Abstract
Machine learning can be used to identify risk factors associated with graft rejection after corneal transplantation for keratoconus. The study included all keratoconus eyes that underwent primary corneal transplantation from 1994 to 2021. Data relating to the recipient, donor, surgery, and postoperative course that might be associated with the occurrence of a graft rejection reaction were compiled. This study used five supervised learning algorithms including artificial neural network, support vector machine, gradient boosting, extra trees classifier, and random survival forests to select the most predictive factors for graft rejection. A total of 1214 consecutive eyes of 985 keratoconus patients were included in the study, and the technique of keratoplasty included penetrating keratoplasty in 574 eyes (47.3%) and deep anterior lamellar keratoplasty in 640 eyes (52.7%). The overall prevalence of first graft rejection was 28.1%. All five models had similar ability in identifying predictive factors for corneal graft rejection. Technique of keratoplasty was associated with an increased risk of graft rejection in all models. Other identified risk factors included patient age, keratoplasty in the fellow eye, donor age, graft endothelial cell density, duration of corticosteroid application, time from keratoplasty to complete suture removal, and suture-associated complications. It is advisable that in the absence of any contraindication, post-transplant keratoconus eyes receive a low dose topical corticosteroid until all sutures are removed.
Similar content being viewed by others
Introduction
Corneal graft survival is excellent in keratoconus eyes as more than 90% of the grafts are clear at 5-year postoperative follow-up1,2. Although cornea is an immune privileged tissue, immunologic reaction is the primary cause of graft failure after keratoplasty3. Several risk factors have been reported for corneal graft rejection, including recipient and donor’s characteristics, surgical technique, and postoperative events and complications. Prediction of graft rejection and its risk factors can optimize graft survival through consoling the patients to attain close follow up and adjusting the pretransplant strategy, surgical technique, posttransplant care, and other potentially modifiable factors. Several studies have used the regression-based model to investigate the risk factors for graft rejection4,5,6,7. However, accurate prediction of corneal graft rejection is challenging due to the numerous influential variables and complex interactions among the risk factors. Machine learning can identify high-dimensional associations and nonlinear relationships between variables and is suggested as an alternative to regression modeling8,9. These associations can help ophthalmologists identify risk factors for corneal graft rejection and make appropriate changes in their routine practice. To the best of our knowledge, no previous studies used machine learning methods to investigate potential risk factors for corneal graft rejection. The present study evaluated the performance of different machine learning methods in identifying the most predictive subset of variables for graft rejection after corneal transplantation for keratoconus.
Results
Patient characteristics
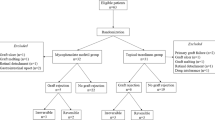
The study included 1214 eyes (585 right eyes, 48.2%) of 985 keratoconus patients (682 male subjects, 69.2%) who underwent corneal transplantation (Table 1). The mean age of recipients was 29.4 ± 8.7 years (range, 12 to 63 years) at the time of keratoplasty. A history of VKC was present in 134 eyes (11.0%) which was inactive at the time of keratoplasty. Ninety-eight patients (8.1%) had a history of atopic diseases, including food intolerance, allergic conjunctivitis, rhinitis, asthma, or dermatitis. PK was performed in 574 eyes (47.3%) and DALK in 640 eyes (52.7%). Corneal transplantation was performed in the fellow eyes of 229 patients (23.3%). The mean duration of follow-up after keratoplasty was 7.5 ± 5.5 years (range, 1 to 26 years). Tables 2, 3 present donor characteristics and operative data.
Graft rejection events
Overall, 341 eyes (28.1%) had at least one episode of immunologic graft reaction. Of these, 174 eyes had 1, 87 had 2, 26 had 3, 11 had 4, and 43 had 5 or more rejection episodes. The incidence of graft rejection was relatively stable during the study period, ranging from 25.1 to 30.2% (Fig. 1). The first episode of graft rejection was classified as epithelial in 3, subepithelial in 163, stromal in 24, endothelial in 96, and mixed in 55 eyes. Time to first rejection episode was 17.1 ± 31.5 months (range, 19 days to 311 months), and 296 episodes (86.8%) occurred within the first 2 years after keratoplasty.
The recipients with graft rejection were significantly older than those without graft rejection, whereas two groups were comparable for sex and history of VKC (Table 1). Donors of the rejection group were significantly younger and had better quality while donor sex and HY compatibility were comparable (Table 2). PK procedure was significantly more common in rejection group (Table 3). Separate and running sutures were more frequently used in eyes with graft rejection, whereas graft size was comparable between the two groups of graft rejection (Table 3).
Treatment of graft rejection was frequent application of topical corticosteroids in all patients and oral prednisolone in 23 patients. Seven episodes of graft rejection resulted in immunologically mediated graft failure yielding an irreversibility rate of 2.1%; diffuse endothelial graft rejection resulted in two PK graft failures, whereas stromal graft rejection led to graft failure in five DALK eyes.
Postoperative course
Duration of corticosteroid administration
The mean duration of corticosteroid treatment after keratoplasty was 3.8 ± 1.5 months (range, 2.5 to 14 months), and was significantly shorter in the rejection group compared to the group without rejection (3.9 ± 1.6 months versus 4.4 ± 1.7 months, respectively, P = 0.005). Eyes that received topical corticosteroid for < 4 months had a significantly higher rate of graft rejection compared to those receiving the medication ≥ 4 months (31.8% versus 25.0%, respectively, P = 0.01). Three hundred and six eyes (89.7%) were off corticosteroid at the time of first graft rejection. Time from corticosteroid discontinuation to graft rejection was 15.1 ± 33.3 months (range, 1 day to 309 months).
VKC reactivation
Reactivation of VKC occurred in 36 eyes (3.0%), indicating that the ocular surface inflammation was still active in a small subset of patients postoperatively. Although VKC reactivation was more frequently observed in eyes with graft rejection compared to those without (4.1% versus 2.5%, respectively), the difference did not reach a significant level (P = 0.14).
Suture management and complications
Suture removal was performed selectively for the management of graft astigmatism or suture-related complications. The median interval from keratoplasty to initial suture removal was 8 months (interquartile range, 4 to 17 months). Suture removal was completed 19.9 ± 11.9 months (range, 8 to 34 months) postoperatively. Time to complete suture removal was longer in eyes with graft rejection (20.2 ± 11.7 months) compared to the group without rejection (19.3 ± 12.2 months), however, the difference was not statistically significant (P = 0.27). Two hundred and seventy-five eyes had corneal sutures at the time of first graft rejection.
Suture-related complications were observed in 698 eyes (57.5%). Sixty-one eyes had suture-related complications within 3 months prior to first graft rejection including suture tract vascularization (40 eyes), loose suture (17 eyes), sterile suture abscesses (13 eyes), spontaneous suture rupture (6 eyes), and suture cheese wiring (1 eye).
Further surgical interventions
Secondary surgical intervention was performed in 482 eyes (39.7%) during the course of the study. Four eyes had secondary surgical intervention within 3 months prior to first graft rejection, including relaxing incision (3 eyes) and resutruing (1 eye).
Predictive performance of different machine learning models
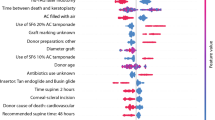
Table 4 compares the performance of five models in differentiation ability, calibration, precision, and accuracy. As demonstrated all models had similar ability in identifying predictive factors for corneal graft rejection. Figures 2, 3, 4, 5, 6 demonstrate the predictors of corneal graft rejection in descending order of importance predicted by different models. Technique of keratoplasty was associated with an increased risk of graft rejection in all five models (Fig. 7). Four models identified keratoplasty in the fellow eye, donor age and graft endothelial cell density, suturing technique, duration of corticosteroid application, time from keratoplasty to complete suture removal, and suture-associated complications as risk factors for graft rejection (Fig. 7). Three models revealed that patient age, history of vernal keratoconjunctivitis, donor sex, graft quality, and graft size increased the risk of graft rejection. The risk of graft rejection was reduced by patient age, HY-mismatch, and graft size in artificial neural network model and by secondary surgical intervention in the random survival forests model (Fig. 7).
Variable importance plot showing variables for predicting graft rejection after corneal transplantation for keratoconus using artificial neural network. Predictors with variable importance above 0.00 contribute to the prediction accuracy of the model. The factor with the most discriminative power in this model was time from keratoplasty to complete suture removal, graft quality, technique of corneal transplantation, and duration of corticosteroid application in descending order.
Variable importance plot showing variables for predicting graft rejection after corneal transplantation for keratoconus using support vector machine. Predictors with variable importance above 0.00 contribute to the prediction accuracy of the model. The factor with the most discriminative power in this model was donor endothelial cell density and patient age in descending order.
Variable importance plot showing variables for predicting graft rejection after corneal transplantation for keratoconus using gradient boosting model. Suture-related complications was ranked as the most predictive factor of graft rejection, followed by discontinuation of corticosteroid at the time of graft rejection, technique of corneal transplantation, and size of corneal graft.
Variable importance plot showing variables for predicting graft rejection after corneal transplantation for keratoconus using extra trees classifier. Predictors with variable importance above 0.00 contribute to the prediction accuracy of the model. Time from keratoplasty to complete suture removal was ranked as the most significant variable, followed by patient and donor age, and duration of corticosteroid application.
Variable importance plot showing variables for predicting graft rejection after corneal transplantation for keratoconus using random survival forests machine learning algorithm. Predictors with variable importance above 0.00 contribute to the prediction accuracy of the model. Discontinuation of corticosteroid at the time of graft rejection was ranked the most important predictor of graft rejection followed by suture-related complications, and size of corneal graft.
Discussion
The results of the present study showed similar and acceptable predictive power among all five models. The five machine learning models identified different sets of variables predicting graft rejection, although several risk factors overlapped. Type of corneal transplantation was a predictor of graft rejection in all models. In line with the previous studies, the present study found a lower rate of graft rejection after DALK10,11. Elimination of endothelial graft rejection is the primary advantage of DALK over PK. Other advantages of the DALK procedure include better long-term graft survival, reduced corticosteroid-related complications, and the ability to transplant donors with lower quality donor10,11. Larger grafts can be transplanted without an increased rejection risk during the DALK procedure10,11.
Five DALK eyes had graft failure following stromal graft rejection due to graft or interface vascularization/opacification, which underscores the importance of appropriate management of graft rejection in this type of corneal transplantation. Although 36.4% of PK eyes developed graft rejections, only two eyes had immunologic failure. This could have been due to timely diagnosis and management of rejection episodes as well as normal endothelial cells present in the corneal rim of keratoconus eyes that can act as a potential reserve and migrate on the graft to replace damaged areas.
The duration of corticosteroid application and discontinuation of corticosteroid at the time of graft rejection were identified by the majority of models as predictive factors for corneal graft rejection which is in line with the results of previous studies. Epstein et al.4. found that steroid potency, recent steroid tapering, and duration of time on the current level of steroids had no correlation with graft rejection in 23 keratoconus eyes after PK. However, their sample size was too small to draw any statistical conclusions4.
While the difference in suture retention duration was not statistically significant, it was longer in rejection group. However, four machine learning models identified time interval to complete suture removal as a contributor to graft rejection. This shows that machine learning algorithms can capture nonlinear relationship between input variables and output and detect new risk factors which are not identifiable by conventional statistical methods. In addition, suture-related complications were associated with an increased risk of graft rejection. This finding highlights the importance of timely suture removal when surgical wound heals appropriately and before any suture-related complications develop.
The observation that younger age of donors increased the risk of graft rejection confirms the results of a pervious report that showed higher risk of rejection in donors ≤ 60 years old5. Conversely, other authors reported no difference in the risk of graft rejection related to donor age3,7. The finding that graft rejection was more frequently observed in older recipients is in contrast with that of several studies showing that recipient age is inversely correlated with the likelihood of graft rejection due to a more aggressive immune reaction in young patients6,12,13,14. The reasoning for this counterintuitive finding is uncertain and remains unsubstantiated. Guilbert et al.15 found a higher rate of rejection events in recipients aged 0 to 10 years and 41 to 50 years compared to other age groups. Other investigators failed to demonstrate any association between the patient age and the risks of graft rejection after PK for keratoconus3.
Gradient boosting and extra trees classifier indicated that graft rejection was associated with donor and recipient sex as well as HY compatibility. However, donor sex and HY mismatch decreased the risk of graft rejection in artificial neural network. Literature review is inconclusive for the effect of sex and HY histocompatibility on graft rejection. Stulting et al.7 reported that female recipients had a greater risk of graft rejection than male recipients. Jonas et al.3 found no correlation of recipient sex with rejection events in keratoconus. A retrospective study showed that HY mismatched corneas were more likely to have rejection episodes compared to matched corneas16. In that report, male recipients with keratoconus who received cornea from male donors were 20% less likely to develop graft rejection compared to female recipients who were transplanted with cornea from male donors16. This finding, however, was not confirmed by later studies17,18.
Better donor quality and higher endothelial cell density was associated with a higher risk of graft rejection in the present study. One explanation for this observation is the fact that donors with good quality were frequently transplanted during the PK procedure which has a higher risk for immunologic reactions. On the other hand, grafts with good quality contain a higher number of epithelial cells and keratocytes which can elicit an immune response.
Technique of suturing increased the risk of rejection in four models. Combined suturing technique was more frequently used in DALK eyes, whereas separate and continuous suturing techniques were mainly used in PK eyes which explains an association between combined suturing technique and lower rate of graft rejection.
Previous history of VKC and atopic diseases and postoperative VKC reactivation were associated with an increased risk of graft rejection. The results of a nationwide epidemiological study suggest that immune-mediated diseases such as atopic diseases can influence the onset of keratoconus19. The results of the present study demonstrate that activated inflammatory pathways associated with atopic diseases may lead to periocular inflammation and increase risk of graft rejection after keratoplasty in keratoconus.
Increased risk of rejection reactions in larger grafts has been reported in some studies4,6. Host trephination size ≥ 8.25 mm has been found to be associated with higher risk of graft rejection after PK for keratoconus, which is attributed to the proximity of donor antigens to the recipient limbal vasculature4. Jonas et al.3 reported that occurrence of a graft rejection had no association with graft size in keratoconus. In the present study, graft size was one of the most important predictive factors for graft rejection in gradient boosting, extra trees classifier, and random survival forests. Artificial neural network, however, showed larger graft size decreased the risk of graft rejection.
In line with previous reports, the majority of machine learning models showed that additional surgical intervention after keratoplasty did not add to the risk of graft rejection20,21. Random survival forests, however, demonstrated that secondary surgical intervention reduced the risk of graft rejection which can be attributed to the course of topical corticosteroid administered after each surgical procedure. Cornea transplant in the fellow eye added to the risk of graft rejection in the present study. Kirkness et al.22 reported an increased odds of rejection in bilaterally transplanted keratoconus patients compared with unilaterally grafted patients. Alternatively, other investigators found no increased risk in bilaterally grafted keratoconus patients4.
The results of the present study should be interpreted in the light of its limitations. Firstly, the study was conducted over a 27-year period during which many surgical and perioperative protocols might change. However, the incidence of graft rejection remained relatively constant during the course of the study, indicating that changes in protocol had no effects on the occurrence of graft rejection. Secondly, this cohort contains 1214 transplantations. Machine learning methods benefit from large training data such as large national corneal transplant registry data. Despite the advantage of registries for identifying risk factors for corneal graft rejection, data reporting to large national registries can be inconsistent. In addition, not all factors associated with graft rejection are registered, and the correctness and completeness of the registry data could not be validated23. The data used in the present study benefited from longitudinal follow-up of a large number of corneal transplant patients with a high degree of accuracy. Third limitation is inherent to machine learning models, which can identify association but cannot provide explanation. Therefore, additional analyses are required to interpret results in terms of the effect of individual predictors identified by machine learning techniques8,9. Last, we did not validate the models with certain data other those used to train the model architecture.
In conclusion, the results of the present study demonstrated that artificial neural network, support vector machine, gradient boosting, extra trees classifier, and random survival forests had similar predictive power to identify risk factors for graft rejection. Based on these machine learning algorithms, early discontinuation of corticosteroid (< 4 months), full-thickness keratoplasty, longer retention of sutures, and suture-related complications were major predictors of corneal graft rejection in keratoconus. Therefore, it is advisable to continue a low dose topical corticosteroid, in the absence of contraindications, until all sutures are removed, especially in PK eyes.
Methods
The protocol of this retrospective interventional study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Shahid Beheshti University of Medical Sciences, Tehran, Iran. Medical Ethics Committee II of the Ophthalmic Research Center, affiliated with Shahid Beheshti University of Medical Sciences waived the requirement for participant consent for the use of medical records in this retrospective chart review.
Study population
The study enrolled all the patients who underwent primary keratoplasty for keratoconus between February 24, 1994 and January 12, 2021. Indications for keratoplasty included inappropriate rigid gas-permeable contact lens fit, unacceptable corrected distance visual acuity (CDVA < 20/40), and contact lens intolerance. Inclusion criteria required a follow-up duration of ≥ 1 year and complete suture removal. The data of both grafts were included for analysis in patients who received bilateral keratoplasty. Exclusion criteria included the preoperative presence of corneal neovascularization or other ocular pathologies. Patients with active vernal keratoconjunctivitis (VKC) were treated medically for at least 6 months before keratoplasty.
Surgical technique
A single experienced surgeon (M.A.J.) performed all surgeries under general anesthesia. Penetrating keratoplasty (PK) was performed in all keratoconus patients before December 2005. Deep anterior lamellar keratoplasty (DALK) was the main procedure after December 2005 unless history of corneal hydrops or intraoperative extensive tear in Descemet membrane that led to conversion to PK. Recipient trephine size was chosen 2.5 mm less than the vertical white-to-white distance in all cases, and grafts were sutured to the recipient bed using 10 − 0 nylon sutures. Suturing techniques included a 16-bite continuous running suture, 16 separate sutures, or eight separate sutures combined with a 16-bite continuous running suture.
Donor preparation
Fresh donor corneas were stored in corneal preservation media (Optisol-GS preservative; Chiron Vision, Irvine, CA, USA) within 15 h after donor death and transplanted within 14 days of storage. Donor tissue was not required to be HY-, human leukocyte antigen (HLA)-, or ABO-compatible with recipients. Donors with quality ranging from good to excellent were transplanted in PK eyes, whereas graft quality for DALK varied from fair to excellent. The donor quality was determined based on the endothelial cell morphology and density as described previously24. DALK grafts were prepared by stripping the Descemet membrane. All grafts were cut from the endothelial side 0.25 mm larger than the size of the recipient trephine.
Postoperative course
Postoperative examination was performed on days 1, 3, and 7, and at months 1, 3, 6, and 12; and every 6 months thereafter. Interim examinations were done if patients experienced new symptoms such as photophobia and decreased vision. The patients received topical antibiotic (chloramphenicol 0.5%) every 6 h for 14 days and topical corticosteroids (betamethasone 0.1%) every 6 h for two months then tapered off per surgeon’s decision. Graft rejection was treated aggressively with the frequent application of topical corticosteroids. Oral prednisolone, 1 mg/kg, was started for severe graft rejection early postoperatively.
Selective separate suture removal started at least 3 months postoperatively when corneal astigmatism was > 4 D. Otherwise, sutures would stay unless they degraded and needed to be removed or any suture complications developed. Management of suture-related complications included application of topical corticosteroids for sterile suture abscess and suture removal for suture tract vascularization, broken, or loose suture. Patients received topical antibiotic and corticosteroids every 6 h for 1 week after suture removal.
Outcome measures
The main outcome measures were the incidence and risk factors of graft rejection. Eyes were categorized to those with at least one episode of graft rejection and those with no graft rejection. The time interval from keratoplasty to the first rejection episode was considered for analysis. Graft rejection was considered irreversible when it resulted in a persistent graft stromal edema or opacity with CDVA < 20/40 for a minimum of 3 months despite intensive treatment.
This study included 19 recipient, donor, operative, and postoperative variables that were suggested to be associated with graft rejection based on previous studies. Recipient characteristics were sex, age at the time of keratoplasty, keratoplasty in the fellow eye, and previous history of VKC and atopic diseases. Donor characteristics included sex, age, endothelial cell density, graft quality, and HY compatibility. A graft from male donor transplanted in female recipient results in HY mismatch. Operative data included the technique of corneal transplantation, size of corneal graft, and suturing technique. Postoperative events were duration of corticosteroid application, discontinuation of corticosteroid at the time of graft rejection, time from keratoplasty to complete suture removal, suture-associated complications, VKC reactivation, and secondary surgical intervention. Suture-related complications and secondary surgical intervention were considered as risk factors if occurred within 3 months prior to graft rejection.
Binary variables, including history of VKC and atopic diseases, keratoplasty in the fellow eye, HY compatibility, suture-associated complications, VKC reactivation, and secondary surgical intervention, had a “Yes” or “No” value. Graft quality was assigned to “excellent”, “very good”, “good”, or “fair”. The technique of corneal transplantation was categorized to “PK” or “DALK”, and suturing technique included “separate”, “continuous”, or “combined”.
Prediction model development
This research used supervised learning algorithms including artificial neural network, support vector machine, gradient boosting, extra trees classifier, and random survival forests for the prediction of graft rejection. Supervised learning algorithms learn from data that already have the correct answers and are often useful for classification purposes which can be categorical values such as “rejection” or “no rejection”, or continuous variables such as height or weight. Support vector machine was a sparse kernel model that predicted unknown class labels based on a subset of the data. This algorithm used a good-fitting hyperplane to separate input data. Kernels were used to convert this hyperplane into a non-linear input separator. Among all possible hyperplanes that satisfied this condition, the hyperplane with the largest margin between selected hyperplane and marginal samples was chosen. Based on this algorithm, largest margin could be achieved by reaching the least value from an edited version of hinge loss function25.
Gradient boosting implemented gradient boosted trees algorithm for supervised learning, using merging multiple simpler models. This model recognized process by minimizing a regularized loss function that combined a convex loss function and penalty term as a presenter of complexity26. In this research, the algorithm was trained for 100 boosting rounds.
Extra trees classifier randomized cut-point choice and attribute while splitting a tree node in tree-based model. This model used multiple decision trees and chose features on the basis of their importance scores27. In this study, the model used 100 estimators to learn training data.
Random survival forests were random forests with a survival outcome such as graft rejection. This algorithm obtained cumulative hazard functions for each tree on the basis of 36.8% of the data that were not used to grow it for greater precision. The final forest cumulative hazard function for each observation was the average of the predictions of decision trees28.
These models have been chosen for the following reasons. Support vector machine performs well with small datasets and has the ability to model non-linear decision boundaries. Gradient boosting is able to handle complex relationships in data, protect against overfitting, and improve the predictive accuracy. The extra trees classifier is less sensitive to noise and irrelevant features. In addition, the random selection of subsets and random splitting points in this model help to decrease the bias that can result from utilizing a single decision tree. The random survival forests analysis is a nonparametric method that can model nonlinear effects and interactions. Due to multiple trees contributing to the results, this analysis accommodates various sorts of predictors and interactions among them and makes reliable prediction on time-to-event outcomes.
The patients were categorized into 80% training set (971 eyes) and 20% test set (243 eyes). The training and test sets were used to train and assess machine learning models, respectively. All simulations were performed using Python (Version 3.10, Van Rossum, Scotts Valley, CA, USA).
Comparison of predictive performance among different models
The predictive performance of the machine learning models was compared using C-statistics, the Brier score, accuracy, precision, mean squared error, root mean squared error, and K-fold cross-validation. The concordance index (C-statistics) is a measure of discrimination ability, which determines if the machine learning model correctly allocates higher predicted risk to patients with graft rejection versus those without rejection. A C-statistics is the area under the receiver operating characteristic curve for sensitivity and specificity, and values closer to unity indicate better discrimination ability29. The Brier score is a measure of calibration to investigate the agreement between predicted and actual risk. A smaller difference between these two risks results in a lower Brier score, hence better calibration. The model is considered to have favorable calibration when the Brier score is ≤ 0.2530. Mean squared error measures the average squared difference between the predicted values generated by a model and the actual values from the data. A lower mean squared error specifies that the predictions of the model are closer to the actual values. Root mean squared error is the square root of the mean squared error. This index allows to understand how much the predicted values deviate from the actual values on average. A lower root mean squared error indicates better model performance. K-fold cross-validation is a model validation method that can evaluate how well a machine learning model will generalize to an independent dataset. This method helps in mitigating overfitting and provides a more reliable evaluation by ensuring that every data point is utilized for both training and validation.
Statistical analysis
Data analyses were performed using SPSS statistical software version 25 (IBM Corp., Armonk, New York, USA). Normality of the data was investigated with the Kolmogorov-Smirnov test and Q-Q plot. Continuous data were presented as range and mean ± standard deviation, and categorical variables were presented as percentages and frequencies. The two groups including eyes without versus with graft rejection were compared using Student t-test for continuous and χ2 test for categorical variables. Two-tailed p values < 0.05 were considered to be statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article (Supplementary file/Main data.sav).
References
Jensen, L. B., Hjortdal, J. & Ehlers, N. Longterm follow-up of penetrating keratoplasty for keratoconus. Acta Ophthalmol. 88, 347–351 (2010).
Guan, M. et al. Graft survival rate of deep anterior lamellar keratoplasty for keratoconus: a meta-analysis. Medicine (Baltimore) 97, e11404 (2018).
Jonas, J. B., Rank, R. M. & Budde, W. M. Immunologic graft reactions after allogenic penetrating keratoplasty. Am. J. Ophtalmol. 133, 437–443 (2002).
Epstein, A. J., de Castro, T. N., Laibson, P. R., Cohen, E. J. & Rapuano, C. J. Risk factors for the first episode of corneal graft rejection in keratoconus. Cornea 25, 1005–1011 (2006).
Inoue, K., Amano, S., Oshika, T. & Tsuru, T. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol. Scand. 79, 251–255 (2001).
Rahman, I. et al. The influence of donor and recipient factors in allograft rejection of the human cornea. Eye (London) 24, 334–339 (2010).
Stulting, R. D. et al. Effect of donor and recipient factors on corneal graft rejection. Cornea 31, 1141–1147 (2012).
Ang, M. et al. Machine learning to analyze factors associated with ten-year graft survival of keratoplasty for cornea endothelial disease. Front. Med. (Lausanne) 9, 831352. https://doi.org/10.3389/fmed.2022.831352 (2022).
O’Brien, R. C., Ishwaran, H., Szczotka-Flynn, L. B. & Lass, J. H. Cornea preservation time study (CPTS) group. Random survival forests analysis of intraoperative complications as predictors of descemet stripping automated endothelial keratoplasty graft failure in the cornea preservation time study. JAMA Ophthalmol. 139, 191–197 (2021).
Song, Y., Zhang, J. & Pan, Z. Systematic review and meta-analysis of clinical outcomes of penetrating keratoplasty versus deep anterior lamellar keratoplasty for keratoconus. Exp. Clin. Transpl. 18, 417–428 (2020).
Arundhati, A. et al. Comparative study of long-term graft survival between penetrating keratoplasty and deep anterior lamellar keratoplasty. Am. J. Ophthalmol. 224, 207–216 (2021).
Armitage, W. J. et al. Corneal transplant follow-up study II (CTFS II): a prospective clinical trial to determine the influence of HLA class II matching on corneal transplant rejection: baseline donor and recipient characteristics. Br. J. Ophthalmol. 103, 132–136 (2019).
Stulting, R. D. et al. Factors associated with graft rejection in the cornea preservation time study. Am. J. Ophthalmol. 196, 197–207 (2018).
Wajnsztajn, D., Hopkinson, C. L. & Larkin D.F.P. Keratoplasty for Keratoconus in young patients: demographics, clinical features, and post-transplant outcomes. Am. J. Ophthalmol. 226, 68–75 (2021).
Guilbert, E. et al. Long-term rejection incidence and reversibility after penetrating and lamellar keratoplasty. Am. J. Ophthalmol. 155, 560–569 (2013).
Hopkinson, C. L. et al. The influence of donor and recipient gender incompatibility on corneal transplant rejection and failure. Am. J. Transpl. 17, 210–217 (2017).
Kim, M. J., Kim, J. H., Jeon, H. S., Wee, W. R. & Hyon, J. Y. Effect of histocompatibility Y antigen matching on graft survival in primary penetrating keratoplasty. Cornea 37, 33–38 (2018).
Fasolo, A. et al. Gender medicine in corneal transplantation: influence of sex mismatch on rejection episodes and graft survival in a prospective cohort of patients. Cell. Tissue Bank. 22, 47–56 (2021).
Claessens, J. L. J., Godefrooij, D. A., Vink, G., Frank, L. E. & Wisse, R. P. L. Nationwide epidemiological approach to identify associations between keratoconus and immune-mediated diseases. Br. J. Ophthalmol. 106, 1350–1354 (2022).
Nagra, P. K. et al. Cataract extraction following penetrating keratoplasty. Cornea 23, 377–379 (2004).
Yu, A. L. et al. Perioperative and postoperative risk factors for corneal graft failure. Clin. Ophthalmol. 8, 1641–1647 (2014).
Kirkness, C. M., Ficker, L. A., Steele, A. D. & Rice, N. S. The success of penetrating keratoplasty for keratoconus. Eye (London) 4, 673–688 (1990).
Muijzer, M. B., Hoven, C. M. W., Frank, L. E., Vink, G. & Wisse, R. P. L. Netherlands corneal transplant network (NCTN). A machine learning approach to explore predictors of graft detachment following posterior lamellar keratoplasty: a nationwide registry study. Sci. Rep. 12, 17705 (2022).
Feizi, S., Javadi, M. A., Kanavi, M. R. & Javadi, F. Effect of donor graft quality on clinical outcomes after deep anterior lamellar keratoplasty. Cornea 33, 795–800 (2014).
Suthaharan, S. Support vector machine. In: Machine Learning Models and Algorithms for Big Data Classification. Integrated Series in Information Systems 36. https://doi.org/10.1007/978-1-4899-7641-3_9 (Springer, Boston, MA, 2016).
Li, Y., Li, M., Li, C. & Liu, Z. Forest aboveground biomass estimation using landsat 8 and Sentinel-1A data with machine learning algorithms. Sci. Rep. 10, 9952 (2020).
Geurts, P., Ernst, D. & Wehenkel, L. Extremely randomized trees. Mach. Learn. 63, 3–42 (2006).
Ishwaran, H. et al. Random survival forests for competing risks. Biostatistics 15, 757–773 (2014).
Pencina, M. J. & D’Agostino, R. B. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat. Med. 23, 2109–2123 (2004).
Alba, A. C. et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 318, 1377–1384 (2017).
Author information
Authors and Affiliations
Contributions
S.F. designed, conducted the study, prepared the data, and wrote the manuscript. M.A.J. provided patients, materials and resources. K.B. and M.A. collected the data. A.R. and M.J.A. analyzed and interpreted the data. M.A.J. revised and approved the article. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.ORCREC.11786.151), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feizi, S., Javadi, M.A., Bayat, K. et al. Machine learning methods to identify risk factors for corneal graft rejection in keratoconus. Sci Rep 14, 29131 (2024). https://doi.org/10.1038/s41598-024-80967-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80967-1