Abstract
Sepsis is a severe infectious disease with high mortality. However, the indicators used to evaluate its severity and prognosis are relatively complicated. The systemic inflammatory response index (SIRI), a new inflammatory indicator, has shown good predictive value in chronic infection, stroke, and cancer. The purpose of this study was to investigate the connection between sepsis and SIRI and evaluate its predictive usefulness. A total of 401 patients with sepsis were included in this study. Multiple linear regression and logistic regression analyses were performed to evaluate the relationship between SIRI and sepsis. The restricted cubic spline (RCS) method was employed to illustrate the dose-response relationship. The area under the curve (AUC) and decision curve analysis (DCA) were used to evaluate the prognostic value of SIRI. Multiple linear regression analysis revealed a significant positive correlation between SIRI and both blood cell count and Sequential Organ Failure Assessment (SOFA) score. Additionally, higher SIRI levels were significantly linked to a higher risk of sepsis worsening, according to logistic regression analysis. The RCS curve demonstrated that the risk of poor prognosis rose with increasing SIRI, particularly when SIRI exceeded 6.1. Furthermore, AUC and DCA results showed that SIRI had superior predictive value compared to traditional indicators. A higher SIRI is linked to a worse prognosis and more severe sepsis. SIRI may serve as a novel prognostic indicator in sepsis, though further clinical studies are necessary to confirm these findings.
Similar content being viewed by others
Introduction
Sepsis, characterized by an uncontrolled inflammatory response leading to organ dysfunction, presents a significant global health challenge1,2,3. In 2017, approximately 48.9 million sepsis cases were recorded worldwide, resulting in 11.0 million deaths, accounting for 19.7% of all global fatalities4,5. This health crisis not only impacts patient outcomes but also imposes substantial economic burdens, with an estimated $24 billion in annual expenses in the United States alone6,7,8. Similarly, China reported a significant burden, with a standardized incidence rate of 328.25 per 100,000 cases in 20178,9. Sepsis’s high rates of morbidity and mortality place a significant financial burden on healthcare systems around the world1,6,9.
Currently, healthcare professionals evaluate sepsis severity and prognosis using tools such as the Sequential Organ Failure Assessment (SOFA) and the Acute Physiology and Chronic Health Evaluation-II (APACHE II) scores10,11. These assessments incorporate various parameters, including blood gases, liver and kidney function tests, blood counts, the Glasgow Coma Scale (GCS), age, and patients’ prior health status10,11. Rapid evaluation of the severity and consequences of sepsis is frequently hampered by the intricacy of these markers. Thus, there is an urgent need for a simpler method to effectively evaluate the condition and prognostic outcome of sepsis patients.
According to earlier research, organ failure results from sepsis, which is caused by an imbalance between pro- and anti-inflammatory reactions1,12. Commonly used indicators for assessing infection include leukocytes, neutrophils, neutrophil ratio, procalcitonin (PCT), and C-reactive protein13,14,15. Recent research has introduced emerging indicators for inflammation assessment, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), systemic immunoinflammatory index (SII), and systemic inflammatory response index (SIRI)16,17,18. Compared to conventional single-factor indicators, these innovative indicators—which integrate several laboratory results—are found to be more representative of overall body inflammation and metabolic abnormalities16,17,19,20.
Notably, SIRI, which links monocytes and lymphocytes, has shown a strong association with stroke and tumor chemotherapy prognosis, indicating its broader clinical relevance21,22,23,24,25. The pathophysiology of sepsis involves intricate interactions between inflammatory and anti-inflammatory responses, including the suppression of T and B lymphocyte functions in advanced stages26,27,28. SIRI, by incorporating these cellular components, offers a more comprehensive and accurate assessment of the inflammatory process. SIRI’s potential as a prognostic tool for inflammation, ischemic stroke, and tumor prognosis has been highlighted by recent research21,22,23,29. However, its reliability and superiority over traditional indicators in severe sepsis cases remain uncertain, necessitating further investigation.
The primary objective of this study is to explore the correlation between SIRI and the severity of sepsis. Additionally, the study aims to evaluate SIRI’s potential as a novel predictive marker for both the severity and prognosis of sepsis patients.
Materials and methods
Study population
Patients admitted to the intensive care unit (ICU) of the Affiliated Hospital of Nantong University for sepsis between January 1, 2021, and March 2023 were included in this study. The inclusion criteria were: admission to the ICU for sepsis for more than 24 h, age over 18 years, and a SOFA score of ≥ 2. Exclusion criteria included: pregnancy, age less than 18 years, insufficient clinical information, organ transplantation within the previous year, brain death or vegetative state, and severe craniocerebral trauma with a Glasgow Coma Scale (GCS) score of less than 8. Patients with pre-existing organ transplants, end-stage tumors, or severe craniocerebral injuries were excluded due to the significant impact of their primary disease on clinical outcomes, which could introduce bias into the study. Ultimately, 401 patients were included in our analysis (Fig. 1).
The study adhered to the Declaration of Helsinki and received approval from the Ethics Committee of Nantong University Hospital (z2020034). All participants’ and families gave informed consent and signed informed consent forms.
Data collection and definitions
Data for this study were obtained from patients’ electronic medical records. Baseline blood samples were collected upon ICU admission, including routine blood tests, blood biochemistry, blood gas analysis, PCT, and NT-proBNP. Test results were recorded within 6 h of admission. Blood lactate, pH, and oxygenation index were measured using an automatic blood gas analyzer; prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen levels were detected using a coagulometer; blood creatinine, bilirubin, and albumin were measured using an automated biochemistry analyzer, leukocytes, neutrophils, lymphocytes, and monocytes were measured using a blood cell analyzer. All blood samples were collected by trained nurses and analyzed by professional examiners following current guidelines and standards. A standardized procedure was used to record vital signs such as respiration, heart rate, and blood pressure upon ICU admission. The axillary temperature was also measured and recorded using a standard method. Mean arterial pressure (MAP) was calculated as diastolic blood pressure plus one-third of the pulse pressure, where pulse pressure equals systolic minus diastolic blood pressure. Details on comorbid conditions and prior medical histories are provided in the Supplementary Material. Sepsis was defined according to the Sepsis Campaign Guidelines version 3.0, i.e., a SOFA score of ≥ 2 with concurrent severe infection30,31. The presence of invasive mechanical ventilation, continuous renal replacement therapy (CRRT), and the use of vasoactive drugs were categorized as dichotomous variables. Vasoactive drug use was defined as the administration of norepinephrine, epinephrine, dopamine, dobutamine, or vasopressin during ICU hospitalization. Baseline information was collected within the first 24 h of ICU admission. The formulae for the inflammation indices discussed are as follows: SIRI = (neutrophil count * monocyte count) / lymphocyte count; LMR = lymphocyte count/monocyte count; PLR = platelet count/lymphocyte count; NLR = neutrophil count/lymphocyte count; SII = (platelet count * neutrophil count)/lymphocyte count32,33,34.
Main outcome
The primary outcomes in this study were based on the severity of the patient’s condition upon ICU admission. Endpoints were categorized into improvement or deterioration of the condition during the ICU stay. Improvement criteria included maintaining a finger-pulse oxygen saturation of over 95% for 48 h after switching from mechanical ventilation to regular oxygen and maintaining a mean arterial pressure of over 65 mmHg for 24 h after weaning off vasoactive drugs35,36. The criteria for worsening sepsis were: the patient’s mean arterial pressure was less than 65 mmHg for a duration of greater than 48 h, and the affected party decided to forgo further treatment36,37. Or within 28 days of hospitalization in the ICU, the patient has died38.
Statistical analysis
Continuous variables are presented as means ± standard deviation (SD), as appropriate, while categorical variables are expressed as proportions. To compare differences between groups, we used the χ2 test for categorical variables. For continuous variables, we first evaluate normality. For variables that fit the normal distribution, we used univariate analysis of variance (ANOVA). Conversely, for variables that do not conform to the normal distribution, we use the Kruskal-Wallis test. Multicollinearity was assessed using the variance inflation factor (VIF), with a VIF value of less than 5 for each variable indicating the absence of multicollinearity (Table S1). Multiple linear regression was employed to assess the relationship between SIRI, SOFA score, and leukocyte count. Additionally, multifactorial logistic regression was used to explore the association between SIRI and sepsis, with results expressed as odds ratios (OR) and 95% confidence intervals (CI). At the same time, the SIRI-based tertiles were included in the model for trend testing. Restricted cubic splines (RCS) were utilized to evaluate the dose-response relationship between SIRI and sepsis severity. To further validate the adverse effects of high SIRI levels on disease progression, a two-stage analysis was conducted based on the turning point. Subgroup analyses were performed to assess the impact of SIRI on outcomes across different populations, with group variances compared based on multiplicative interaction. Finally, the predictive advantages of SIRI over traditional sepsis prognostic indicators were comparatively analyzed using receiver operating characteristics (ROC) and decision curve analysis (DCA).
All statistical tests were two-sided, with a significance level set at 0.05. Analyses were conducted using R (version 4.2.2).
Results
Basic characteristics of participants
Among the 401 participants, those with higher SIRI levels tended to have relatively higher white blood cell counts, neutrophil counts, monocyte counts, N-terminal pro-B-type natriuretic peptide (NT-proBNP), albumin, fibrinogen, and SOFA scores, while body temperature and blood lactate levels were relatively lower. In terms of patients’ medical history, the high SIRI group had a greater prevalence of hypertensive disorders compared to the low SIRI group. Additionally, they exhibited a higher incidence of infectious shock and were more likely to require vasoactive medications. Other clinical parameters showed no significant differences between the groups (Table 1). Furthermore, when regrouping according to the prognostic status of the disease, we found that the results were largely consistent, with significantly higher SIRI levels observed in the deteriorating group (Table S2).
Relationship between SIRI and white blood cell count
Table 2 illustrates the relationship between SIRI and white blood cell counts, revealing a strong positive correlation. This positive association persisted even after full adjustments in Model 4. Additionally, the consistency of these results was observed when SIRI was analyzed as a categorical variable. Specifically, in the fully adjusted Model 4, the β-values for the T2 and T3 groups were 5.486 and 11.870, respectively, compared to the T1 group. These findings suggest that SIRI may serve as a reliable marker for reflecting inflammation within the organism.
Relationship between SIRI and SOFA scores
Similar to the relationship observed with white blood cell counts, there was a positive correlation between SIRI—both as a continuous and categorical variable—and the SOFA score. SOFA scores were significantly higher in the T2 group (β: 2.259, 95% CI: 1.564, 3.354) and the T3 group (β: 3.094, 95% CI: 2.201, 3.984) compared to the T1 group. This relationship remained consistent across both Model 1 and the fully adjusted Model 4 (Table 3). These findings suggest that SIRI may serve as a potentially reliable marker for assessing disease severity in patients with sepsis.
Relationship between SIRI and prognosis in patients with sepsis
Logistic regression analysis revealed a strong correlation between SIRI levels and prognosis in patients with sepsis (OR, 1.015; 95% CI, 1.003–1.028). After adjusting for all covariates, this correlation remained significant in Model 4 (OR, 1.009; 95% CI, 1.004–1.030). Additionally, the risk of disease progression was 1.38 times higher in the T2 group and 1.879 times higher in the T3 group compared with the T1 group. These findings suggest that SIRI may be associated with the prognosis of septic patients. Therefore, reducing SIRI could potentially improve the condition of these patients to some extent (Table 4).
Dose-response relationship and two-stage comparative analysis
RCS curves were employed to explore the dose-response relationship between SIRI and sepsis prognosis. Figure S1 illustrates the trend between these two variables. To further emphasize the noteworthy influence of SIRI, we performed a two-stage comparative study after determining the turning point based on the curve. The results indicated that the risk of sepsis increased significantly when SIRI exceeded 6.1. Moreover, the risk of progression was 98.1% higher in the group with a SIRI above 6.1 compared to the group with a SIRI of 6.1 or less (Table 5).
Subgroup analysis
To further investigate the impact of SIRI on the prognosis of sepsis patients across different stratification factors, we conducted a preliminary stratification based on baseline characteristics, sepsis with concomitant organ dysfunction, and patient origin. The results aligned with the overall trend of increasing sepsis deterioration risk as SIRI levels increased (Fig. 2). These findings reinforce our conclusion that the relationship between SIRI and prognosis in sepsis patients is not significantly influenced by other stratification factors. Notably, an intriguing observation emerged: patients with a surgical origin exhibited a higher risk of deterioration associated with elevated SIRI compared to those with a general medicine origin. This suggests that clinicians should be particularly vigilant about the potential for poor prognosis in surgical patients with elevated SIRI levels.
Comparison of the ability of three indicators to predict the prognosis of patients with sepsis
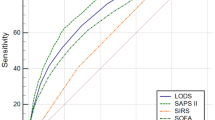
To evaluate the prognostic predictive value of SIRI compared to other indicators in sepsis patients, we first utilized ROC analysis to assess the predictive performance of each index. The results demonstrated that when various indicators, including SIRI, were added individually to the fully adjusted Model 4, SIRI exhibited the largest area under the curve (AUC = 0.682) (Fig. 3; Table 6). To further validate the superiority of SIRI, we conducted additional analysis using the DCA curve, which confirmed that SIRI provided the best incremental value (Fig. 4). Furthermore, we validated these findings with the net reclassification index, and the results remained consistent (Fig. S2). Finally, to further verify that the SIRI has the greatest predictive value, we performed a Boruta variable importance analysis, and the results remain consistent (Fig. S3). Overall, these results indicate that SIRI offers the best predictive performance compared to other metrics.
Discussion
SIRI has been recognized as a predictive marker in various conditions, including inflammation, tumor prognosis, and ischemic stroke18,21,23,24,39. However, its association with sepsis has not yet been explored. This study demonstrates the prognostic utility of SIRI in this setting by establishing a strong correlation between it and sepsis. We observed a strong positive correlation between SIRI and SOFA scores. The RCS analysis further indicated that the risk of adverse outcomes in sepsis patients increases with rising SIRI levels, particularly when SIRI exceeds 6.1. This relationship persisted even after adjusting for various covariates. Moreover, SIRI’s predictive value in sepsis is superior to that of conventional indicators, as shown by ROC and DCA studies. In summary, these findings suggest that closely monitoring SIRI, which represents immunity and inflammation, and maintaining it at lower levels in critically ill septic patients may improve their prognosis.
Traditional indicators for evaluating inflammation in sepsis include NLR, PLR, LMR, PCT, and C-reactive protein, as referenced in various studies16,17,20,40,41. However, these indicators often overlook the role of lymphocytes, which are crucial for understanding the high morbidity and long-term mortality associated with sepsis. In contrast, SIRI, which includes lymphocytes in its calculation, may provide a more comprehensive assessment of the inflammatory response in sepsis patients.
Previous research has consistently shown that SIRI is linked to inflammatory responses and mortality rates in various conditions18,22,42,43. For example, a study on in-hospital mortality in patients with novel coronavirus pneumonia revealed that higher SIRI values were associated with increased mortality, as shown by the Kaplan-Meier survival curve, which indicated lower survival rates for patients with elevated SIRI levels18. Similarly, in the context of ischemic stroke accompanied by pneumonia, a significant increase in SIRI values was observed in patients with pneumonia compared to a control group, underscoring the index’s relevance in different clinical scenarios42. A case-control study on adverse pregnancy outcomes in patients with systemic lupus erythematosus (SLE) revealed that SIRI values were significantly higher in women with SLE than in healthy controls. Among pregnant women with lupus, those exhibiting clinical symptoms had significantly higher SIRI values compared to their asymptomatic counterparts43. Extending these findings, our study specifically investigated the role of SIRI in sepsis. We found that patients with higher SIRI scores tended to have worse prognoses, often accompanied by infectious shock. Diverging from earlier studies, our focus expanded to include the relationship between SIRI and circulatory disturbances. We observed that higher SIRI levels not only correlated with sepsis severity but also significantly increased the likelihood of concurrent infectious shock, highlighting the broader clinical implications of elevated SIRI.
One important finding from our subgroup analysis was that SIRI had a higher predictive impact on patients who had surgery than on those who had medicinal treatment. The underlying reasons for this are multifaceted. Firstly, surgery often results in soft tissue injury, leading to both hyperinflammation and immunosuppression44,45,46. Secondly, surgery activates neutrophils within 24 h, increasing the risk of postoperative infections47,48,49. Thirdly, surgical procedures can impair the functions of monocytes and natural killer cells, decreasing the monocytes’ ability to produce TNF and shifting the immune response towards one mediated by helper T cells, which elevates infection risks47,50. Fourthly, the stress response induced by surgery activates the hypothalamo-pituitary-adrenal axis, potentially leading to a state of relative immunosuppression post-surgery51,52,53. Finally, the overall weakened state of patients post-surgery further escalates their susceptibility to infections and related complications54,55.
The pathogenesis of sepsis is complex, involving innate and adaptive immune responses, activation of co-stimulatory molecules, diverse signaling pathways, and abnormal apoptotic and necrotic mechanisms56,57,58,59. Upon pathogen invasion, macrophages engulf the pathogens and secrete cytokines such as interleukin 1-β, tumor necrosis factor, and interleukin-6, triggering the innate immune response59. Concurrently, neutrophils converge at the infection site, eliminating pathogens through phagocytosis and the formation of neutrophil extracellular traps56,59. The immune response is further amplified when antigen-presenting cells activate CD4 + T lymphocytes via co-stimulatory molecules like CD40, leading to the release of interferon-gamma, which enhances the activity of phagocytes and B lymphocytes59. In sepsis patients, distinct necro-apoptotic processes are observed. Autopsies have revealed significant decreases in gastrointestinal epithelial cells and lymphocytes. Studies indicate that B-lymphocytes and CD4 + T-lymphocytes are particularly susceptible to apoptosis in sepsis, contributing to immunosuppression and increasing the risk of subsequent infections. Neutrophils’ controlled apoptosis helps to avoid excessive inflammation, but sepsis can postpone this process, which may result in organ malfunction59. Additionally, co-stimulatory molecules play a significant role in the pathogenesis of sepsis59,60. Members of the B7 family, such as CD80/CD86, CD40, and PDL1/PDL2, influence the functional status of macrophages, neutrophils, and lymphocytes through various signaling pathways. Sepsis patients’ conditions may become even more complicated as a result of this interaction, which may cause an imbalance in inflammatory responses59,60,61.
The innovation of this study lies in our comprehensive use of statistical methods to explore the groundbreaking relationship between SIRI and sepsis severity. To further illustrate SIRI’s dependable and consistent performance, we conducted threshold analysis and comparative analysis. However, alongside these strengths, certain limitations should be acknowledged. First, as an observational study, we can only establish an association, not causation. Second, this study is a single-center study, which necessitates caution when generalizing the results. In addition, although we adjusted for as many confounders as possible, some potential confounders may still exist. Finally, since we only collected test parameters at the time of patient admission to the ICU, these data may change dynamically during hospitalization, potentially affecting the results. Therefore, further studies are needed to verify the impact of dynamic changes in SIRI on sepsis prognosis.
Conclusion
Our research identified a significant positive correlation between SIRI levels and the severity of sepsis. Notably, SIRI demonstrated superior predictive capabilities compared to traditional predictors commonly used in this context. However, given the observational nature of our study, further prospective studies are essential to substantiate and confirm these findings.
Data availability
The manuscript contains all the evidence that supports the findings. The datasets used and/or analyzed in this study are available from the corresponding author on request if necessary.
References
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810. https://doi.org/10.1001/jama.2016.0287 (2016).
Morgan, P. & Majeed, A. Sepsis: Getting the balance right. BMJ (Clin. Res. ed.). 367, l6700. https://doi.org/10.1136/bmj.l6700 (2019).
Nedeva, C. Inflammation and cell death of the Innate and adaptive immune system during sepsis. Biomolecules 11 https://doi.org/10.3390/biom11071011 (2021).
Evans, L. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247. https://doi.org/10.1007/s00134-021-06506-y (2021).
Wagenlehner, F. M. E. & Dittmar, F. Re: Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Eur. Urol. 81 213 https://doi.org/10.1016/j.eururo.2021.11.014 (2022).
Rudd, K. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet (Lond. Engl.). 395, 200–211. https://doi.org/10.1016/s0140-6736(19)32989-7 (2020).
Dellinger, R. P. et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637. https://doi.org/10.1097/CCM.0b013e31827e83af (2013).
Gu, X., Zhou, F., Wang, Y., Fan, G. & Cao, B. Respiratory viral sepsis: Epidemiology, pathophysiology, diagnosis and treatment. Eur. Respir. Rev. Off. J. Eur. Respirat. Soc. 29 https://doi.org/10.1183/16000617.0038-2020 (2020).
Weng, L. et al. National incidence and mortality of hospitalized sepsis in China. Crit. Care. (Lond. Engl.). 27, 84. https://doi.org/10.1186/s13054-023-04385-x (2023).
Seymour, C. et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 762–774. https://doi.org/10.1001/jama.2016.0288 (2016).
Su, X. et al. Comparison of four early warning scores in predicting the prognosis of critically ill patients in secondary hospitals. Zhonghua Wei Zhong Bing Ji jiu Yi Xue. 35, 1093–1098. https://doi.org/10.3760/cma.j.cn121430-20230614-00441 (2023).
Gotts, J. & Matthay, M. Sepsis: Pathophysiology and clinical management. BMJ (Clin. Res. ed.). 353, i1585. https://doi.org/10.1136/bmj.i1585 (2016).
Barreiro, O., Martín, P., González-Amaro, R. & Sánchez-Madrid, F. Molecular cues guiding inflammatory responses. Cardiovasc. Res. 86, 174–182. https://doi.org/10.1093/cvr/cvq001 (2010).
Limper, M., de Kruif, M. D., Duits, A. J., Brandjes, D. P. & van Gorp, E. C. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J. Infect. 60, 409–416. https://doi.org/10.1016/j.jinf.2010.03.016 (2010).
Verbakel, J. Y., Van den Bruel, A. & Thompson, M. C-Reactive protein for antibiotic use in COPD exacerbations. N. Engl. J. Med. 381, 2371. https://doi.org/10.1056/NEJMc1912624 (2019).
Djordjevic, D. et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically Ill and injured patients: Which ratio to choose to predict outcome and nature of bacteremia? Mediat. Inflamm. 3758068. https://doi.org/10.1155/2018/3758068 (2018).
Huang, Z., Fu, Z., Huang, W. & Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 38, 641–647. https://doi.org/10.1016/j.ajem.2019.10.023 (2020).
Fois, A. G. et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules 25 https://doi.org/10.3390/molecules25235725 (2020).
Mangalesh, S., Dudani, S. & Malik, A. The systemic immune-inflammation index in predicting sepsis mortality. Postgrad. Med. 135, 345–351. https://doi.org/10.1080/00325481.2022.2140535 (2023).
Russell, C. et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J. Infect. 78, 339–348. https://doi.org/10.1016/j.jinf.2019.02.006 (2019).
Xia, Y. et al. Systemic Immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J. Clin. Med. 12 https://doi.org/10.3390/jcm12031128 (2023).
Zhang, Y., Xing, Z., Zhou, K. & Jiang, S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin. Interv. Aging. 16, 1997–2007. https://doi.org/10.2147/cia.S339221 (2021).
Schietroma, M. et al. Systemic inflammation response index (SIRI) as predictor of anastomotic leakage after total gastrectomy for gastric cancer. Surg. Oncol. 43, 101791. https://doi.org/10.1016/j.suronc.2022.101791 (2022).
Xin, Y. et al. A systemic inflammation response index (SIRI)-based nomogram for predicting the recurrence of early stage hepatocellular carcinoma after radiofrequency ablation. Cardiovasc. Interv. Radiol. 45, 43–53. https://doi.org/10.1007/s00270-021-02965-4 (2022).
Mureşan, A. et al. Prognostic nutritional index, controlling nutritional status (CONUT) score, and inflammatory biomarkers as predictors of deep vein thrombosis, acute pulmonary embolism, and mortality in COVID-19 patients. Diagnostics (Basel Switzerland). 12 https://doi.org/10.3390/diagnostics12112757 (2022).
van der Poll, T., van de Veerdonk, F., Scicluna, B. & Netea, M. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17, 407–420. https://doi.org/10.1038/nri.2017.36 (2017).
Hotchkiss, R., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874. https://doi.org/10.1038/nri3552 (2013).
Cohen, J. The immunopathogenesis of sepsis. Nature 420, 885–891. https://doi.org/10.1038/nature01326 (2002).
Ma, H. et al. Association of systemic inflammatory response index with bone mineral density, osteoporosis, and future fracture risk in elderly hypertensive patients. Postgrad. Med. 136, 406–416. https://doi.org/10.1080/00325481.2024.2354158 (2024).
Cecconi, M., Evans, L., Levy, M. & Rhodes, A. Sepsis and septic shock. Lancet (Lond. Engl.). 392, 75–87. https://doi.org/10.1016/s0140-6736(18)30696-2 (2018).
Moreno, R. et al. The sequential organ failure assessment (SOFA) score: Has the time come for an update? Crit. Care. (Lond. Engl.). 27, 15. https://doi.org/10.1186/s13054-022-04290-9 (2023).
Ma, F. et al. The relationship between systemic inflammation index, systemic immune-inflammatory index, and inflammatory prognostic index and 90-day outcomes in acute ischemic stroke patients treated with intravenous thrombolysis. J. Neuroinflamm. 20, 220. https://doi.org/10.1186/s12974-023-02890-y (2023).
Wang, R. H. et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front. Immunol. 14 https://doi.org/10.3389/fimmu.2023.1115031 (2023).
Russell, C. D. et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J. Infect. 78, 339–348. https://doi.org/10.1016/j.jinf.2019.02.006 (2019).
Perner, A. et al. Sepsis: Frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. 42, 1958–1969. https://doi.org/10.1007/s00134-016-4577-z (2016).
Evans, L. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 49, e1063–e1143. https://doi.org/10.1097/ccm.0000000000005337 (2021).
Prescott, H. C. et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am. J. Respir. Crit Care Med. 200, 972–981. https://doi.org/10.1164/rccm.201812-2383CP (2019).
Ibarra-Estrada, M. et al. Early adjunctive methylene blue in patients with septic shock: A randomized controlled trial. Crit. Care. (Lond. Engl.). 27, 110. https://doi.org/10.1186/s13054-023-04397-7 (2023).
Cai, X. et al. Systemic inflammation response index as a predictor of stroke risk in elderly patients with hypertension: A cohort study. J. Inflamm. Res. Volume. 16, 4821–4832. https://doi.org/10.2147/jir.S433190 (2023).
Póvoa, P. et al. How to use biomarkers of infection or sepsis at the bedside: Guide to clinicians. Intensive Care Med. 49, 142–153. https://doi.org/10.1007/s00134-022-06956-y (2023).
Reinhart, K., Bauer, M., Riedemann, N. & Hartog, C. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 25, 609–634. https://doi.org/10.1128/cmr.00016-12 (2012).
Wang, R. et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front. Immunol. 14 https://doi.org/10.3389/fimmu.2023.1115031 (2023).
Sahin, R. et al. The role of first-trimester NLR (neutrophil to lymphocyte ratio), systemic immune-inflammation index (SII), and, systemic immune-response index (SIRI) in the prediction of composite adverse outcomes in pregnant women with systemic lupus erythematosus. J. Reprod. Immunol. 158, 103978. https://doi.org/10.1016/j.jri.2023.103978 (2023).
Toft, P., Dagnaes-Hansen, F., Tønnesen, E. & Petersen, M. Influence of surgery and endotoxin-induced sepsis combined on natural killer cell activity, oxidative burst of granulocytes and antigen presentation capability of monocytes. Acta Anaesthesiol. Scand. 46, 405–410. https://doi.org/10.1034/j.1399-6576.2002.460413.x (2002).
Jiménez-Sousa, M. A. et al. Mitochondrial DNA haplogroups are associated with severe sepsis and mortality in patients who underwent major surgery. J. Infect. 70, 20–29. https://doi.org/10.1016/j.jinf.2014.07.005 (2015).
Arias, J. I., Aller, M. A. & Arias, J. Surgical inflammation: A pathophysiological rainbow. J. Transl. Med. 7 https://doi.org/10.1186/1479-5876-7-19 (2009).
Fried, E., Weissman, C. & Sprung, C. Postoperative sepsis. Curr. Opin. Crit. Care. 17, 396–401. https://doi.org/10.1097/MCC.0b013e328348bee2 (2011).
Howe, C. W. Prevention and control of postoperative wound infections owing to Staphylococcus aureus. N. Engl. J. Med. 255, 787–794. https://doi.org/10.1056/nejm195610252551701 (1956).
Bassetti, M., Eckmann, C., Giacobbe, D. R., Sartelli, M. & Montravers, P. Post-operative abdominal infections: Epidemiology, operational definitions, and outcomes. Intensive Care Med. 46, 163–172. https://doi.org/10.1007/s00134-019-05841-5 (2020).
Albertsmeier, M. et al. Monocyte-dependent suppression of T-Cell function in postoperative patients and abdominal sepsis. Shock (Augusta Ga). 48, 651–656. https://doi.org/10.1097/shk.0000000000000924 (2017).
Desborough, J. The stress response to trauma and surgery. Br. J. Anaesth. 85, 109–117. https://doi.org/10.1093/bja/85.1.109 (2000).
Desborough, J. P. The stress response to trauma and surgery. Br. J. Anaesth. 85, 109–117. https://doi.org/10.1093/bja/85.1.109 (2000).
Matzner, P. et al. Deleterious synergistic effects of distress and surgery on cancer metastasis: Abolishment through an integrated perioperative immune-stimulating stress-inflammatory-reducing intervention. Brain. Behav. Immun. 80, 170–178. https://doi.org/10.1016/j.bbi.2019.03.005 (2019).
Brakenridge, S. et al. Current epidemiology of surgical sepsis: Discordance between inpatient mortality and 1-year outcomes. Ann. Surg. 270, 502–510. https://doi.org/10.1097/sla.0000000000003458 (2019).
Walton, B. Effects of anaesthesia and surgery on immune status. Br. J. Anaesth. 51, 37–43. https://doi.org/10.1093/bja/51.1.37 (1979).
Zhang, H. et al. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin. Translational Med. 13, e1170. https://doi.org/10.1002/ctm2.1170 (2023).
Bosmann, M. & Ward, P. The inflammatory response in sepsis. Trends Immunol. 34, 129–136. https://doi.org/10.1016/j.it.2012.09.004 (2013).
Joffre, J., Hellman, J., Ince, C. & Ait-Oufella, H. Endothelial responses in sepsis. Am. J. Respir. Crit Care Med. 202, 361–370. https://doi.org/10.1164/rccm.201910-1911TR (2020).
Stearns-Kurosawa, D., Osuchowski, M., Valentine, C., Kurosawa, S. & Remick, D. The pathogenesis of sepsis. Annu. Rev. Pathol. 6, 19–48. https://doi.org/10.1146/annurev-pathol-011110-130327 (2011).
Lu, Y., An, L., Liu, Q. & Li, C. Expression and clinical correlations of costimulatory molecules on peripheral T lymphocyte subsets of early-stage severe sepsis: A prospective observational study. Shock (Augusta Ga). 49, 631–640. https://doi.org/10.1097/shk.0000000000001017 (2018).
Carli, F. et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: A randomized clinical trial. JAMA Surg. 155, 233–242. https://doi.org/10.1001/jamasurg.2019.5474 (2020).
Funding
This study was supported by the Jiangsu Provincial Health Commission. Project number: Z2020034.
Author information
Authors and Affiliations
Contributions
Shu Lu provided the idea for this study and oversaw the entire study. Tuo Xu collected data and wrote the manuscript. Tuo Xu, Shuaiwei Song, and Ke Zhu performed data counting and manuscripts written. Naixue Wang, and Yin Yang performed literature search and protocol design. Chengyu Wu helped in the manuscript revision process. All authors have contributed significantly to this work, have read the manuscript, and have approved the submission of the manuscript in its current form.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, T., Song, S., Zhu, K. et al. Systemic inflammatory response index improves prognostic predictive value in intensive care unit patients with sepsis. Sci Rep 15, 1908 (2025). https://doi.org/10.1038/s41598-024-81860-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81860-7