Abstract
Transfusion of donor-derived red blood cells (RBC) depends on donor availability. Alloimmunization can limit the availability of transfusion units, particularly for chronically transfused patients. In vitro cultured, customizable RBC (cRBC) would negate these concerns and provide infinite RBC products. Previously, we developed a defined medium based on good manufacturing practice (GMP) requirements. To optimize medium conditions with regards to reproducibility and cost effectiveness, we tested the requirement for plasma during the differentiation phase and the replacement of HSA by polyvinyl alcohol (PVA) during the expansion and differentiation phase. We show that 5% plasma is essential to enhance cell count, enucleation% and mostly stability of cRBC during the differentiation phase. During the expansion phase HSA could be replaced by PVA without compromising the expansion capacity. Substitution of HSA by PVA even increased the number of cells at the end of the expansion phase. During the differentiation phase PVA could also replace HSA, but only in the presence of plasma. Plasma is still essential to achieve an optimum yield of enucleated cRBC, likely by stabilizing enucleated cRBC. Substitution of HSA by PVA is a new advancement in the development of a, defined, cost-effective culture medium to culture cRBC for all.
Similar content being viewed by others
Introduction
Discovery of the molecular basis of blood groups systems (e.g. A, B, O and AB) enabled physicians to adopt effective and safe blood transfusions as a routine procedure. Nowadays, more than 100 million units of blood are collected worldwide each year1. Currently 47 blood group systems exist including the AB0 and Rh systems2. Although many of these blood group antigens possess low immunogenicity, repeated transfusion may result in alloimmunization. The current available RBC’s concerning blood group compatibility does not adequately serve the needs of chronically transfused, allo-immunized individuals, such as patients with sickle cell disease. It can be very challenging to find transfusion units for allo-immunized patients that require RBCs with matched blood groups. The production of cultured red blood cells (cRBCs) may provide matched transfusion units. Thus, the dependence on existing RBC supply systems may be overcome by eliminating the current sporadic shortages, securing the supply lines, and providing back-up capability. In 2011 a proof of principle autologous transfusion product of 1 ml of (51)Cr labelled cRBC was infused in one human volunteer. After 26 days, approximately half of the transfused cells could still be detected in the circulation, which compares favorably with 28 days for native RBC3. A major challenge of cRBC production for transfusion purposes is the large number of cells in a single transfusion unit, 2 × 1012 erythrocytes. This requires massive expansion of progenitor cells and, subsequently, efficient differentiation into mature and functional cRBC.
In recent years several significant contributions were made in in vitro production of cRBC, optimizing the different phases of erythroid commitment, expansion, and terminal differentiation albeit with variable culture conditions that are not standardized between the laboratories3,4,5,6,7,8,9,10,11,12. An additional emphasis of compliance with good manufacturing procedures (GMP), to produce robust cRBC, and be cost-effective is also important13,14,15,16,17. Whereas the use of undefined products is minimized, most protocols use human plasma or serum-enriched media. We employed a 3-stage GMP (good manufacturing practice)-grade culture protocol with high expansion and efficient enucleation to generate PBMC-derived cRBC16. Our Cellquin medium is based on HEMAdef medium4,18 with modifications of the lipid fraction, omission of nucleosides, and reduction of HSA to 0.1%14,16. Plasma has to be added to Cellquin during the terminal differentiation stage of erythroblasts to obtain enucleated cRBC3,18,19,20.
Differentiation of erythroblasts to erythrocytes is characterized by major changes in gene expression that allow hemoglobin accumulation and adaptation in the cytoskeleton21,22.
Regulation of the final steps of maturation, including enucleation is still ill-defined. Late in differentiation the orthochromatic erythroblasts change their membrane-cytoskeleton interactions. Membrane proteins are sorted to end up with pyrenocyte- or reticulocyte membranes. Vesicle trafficking trims the cRBC from organelles and proteins that need to be removed. These vesicles can be excreted or be removed by autophagy. Enucleation itself is an active process that involves vesicle transport and components of the cytokinetic machinery23.
Following enucleation, the stability of the reticulocyte membrane is dependent on the attachment between spectrin and ankyrin24. The proteins that provide anchoring points between the membrane and cytoskeleton for mechanical stability (e.g. band 3, Rh, RHAG, GPC and XK) increase after maturation25,26. Compared to red blood cells, reticulocytes are less stable. The membrane composition of reticulocytes differs from circulating erythrocytes in protein levels and in their phosphorylation25,27,28,29. Vesiculation occurring during the circulation contributes to the maturation of reticulocytes to erythrocytes30. It is currently unknown if and to what extent these processes are controlled by the shear stress associated with circulation or by extracellular factors, and which medium components affect the stability of enucleated cRBC.
Ideally cRBCs are cultured in medium without any biological (human) components. Therefore, we systematically narrowed down possible (human) plasma factors that sustain enucleation. Furthermore, we reviewed the use of Human Serum Albumin (HSA) 0.1% in Cellquin medium by replacing HSA by Polyvinyl alcohol (PVA). PVA can replace albumin in cultures that support the expansion of mouse hematopoietic stem cells and chimeric antigen receptor T-cells31,32. Similar to HSA, PVA binds medium components such as proteins, lipids, and metal ions. Moreover, PVA is GMP compatible, non-toxic, biodegradable and inexpensive. In this study we investigated the need for HSA and human plasma components during enucleation. We demonstrate that PVA can replace HSA in the culture of erythroblasts but that terminal differentiation to cRBC requires plasma addition. Plasma seems to have at least two functions: (i) it increases the cell numbers at the start of terminal differentiation, which could be replaced by HSA, and (ii) most importantly, it promoted the stability of cRBC in the enucleation process, a function that could not be replaced by HSA.
Materials and methods
Ethical statements
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by Stichting Sanquin Bloedbank, Department of Research and Sanquin Ethical Advisory Board. Written informed consent was obtained from all subjects.
Cell culture
Human adult peripheral blood mononuclear cells (PBMCs) were isolated from donor derived buffy coats by density centrifugation using Ficoll-Paque (density = 1.077 g/mL; 600 g, 30 min; GE Healthcare). In accordance with the Declaration of Helsinki and the Sanquin Ethical Advisory Board, and in line with Sanquin’s Not-For-Transfusion (NVT) project NVT0258), waste material was used for research purposes upon written informed consent.
Erythroid cells were cultured from human PBMCs in serum-free Cellquin medium, based on IMDM (PAN Biotech, Aidenbach, Germany) as previously described16,33. Unless mentioned otherwise Cellquin contained 0.1% (w/v) human serum albumin (HSA, Akron BioProducts LLC, Boca Raton, USA). Alternatively, we added recombinant Human Albumin (rHu Albumin, Akron BioProducts LLC), or Polyvinyl Alcohol (PVA, Sigma-Aldrich, Merck, Darmstadt Germany). PVA was dissolved in IMDM (PAN Biotech, #P04-20250) at 0.1% or 0.3% (w/v) either as low or as high molecular weight PVA (Sigma-Aldrich, cat#p8136-250G, MW 30-70 kD, and cat#363081-25G, MW 85–124 kD, respectively), or we used EryPlus medium which is Cellquin medium with PVA 0.1% (Pan Biotech, Erythroplus #P04-20251 K). In the first phase, (pro)erythroblast cultures were established from PBMC in the presence of erythropoietin (Epo, 1 U/mL; EPREX®, Janssen-Cilag, Breda, Netherlands), hSCF (30 ng/mL, produced in HEK293T cells), dexamethasone (1 µmol/L; Sigma-Aldrich), and IL-3 (1 ng/mL first day only; Stemcell Technologies; Vancouver, Canada) using culture dishes. L-glutamine (2 mM, Sigma Aldrich) and Penicillin/streptomycin (100 ug/mL, Sigma-Aldrich) were added to the medium. From day 6 erythroblast cultures were expanded in the same medium minus IL-3, and the cell density was maintained between 0.7 and 2.0 × 106 cells/mL (CASY Model TCC; OLS OMNI Life Science GmbH & Co KG, Bremen, Germany).
To induce differentiation, cells were washed and reseeded at 1–2 × 106 cells/ml in the presence of EPO (5 U/mL); plasma 5% (Octapharma Biopharmaceuticals GmbH, Frankfurt, Germany) and heparin (5 U/mL; Leo Pharma A/S, Ballerup, Denmark). During this differentiation phase the cells undergo 3–4 divisions before they mature to enucleated reticulocytes. Unless otherwise stated most experiments were performed on cells differentiated for 11 days.
Plasma
Omniplasma® (Prothya Biosolutions, Amsterdam, the Netherlands) was used throughout the study which is a pooled, detergent-treated and prion reduced plasma; made from plasma of voluntary and unpaid Dutch donors (approximately 600–1200 donations). The average total protein content is 58 mg/ml, albumin accounts for 50% (29 mg/ml)34 (SPC Octaplas (prothya.com)).
Plasma treatments
Charcoal treatment to remove fatty acids and steroids35,36: Dextran-coated charcoal (DCC) was added to plasma (1–2 ml DCC per 45 ml Omniplasma), mixed over night at 4 °C and for 1 h at 50 °C, centrifuged and sterile filtered. 10 kD Dialysis: plasma (12 ml) was dialysed for 24 h at 4 °C against PBS (1200 ml) in a sterile Slide-A-Lyzer (10 K MWCO; ThermoScientific Thermo-Fisher). Heat Inactivation: plasma heated for 30 min at 56 °C with mixing to inactivate complement proteins that are part of the immune response. Polyethylene glycol precipitation (PEG) precipitation: PEG (Polyethylene glycol 6000, Sigma-Aldrich, CAS 253222-68-3, pH 5–7, 66% w/v) was added to plasma at a final concentration of 3% and 22%37. NaCl was added to a final concentration of 0.15 M. The PEG/plasma mix was incubated for 8 h at 4 °C, centrifuged at 4500 g for 30 min. The pellet was resuspended in RPMI medium in the original plasma volume. The resuspended pellet and the supernatant were filter sterilized and the equivalent of 5% plasma was added to the erythroid differentiation culture.
Flow cytometry
Cells were washed and resuspended in N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES) buffer supplemented with 0.5% Bovine Serum Albumin (BSA). Cells were incubated with primary antibodies for 30 min at 4 °C, measured on FACS Canto II or LSRFortessa (both BD Biosciences, Oxford, United Kingdom) and analyzed using FlowJo software (FlowJo v10, Ashland, USA). Antibodies used: anti-CD235a (PE Acris 1:2500); anti-CD71 (Miltenyi Biotec Vio Blue 1:200). DNA was stained with Deep Red Anthraquinone 5 (DRAQ5; APC Abcam 1:2500).
Protein quantification|Bradford Coomassie brilliant blue assay
The protein quantities (microgram) in the PEG treated plasma supernatants and precipitates were determined by Bradford Coomassie brilliant blue assay and absorbance was measured at 620 nm. The calibration line was based on Bovine Serum Albumin (BSA) in Carin lysis buffer.
Silver stain
To qualitatively evaluate the protein content of both supernatants and precipitates, equal amounts of protein (according to the Bradford measurement) were separated by two-dimensional Sodium Dodecyl Sulfate (SDS) electrophoresis in polyacrylamide gel and visualized by Silver-staining (Thermo Fisher Scientific; Invitrogen™ SilverQuest™ Silver Staining Kit; Catalog number: LC6070 Product code: 10543053, Carlsbad, USA).
Statistical analysis
Statistical analyses were performed using Analysis of Variance (ANOVA). Post hoc multiple comparison analysis were performed using Dunnett’s test to identify the pair with significant differences between the treatment group and the control group. Tukey’s multiple comparison test was used to compare the difference between each pair of means with appropriate adjustment for the multiple testing. All data in figures are displayed as mean ± the standard deviation of the measurements. The number of replicates is n ≥ 3 for all experiments, unless indicated differently. Significance is expressed as: ns for not significant differences, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001. All measurements and the statistical analysis are added as supplemental data.
Results
Production of enucleated cRBC requires 5% plasma
To determine the optimal plasma concentration for the production of enucleated cRBC, we terminally differentiated immature (pro)-erythroblast cultures (day 10 after seeding PBMC; Supplementary Fig. 1A) in the absence or presence of 1.0, 2.5, 5.0 or 10% plasma for 11 days. Overall cell count (cells > 4.5 μm) increased with increasing plasma percentages, which plateaued at 5% and did not further increase at 10% plasma (Fig. 1A). Terminal differentiation and maturation into enucleated and mature CD71−/CD235+ cells was monitored by flow cytometry (gating strategy in Supplementary Fig. 1B). The percentage of enucleated cells was determined by the fraction of all DRAQ5 negative cells among the total of DRAQ5 negative plus large DRAQ5 positive cells (nucleated cRBC, excluding the pyrenocytes) (Fig. 1C, Supplementary Fig. 1). The stability of enucleated cRBC is measured by the ratio between DRAQ5 negative cRBC to small DRAQ5 positive events (the pyrenocytes) (Fig. 1D, Supplementary Fig. 1). The yield of reticulocytes at the end of differentiation is dependent on the production (cRBC maturation and enucleation) of reticulocytes and reticulocyte stability. Compared to absence of plasma, the addition of 5% plasma significantly increased the number of enucleated cells (from 42 ± 9 to 84 ± 5%) and stability (from 0.2 ± 0.1 to 3.8 ± 1.5). Enhancement to 10% plasma gave no further significant increase. Thus, plasma addition during terminal differentiation is crucial for efficient cRBC production and addition of 5% plasma was required and sufficient for optimal production of enucleated cRBC.
Effect of various plasma concentrations on the production of cRBC. (A–D) Erythroblast expanded from PBMC were differentiated for 11 days in presence of increasing concentrations of HSA (v/v volume plasma percentage). Differentiation was monitored as cell count, developmental stage, enucleation percentage and stability. (A) Cell count (> 4 μm) measured on CASY® cell counter (Model TCC; OLS OMNI Life Science; Germany). (B) Developmental stage based on expression of transferrin receptor (CD71) and Glycophorin A (CD235). Q1: CD71−/CD235−, Q2: CD71+/CD235a−, Q3: CD71+/CD235a+, Q4: CD71−/CD235a+. (C) Enucleation % calculated as the percentage of DRAQ5− (enucleated) cells among the total of Draq5− plus large Draq5 + cells (see Supplementary Fig. 1). (D) Stability is calculated as the ratio between Draq5− and small Draq5 + cells (pyrenocytes). n = 4, for original data and statistical analysis see supplemental excel file. Error bars represent standard Deviation (SD), asterixis indicate probability (see “Materials and methods” section).
Plasma factors affect cRBC production early and late in terminal differentiation
Next, we investigated when plasma needs to be added during terminal differentiation. Plasma (5%) was added from the start of terminal differentiation or at day + 1 through + 5 when enucleation initiates. CRBC production was analyzed at day 11 similar to Fig. 1. Plasma added from start or day + 1 increased the total number of cells by ~ 40% compared to additions at later timepoints (Fig. 2A). Strikingly, the addition of plasma at day4 or day5 had the same positive effect on enucleation efficiency and on the stability of enucleated cRBC as addition at the start of differentiation. Thus, timing is less relevant for plasma to exert its positive effect on the production of enucleated cRBC, (Fig. 2B, C). The total yield of enucleated cRBC as a product of total cell count and enucleation percentage shows that the addition of plasma is more important than the timing of addition (Fig. 2C). The benefits of the addition of plasma to the differentiation mix also applies to the stability (Fig. 2D).
Timing of plasma addition in differentiating cRBC cultures. (A–D) Plasma was added from start of differentiation or from day + 1, +2, + 3, +4, + 5 after start of differentiation, or omitted. On day 11 of differentiation total cell count (A) was measured and cells were stained with Draq5 to calculate enucleation % (B) and Stability (C) as defined in the legend of Fig. 1. Yield is calculated as the number of enucleated cells (total cell number multiplied with enucleation percentage). n = 3. Error bars represent standard Deviation (SD), asterixis indicate probability (see “Materials and methods” section).
The enucleation promoting factor in plasma is larger than 10 kD and precipitated by PEG
To understand how plasma may support the production of enucleated cRBC, insights into the nature of the plasma components that mediate these effects are warranted. Therefore, we tested the production of enucleated cRBC in the presence of plasma depleted for specific components. Specifically, we depleted (i) non-polar/lipophilic components by activated charcoal treatment (i.e., steroids38, growth factors and some cytokines), (ii) small molecules < 10 kDa by dialysis, and (iii) instable proteins by heat inactivation at 56 °C. Compared to the negative control, all plasma conditions treated or untreated retained the capacity to enhance production of enucleated cRBC.
Compared to the addition of untreated plasma, or lack of plasma, all treated plasma fractions yielded intermediate enucleation percentages. Whereas the difference between plasma and no plasma is clear and significant, heat inactivation yields enucleation percentage that is not significantly different from either plasma or no plasma, whereas enucleation in presence of charcoal treatment is significantly different from both untreated plasma and no plasma conditions. This may imply that a labile and/or lipophilic component is involved, but not as sole factor. Enucleation in presence of plasma dialyzed in a 10 kDa membrane is significantly different from enucleation in presence of untreated plasma and not different from the negative control. The values, however, are also intermediate (Fig. 3A and Supplemental Excel file tab).
The role of plasma components. (A) Plasma (P) was added at the start of the 11 day terminal differentiation assay. For comparison plasma was pretreated with activated Charcoal (CT), dialysed over a 10 kD filter (> 10kD), or heat inactivation (HI, 56 °C, 30 min) before addition. Cells were stained with Draq5 and the percentage enucleated cRBC was calculated as defined in Fig. 1. (B, C) Plasma fraction were precipitated by 3 or 22% PEG. Following centrifugation pellets were dissolved in medium and the equivalent of 5% plasma of both pellet (p) and supernatant (s) was added to the differentiation assay. (B) Bradford protein quantification of individual fractions. (C) Enucleation percentage of cRBC at end of differentiation (day 13). (D) Two dimensional SDS-PAGE gel electrophoresis and Silver staining of individual fractions. P = plasma; s3 = supernatant of PEG 3% treated plasma; s22 = supernatant PEG 22% treated plasma; p3 = precipitate PEG 3% treated plasma; p22 = precipitate PEG 22% treated plasma. Sections are cut from original gel (see supplemental Fig. 3). Error bars represent standard Deviation (SD), asterixis indicate probability (see “Materials and methods” section).
To further delineate plasma components, we performed a size separation of plasma components using Polyethylene Glycol (PEG) at concentrations of 3 and 22% (w/v) to produce fractions with low molecular weight components (supernatant 22%), general proteins (pellet 22% and supernatant 3%) and large protein complexes (pellet 3%)37. PEG 22% was tested for possible toxicity in cRBC culture. It did not affect cell count or enucleation capacity (Supplementary Fig. 2). Bradford protein quantification of the supernatants and precipitates shows the differential ability to precipitate proteins in plasma (Fig. 3B). These fractions were tested on the capacity to terminal differentiate erythroblasts to cRBC. The enucleation promoting plasma factor disappears in the supernatants of 22% PEG and is not present in the PEG 3% precipitate (Fig. 3C). To qualitatively evaluate the protein content of both supernatants and precipitates, equal amounts of protein (according to the Bradford measurement) were analysed using two dimensional SDS electrophoresis in polyacrylamide gel and visualized by Silver-staining. An abundant protein at the size of Albumin (65–70 kDa) clearly disappeared in the supernatants and appeared in the precipitates (Fig. 3D, Supplementary Fig. 3).
Polyvinyl alcohol (PVA) supports cRBC production in the presence of plasma
Mass spectrometry and comparison of the protein content of PEG fractions that do or do not support production of enucleated cRBC did not reveal candidate factors that could be validated as enucleation promoting factor (data not shown). However, Albumin is a multipurpose plasma carrier of among others signaling molecules. Thus, albumin may be the carrier of the candidate enucleation promoting plasma factor. We investigated whether HSA and plasma could be substituted by polyvinyl alcohol (PVA), as was previously described for mouse HSC39,40,41. We first tested whether high or low MW PVA can replace HSA under conditions that do not require plasma, i.e. in the expansion phase of (pro-) erythroblast cultures. PVA (both high and low MW) and HSA supported the proliferation of immature erythroblasts equally well under conditions that induce transient renewal divisions (medium supplemented with Epo, hSCF, dexamethasone) (Fig. 4A). Subsequently, the expanded erythroblast cultures were reseeded in 3 different differentiation media, one containing the standard HSA plus plasma, one containing PVA plus plasma and one containing PVA alone. Differentiation parameters were measured at day 11 following differentiation induction (Fig. 4B–E). There was a significant reduction in cell count in the conditions expanded in PVA and differentiated in PVA alone (1.53 ± 0.29 and 1.66 ± 0.28 × 106/ml) versus HSA plus plasma (2.36 ± 0.45 and 2.44 ± 0.68 × 106/ml) or PVA plus plasma (2.67 ± 0.45 and 2.74 ± 0.51 × 106/ml) (Fig. 4B). There was a significant decrease in the number of enucleated cells in the condition with PVA alone compared to PVA or HSA plus plasma, which was most pronounced for cells expanded in PVA-containing medium (Fig. 4C). The low overall yield of enucleated cells in the presence of PVA and absence of plasma was associated with a very low stability of enucleated cells (Fig. 4D, E). Thus, we conclude that HSA can be replaced by PVA in the differentiation phase, regardless of expansion in HSA or PVA, but plasma cannot be omitted.
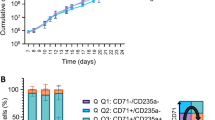
HSA can be replaced by polyvinylalcohol (PVA). (A) cRBC cultures of 3 independent donors were expanded for 31 days in expansion media containing Human Serum Albumin (HSA, default) or in medium in which HSA was replaced by low or by high molecular weight PVA (PVA low, 30–70 kD; PVA high, 85–124 kD). Cell density was maintained at 0.7–1.5 million/ml. Cumulative cell numbers were calculated. Floating bars indicate min. to max. (B–E) Cells from each culture, expanded for 13 days, were differentiated in culture medium containing HSA plus plasma (P), or PVA (high/low) plus plasma, or PVA (high/low) alone. On day 13 of differentiation the cultures were harvested to determine total cell count (B), Enucleation percentage (C), Stability (D) and Yield (E) as defined in Figs. 1 and 2. Error bars represent standard Deviation (SD), asterixis indicate probability (see “Materials and methods” section). PVA high polyvinyl alcohol high molecular weight, PVA low polyvinyl alcohol low molecular weight.
HSA cannot replace plasma to produce enucleated cRBC
Knowing that PVA allows differentiation in the presence of plasma, we investigated whether HSA could be the essential component in plasma. We questioned if there is a difference between fresh HSA in the differentiation phase or stored HSA (old; 1 year, 4 °C) that could have lost biologically active metabolites. Therefore, we compared the addition of plasma to the addition of PVA without biological metabolites. We also tested whether plasma could be substituted by increasing levels of albumin. There were no differences between the addition of freshly isolated HSA versus HSA that was stored at 4 °C for a year (Fig. 5A–E). The stabilizing effect of plasma on the yield of enucleated cells is immediately clear from Flowcytometry dot plots (Fig. 5C). The two or fourfold increase in HSA did not alter differentiation parameters compared to standard HSA levels. However, there was a significantly inferior outcome of the condition without any form of albumin (PVA 0.1%, negative control) on cell count, enucleation percentage and yield (third panel, lane 2 in Fig. 5A, B,E). Addition of HSA improved the yield and stability of enucleated cells compared to PVA (by 3.5 and 3-fold, respectively), but not to the levels obtained with plasma (7 and 35-fold). The addition of HSA to PVA conditions was sufficient to restore cell count (Fig. 5A), but it did not restore the yield and stability of enucleated cells, also not when added at a 2- or 4-fold increase. This means a different plasma factor than HSA is probably responsible for the increased number of enucleated cells.
HSA cannot replace plasma. (A–E) Expanded erythroblasts were differentiated in freshly isolated HSA 0.1% (1 mg/ml) or substituted by old HSA 0.1% (1 mg/ml; kept > 1 year at 4 °C before use) or PVA 0.1% (1 mg/ml). The standard addition of plasma 5% was substituted by a negative control or by HSA 2 × (2.9 mg/ml) or HSA 4 × (5.8 mg/ml) (relative to the amount added through plasma (1.45 mg/ml)). The effect of the individual differentiation media on cell count (A), enucleation percentage (B), stability (D) and yield (E) was determined. (C) FlowCytometry dot plots to discriminate Enucleated cRBC, Nucleated cRBC and Pyrenocytes on the basis of Forward Scatter (FSC) (x-axis) and DRAQ5 (nucleic stain) (Y-axis). Gating strategy: live cells FSC versus SSC, Glycophorin A and/or Transferrin positive cells (CD235apos and/or CD71pos), Forward scatter (cell size) versus DRAQ5 (nucleic stain). Error bars represent standard Deviation (SD), asterixis indicate probability (see “Materials and methods” section).
Finally, we examined whether 5% plasma was sufficient also in the presence of PVA, and whether 0.1% PVA is needed and sufficient to promote differentiation. To investigate whether the difference between PVA and HSA is due to small molecules that remain associated with HSA, we compared the addition of HSA with recombinant human albumin. In the presence of PVA both 5% and 10% plasma enhanced the yield of enucleated cells equally compared to no plasma. Strikingly, HSA or PVA could be suspended from the media in presence of plasma (Fig. 6A–D). The addition of 0, 0.1, or 0.3% PVA resulted in similar cell counts (Fig. 6A), enucleation percentages (Fig. 6B), yield of enucleated cells (Fig. 6C) and stability of enucleated cells (Fig. 6D). In the conditions without plasma (0%), there was a significant difference in cell count from recombinant human albumin versus negative control and the PVA 0.1% and PVA 0.3% condition but not with the HSA (Fig. 6A). A similar trend was observed on enucleation capacity (Fig. 6B), yield (Fig. 6D) and stability (Fig. 6C) and yield (Fig. 6D) but these were all not statistically significant.
Requirement for 5% plasma during differentiation is independent of HSA or PVA. (A–D) Expanded erythroblast cultures were differentiated for 13 days in separate differentiation mixes to observe the effect of plasma 0%, 5% or 10% versus HSA 0.1%, Negative control, PVA 0.1%, PVA 0.3% and Recombinant Human Albumin 0.1% on Cell count (A), Enucleation (B), Stability (C) and Yield (D) were determined as described in Fig. 1. Error bars represent standard deviation (SD), asterixis indicate probability (see “Materials and methods” section).
In conclusion, PVA is a safe and cost-effective supplement that can replace HSA in culture medium for the production of cRBC, provided that 5% plasma is added during terminal erythroid differentiation. HSA can not replace plasma to produce stable enucleated cRBC (Fig. 7).
Schematic overview of the cRBC culture system. HSA 0.1% is essential in the first week of culture establishment and can be added to PVA 0.1% culture medium. PVA 0.1% solely can replace HSA 0.1% during expansion as the erythroblast (EBL) culture is established and during differentiation in the presence of plasma. Plasma is required for optimal differentiation. PBMC peripheral blood mononuclear cells, EBL erythroblasts, RBC red blood cells, HAS human serum albumin, PVA polyvinyl alcohol, FSC forward scatter, SSC side scatter, CD71 transferrin receptor, CD235 glycophorin A, DRAQ5 nucleic stain.
Discussion
The in vitro production of red blood cells for transfusion is possible but requires fully defined GMP compliant culture medium preferably at low costs. Here, we investigated the need of HSA and plasma for the expansion and differentiation of erythrocyte precursors. We found that PVA can replace HSA during expansion of erythroblast cultures. However, terminal differentiation to stable enucleated red blood cells critically depends on the addition of 5% plasma. At the start of terminal differentiation plasma supports the production of cells a function that can largely be replaced by HSA. However, a plasma factor is required for the stability and yield of enucleated cells. This factor cannot be replaced by PVA or (recombinant) HSA.
The plasma used in these experiments was solvent/detergent (SD)-treated plasma (Omniplasma®). Pools of ~ 1000 donors are solvent/detergent-treated (tri-n-butyl phosphate/Tritonx100) to eliminate lipid enveloped viruses42,43. To identify the plasma factor required for optimal enucleation and stability of cRBC, we performed treatment with activated charcoal, size fractionation on a 10 kD filter, and heat inactivation, which only moderately impaired the plasma’s ability to enhance the yield of enucleated cRBC. This may imply (i) that we need a combination of factors, and (ii) that at least one of the plasma factor we aim to identify is a stable, non-lipophilic molecule larger than 10 kD. Using PEG precipitation we could separate distinct protein fractions that were analyzed by mass spectrometry. Differentially expressed proteins hinted to a potential role for signaling pathways, none of which could be functionally validated or rejected. A more comprehensive analysis of plasma and plasma fractions is required using multi-omics as a non-protein moiety may also be involved (metabolomics, lipidomics, proteomics).
However, plasma is a rich source of biologically active molecules. It is also possible that plasma contains several factors that are able to stabilize enucleated cRBC in a concerted action. Our experiments dissected two functions. First, plasma enhances the number of cells produced during terminal differentiation, for which plasma had to be added at the start of differentiation (Figs. 1, 2), and which could be substituted by HSA (Fig. 5). Second, the plasma component that increased the number of enucleated cells could be added 5 days after differentiation induction (Figs. 1, 2) and could not be replaced by HSA (Fig. 5).
Albumin can be a carrier of many biologically active molecules44,45,46,47. However, recombinant albumin was equally active to increase the number of cells during terminal differentiation. It is possible that HSA does not need to present biologically active molecules, but that it binds and quenches metabolites produced by the cells. This could explain why pharmaceutical HSA that is blocked with caprylic acid is not functional in the medium16. Similar to HSA, PVA has the ability to adsorb proteins, lipids and metal ions, with various association and de-association kinetics over time48.
Plasma is eventually required, at the time of enucleation, to increase the yield of enucleated cells. The enucleation process is not fully understood, but sorting of proteins and lipids and the formation of vesicles is important23. The plasma factor may be involved in this process. To increase the yield of enucleated cRBC, plasma can be added on day 5. At this stage, the nucleus is fully condensed, indicating that plasma is required for posttranscriptional processes16,49. In absence of plasma, many pyrenocytes (small DRAQ5 positive cells) are formed. Thus, it is mainly the stability of enucleated cells that is severely impaired in the absence of plasma. Most likely the membrane structure of the enucleated cRBC lacks a factor that is supplied by plasma, or one or more factors are missorted or misfolded in absence of plasma. Previously we showed that additional cholesterol is needed to enhance the yield of enucleated cRBC after filtration33. Cholesterol is an important component of plasma, and the requirement for cholesterol to produce cRBC is well documented12,33,50. Plasma is a rich source of cholesterol and apolipoproteins. However, cholesterol inhibited enucleation itself and mainly affected the filtration process33. Thus cholesterol is not the contributing factor. A strong barrier against oxidative stress is crucial for erythrocyte stability. Lack of important players in the defense against oxidative stress such as Periredoxin-2 or GPx4 results in hemolytic anemia51,52,53,54,55,56. Conversely, an increase in periredoxins or GPx proteins enhances the stability of cultured reticulocytes54. Plasma may also provide elements to counteract oxidative stress57.
Both the replacement of HSA by PVA and the addition of plasma at day 5 following differentiation induction enhance the (cost)efficiency to produce cultured RBC. First because PVA is produced chemically and can be made in large amounts and at very low costs whereas HSA is donor dependent or recombinantly produced in cellular models, inherently more expensive. HSA currently contributes to at ~ 15$ per liter of medium. For basic medium (without growth factors) the use of PVA reduces the cost by at least 12%. To produce large numbers of cRBC, we culture cells in stirred bioreactors and we use medium perfusion14. When we do not need plasma during medium perfusion from the start of terminal differentiation induction up to day 5, this provides a 5 to 10-fold reduction in the use of plasma. Replacement of holotransferrin by deferriprone58, and HSA by PVA, together with the 5-fold reduction in plasma, reduces the costs of differentiation medium roughly by 60%.
In conclusion, we show that PVA is equivalent to HSA in the capacity to expand cRBC during the expansion phase. Only during the establishment of erythroid cultures we need to add HSA (data not shown). During terminal differentiation, PVA is equally potent as HSA to produce stable cRBC in the presence of plasma. Plasma, however, is essential to achieve an optimum yield of enucleated cRBC. Plasma containing HSA moderately increases cell production at the start of differentiation but a different factor, or different factors, are required to stabilize the enucleated cRBC.
Data availability
For original data, please contact e.vandenakker@sanquin.nl. FAIR website Sanquin.
Abbreviations
- cRBC:
-
Cultured red blood cells
- DRAQ5:
-
Deep Red Anthraquinone 5
- EPO:
-
Erythropoietin
- FSC:
-
Forward scatter
- GMP:
-
Good manufacturing practice
- HSA:
-
Human serum albumin
- HSC:
-
Hematopoietic stem cells
- hSCF:
-
Human stem cell factor
- kDa:
-
kiloDalton
- MW:
-
Molecular weight
- PBMC:
-
Periferal blood mononuclear cells
- PEG:
-
PolyEthyleen glycol
- PVA:
-
Polyvinyl alcohol
- RBC:
-
Red blood cells
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel
- SSC:
-
Side scatter
References
Carson, J. L. et al. Clinical practice guidelines from the AABB. JAMA 316(19), 2025. https://doi.org/10.1001/jama.2016.9185 (2016).
Hyland, C. & Gassner, C. ISBT Working Parties: Red Cell Immunogenetics and Blood Group Terminology. https://www.isbtweb.org/isbt-working-parties/rcibgt.html
Giarratana, M. C. et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood 118(19), 5071–5079. https://doi.org/10.1182/blood-2011-06-362038 (2011).
van den Akker, E., Satchwell, T. J., Pellegrin, S., Daniels, G. & Toye, A. M. The majority of the in vitro erythroid expansion potential resides in CD34– cells, outweighing the contribution of CD34+ cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica 95(9), 1594–1598. https://doi.org/10.3324/haematol.2009.019828 (2010).
Migliaccio, G. et al. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol. Dis. 28(2), 169–180. https://doi.org/10.1006/bcmd.2002.0502 (2002).
Migliaccio, G. et al. Humanized culture medium for clinical expansion of human erythroblasts. Cell. Transplant. 19(4), 453–469. https://doi.org/10.3727/096368909X485049 (2010).
Giarratana, M. C. et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat. Biotechnol. 23(1), 69–74. https://doi.org/10.1038/nbt1047 (2005).
Leberbauer, C. et al. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood 105(1), 85–94. https://doi.org/10.1182/blood-2004-03-1002 (2005).
Miharada, K., Hiroyama, T., Sudo, K., Nagasawa, T. & Nakamura, Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat. Biotechnol. 24(10), 1255–1256. https://doi.org/10.1038/nbt1245 (2006).
Griffiths, R. E. et al. Maturing reticulocytes internalize plasma membrane in glycophorin A–containing vesicles that fuse with autophagosomes before exocytosis. Blood 119(26), 6296–6306. https://doi.org/10.1182/blood-2011-09-376475 (2012).
Kupzig, S., Parsons, S. F., Curnow, E., Anstee, D. J. & Blair, A. Superior survival of ex vivo cultured human reticulocytes following transfusion into mice. Haematologica 102(3), 476–483. https://doi.org/10.3324/haematol.2016.154443 (2017).
Wang, E. et al. An optimized human erythroblast differentiation system reveals cholesterol-dependency of robust production of cultured red blood cells ex vivo. Adv. Sci. https://doi.org/10.1002/advs.202303471 (2024).
Rousseau, G. F., Giarratana, M. C. & Douay, L. Large-scale production of red blood cells from stem cells: what are the technical challenges ahead? Biotechnol. J. 9(1), 28–38. https://doi.org/10.1002/biot.201200368 (2014).
Gallego-Murillo, J. S. et al. Expansion and differentiation of ex vivo cultured erythroblasts in scalable stirred bioreactors. Biotechnol. Bioeng. 119(11), 3096–3116. https://doi.org/10.1002/bit.28193 (2022).
Bayley, R. et al. The productivity limit of manufacturing blood cell therapy in scalable stirred bioreactors. J. Tissue Eng. Regen. Med. 12(1), e368–e378. https://doi.org/10.1002/term.2337 (2018).
Heshusius, S. et al. Large-scale in vitro production of red blood cells from human peripheral blood mononuclear cells. Blood Adv. 3(21), 3337–3350. https://doi.org/10.1182/bloodadvances.2019000689 (2019).
Pellegrin, S., Severn, C. E. & Toye, A. M. Towards manufactured red blood cells for the treatment of inherited anemia. Haematologica 106(9), 2304–2311. https://doi.org/10.3324/haematol.2020.268847 (2021).
Migliaccio, G. et al. Humanized culture medium for clinical expansion of human erythroblasts. Cell. Transplant. 19(4), 453–469 (2010).
van den Akker, E., Satchwell, T. J., Pellegrin, S., Daniels, G. & Toye, A. M. The majority of the in vitro erythroid expansion potential resides in CD34– cells, outweighing the contribution of CD34+ cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica 95(9), 1594–1598 (2010).
Hu, J. et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood 121(16), 3246–3253. https://doi.org/10.1182/blood-2013-01-476390 (2013).
Chiabrando, D., Mercurio, S. & Tolosano, E. Heme and erythropoieis: more than a structural role. Haematologica 99(6), 973–983. https://doi.org/10.3324/haematol.2013.091991 (2014).
Liu, S., McConnell, S. C. & Ryan, T. M. Erythropoiesis in the absence of adult hemoglobin. Mol. Cell. Biol. 33(11), 2241–2251. https://doi.org/10.1128/MCB.01734-12 (2013).
Keerthivasan, G., Wickrema, A. & Crispino, J. D. Erythroblast enucleation. Stem Cells Int. 2011, 1–9. https://doi.org/10.4061/2011/139851 (2011).
Alaarg, A., Schiffelers, R. M., van Solinge, W. W. & van Wijk, R. Red blood cell vesiculation in hereditary hemolytic anemia. Front. Physiol. 4. https://doi.org/10.3389/fphys.2013.00365 (2013).
Gautier, E. F. et al. Absolute proteome quantification of highly purified populations of circulating reticulocytes and mature erythrocytes. Blood Adv. 2(20), 2646–2657. https://doi.org/10.1182/bloodadvances.2018023515 (2018).
Wilson, M. C. et al. Comparison of the proteome of adult and cord erythroid cells, and changes in the proteome following reticulocyte maturation. Mol. Cell. Proteom. 15(6), 1938–1946. https://doi.org/10.1074/mcp.M115.057315 (2016).
Malleret, B. et al. Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PLoS ONE 8(10), e76062. https://doi.org/10.1371/journal.pone.0076062 (2013).
Li, H. et al. Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders. Proc. Natl. Acad. Sci. 115(38), 9574–9579. https://doi.org/10.1073/pnas.1806501115 (2018).
Chasis, J., Prenant, M., Leung, A. & Mohandas, N. Membrane assembly and remodeling during reticulocyte maturation. Blood 74(3), 1112–1120. https://doi.org/10.1182/blood.V74.3.1112.1112 (1989).
Moura, P. L. et al. Reticulocyte and red blood cell deformation triggers specific phosphorylation events. Blood Adv. 3(17), 2653–2663. https://doi.org/10.1182/bloodadvances.2019000545 (2019).
Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571(7763), 117–121. https://doi.org/10.1038/s41586-019-1244-x (2019).
Nishimura, T. et al. Use of polyvinyl alcohol for chimeric antigen receptor T-cell expansion. Exp. Hematol. 80, 16–20. https://doi.org/10.1016/j.exphem.2019.11.007 (2019).
Claessen, M. J. A. G. et al. Production and stability of cultured red blood cells depends on the concentration of cholesterol in culture medium. Sci. Rep. 14(1), 15592. https://doi.org/10.1038/s41598-024-66440-z (2024).
Omniplasma Summary of Product Characteristics. https://prothya.com/storage/uploads/2022/04/Omniplasma-SmPC-Netherlands.pdf
Mardomi, A. et al. Human charcoal-stripped serum supplementation enhances both the stearoyl-coenzyme a desaturase 1 activity of cumulus cells and the in vitro maturation of oocytes. Hum. Fertil. 22(3), 212–218. https://doi.org/10.1080/14647273.2018.1466400 (2019).
Chen, R. F. Removal of fatty acids from serum albumin by charcoal treatment. J. Biol. Chem. 242(2), 173–181. https://doi.org/10.1016/S0021-9258(19)81445-X (1967).
Liu, Z. et al. Enhanced detection of low-abundance human plasma proteins by integrating polyethylene glycol fractionation and immunoaffinity depletion. PLoS ONE 11(11), e0166306. https://doi.org/10.1371/journal.pone.0166306 (2016).
Burchardt, T. et al. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J. Urol. 162(5), 1800–1805. https://doi.org/10.1016/S0022-5347(05)68241-9 (1999).
Wilinson, A. C., Ishida, R., Yamazaki, S. & Nakauchi, H. Ex Vivo Mouse Hematopoietic Stem Cell. Expansion Using Polyvinyl Alcohol. https://doi.org/10.21203/rs.2.9270/v1 (2019).
Igarashi, K. J. et al. Physioxia improves the selectivity of hematopoietic stem cell expansion cultures. Blood Adv. 7(14), 3366–3377. https://doi.org/10.1182/bloodadvances.2023009668 (2023).
Sudo, K., Yamazaki, S., Wilkinson, A. C., Nakauchi, H. & Nakamura, Y. Polyvinyl alcohol hydrolysis rate and molecular weight influence human and murine HSC activity ex vivo. Stem Cell. Res. 56, 102531. https://doi.org/10.1016/j.scr.2021.102531 (2021).
Mack, J. et al. FAQ: Solvent Detergent (S/D) Treated Plasma (Octaplasma). https://professionaleducation.blood.ca/en/transfusion/publications/faq-solvent-detergent-sd-treated-plasma-octaplasma
Cunha, J. P. RxList OCTAPLAS. https://www.rxlist.com/octaplas-drug.htm
Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 5(11), e02879. https://doi.org/10.1016/j.heliyon.2019.e02879 (2019).
Al-Harthi, S., Lachowicz, J. I., Nowakowski, M. E., Jaremko, M. & Jaremko, Ł. Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin. J. Inorg. Biochem. 198, 110716. https://doi.org/10.1016/j.jinorgbio.2019.110716 (2019).
Fanali, G. et al. Human serum albumin: from bench to bedside. Mol. Aspects Med. 33(3), 209–290. https://doi.org/10.1016/j.mam.2011.12.002 (2012).
Peters, T., Hassan, M., Azzazy, E. & Christenson, R. All About Albumin: Biochemistry, Genetics, and Medical Applications (1996).
Ryu, K. E. et al. Plasma protein adsorption to anion substituted poly(vinyl alcohol) membranes. Macromol. Res. 11(6), 451–457. https://doi.org/10.1007/BF03218975 (2003).
Iacono, G. et al. Differentiating erythroblasts adapt to turbulent flow by accelerating maturation and activating cholesterol biosynthesis. BioRxiv. https://doi.org/10.1101/2023.12.08.570773
Bernecker, C. et al. Cholesterol deficiency causes impaired osmotic stability of cultured red blood cells. Front. Physiol. 10, 1–14 (2019).
Orrico, F. et al. Oxidative stress in healthy and pathological red blood cells. Biomolecules 13(8), 1262. https://doi.org/10.3390/biom13081262 (2023).
Sadowska-Bartosz, I. & Bartosz, G. Peroxiredoxin 2: an important element of the antioxidant defense of the erythrocyte. Antioxidants 12(5), 1012. https://doi.org/10.3390/antiox12051012 (2023).
Stolwijk, J. M. et al. Red blood cells contain enzymatically active GPx4 whose abundance anticorrelates with hemolysis during blood bank storage. Redox Biol. 46, 102073. https://doi.org/10.1016/j.redox.2021.102073 (2021).
Langlands, H. D., Shoemark, D. K. & Toye, A. M. Modulation of antioxidant enzyme expression of in vitro culture-derived reticulocytes. Antioxidants 13(9), 70. https://doi.org/10.3390/antiox13091070 (2024).
Altamura, S. et al. Glutathione peroxidase 4 and vitamin E control reticulocyte maturation, stress erythropoiesis and iron homeostasis. Haematologica 105(4), 937–950. https://doi.org/10.3324/haematol.2018.212977 (2020).
Low, F. M., Hampton, M. B., Peskin, A. V. & Winterbourn, C. C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood 109(6), 2611–2617. https://doi.org/10.1182/blood-2006-09-048728 (2007).
Obeagu, E. I., Igwe, M. C. & Obeagu, G. U. Oxidative stress’s impact on red blood cells: unveiling implications for health and disease. Medicine (Baltim.) 103(9), e37360. https://doi.org/10.1097/MD.0000000000037360 (2024).
Gallego-Murillo, J. S. et al. Iron-loaded deferiprone can support full hemoglobinization of cultured red blood cells. Sci. Rep. 13(1), 6960. https://doi.org/10.1038/s41598-023-32706-1 (2023).
Acknowledgements
We thank Carmen van der Zwaan (Molecular Hematology, Research Facility and Mass Spectrometry) for the proteomic analysis on the untreated and treated plasma samples.
Funding
This study was funded by The Dutch Organisation for Health Research and Development (ZonMw; TAS project 116003004), the Top Knowledge Institute Life, Science & Health (Health Holland; project EMCLSH20025 (FERTUS)), and the ministry of health (PPOC19-14).
Author information
Authors and Affiliations
Contributions
M.J.A.G.C., N.Y., E.v.d.A. performed experiments; M.J.A.G.C., G.v.M., M.J.K., M.v.L. and E.v.d.A. planned and evaluated experiments. M.J.A.G.C., M.v.L. and E.v.d.A. wrote the manuscript. All Authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Claessen, MJ.A.G., Yagci, N., van Mierlo, G. et al. Human serum albumin or polyvinyl alcohol can only partially replace human plasma during in vitro red cell production from PBMC. Sci Rep 15, 12058 (2025). https://doi.org/10.1038/s41598-024-81341-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81341-x