of DCF for measuring ROS levels. Compared with those in the scopolamine group, ROS levels were reduced in a concentration-dependent manner. The fold change in ROS levels compared with those in the control group is shown. The data are expressed as the mean ± standard deviation (SEM) (n = 3). The significance of P values < 0.05 was determined, #p < 0.05, ##p < 0.01, ###p < 0.001 vs the control, *p < 0.05, **p < 0.01, ***p < 0.001 vs the scopolamine group.
Effect of TZ4M on the incidence density of mAChRM1 in scopolamine-treated cells
The M1 subtype of the mAChR is abundantly expressed in the CNS, constituting up to 50–60% of the total mAChR expression26. mAChRM1 activation improved memory deficits in models of neurodegeneration and patients with CNS disorders15,27,28. Figure 4A displays mAChRM1 immunohistochemical staining of treated cells, and Fig. 4B presents quantitative data (F(4, 15) = 119.5, P < 0.0001, ANOVA) for groups. Treatment with scopolamine, an antagonist of mAChRs, resulted in a weaker presence (0.16-fold) of mAChRM1 in PC12 cells than in nontreated cells (control). An increase (0.54-fold) in the density of mAChRM1 after treatment with 3 µM TZ4M was detected. However, compared with scopolamine treatment, treatment with 5 µM TZ4M significantly increased (1.02-fold) the number of immunostained cells. Additionally, a comparison between the staining of mAChRM1 in PC12 cells in the TZ4M-treated group and the staining of cells in the rivastigmine (10 µM)-treated group indicated that TZ4M increased (0.83-fold) the number of mAChRM1-expressing cells among scopolamine-treated differentiated PC12 cells.
The effect of TZ4M on the effect of mAChRM1 against scopolamine-induced neurotoxicity in PC12 cells. To investigate the protective effect of TZ4M on mAChRM1 against scopolamine-induced neurotoxicity in PC12 cells, Immunocytochemical staining was used. (A) Representative images of immunocytochemistry from the control, scopolamine (3 µM), scopolamine + 3 µM TZ4M, scopolamine + 5 µM TZ4M, and rivastigmine groups at a magnification of × 200. The scale bar is 50 µm. (B) The graph depicts the fold change in the density of muscarinic acetylcholine receptor M1 in the control and treated groups. The data are expressed as the mean ± standard deviation (SEM) (n = 4). The significance of P values < 0.05 was determined, #p < 0.05, ##p < 0.01, ###p < 0.001 vs the control, *p < 0.05, **p < 0.01, ***p < 0.001 vs the scopolamine group.
Protective effect of TZ4M against cell death in scopolamine-treated cells
Scopolamine can cause apoptosis via oxidative stress induced in the brain, leading to the death of hippocampal neurons16. The TUNEL assay was used to evaluate cell apoptosis during death in differentiated scopolamine-treated PC12 cells (Fig. 5A). The number of apoptotic bodies in the control and scopolamine-treated cells in each group is displayed in Fig. 5B (F(4, 15) = 47.18, P < 0.0001, ANOVA). Apoptotic bodies were more abundant (3.53-fold) in the scopolamine-treated cells than in the nontreated (control) cells. These results confirmed that scopolamine treatment or M1 receptor deficiency caused an increase in apoptosis. Pretreatment with TZ4M caused a decrease in the number of TUNEL-positive PC12 cells in the 3- and 5 µM-treated groups compared with that in the scopolamine-treated group. There were noticeably fewer (1.16-fold) apoptotic bodies in the 5 µM TZ4M-treated cells than in the scopolamine-treated cells. This decrease (1.3-fold) was also observed in the 3 µM treatment group. Additionally, the number of apoptotic bodies was markedly lower in the TZ4M (3 and 5 µM)-treated group than in the rivastigmine (10 µM)-treated group. Compared with those in the scopolamine group (1.75-fold), the scopolamine-treated cells in the TZ4M pretreatment groups were protected from apoptosis at different doses.
The protective effect of TZ4M against apoptosis induced by scopolamine neurotoxicity in PC12 cells. To investigate the protective effect of TZ4M on apoptosis induced by scopolamine neurotoxicity in PC12 cells, TUNEL staining was used. (A) Representative TUNEL images of the control, scopolamine (3 µM), scopolamine + 3 µM TZ4M, scopolamine + 5 µM TZ4M, and rivastigmine groups at a magnification of × 200. The scale bar is 50 µm. (B) The graph depicts the fold change in TUNEL positive cells in the control and treated groups. The data are expressed as the mean ± standard deviation (SEM) (n = 4). The significance of P values < 0.05 was determined, #p < 0.05, ##p < 0.01, ###p < 0.001 vs the control, *p < 0.05, **p < 0.01, ***p < 0.001 vs the scopolamine group.
Effect of TZ4M on mAChRM1, ChAT, and PKC protein expression in scopolamine-treated cells
Figure 6A showes images of Western blot gels for mAChRM1 (original image in Supplementary material Fig. S4), ChAT (original image in Supplementary material Fig. S5), PKC (original image in Supplementary material Fig. S6), and β-actin (original image in Supplementary material Fig. S7) Studies have demonstrated that PKC leads to mAChRM1 activation through PKA/PI3K/Akt signaling29,30. The results indicated that scopolamine treatment decreased mAChRM1 (0.53-fold) (F(4, 10) = 199.1, P < 0.0001, ANOVA) and PKC (0.87-fold) (F(4, 10) = 79.43, P < 0.0001, ANOVA) protein expression in scopolamine-induced PC12 cells compared with that in the control group. The results showed that 3 µM TZ4M increased the protein levels of mAChRM1 (0.62-fold) and PKC (1.12-fold). However, 5 µM TZ4M significantly increased the mAChRM1 (0.98-fold) and PKC (1.01-fold) levels compared with those in the scopolamine group. Additionally, compared with those in the scopolamine group, the levels of the mAChRM1 and PKC proteins in the rivastigmine treatment group (10 µM) increased by 1.05-fold and 1.01-fold, respectively (Fig. 6 B,C).
A Representative Western blot gel and quantitative analysis of protein expression in cells pretreated with TZ4M. (A) Western blot images of mAChRM1 (52 kDa), ChAT (69 kDa), PKC (80 kDa), and β-actin (43 kDa) and an analysis diagram of (B) mAChRM1, (C) ChAT, and (D) PKC in PC12 cells pretreated with TZ4M (3 and 5 µM) and exposed to scopolamine (3 µM). Rivastigmine (10 µM) was utilized as a positive control. The data are expressed as the mean ± standard deviation (SEM) (n = 3). The significance of P values < 0.05 was determined, #p < 0.05, ##p < 0.01, ###p < 0.001 vs the control, *p < 0.05, **p < 0.01, ***p < 0.001 vs the scopolamine group. The original Western blot images are displayed in Figs. S4-S7 in Supplementary material file.
ChAT-catalyzed acetylcholine (ACh) is released into synapses and binds to the G protein-coupled receptor mAChRM1 to transfer signals from one neuron to another31. The data showed that scopolamine treatment decreased (0.59-fold) (F(4, 10) = 189.7, P < 0.0001, ANOVA) ChAT compared with that in the control group. However, treatment with TZ4M at 3 µM and 5 µM significantly increased the level of ChAT protein expression (0.67-fold and 1.04-fold, respectively). Compared with scopolamine, rivastigmine increased the PKC level 1.00-fold (Fig. 6D).
Molecular docking study of TZ4M as an mAChRM1 agonist
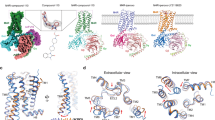
To investigate the binding affinity of TZ4M for mAChRM1, GSK1034702 (an M1 agonist) was used as the positive control. The position of GSK1034702 (a crystal ligand), which is considered an M1 agonist, was primarily maintained during molecular docking studies because of its interaction with the negatively charged aspartate (Asp) 10515,32. This investigation revealed that the charge‒charge interaction between the protonated nitrogen and the negatively charged aspartate (Asp105) in the amine pocket is the cause for the retention of the position of TZ4M. TZ4M exhibited a similar rearrangement of tyrosine to that of GSK1034702, with the nitrogen of the piperidine ring driving contact with Tyr404. However, a few minor dissimilarities were detected in the binding state of TZ4M compared with that of GSK1034702, indicating that the selectivity of TZ4M against M1 receptor agonism was not strongly affected (GSK1034702) because of the identical amino acid sequences in the orthosteric site (acetylcholine site). The important residues involved in the interaction of TZ4M with 6ZG9 are Leu81, Tyr82, Tyr85, Trp91, Asp105, Cys178m, Tyr404, and Tyr408, which are also common in the interaction of GSK1034702 with 6ZG9. The other residues that play important roles in the interaction between TZ4M and 6ZG9 are Ser78 and Val84. The affinities of TZ4M and GSK1034702 for mAChRM1 were calculated to be − 8.8 and − 11.2 kcal/mole, respectively. To obtain additional details about the binding interactions of the ligands with receptor M1, which were evaluated by UCSF Chimera and LigPlot, Fig. 7 depicts views of the M1 extracellular region proximal to the ACh binding site, and 3D and 2D images of the interactions of these interactions.
Molecular docking of TZ4M to the extracellular region of mAChRM1 proximal to the ACh binding site. 2D and 3D representations of the complexes of M1-StaR-T4L with GSK1034702 and TZ4M. The figure shows the binding locations of (A-1) GSK1034702 and (B-1) TZ4M on the extracellular side of the M1 receptor. 2D (left) and 3D (right) interactions of (A-1) GSK1034702 and (B-2) TZ4M with the orthosteric binding site residues of the M1 receptor. In the 2D and 3D interaction images, GSK1034702 and T4M are colored pink and green, respectively (ball and stick), and the residues are colored brown. In the 2D interaction picture, the ligand bonds are colored gray. The crescents with bristles represent hydrophobic interactions. The hydrophobic atoms are colored black. The polar atoms are in red (oxygen) or blue (nitrogen). The dashed lines represent hydrogen bonds (blue).
Discussion
Five-membered heterocyclic rings, thiazole moieties, have been extensively studied in AD research20,33,34. Thiazolidine- and thiazolidinedione-based compounds have been investigated extensively as potential M1 receptor agonists and inhibitors of choline esterase and other factors affecting the pathogenesis of AD21,35. Xanomeline, with a thiazole base, is an orthosteric agonist of mAChR, often introduced as the M1/M4 preferring receptor in the cerebral cortex and hippocampus, and effectively reduces behavioral disturbances in patients with AD36 The administration of xanomeline has been shown to reduce TNF levels in the blood and alleviate behavioral disorders37
Recently, novel derivatives of TZD have been designed and synthesized to penetrate the blood–brain barrier via cholinergic targeting. Previous results demonstrated that these derivatives decreased serum proinflammatory cytokines and ameliorated memory deficits in scopolamine-treated rats16. To this end, in the present study, TZ4M was selected among the synthesized TZD derivatives to target muscarinic receptors. Many ligands targeting mAChR, specifically M1, have been extensively studied in clinical trials and have shown signs of efficacy. Numerous orthosteric agonists use the conserved acetylcholine binding site in the mAChRM1 structure site of the ligand. In these models, residues Y82, L102, D105, and Y408 form the lining of the orthosteric pocket15,26,38,39. The interaction profile of TZ4M was evaluated through molecular docking studies, which confirmed the interaction of the TZ4M-mAChRM1 complex. The molecular docking study conducted on mAChRM1 has demonstrated that TZ4M exhibited appropriate binding affinity at the ACh site, which can be recognized as a novel orthosteric agonist for this receptor and interacting with the key residues. This interaction is significant as it engages the critical residues, which play a pivotal role in the receptor’s function. The analysis revealed a bond between TZ4M and Asp105, along with interactions with Tyr82 and Tyr408, enhancing the binding affinity observed in the docking study. In order to accurately identify and characterize this specific binding pocket, the predicted binding mode of a known mAChRM1 orthosteric agonist, GSK1034702, was analyzed during the docking study.
Several studies have indicated that cholinergic receptor agonists can enhance the cholinergic pathway in neuron-like cells, while their antagonists can inhibit it40,41. Scopolamine, an antagonist of the cholinergic-muscarinic system, causes disturbances in this pathway similar to those observed in older people with central nervous system disorders11. To date, many studies of cholinergic treatments involving M1 muscarinic agonists as disease-modifying agents for AD have been conducted. Activation of mAChRM1 improves memory in neurodegenerative models and CNS disorder patients36,42. The ChAT enzyme is being investigated as a potential biomarker for identifying the early stages of neurodegenerative disorders. ACh synthesis and ChAT activity are correlated with M1 activation3. In addition PKC signaling pathways regulate crucial molecular events in the neurodegenerative pathophysiology of AD. M1 receptor activation decreases p-Tau levels by activating PKC and inhibiting GSK-3β43. This study investigated the cholinergic pathway in cells exposed to scopolamine, focusing on the expression of mAChRM1, PKC, and ChAT, which are important for ACh release in neurons Immunocytochemical staining revealed that scopolamine treatment reduced the level of mAChRM1 in scopolamine-treated differentiated PC12 cells. However, TZ4M pretreatment increased the presence of the receptors in the scopolamine-treated cells. M1 presence was identified using a monoclonal antibody in immunocytochemistry experiments, where TZ4M-pretreated cells were stained positive for M1. In addition to the receptor staining results and docking studies, the Western blot results showed that pretreatment with 5 µM TZ4M significantly increased mAChRM1 protein expression (0.98-fold) compared with that in the scopolamine group (0.53-fold). Several studies have synthesized and investigated potent thiazolidinone-based mAChRM1 agonists and confirmed their effectiveness21,22,35. Jones et al. (2008) identified BPB (1-(1′-2-methylbenzyl)-1,4′-bipiperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one), which contains an imidazole ring, as a selective allosteric agonist at the M1 receptor, that can treat symptoms linked to schizophrenia and AD44.
Previous studies have demonstrated that TZ4M markedly decreases AChE activity and protein expression while enhancing the levels of mAChRM1 and its protein expression in the hippocampus and cortex of rats treated with scopolamine. This ultimately improved the cholinergic pathway and alleviated memory impairments in a rat model of dementia16. Cognitive deficits may be caused by basal forebrain cholinergic neuron malfunction, AChE activation, and reduced levels of ChAT and ACh5. Many documents have proven that M1 agonists (cholinergic therapies) improve cognitive deficits by activating muscarinic pathways26,42,45,46.
mAChRM1-mediated activation of one of the transduction pathways, the PKC pathway, leads to the activation of α-secretase and an increase in α-APP secretion, which can reduce tau and Aβ accumulation and improve behavioral symptoms42. Western blot analysis demonstrated a significant increase in PKC expression, indicating activation of the M1 receptor pathway upon pretreatment with TZ4M. Moreover, western blot results showed that cells treated with TZ4M exhibited increased levels of ChAT protein expression. Compared with the positive control rivastigmine, which was used to determine scopolamine damage, TZ4M had a more significant effect. ChAT induces the expression of neuronal-related proteins and muscarinic receptors, and the production of ACh, and subsequently regulates cell proliferation and apoptosis40,47.
In neurodegenerative disorders, high concentrations of ROS may cause chronic oxidative stress, leading to neuronal degeneration and synaptic failure48. ROS play a vital role in cell death and control cell death in neuronal cells. ROS can react with major cellular biomolecules through oxidation and cause cells to undergo necrosis or apoptosis. ROS regulation can be fundamental to AD25. The levels of ROS in cells treated with 3 and 5 µM TZ4M were significantly lower than those in cells treated with 10 µM rivastigmine. Decreased ROS levels may be another indication of the neuroprotective effects of TZ4M against scopolamine-induced toxicity. TUNEL staining demonstrated that pretreatment with TZ4M at both 3 µM and 5 µM protected cells from scopolamine-induced apoptosis and death, and this effect was greater than the protection provided by 10 µM rivastigmine. Overall, TZ4M treatment markedly protected scopolamine-induced cells, as revealed by the MTT assay. These results confirm previous findings that TZ4M treatment significantly reduced scopolamine-induced apoptosis in the cortex and hippocampus of rats16. According to these findings, the activation of neurotransmitter receptors can significantly support cellular survival mechanisms through increased PKC and ChAT expression and reduced ROS levels.
Clinical studies indicate that AChE inhibitors and xanomeline as an M1/M4-preferring agonist can enhance cognition and provide antipsychotic effects in patients28,49,50. Our previous ex vivo study indicated that TZ4M influences anti-human AChE (anti-huAChE). Also, in vivo results demonstrated its anti-AChE activity and improved memory impairment in an Alzheimer’s model using adult male Wistar rats16. Furthermore, the current in vitro investigation found that TZ4M acts as an mAChRM1 agonist. These findings suggest that TZ4M acts as a novel dual ligand targeting system. These studies developing a new approach suggest the potentially functional role of TZ4M compared with other mAChRM1 agonists. On other hand, this study suggested that the loss of synaptic input can increase the vulnerability of cells to programmed cell death caused by insults. Overall, mAChRM1 and cholinergic impairment, in addition to being essential pathological biomarkers for AD, can also serve as promising therapeutic targets. The results indicated that the use of TZ4M as an M1 muscarinic agonist can be researched as a therapy for AD and other neurodegenerative diseases.
Methods
Materials
The chemicals and necessary kits were acquired from the related sites of their respective representatives. mAChRM1 (G-9): (sc-365966), β-Actin (C4): (sc-47778), PKC (A-3): (sc-17769), m-IgGκBP-HRP: (sc-516102), and mouse anti-rabbit IgG-HRP: (sc-2357) were obtained from Santa Cruz Biotechnology, Inc.; CHAT polyclonal antibody (catalog no. E-AB-30920) and TUNEL assay kit (enhanced FITC) (catalog no: E-CK-A334) were obtained from Elabscience Biotechnology, Co.; goat anti-mouse IgG H&L (FITC) ab6785 was obtained Abcam, and SuperSignal Molecular Weight Protein Ladder (20–150 K) was obtained from Thermo Fisher Scientific, Inc. The TZD derivative TZ4M was obtained from the Biochemistry Lab at the University of Guilan. The synthesis technique and structural features (Supplementary material Table S1), including elemental analysis, FT-IR (Supplementary material Fig. S1), 1H NMR (Supplementary material Fig. S2), and 13C NMR (Supplementary material Fig. S3), were established in an earlier study16. TZ4M was dissolved in a solution containing 10% DMSO and 0.12% carboxymethylcellulose (CMC) and then suspended in PBS.
Cell culture procedure for differentiation
Undifferentiated PC12 cells (code number: C153), derived from rat pheochromocytoma cells, were procured from the Pasteur Institute (Iran) and cultured at a density of 6 × 105 cells/mL in RPMI 1640 (Bio Idea Co., Iran) medium supplemented with 10% heat-inactivated FBS (hiFBS) (GIBCO/BRL), 11 × 105 U/L penicillin, and 100 mg/L streptomycin antibiotic (GIBCO/BRL) for 24 h at 37 °C, 5% CO2, and 95% humidity. All methods were carried out in accordance with relevant guidelines and regulations.
After 72 h, undifferentiated PC12 cells were differentiated with 50 ng/mL nerve growth factor (NGF; Sigma‒Aldrich Co. St Louis, USA). The cell culture medium was refreshed after 3 days of incubation with NGF until the cells reached 80–90% confluence. After noticing changes in the PC12 cells after differentiation, the effects of scopolamine (Sigma‒Aldrich Co., St Louis, USA) on cell viability were investigated to determine the dose required for the AD model. To determine the dose of scopolamine, PC12 cells were treated with different concentrations of TZ4M for 1 h and then incubated with one dose of scopolamine for 48 h at 37 °C.
MTT assay
To evaluate the viability of PC12 cells treated with scopolamine and TZ4M, an MTT (Thermo Fisher Scientific) assay was utilized, as previously described51. Different concentrations of scopolamine (0.01, 0.5, 1, 3, 5, and 10 µM) were applied to PC12 cells for 24 h to determine the optimal dose of scopolamine for the next tests. Additionally, the cytotoxicity of TZ4C at various concentrations (0.1, 1, 3, 5, 10, 20, 50, 100, 500, and 1000 µM) on PC12 cells was evaluated using an MTT assay.
The protective effect of TZ4M (0.1, 1, 3, 5, 10, 20, 50, and 100 µM) against scopolamine toxicity was determined by examining the viability of the pretreated cells compared with that of the untreated cells (control). After 1 h, the cells were exposed to scopolamine (3 µM), and an MTT assay was carried out 48 h later. Additionally, 10 µM rivastigmine was used as a control drug. MTT was added to the wells (at a final concentration of 1 mg/mL) and the samples were incubated at 37 °C for 4 h. After adding 200 µL of DMSO, the plates were shaken. The final aim of the investigation was to estimate the optical density at 570 nm with the assistance of a microplate reader (MPR + Hiperion, Germany). The percentage of viable cells was determined using following Eq. (1):
The viability of the pretreated cells was compared with that of the control cells, and the results are expressed as a percentage of the control ± SEM. The experiments were performed in triplicate, with three wells per experiment. Next, the cells treated with two doses of the IC50 were prepared for further tests.
ROS assay
The dichlorofluorescein (DCF) assay (Abcam, ab113851) was used to estimate the level of ROS. A total of 3–4 × 104 cells were seeded and incubated overnight on 8-well plates. The cells were treated with 3 and 5 µM TZ4M for 1 h, followed by 3 µM scopolamine for 24 h. The cells were incubated in the dark at 37 °C with DCFDA for 45 min, followed by the addition of DCFDA at a final concentration of 25 µM. After each treatment, the cells were washed with PBS. The ROS levels were analyzed using a spectrophotometer with a 450 nm excitation filter and a 535 nm emission filter. The results are presented as the fold change in fluorescence intensity compared with that of the control.
Immunocytochemistry staining
For immunocytochemistry staining, PC12 cells were grown on poly-L-lysine cover slipped slides and washed with buffer containing 0.1% PBS and Tween 20. After treatment, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature (25 °C) and then blocked. The cells were then washed three times with an ice-cold solution. Subsequently, nonspecific binding of the antibodies was blocked by incubating the cells in PBST (PBS + 0.1% Tween 20) supplemented with 1% BSA and 22.52 mg/mL glycine for 30 min. In a humidified chamber for 1 h at room temperature, the cells were incubated with the primary antibody against mAChRM1. Finally, after incubating the cells with 1% BSA for 1 h at RT, the cells were mounted with an antifade reagent containing 0.1–1 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) (DNA stain) for 1 min and then covered with a drop of mounting medium. The slides were rinsed three times with PBS buffer after each stage. The slides were observed and photographed (Olympus DP72) with a fluorescence microscope (Olympus BX50).
TUNEL test
The TUNEL assay was conducted with an enhanced FITC-conjugated TUNEL apoptosis assay kit. The cells were initially rinsed with PBS. After removing the slides with fixative buffer, the slides were fixed for 15 min at RT (25 °C) using polyformaldehyde (dissolved in PBS at a final concentration of 4%). The cell slides were washed in PBS and incubated in proteinase K for 20 min. The subsequent procedure involved incubation with TdT equilibration buffer at RT for 30 min, and later with TdT enzyme working solution at RT for 60 min (with the exception of the negative control). The cell nuclei were stained at RT for 5 min. Finally, the slides were sealed with mounting medium (containing FITC-12-dUTP mounting media). After every stage, the slides were rinsed with buffer PBS 3 times for 5 min. The cells were monitored using a fluorescence microscope.
Western blotting technique
PC12 cells were seeded in a 6-well plate and treated with TZ4M (3 and 5 µM) for 1 h, followed by incubation with scopolamine (3 µM). After 24 h of treatment, the cells were washed with cold PBS and lysed with lysis buffer for 30 min on ice. Lysed cells were centrifuged at 12,000 × g for 10 min at 4°C. A Bradford assay was used to estimate the protein concentration16,52. Blots were detected and separated by subsequent primary antibodies (incubated overnight at 4 °C) against mouse anti-PKC (1:1000), mouse anti-ChAT (1:2000), mouse anti-mAChRM1 (1:1000), and β-actin (1:1000). Mouse IgGκBP-HRP (1:1000) or mouse anti-rabbit IgG-HRP (1:1000) secondary antibodies were used for detection and incubated for 2 h at RT. Protein band density was estimated using the densitometric scanning technique in the ImageJ program. β-Actin expression was used to normalize the specific protein expression levels.
Molecular docking process
A molecular docking study was carried out to determine the binding energy and residues of the binding site. The crystal structure of M1-StaR-T4L (Homo sapiens, Escherichia virus T4, PDB ID: 6ZG9) at 2.5 Å resolution was obtained from the Protein Data Bank. M1-StaR-T4L (6ZG9, as a receptor), TZ4M (TZD ligand), and GSK1034702 (the crystal ligand of 6ZG9, as a positive control) were prepared using ICM-Pro, (Molsoft, L.L.C.) (http://www.molsoft.com/). AutoDockTools-1.5.653 was used to determine the grid box coordinates in the x, y, and z directions with dimensions of 40 × 40 × 40, which were then defaults with other parameters. These compounds were subsequently docked to mAChRM1. The hydrophobic and hydrogen bonds in the protein‒ligand complex were analyzed using UCSF Chimera54 and LigPlot55 software.
Statistical
All the statistical analyses were performed using GraphPad Prism 8 (GraphPad, La Jolla, CA, USA). The tests were conducted (n = 3 for biochemical tests and n = 4 for stained cell counting) under three independent conditions. The means ± SEMs were calculated. One-way ANOVA and Dunnett’s multiple comparisons test were used to identify significant differences from the controls. A P value < 0.05 indicated an important difference.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Peter, J. et al. The relationship between cholinergic system brain structure and function in healthy adults and patients with mild cognitive impairment. Sci. Rep. 11(1), 16080 (2021).
Taheri, M. et al. Synthesis, in vitro biological evaluation and molecular modelling of new 2-chloro-3-hydrazinopyrazine derivatives as potent acetylcholinesterase inhibitors on PC12 cells. BMC Chem. 16(1), 7 (2022).
Ferreira-Vieira, T. H. et al. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 14(1), 101–115 (2016).
Sharma, K. et al. Role of receptors in relation to plaques and tangles in Alzheimer’s disease pathology. Int. J. Mol. Sci. 22(23), 12987 (2021).
Chen, Z.-R. et al. Role of cholinergic signaling in Alzheimer’s disease. Molecules 27(6), 1816 (2022).
May, L. T. et al. Allosteric modulation of G protein–coupled receptors. Annu. Rev. Pharmacol. Toxicol. 47, 1–51 (2007).
Lebois, E. et al. Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer’s disease. Neuropharmacology 136, 362–373 (2018).
Davie, B. J., Christopoulos, A. & Scammells, P. J. Development of M1 mAChR allosteric and bitopic ligands: Prospective therapeutics for the treatment of cognitive deficits. ACS Chem. Neurosci. 4(7), 1026–1048 (2013).
Lalut, J. et al. Rational design of novel benzisoxazole derivatives with acetylcholinesterase inhibitory and serotoninergic 5-HT4 receptors activities for the treatment of Alzheimer’s disease. Sci. Rep. 10(1), 3014 (2020).
Assi, A.-A. et al. Effect of ivabradine on cognitive functions of rats with scopolamine-induced dementia. Sci. Rep. 12(1), 16970 (2022).
Johnson, C. R. et al. Drug design targeting the muscarinic receptors and the implications in central nervous system disorders. Biomedicines 10(2), 398 (2022).
Budzik, B. et al. Novel N-substituted benzimidazolones as potent, selective, CNS-penetrant, and orally active M1 mAChR agonists. ACS Med. Chem. Lett. 1(6), 244–248 (2010).
Russell, J. K. et al. M1/M4-preferring muscarinic cholinergic receptor agonist xanomeline reverses wake and arousal deficits in nonpathologically aged mice. ACS Chem. Neurosci. 14(3), 435–457 (2023).
Brown, A.J. et al., From structure to clinic: Discovery of A M1 muscarinic acetylcholine receptor agonist for the treatment of memory loss in Alzheimer’s disease.
Brown, A. J. et al. From structure to clinic: Design of a muscarinic M1 receptor agonist with the potential to treat Alzheimer’s disease. Cell 184(24), 5886–5901 (2021).
Taheri, M. et al. Neuroprotective effect of thiazolidine-2, 4-dione derivatives on memory deficits and neuropathological symptoms of dementia on a scopolamine-induced Alzheimer’s model in adult male wistar rats. ACS Chem. Neurosci. 14(17), 3156–3172 (2023).
Sethi, N. S., Prasad, D. N. & Singh, R. K. An insight into the synthesis and SAR of 2, 4-thiazolidinediones (2, 4-TZD) as multifunctional scaffold: A review. Mini Rev. Med. Chem. 20(4), 308–330 (2020).
Naim, M. J. et al. Therapeutic journey of 2, 4-thiazolidinediones as a versatile scaffold: An insight into structure activity relationship. Euro. J. Med. Chem. 129, 218–250 (2017).
Jain, A. K. et al. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorg. Med. Chem. 20(11), 3378–3395 (2012).
Abdollahi, Z. et al. The therapeutic value of thiazole and thiazolidine derivatives in Alzheimer’s disease: A systematic literature review. Res. Pharmaceut. Sci. 19(1), 1–12 (2024).
Chandra, J. N. S. et al. Effect of novel arecoline thiazolidinones as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Neurochem. Int. 52(3), 376–383 (2008).
Kumar, Y. S. et al. Effect of novel N-aryl sulfonamide substituted 3-morpholino arecoline derivatives as muscarinic receptor 1 agonists in Alzheimer’s dementia models. Bioorg. Med. Chem. 16(9), 5157–5163 (2008).
Chen, W. N. & Yeong, K. Y. Scopolamine, a toxin-induced experimental model, used for research in Alzheimer’s disease. CNS & Neurological Disorders-Drug Targets. Former. Curr. Drug Targets CNS Neurol. Disord. 19(2), 85–93 (2020).
Silva-Abreu, M. et al. Thiazolidinedione as an alternative to facilitate oral administration in geriatric patients with Alzheimer’s disease. Euro. J. Pharmaceut. Sci. 129, 173–180 (2019).
Villalpando-Rodriguez, G. E. & Gibson, S. B. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid. Med. Cell. Longev. 2021, 9912436 (2021).
Dwomoh, L., Tejeda, G. S. & Tobin, A. B. Targeting the M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neuronal Sign. 6(1), NS20210004 (2022).
Melancon, B. J. et al. Allosteric modulation of the M1 muscarinic acetylcholine receptor: Improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discov. Today 18(23–24), 1185–1199 (2013).
Moran, S. P., Maksymetz, J. & Conn, P. J. Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends Pharmacol. Sci. 40(12), 1006–1020 (2019).
Chen, Y. et al. Endogenous Gαq-coupled neuromodulator receptors activate protein kinase A. Neuron 96(5), 1070–1083 (2017).
Chen, M.-W. et al. PKC and Ras are involved in M1 muscarinic receptor-mediated modulation of AMPA receptor GluA1 subunit. Cell. Mo. Neurobiol. 40, 547–554 (2020).
Winek, K., Soreq, H. & Meisel, A. Regulators of cholinergic signaling in disorders of the central nervous system. J. Neurochem. 158(6), 1425–1438 (2021).
Abdul-Ridha, A. Pharmacological and Structure-Function Studies of M₁ Muscarinic Acetylcholine Receptor Allosteric Modulation (Monash University, 2015).
Mishra, C. B., Kumari, S. & Tiwari, M. Thiazole: A promising heterocycle for the development of potent CNS active agents. Euro. J. Med. Chem. 92, 1–34 (2015).
Martorana, A. et al. Heterocyclic scaffolds for the treatment of Alzheimer’s disease. Curr. Pharmaceut. Des. 22(26), 3971–3995 (2016).
Sadashiva, C. et al. Synthesis and pharmacological evaluation of novel N-alkyl/aryl substituted thiazolidinone arecoline analogues as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Euro. J. Med. Chem. 44(12), 4848–4854 (2009).
Scarpa, M., Hesse, S. & Bradley, S. J. M1 muscarinic acetylcholine receptors: A therapeutic strategy for symptomatic and disease-modifying effects in Alzheimer’s disease?. Adv/. Pharmacol. 88, 277–310 (2020).
Metz, C. N. & Pavlov, V. A. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J. Neurochem. 158(6), 1359–1380 (2021).
Moreau, E. et al. Orthosteric muscarinic receptor activation by the insect repellent IR3535 opens new prospects in insecticide-based vector control. Sci. Rep. 10(1), 6842 (2020).
Bradley, S. J. et al. Bitopic binding mode of an M1 muscarinic acetylcholine receptor agonist associated with adverse clinical trial outcomes. Mol. Pharmacol. 93(6), 645–656 (2018).
Resende, R. R. & Adhikari, A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun. Signal. 7, 1–20 (2009).
Racké, K., Juergens, U. R. & Matthiesen, S. Control by cholinergic mechanisms. Euro. J. Pharmacol. 533(1–3), 57–68 (2006).
Fisher, A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer’s disease. Neurotherapeutics 5(3), 433–442 (2008).
Alkon, D. L., Sun, M.-K. & Nelson, T. J. PKC signaling deficits: A mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol. Sci. 28(2), 51–60 (2007).
Jones, C. K. et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. 28(41), 10422–10433 (2008).
Clader, J. W. & Wang, Y. Muscarinic receptor agonists and antagonists in the treatment of Alzheimer’s disease. Curr. Pharm. Des. 11(26), 3353–3361 (2005).
Giacobini, E., Cuello, A. C. & Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain 145(7), 2250–2275 (2022).
Mashimo, M. et al. Regulation of immune functions by non-neuronal acetylcholine (ACh) via muscarinic and nicotinic ACh receptors. Int. J. Mol. Sci. 22(13), 6818 (2021).
Teleanu, D. M. et al. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 23(11), 5938 (2022).
Massoud, F. & Gauthier, S. Update on the pharmacological treatment of Alzheimer’s disease. Curr. Neuropharmacol. 8(1), 69–80 (2010).
Leber, A. et al. Efficacy, safety, and tolerability of xanomeline for schizophrenia spectrum disorders: A systematic review. Exp. Opin. Pharmacother. 25(4), 467–476 (2024).
Pirali, M. et al. Artesunate, as a HSP70 ATPase activity inhibitor, induces apoptosis in breast cancer cells. Int. J. Biol. Macromol. 164, 3369–3375 (2020).
Jaffaraghaei, M. et al. Induction of heat shock protein expression in SP2/0 transgenic cells and its effect on the production of monoclonal antibodies. Plos one 19(5), e0300702 (2024).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31(2), 455–461 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25(13), 1605–1612 (2004).
Laskowski, R. A. & Swindells, M. B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery (ACS Publications, 2011).
Acknowledgements
The authors thank the University of Guilan for their generous support.
Funding
The authors declare that no funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Conceptualization; H.G., Analysis, and Interpretation of Data; M.T., M.A.M., Writing the Original Draft; M.T., Reviewing and Editing; H.G., M.T., M.A.M., Funding Acquisition; M.A.M., M.T. All authors have reviewed and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Taheri, M., Afzali Mehr, M. & Ghafouri, H. The novel orthosteric agonist M1 muscarinic acetylcholine receptor reveals anti-Alzheimer’s disease activity. Sci Rep 14, 28824 (2024). https://doi.org/10.1038/s41598-024-80102-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80102-0