Abstract
Development of supercritical carbon dioxide (SC-CO2) dyeing technology for natural fabrics and their blended fabrics is essential for the textile industry due to environmental and economic considerations. Wool (W), polyester (PET) and nylon (N) fabrics and their wool/polyester (W/PET) and wool/nylon (W/N) blended fabrics were dyed in SC-CO2 medium with a synthesized reactive disperse dye containing a vinylsulphone (VS) reactive group, which behaves as a disperse dye for synthetic fibers and a reactive dye for protein fibers. The SC-CO2 dyeing performance of all fabrics was investigated in terms of color strength, fixation, colorimetric and fastness measurements and compared with the conventional aqueous dyeing method. The results obtained indicate that the VS reactive disperse dye structure and non-polar PET component mainly improved colour strength (K/S) values of the dyed PET fabric and W/PET blended fabrics in SC-CO2 compared with those in the aqueous medium. Also, SC-CO2 dyeing has a notable influence on a*, b* and C* values of the dyed PET, N and W/PET fabrics and showed that the uptake of the VS reactive disperse dye and their appearance colors are higher and more saturated than the aqueous dyed samples. The levelling and fastness properties of all dyed fabrics in SC-CO2 medium are similar to those obtained in the aqueous medium. It was observed that VS reactive disperse dye penetrates well into the PET fabric and is chemically bound with the W fabric using both SC-CO2 and aqueous media and did not display significant color difference (∆E) values of W, PET and W/PET fabrics even after 20 washing cycles. The study claims that the VS reactive disperse dye structure and dyed PET-based wool blended fabric are good candidates for industrially SC-CO2 dyeing technology.
Similar content being viewed by others
Introduction
The fabric blends have continuously grown over recent years. W/PET blend fabric has many superior properties over pure wool fabric for woven apparel goods (e.g. men’s suits and knit dresses). W/N blend fabric is a very popular blend for woven apparel and carpets. It can show complementary properties in terms of crease recovery, durability, abrasion resistance, fast drying, and dimensional stability. Conventional aqueous dyeing methods of blended textiles poses certain obstacles due to the difficulty in selecting compatible dyes and chemicals with better color match, high dyeing performance and the vast color difference of dye up-take on each fiber component. Generally, conventional aqueous dyeing methods of textiles have always involved water intensive processes, with an estimated 100 kg of water required per kg of textiles. Water is a finite resource, and the aqueous dye house effluents contain large amounts of dyes, salts and alkali which are required for the coloration of textiles. Therefore, the importance of eco-friendly dyeing methods of blended textiles is increasing. A non-aqueous dyeing technology using liquid paraffin, siloxane, vegetable oil, etc. as an environmentally friendly dyeing method instead of aqueous dyeing has achieved salt-free dyeing of cotton fibers without discharging wastewater1,2,3,4,5. In continuation of the water-free dyeing technologies, SC-CO2 is the perfect replacement for the aqueous medium to overcome the problems of conventional dyeing6,7,8,9,10,11,12,13,14. Potential applications of SC-CO2 dyeing technology have been efficiently used for dyeing synthetic fibers. Due to the hydrophobic nature of SC-CO2 and its non-polarity, synthetic fibers have been successfully dyed with disperse dyes, which have much better solubility in SC-CO2 than other classes of dyes. Furthermore, SC-CO2 dyeing of PET fabrics as an alternative to aqueous dyeing processes results in environmental advantages, including no fresh water, no wastewater, no auxiliaries, a small amount of hydrophobic disperse dyes, residual hydrophobic disperse dye can be reused and no air pollution as the SC-CO2 can be recycled15,16,17,18,19,20,21,22,23. Importantly, DyeCoo Company has developed a patented and industrially proven dyeing technology based on SC-CO2, instead of water, to dye PET fabric and yarns24. With DyeCoo Company and its 14 industrial machines in Taiwan, Vietnam and Thailand, brands and manufacturers have the opportunity to decrease water and chemical usage significantly, reduce CO2 emissions, reduce energy consumption from 20 to 60% and eliminate air pollution as the SC-CO2 can be recycled25. For further clarification, the industrial DyeCoo SC-CO2 dyeing consumes lower energy than industrial aqueous dyeing due to no drying, no steam and shorter dyeing times. DyeCoo reported that the possible range of reduction of CO2 emissions from the industrial SC-CO2 dyeing process may range from 25 to 40% compared with industrial aqueous dyeing processes. Moreover, there are no other man-made greenhouse gas emissions from the industrial SC-CO2 dyeing process like water vapor. Additionally, Yeh Group in Thailand has used DyeCoo’s machines and reported that the industrial DyeCoo SC-CO2 dyeing process allowed a 50% reduction in energy26. However, SC-CO2 dyeing of natural fabrics has not been widely applied because conventional dyes for natural fabric have water-soluble sulfonic acid groups, which cannot be dissolved in the non-polar SC-CO2 medium27,28. To solve this problem, several reactive disperse dyes have been reported for SC-CO2 dyeing of natural fabrics and their blended fabrics including fluorotriazine, chlorotriazine, bromoacrylic acid, halogenated acetamide and VS reactive groups. One major advantage of using reactive disperse dyes for SC-CO2 dyeing of natural fabrics that they are non-polar and potentially soluble in non-polar SC-CO228,29,30,31,32,33,34,35,36,37,38,39,40,41,42. Some studies have reported on the dyeing of N fabric with reactive disperse dyes under SC-CO243,44.
Many other research activities have applied some temporarily solubilized sulphatoethylsulphone reactive disperse dyes to natural, synthetic and their blended fabrics using conventional aqueous dyeing methods44,45,46,47,48,49,50,51. The hydrophobic/hydrophilic nature of wool fabric makes it promising for SC-CO2 dyeing compared to the hydrophilic nature of cotton fabric, which makes it challenging. In other words, the hydrophobic surface of wool fiber may improve the affinity and diffusion of the VS reactive disperse dye molecules into wool fibers. It is worth mentioning that the amino groups in wool fiber may also contribute to increasing the forces of interactions between the wool fiber and dye molecules in SC-CO2 media. In this context, dyeing of synthetic fiber-based wool blended fabrics in SC-CO2 could be accessible when the dyes are able to react with the amino groups in the wool component along with their affinity towards the synthetic fiber component. As the name implies, the target reactive disperse dyes which have reactive disperse dye structure with reactive group might be beneficial for SC-CO2 dyeing of synthetic fiber-based wool blended fabrics. In continuation of our previous work51, this work provides a simple route to synthesize VS reactive disperse dye, which is also suitable for commercial scale production. The chemistry of the target dye in the present study has been designed to contain β-naphthol as a coupling component to promote the formation of the hydrazo form (intramolecular hydrogen bonding) of the dye chemical structure along with a non-polar VS group, optimizing the dye solubility and uptake in SC-CO2. The main objective of this work is to investigate the dyeing behavior of W, N, PET and their PET/W and N/W blended fabrics in SC-CO2 using the synthesized VS reactive disperse dye and in this regard the different factors that may affect this process are investigated.
Materials and methods
Materials
Four types of mill-scoured and bleached fabrics were supplied by Misr El-Mahala Co. (Egypt). A 100% W fabric, 210 g/m2, had a twill weave (2/2) of equal warp and weft (26 × 24 threads/cm, yarn count Nm 44/2). A 100% PET fabric, 149 g/m2 had a continuous filament plain weave of warp and weft (72 × 32 threads/cm, yarn count tex 150 × 1). The W/PET blend fabric (55/45), 165 g/m2, had a plain weave of warp and weft (21 × 19 thread/cm, yarn count Nm 42/2). The W/N blend fabric (70/30), 165 g/m2, had a plain weave of warp and weft (32 × 32 thread/cm, warp yarn count Nm 36/2, weft yarn count Nm 18/1). Also, mill-scoured and bleached single jersey knitted N fabric, 114 g/m2, 135d/30f was obtained from El-Shourbagy Co. (Egypt). All the fabrics were treated in an aqueous solution containing 2 g/l nonionic detergent (Sera Wash M-SF, DyStar, Egypt) for 1 h at 80 °C and at a 50:1 liquor ratio, then washed thoroughly with water to ensure the removal of any undesirable contaminants, such as oils that may exist in all types of fabrics and air-dried at room temperature. 1-aminobenzene-4-β-sulphatoethylsulphone was obtained from Amar Impex, India. Other chemicals used in the study were of laboratory reagent grade.
Methods
Preparation of model VS reactive disperse dye
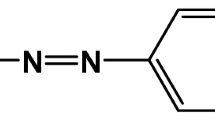
The reactive disperse dye, 2-Hydroxy-1-((4-vinylsulfonyl) phenyl) naphthalene, of VS dye structure was synthesized using a similar procedure previously described in literature44. As presented in Fig. 1, 1-aminobenzene-4-β-sulphato-ethylsulphone was dissolved in water at room temperature and treated with sodium hydroxide solution at 0–5 °C to form the vinylsulphone analogue, which was diazotized and coupled with β-naphthol in alkaline condition according to the usual procedure. A percent yield of 84% was obtained. The prepared VS dye was used in SC-CO2 and aqueous dyeing of W, PET and N fabrics and their W/PET and W/N blend fabrics.
SC-CO2 dyeing
The SC-CO2 apparatus, as shown in Fig. 2, consists of the main parts: a cylinder of carbon dioxide, chiller (model Julabo FL601), a semi-preparative CO2 pump (model JASCOPU-4386), RHPLC pump for extraction (model JASCO PU-4180), a back pressure regulator (model JASCO BP-4340) with a maximum pressure rate of 300 bar, a heater controller (model HC-2068-01), a dyeing autoclave, a temperature controller and a speed controller (model EYELARCX-1000 H) with a maximum temperature rate of 130 °C, and an internal capacity of approximately 50 ml. The maximum CO2 flow rate of the circulation pump is 10 mL/min, but it can reach 30 ml/min with an optional heater42.
The appropriate amount of VS dye (2% owf shade) was placed inside the SC-CO2 dyeing vessel without a dispersing agent. The W, PET and N fabrics, and blends of W/PET and W/N fabrics were added into the SC-CO2 dyeing vessel. The CO2 fluid preheated, and then charged into the dyeing vessel. The dyeing was continued at both temperature and pressure required for 1 h. After the dyeing cycle is completed, the machine automatically cooled down to a safe temperature and pressure before the vessels can be opened and the dyed fabrics were then soaped to remove the surface dye, rinsed thoroughly for 2 min with cold water and air-dried for colour measurements and color fastness.
Aqueous dyeing
The same amount of VS dye (2% owf shade) was initially premixed with an appropriate amount of nonionic dispersing agent (Sera Gal P-LP; DyStar, Egypt) in a ratio of 1:1, which was then added with stirring to the dye bath at a liquor ratio of 50:1. In the case of the PET and W/PET, the dye bath pH was adjusted to 7 in the presence of 2 g/L low-odour carrier (Sera Gal P-EW; DyStar, Egypt). For the W, N and W/N fabrics, the dye bath pH was adjusted to 7 and the addition of 1 g/L levelling agent (Lyogen NH liquid; Clariant, Egypt). All fabrics were dyed in a Pyrotec infrared laboratory dyeing unit (Roaches International) using 2 g samples51. Dyeing started at 40 °C and the temperature was then raised to 100 °C over 45 min. Dyeing continued at the desired temperatures for a further 45 min (90 min. total dyeing time). After dyeing, the PET dyed samples were removed, rinsed in warm water, and subjected to reduction clearing in a solution comprising 2 g /L of sodium hydrosulphite and 2 g /L of sodium hydroxide for 10 min at 60 °C, with a liquor ratio of 1:40. The reduction-cleared sample was then rinsed thoroughly in water. All the dyed samples were soaped with 2 g/l nonionic detergent (Sera Wash M-SF, DyStar, Egypt) for 30 min at 60 °C and at a 50:1 liquor ratio, then thoroughly rinsed in water and air-dried for color measurements and color fastness evaluation (Fig. 3).
Color measurements
The K/S values and color coordinates (L*, a*, b*, C* and h°) of the dyed W, PET and N fabrics and their W/PET and W/N blend fabrics were measured using an UltraScan PRO spectrophotometer (Hunter Lab, USA). L* represents the lightness coordinate, a* represents the redness/greenness coordinate, with + a* indicating red, and -a* indicating green, b* represents the yellowness/blueness coordinate, with + b* indicating yellow, and -b* indicating blue, C* represents the chroma coordinate and h° represents the hue angle, expressed in degrees, with 0° being a location on the + a* axis (red), continuing to 90° for the + b* axis (yellow), 180° for - a* (green), 270° for - b* (blue), and back to 360° = 0°. The levelling of the all dyed fabrics in SC-CO2 and aqueous medium was evaluated by measuring the ∆E within each fabric sample at five separate points and the average ∆E between these points was determined52,53.
The fixation value of VS dye (%F), which is the percentage of the dye fixed on W and N fabrics and their W/N blend fabric in SC-CO2 and aqueous medium relative to the exhausted dye, was measured by refluxing the dyed fabrics in 50% aqueous dimethylformamide (DMF) for 15 min (20:1 liquor ratio) to extract the unfixed dye51 using Eq. (1).
where (K/S)1 and (K/S)2 are the color strength of the dyed samples before and after extraction, respectively.
Color fastness testing
The color fastness of the SC-CO2 and aqueous dyed W, PET and N fabrics and W/PET and W/N blended fabrics were assessed according to ISO standard methods54,55,56,57. Fastness to washing was carried out according to ISO 105-C06 B2S. The dyed W, PET and W/PET samples were also subjected to reapt washing cycles (5, 10 and 20) to determine the durability color against successive laundering by evaluating the color difference (ΔE) values relative to the samples with zero washing cycle. Fastness to an acidic and alkaline perspiration was carried out according to ISO 105-E04. The colour changing of PP fabrics and colour staining of the adjacent multi-fiber were then assessed with the ISO grey scales. Light fastness was also assessed according to ISO105-B02 using a Xenon arc lamp test.
Results and discussion
Characterization of VS dye
The UV-Vis absorption spectrum (Fig. 4) of the synthesized VS dye, recorded in a 50/50 w/w aqueous DMF solution, showed a maximum absorption peak at 483 nm and the molar excitation coefficient of 2.5 × 10− 5 M/L dye concentration was 16,940 L mol− 1 cm− 1.
The IR spectrum of the synthesized VS dye (Fig. 5) showed an absorption band at 3485 –3401 cm− 1, corresponding to the characteristic N-H group. The bands at 3057 and 2970 cm-1 were assigned to the aromatic (= C–H) and aliphatic C-H groups, respectively. Also, the appearance of strong peaks at 1591 and 1499 revealed to the existence of = N-NH and –C = N groups, indicating to the hydrazo form. The characteristic absorption band corresponding to the SO2 group appeared at 1126 cm− 1. The 1H-NMR spectrum of VS dye (Fig. 6) was characterized by two peaks at 6.23 and 6.52 ppm, assignable to the protons of the VS group, respectively. The spectrum exhibited multiplet peaks at 7.1–8.18 ppm, integrated to 10 protons assignable to the aromatic naphthyl and phenyl protons. The band at 15.53 ppm was observed corresponding to the hydrazone NH proton.
Effect of SC-CO2 dyeing temperature
Pressure and temperature are two important factors that determine dye solubility in SC-CO2. An investigation of the effect of dyeing temperature on K/S values of W, PET and N fabrics and their W/PET and W/N blended fabrics dyed in SC-CO2 was performed with the VS dye at a concentration of 2% owf and at constant pressure of 250 bars as shown in Fig. 7. It is clear that increasing the temperature from 90 to 100 °C results in an improvement in the K/S values of all the dyed fabrics. The increase in K/S values is a direct consequence of increasing the VS dye solubility and fiber swelling, thereby enhancing the VS dye diffusion into the fabrics, as more pores and channels are formed in the interior of the fabrics. However, a further increase in SC-CO2 dyeing temperature, i.e., beyond 100 °C, has practically a marginal increasing effect on the K/S values of all fabrics. Accordingly, the optimum temperature condition was tailored to the wool fabric types at a relatively mild and promising dyeing temperature (100 °C). The results also reveal that the K/S values of the blends dyeing are mainly determined by the polarity and crystallinity of the fiber components. These findings were further studied to investigate the effect of SC-CO2 pressure on the dyeing performance of all fabrics with VS dye at 100 °C.
Effect of SC-CO2 dyeing pressure
The effect of SC-CO2 dyeing pressure on the dyeing performance of W, PET and N fabrics as well as their W/PET and W/N blended fabrics with the VS dye was investigated at pressures ranging from 150 to 250 bar at a constant temperature of 100 °C and 2% owf dye concentration, as shown in Fig. 8. It is clear that the K/S values of all dyed fabrics were significantly and linearly improved with system pressures of 150–250 bar. The density of the SC-CO2 fluid increases with an increase in pressure, resulting in an enhancement of the VS dye solubility and promoting more interactions between non-polar SC-CO2 medium and the intermolecular chains of the fabrics under investigation. Consequently, the adsorption opportunities between VS dye molecules and the fabrics are increased, leading to higher K/S values.
Furthermore, it is clear from both Figs. 7 and 8 that, the K/S values of dyed non-polar PET fabrics at all temperatures and pressures were higher than those of the other dyed fabrics. This is because the VS dye solubility and its affinity to PET fabric become larger, which is in agreement with the dyeing behavior of PET fabrics with disperse dyes in SC-CO216,58,59. Theoretically, as shown in scheme 1, the VS dye molecule was designed with a β-naphthol as coupling component, which can predominate the hydrazo form (intramolecular hydrogen bonding) of the dye chemical structure60. This resulting in no dye-dye interaction taking place in the SC-CO2 medium and consequently can significantly improve the VS dye solubility in the SC-CO2 medium. The VS dye behaves as a disperse dye for SC-CO2 dyeing of PET fabric and polyester component in W/PET blended fabric as well as a reactive dye for SC-CO2 dyeing of W, N and their W/N blended fabrics. Among the dyed fabrics in SC-CO2, the order of K/S values found was: PET > N > W/PET > W/N > W. The lower K/S values of dyed N fabrics compared with dyed PET fabrics are attributed to the higher degree of crystallinity of N fabric61. The K/S values of dyed W/PET blend fabrics were found to be between those of PET fabrics and those of W fabrics. Also, the K/S values of dyed W/N blend fabrics were found to be between those of N fabrics and those of W fabrics. PET SC-CO2 dyeings were excellent due to the non-polarity of PET fabrics that predominates a variety of intermolecular forces of interactions with the VS dye structure; however, the lowest K/S values assigned to W fabric can be attributed to the lower diffusion and interactions of VS dye with the polar W fabric, although the nucleophilic addition reaction between the VS reactive group of the dye molecules and nucleophilic amino in W is possible under SC-CO2 conditions. Therefore, the effect of the color range of reactive disperse dye structures incorporating different reactive groups and the blend ratios is a crucial point in SC-CO2 blends dyeing and further work will merit investigation.
K/S values of SC-CO2 and aqueous dyeings
K/S (400–700 nm) values of the dyed W, PET and N fabrics and their W/PET and W/N blended fabrics in SCCO2 at 250 bar, 100 °C, 2% shade and 1 h were compared with those in aqueous dyeing at 2% shade, 100 °C, pH 7, and 1 h, as reported in Figs. 9 and 10, respectively.
The comparative results of the dyed PET and W/PET blended fabrics in SC-CO2 medium showed higher K/S values than those dyed in aqueous medium, as shown in Fig. 11. This is mainly due to the effect of both the non-polar PET component in W/PET blended fabrics and the high VS dye solubility. Additionally, SC-CO2 is known to reduce the glass transition temperature Tg of many polymers considerably, which induces a major beneficial role in increasing the dye transfer inside the polymeric matrix62,63. This is also in agreement with that the diffusivity of PET dyeing in the presence of SC-CO2 is approximately three orders of magnitude larger than that in aqueous dyeing64,65. However, the high ratio of polar W component in W/N blended fabrics resulted in relatively lower K/S values in SC-CO2 medium than those dyed in aqueous medium. This is attributed to the fact that, the interactions of the polar W fabric with VS dye are very low in the non-polar SC-CO2 medium. Furthermore, the forces of interactions between the VS dye and W component in W/N blended fabrics in the aqueous dyeing are expected to be more than the only chemical bonding of W amino groups and the dye VS reactive groups.
Generally, the dye-fibre interactions between VS reactive disperse dye and W/N components in SC-CO₂ or aqueous dyeing medium may involve different mechanisms due to the unique properties of the VS dye structure. In an aqueous medium, water is a polar solvent, facilitating strong hydrogen bonding, Van der Waals and ionic interactions. Also, the W/N functional amino groups can easily be chemically bounded with the VS groups of the dye, allowing for effective dye-fiber covalent bonding. While in SC-CO2, the non-polar nature of CO₂ may reduce the ionic interactions. However, the VS groups still react with the nucleophilic sites on the W and N fibers, but potentially at different rates and efficiencies. Bearing this in mind, both the SC-CO2 and aqueous dyed W, N and W/N blended fabrics were subjected to a DMF extraction to ascertain the chemical bonding between the VS dye molecules and the amino groups of the fabrics. The VS dye exhibited high fixation on W fabrics with values of 83% and 84% while those of N fabric show fixation values of 72% and 73%, in both SC-CO2 and aqueous mediums, respectively, as shown in Fig. 12. The fixation values of the dyed W/N blend fabrics were found to be between those on W fabrics and those on N fabrics, inferring that the VS dye secured relatively high fixation values with the amino-based W and N fibres. Theoretically, there is a nucleophilic addition reaction between the VS reactive group of VS dye molecules and the nucleophilic amino groups in W and N fibers; this reaction is responsible for the fixation of VS dye molecules onto the W and N fibers during the dyeing process in both SC-CO2 and aqueous media, as depicted in Fig. 13.
Color coordinates of SC-CO2 and aqueous dyeings
Table 1 shows the values obtained for the color coordinates (L*, a*, b*, C* and h°) of dyed W, PET, N, W/PET and W/N blended fabrics in SC-CO2 medium at (250 bar, 100 °C, 2% shade and 1 h) and aqueous medium at (2% shade, 100ºC and 1 h) with VS dye. The lower value of L* represents the darker hue and the higher value refers to the lighter hue of a dyed fabric. The value a* denotes the redness/greenness quality of the dyed fabric with the positive value of a* indicating redness and the negative value of a* indicating greenness. The value b* represents the yellowness/blueness quality of the dyed fabric with the positive value of b* indicating the yellowness and the negative value of b* denoting blueness. The value C* indicates the chroma and the degree of saturation of the color and the value h° represents the hue angle, expressed in degrees, with 0° being a location on the + a* axis (red), continuing to 90° for the + b* axis (yellow), 180° for - a* (green), 270° for - b* (blue), and back to 360° = 0°.
It is clear from Table 1 that the dyed PET, N and W/PET fabrics in SC-CO2 exhibited darker colors than those dyed in the aqueous medium, as evidenced by the lower values of L*. However, the high L* value of SC-CO2 dyed W fabric indicate its lighter colors compared to that in the aqueous medium. The values of b* > a* and both positive in all SC-CO2 and aqueous dyed fabrics as well as their h° values range from 53.80 to 63.79, indicating the appearance of the orange color of the dye used. The SC-CO2 dyeing has a notable influence on a*, b* and C* values of the dyed PET, N and W/PET fabrics and showed that their appearance colors are higher and more saturated than the aqueous dyed samples. Alternatively, the aqueous dyeing of W and W/N fabrics showed higher values of a*, b* and C* than those of SC-CO2 dyeing, depending on the higher uptake of the VS reactive disperse dye in the aqueous dyeing. All the dyed W, PET and N fabrics and W/PET and W/N blended fabrics show very good levelling properties, indicating that the SC-CO2 dyeing method has a substantially better effect on the ∆E values of the dyed samples, as shown in Table 1. Images of all SC-CO2 dyed fabrics are illustrated in Fig. 14.
Importantly, successive washing cycles (5, 10 and 20 cycles) were carried out for W, PET and W/PET dyed fabrics and ΔE values were evaluated accordingly. The color effects of the dyed fabrics displayed satisfactory ΔE values and both the SC-CO2 and aqueous methods showed no significant color loss in the dyed fabrics as shown in Figs. 15 and 16, respectively.
Fastness properties of SC-CO2 and aqueous dyeings
Table 2 shows data for color fastness to washing, perspiration and light for W, PET, N, W/PET and W/N blended fabrics dyed in SC-CO2 medium at (250 bar, 100 °C,2% shade and 1 h) and aqueous medium at (2% shade, 100 °C and 1 h) with VS dye. As listed in Table 2, W, PET, N, W/PET and W/N blended fabrics dyed in SC-CO2 and aqueous medium present similar fastness ratings with excellent to very good washing, perspiration and light fastness. Also, both the SC-CO2 and aqueous dyed fabrics showed approximately similar rubbing fastness results with very good to good ratings.
Conclusion
A reactive disperse dye containing VS reactive group was successfully designed, prepared and applied to W, PET and N and their W/PET and W/N blended fabrics in SC-CO2 medium. Effects of dyeing temperature and pressure on the K/S values of all dyed fabrics were analyzed. The application of VS dye in SC-CO2 medium exhibited satisfactory dye uptake performance, color characteristics, leveling properties and color fastness on all fabrics. The detection of higher K/S values on both PET and W/PET fabrics in SC-CO2 medium compared with those in aqueous medium can advantageously the application of such type of VS dye structure for SC-CO2 dyeing of W/PET blended fabrics on a semi-pilot or industrial scale. These findings can induce an alternative approach to the conventional aqueous dyeing of blend fabrics, which are mainly carried out in two separate dyebaths, resulting in saving time, chemicals, water and dyes. The SC-CO2 dyeing also found satisfactory for the type of N fabric products. The nucleophilic addition reaction between the VS reactive group of the dye molecules and nucleophilic sites in W and N fabrics is possible under SC-CO2 conditions. The SC-CO2 dyeing has a notable influence on a*, b* and C* values of the dyed PET, N and W/PET fabrics and showed that their appearance colors are higher and more saturated than the aqueous dyed samples. All the dyed W, PET and N fabrics and W/PET and W/N blended fabrics show very good levelling properties, indicating that the SC-CO2 dyeing method has a substantially better effect on the ∆E values of the dyed samples. The results of successive washing of the dyed W, PET and W/PET blended fabrics showed no significant color change during domestic laundering, even up to 20 cycles. These results can favour this model of VS reactive disperse dye for dyeing PET fabric and its W/PET blend fabrics in SC-CO2 medium. Therefore, the effect of the color range of reactive disperse dye structures incorporating different reactive groups and the blend ratios is a crucial point in SC-CO2 blends dyeing and further work will merit investigation.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hu, Q., Pei, L., Shen, J., Sun, S. & Wang, J. Level dyeing property and aggregation morphology of reactive black 5 on cotton fabric in a salt-free and less-water dyeing system. Text. Res. J. 94 (5–6), 598–608 (2024).
Pei, L., Li, H., Shen, J., Zhang, H. & Wang, J. Saltfree dyeing of cotton fabric and adsorption of reactive dyes in nonaqueous dyeing system: equilibrium, kinetics, and thermodynamics. Cellulose 29, 4753–4765. https://doi.org/10.1007/s10570-022-04576-9 (2022).
Pei, L., Gu, X. & Wang, J. Sustainable dyeing of cotton fabric with reactive dye in silicone oil emulsion for improving dye uptake and reducing wastewater. Cellulose 28, 2537–2550. https://doi.org/10.1007/s10570-020-03673-x (2021).
Zhu, H. et al. Efficient zero wastewater and salt-free of cotton fiber dyeing via hydrophobic organic chemical/water two-phase dyeing system. J. Clean. Prod. 451, 141886. https://doi.org/10.1016/j.jclepro.2024.141886 (2024).
Pei, L. et al. Comprehensive analysis of dye adsorption and supramolecular interaction between dyes and cotton fibers in non-aqueous media/less water dyeing system. Ind. Crops Prod. 222, 119955. https://doi.org/10.1016/j.indcrop.2024.119955 (2024).
Abou Elmaaty, T., Mousa, A., Gaafar, H., Hebeish, A. & Sorour, H. Development of Textile Dyeing using the Green Supercritical Fluid Technology, a review. Mater. Int., 373–390 (2020).
Ma, Y. et al. Effect of swelling on dyeing of cotton fabric in supercritical CO2 with ionic liquid domain reverse micelles. Cellulose 30 (5), 1–13. https://doi.org/10.1007/s10570-023-05565-2 (2023).
Ma, Y. et al. Dyeing of linen fabrics in supercritical CO2 using a reverse Micellar System with Ionic Liquid domains. J. Nat. Fibers. 20 (2), 2222555. https://doi.org/10.1080/15440478.2023.2222555 (2023).
Broadbent, P. J. et al. Supercritical carbon dioxide (SC-CO2) dyeing of cellulose acetate: an opportunity for a greener circular textile economy. Color. Technol. 139 (4), 475–488. https://doi.org/10.1111/cote.12690 (2023).
Cheng, Y. W. et al. Synthesis of Azo disperse dyes with high absorption for efficient polyethylene terephthalate dyeing performances in Supercritical Carbon Dioxide. Polymers 14, 3020. https://doi.org/10.3390/polym14153020 (2022).
Cheng, Y. W. et al. Red disperse azo dye side chains influence on Polyethylene Terephthalate Dyeing performances in Supercritical Carbon Dioxide Media. Polymers 14, 5487. https://doi.org/10.3390/polym14245487 (2022).
Abate, M. T. et al. Supercritical CO2 dyeing of polyester fabric with photochromic dyes to fabricate UV sensing smart textiles. Dyes Pigm. 183, 108671. https://doi.org/10.1016/j.dyepig.2020.108671 (2020).
Prokein, M., Dyes, T., Renner, M. & Weidner, E. Waterless leather dyeing with dense carbon dioxide as solvent for dyes. J. Supercrit. Fluids. 178, 105377. https://doi.org/10.1016/j.supflu.2021.105377 (2021).
Goñi, M. L., Gañán, N. A. & Martini, R. E. Supercritical CO2-assisted dyeing and functionalization of polymeric materials: a review of recent advances (2015–2020). J. CO2 Utilization. 54, 101760. https://doi.org/10.1016/j.jcou.2021.101760 (2021).
Schollmeyer, E., Knittel, D., Buschmann, H-J., Schneider, G. M. & Poulakis, K. DE. 39 06 724 A1, D 06 P 1/00. Germany: 13.09 (1990).
Saus, W., Knittel, D. & Schollmeyer, E. Dyeing of textiles in supercritical carbon dioxide. Text. Res. J. 63, 135–142. https://doi.org/10.1177/004051759306300302 (1993).
Knittel, D., Saus, W. & Schollmeyer, E. Application of supercritical Carbon Dioxide in finishing processes. J. Text. Inst. 84, 534–552. https://doi.org/10.1080/00405009308658986 (1993).
Saus, W., Knittel, D. & Schollmeyer, E. Dyeing with supercritical carbon dioxide-an alternative to high temperature dyeing of polyester. Textile-Praxis 47, 1052–1054 (1992).
Bach, E., Cleve, E. & Schollmeyer, E. Past, present and future of supercritical fluid dyeing technology-an overview. Rev. Prog. Color. Relat. Top. 32, 88–102. https://doi.org/10.1111/j.1478-4408.2002.tb00253.x (2002).
Montero, G. A., Smith, C. B., Hendrix, W. A. & Butcher, D. L. Supercritical fluid technology in textile processing: an overview. Ind. Eng. Chem. Res. 39, 4806–4812 (2000).
Perrut, M. Supercritical fluid applications: Industrial Developments and Economic issues. Ind. Eng. Chem. Res. 39, 4531–4535 (2000).
Banchero, M. Supercritical fluid dyeing of synthetic and natural textiles – a review. Color. Technol. 129, 2–17 (2013).
Knittel, D. & Schollmeyer, E. Environmentally friendly dyeing of synthetic fibres and textile accessories. Int. J. Cloth. Sci. Technol. 7, 17–35. https://doi.org/10.1108/09556229510080701 (1995).
DyeCoo. https://dyecoo.com/. Accessed 28th December (2022).
http://www.dyecoo.com/co2-dyeing and http://colourtex.co.in/product-category/textiledyes/polyester/corangar-pe/.
Yeh Group. DryDye Technology. https://amyyehyehyeh.wordpress.com/2014/11/28/drydye-waterless-dyeing/ (2014).
Long, J. J., Cui, C. L., Zhang, Y. Q. & Yuan, G. H. Clean fixation of dye on cotton in supercritical carbon dioxide with a heterogeneous and phase transfer catalytic reaction. Dyes Pigm. 115, 88–95. https://doi.org/10.1016/j.dyepig.2014.12.018 (2015).
Zhang, Y. Q., Wei, X. C. & Long, J. J. Ecofriendly synthesis and application of special disperse reactive dyes in waterless coloration of wool with supercritical carbon dioxide. J. Clean. Prod. 133, 746–756 (2016).
Schmidt, A., Bach, E. & Schollmeyer, E. The dyeing of natural fibres with reactive disperse dyes in supercritical carbon dioxide. Dyes Pigm. 56 (02), 27–35. https://doi.org/10.1016/S0143-7208 (2003).
Fernandez Cid, M. V. et al. Excellent dye fixation on cotton dyed in supercritical carbon dioxide using fluorotriazine reactive dyes. Green. Chem. 8, 609–616. https://doi.org/10.1039/b503801d (2005).
Patel, D. R. & Patel, K. C. Synthesis, antimicrobial activity and application of some novel quinazolinone based Monoazo reactive dyes on various fibres. Dyes Pigm. 90, 1–10 (2011).
Kraan, M., Cid, M. V. F., Woerlee, G. F., Veugelers, W. J. T. & Witkamp, G. J. Dyeing of natural and synthetic textiles in supercritical carbon dioxide with disperse reactive dyes. J. Supercrit. Fluids. 40, 470–476. https://doi.org/10.1016/j.supflu.2006.07.019 (2007).
Gao, D., Cui, H. S., Huang, T. T., Yang, D. F. & Lin, J. X. Synthesis of reactive disperse dyes containing halogenated acetamide group for dyeing cotton fabric in supercritical carbon dioxide. J. Supercrit. Fluids. 86, 108–114. https://doi.org/10.1016/j.supflu.2013.12.006 (2014).
Long, J. J. et al. Dyeing of cotton fabric with a reactive disperse dye in supercritical carbon dioxide. J. Supercrit. Fluids. 69, 13–20. https://doi.org/10.1016/j.supflu.2012.05.002 (2012).
Ozcan, A., Clifford, A., Bartle, K., Broadbent, P. & Lewis, D. Dyeing of modified cotton fibres with disperse dyes from supercritical carbon dioxide. Color. Technol. 114, 169–173 (1998).
Yang, D. F. et al. Dyeing of cotton fabric with reactive disperse dye contain acyl fluoride group in supercritical carbon dioxide. Dyes Pigm. 139, 566–574. https://doi.org/10.1016/j.dyepig.2016.12.050 (2017).
Luo, X. et al. Novel sustainable synthesis of dyes for clean dyeing of wool and cotton fibres in supercritical carbon dioxide. J. Clean. Prod. 199, 1–10. https://doi.org/10.1016/j.jclepro.2018.07.158 (2018).
Zaghloul, D. N. et al. Influence of additive organic base on dyeing of cotton fabric under supercritical carbon dioxide using fluorotriazine reactive disperse dye and investigation of optimal dyeing conditions. J. Supercrit. Fluids. 174, 105243. https://doi.org/10.1016/j.supflu.2021.105243 (2021).
Zhou, T., Wang, Y., Zheng, H., Du, B. & Zheng, L. Sustainable and eco-friendly strategies for polyester-cotton blends dyeing in supercritical CO2. J. CO2 Utilization. 55, 101816. https://doi.org/10.1016/j.jcou.2021.101816 (2022).
Maeda, S., Kunitou, K., Hihara, T. & Mishima, K. One-bath dyeing of Polyester/Cotton blends with reactive disperse dyes in supercritical Carbon Dioxide. Text. Res. J. 74, 989–994 (2004).
Abou Elmaaty, T., Sofan, M., Ayad, S., Negm, E. & Elsisi, H. Novel synthesis of reactive disperse dyes for dyeing and antibacterial finishing of cotton fabric under scCO2. J. CO2 Utilization. 61, 102053 (2022).
Abou Elmaaty, T. et al. Pilot scale water free dyeing of pure cotton under supercritical carbon dioxide. Carbohydr. Polym. Technol. Appl. 1, 100010. https://doi.org/10.1016/j.carpta.2020.100010 (2020).
Liao, S. K., Ho, Y. C. & Chang, P. S. Dyeing of nylon 6,6 with a disperse–reactive dye using supercritical carbon dioxide as the transport medium. J.S.D.C 116, 403–407 (2006).
Liao, S.K. Dyeing nylon-6,6 with some hydrophobic reactive dyes by supercritical processing. J. Poly. Res 11, 285–291 (2004).
Lee, W. J., Choi, W. H. & Kim, J. P. Dyeing of wool with temporarily solubilized disperse dyes. Color. Technol. 117, 212–216 (2001).
Lee, J. J., Han, N. K., Lee, W. J., Choi, J. H. & Kim, J. P. Synthesis of temporarily solubilized reactive disperse dyes and their application to the polyester/cotton blend fabric. Fibers Polym. 3, 85–90 (2002).
Lee, J. J., Han, N. K., Lee, W. J., Choi, J. H. & Kim, J. P. One-bath dyeing of a polyester/cotton blend with reactive disperse dyes from 2-hydroxypyrid-6-one derivatives. Color. Technol. 119, 134–139. https://doi.org/10.1111/j.1478-4408.2003.tb00163.x (2003).
Lee, J. J., Lee, W. J., Choi, J. H. & Kim, J. P. Synthesis and application of temporarily solubilised azo disperse dyes containing β-sulphatoethylsulphonyl group. Dyes Pigm. 65 (1), 75–81. https://doi.org/10.1016/j.dyepig.2004.06.017 (2005).
Kim, J. P., Kim, J. S., Park, J. S., Jang, S. S. & Lee, J. J. Synthesis of temporarily solubilised azodisperse dyes containing a β -sulphatoethylsulphonyl group and dispersant-free dyeing of polyethyleneterephthalate fabric. Color. Technol. 132, 368–375 (2016).
Park, Y. K., Jo, A. R., Jang, S. S. & Lee, J. J. Dispersant-free dyeing of poly (lactic acid) fabric with temporarily solubilised dispersedyes from azopyridone derivatives. Color. Technol. 132, 361–367 (2016).
Mousa, A. A., Youssef, Y. A., Farouk, R., Ayiesh, T. M. & Arief, M. H. Neutral dyeing of wool and wool /polyester blend fabrics using sulphatoethylsulphone reactive-disperse dyes. Color. Technol. 127, 304–309 (2011).
Lewis, D. M. & Shao, J. Z. A new approach to the dyeing of silk with sulphatoethylsulphone dyes. J. S D C. 111, 146–150. https://doi.org/10.1111/j.1478-4408.1995.tb01712.x (1995).
Kohl, J., Kim, J. D. & Kim, J. P. Synthesis and application of a temporarily solubilized alkali-clearable azo disperse dye and analysis of its conversion and hydrolysis behavior. Dyes Pigm. 56, 17–26. https://doi.org/10.1016/S0143-7208(02)00110-9 (2003).
ISO 105-C06. :2010 Textiles: Tests for Colour Fastness. Part C06: Colour Fastness to Domestic and Commercial Laundering (ISO, 2010).
ISO 105-E04. 2008 Textiles: Tests for Colour Fastness. Part E04: Colour Fastness Toperspiration (BSI: ISO, 2008).
ISO 105-P. 01:1993 Textiles: Tests for Colour Fastness. Part P01: Colour Fastness to dry heat (Excluding Pressing) (BSI (ISO, 1993).
ISO 105-B02. :2013 Textiles: Tests for Colour Fastness. Part B02: Colour Fastness to Artificial Light: Xenon arc Fading lamp test (BSI: ISO, 2013).
Poulakis, K., Spee, M., Knittel, S. G. M., Buschmann, D., Schollmeyer, E. & H. J. & Farbung Von Polyester in Uberkritischem CO2. Chemiefasern/Textilind 41/93, 142–147 (1991).
Saus, W., Knittel, D. & Schollmeyer, E. Farben Aus Uberkritischem Kohlendioxideine Alternative zur HT-Farbung Von Polyester. Textil Prax Int. 47, 1052–1054 (1992).
Schmidt, M. U., Bru¨ning, J., Wirth, D. & Bolte, M. Two azo pigments based on β-naphthol. Acta Cryst. C64, 474–477 (2008).
Park, S. C., Tuma, D., Kim, S., Lee, Y. R. & Shim, J. J. Sorption of C. I. disperse Red 60 in polystyrene and PMMA films and polyester and Nylon 6 textiles in the presence of supercritical carbon dioxide. Korean J. Chem. Eng. 27, 299–309 (2010).
Bach, E., Cleve, E. & Von Schollmeyer, E. Rohr, Treep Ch, editors. High pressure chemical engineering, , p. 581 (Elsevier Science, 1996).
Beltrame, P. L. et al. Morphological changes and dye uptake of poly(ethylene terephthalate) and 2,5-cellulose diacetate immersed in supercritical carbon dioxide. Dyes Pigm. 39, 35–47. https://doi.org/10.1016/S0143-7208(98)00006-0 (1998).
Von Schnitzler, J. & Eggers, R. Mass transfer in polymers in a supercritical CO2-atmosphere. J. Supercrit. Fluids. 16 (1), 81–92. https://doi.org/10.1016/S0896-8446(99)00020-0 (1999).
Fleming, O. S., Kazarian, S. G., Bach, E. & Schollmeyer, E. Confocal Raman study of poly (ethylene terephthalate) fibres dyed in supercritical carbon dioxide: dye diffusion and polymer morphology. Polymers 46, 9, 2943–2949. https://doi.org/10.1016/j.polymer.2005.02.067 (2005).
Acknowledgements
The authors thank the National Research Center for the financial support of the research and development in treatment and production lines of textile industries, in-house project ID: 13010209. They thank the Central Lab Services at Textile Research and Technology Institute for allowing us to use all facilities and apparatus.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.A.M. and Y.A.Y. wrote the main manuscript text and F.A.M. and S.A.A. prepared Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 16; Tables 1 and 2. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mousa, A.A., Mohamed, F.A., Abd El-Megied, S.A. et al. Dyeing of synthetic fiber-based wool blended fabrics in supercritical carbon dioxide. Sci Rep 14, 30604 (2024). https://doi.org/10.1038/s41598-024-81417-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81417-8