Abstract
Insulin resistance (IR) is linked to both the vulnerable plaque and the stroke risk. However, the precise extent of this correlation and its impact on stroke risk in carotid artery stenosis patients remain unclear. Therefore, this study aims to investigate the relationship between vulnerable plaque and IR and stroke risk and the mediating role of vulnerable plaque in patients with carotid artery stenosis. This study included 505 patients with carotid artery stenosis. IR was assessed using the triglyceride-glucose (TyG) index. The association of the TyG index and vulnerable plaque with stroke risk was investigated using the restricted cubic splines (RCS)and adjusted Logistic regression. Additionally, the mediation analysis was used to explore the mediating impact of the vulnerable plaque on the association between the TyG index and stroke risk. A total of 184 (36.4%) stroke events were recorded. The RCS curves revealed a positive linear association between TyG index and risk events among patients with carotid artery stenosis (P-value < 0.001 and P for nonlinear = 0.860). After fully adjusting for covariates, both the TyG index and vulnerable plaque emerged as significant predictors of stroke events. Mediation analysis indicated that the vulnerable plaque mediated 18.3%, 15.8%, 13.9%, and 11.6% of the correlation between the TyG index and stroke risk in different adjusted models, respectively. TyG index and vulnerable plaque are associated with a higher risk of stroke in patients with carotid artery stenosis. In addition, vulnerable plaques partially mediated the relationship between TyG index and stroke risk.
Similar content being viewed by others
Introduction
Globally, stroke is the main cause of adult death and severe long-term disability, while the risk of stroke in Chinese population is the highest in the world, and the incidence of stroke is gradually increasing1,2. Among the inpatients with cerebrovascular diseases in China, about 83% are ischemic stroke, and the recurrence rate within 1 year is about 9.6%~17.7%3,4,5. The main pathological mechanism of stroke is atherosclerosis, and about 7–18% of ischemic stroke are attributed to carotid atherosclerosis6,7,8,9. Therefore, early detection of high-risk patients with carotid atherosclerosis and timely management of related risk factors are crucial to reduce the occurrence of stroke.
Insulin resistance (IR) is considered as a predictor of cerebrovascular diseases, which is closely related to the occurrence and development of atherosclerosis10,11. The triglyceride-glucose (TyG) index was first proposed in 2008, it can be obtained only by detecting fasting blood glucose and fasting triglyceride, and it was found that TyG index is a better alternative index to identify IR than HOMA-IR index, and it is more economical, convenient and effective12,13. Up to now, TyG index has been identified as a reliable alternative biomarker of IR14.A large prospective cohort study from 22 countries on five continents found that the TyG index was significantly associated with the incidence and mortality of cardiovascular disease, and the TyG index helped in the early identification of individuals at high risk of developing cardiovascular disease15. At the same time, there is growing evidence that the increase of TyG index is significantly related to the increased incidence of stroke and the risk of recurrence in both diabetic and non-diabetic patients16,17.
In addition, a number of previous studies have consistently shown that there is a significant correlation between carotid plaque properties and the occurrence of cerebral infarction events18,19. Carotid artery computed tomography angiography (CTA) can delineate and measure the plaque, distinguish the properties of the plaque according to the different CT values, and can be used to evaluate the vulnerability of carotid artery plaque20. Previous studies have shown that IR measured by TyG index is closely related to the properties of atherosclerotic plaques21,22. Nevertheless, further evidence is needed to better understand the complex relationship between TyG, carotid plaque properties, and stroke events.
Therefore, this study aimed to investigate the association between IR assessed by the TyG index and carotid plaque vulnerability assessed by CTA and the incidence of stroke. In addition, we also analyzed whether the relationship between TyG and stroke outcome is partly mediated by vulnerable plaque.
Materials and methods
Study population
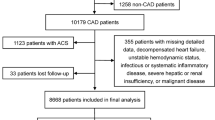
This retrospective study complied with the declaration of Helsinki and was approved by The Affiliated Suzhou Hospital of Nanjing Medical University Ethical Committee. Human participants’ names have been removed from all sections of the manuscript. The Affiliated Suzhou Hospital of Nanjing Medical University Ethical Committee waived the need for informed consent. The carotid artery stenosis rate was measured by North American Symptomatic Carotid Endarterectomy Trial (NASCET) method. Screening was conducted from January 2012 to January 2023 in our hospital. Inclusion criteria: (1) complete data for carotid CTA examination; (2) carotid artery stenosis ≥ 30%; (3) available stroke data. Exclusion criteria: (3) Participants with missing triglycerides (TG) and fasting blood glucose (FBG); (2) Individuals with incomplete clinical data. A total of 505 patients were included in this study, and were divided into stroke group (n = 184) and non-stroke group (n = 321) according to whether stroke had occurred. The subject enrollment flowchart is illustrated in Fig. 1.
Data collection and definition
The patient’s details were obtained from the patient’s electronic health records and telephone follow-up. Include: sex, age, hypertension, hyperlipidemia, diabetes mellitus (DM), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), fasting blood glucose (FBG), high density lipoprotein cholesterol (HDL-C), uric acid, TyG index, NASCET, body mass index (BMI), lost area (It means the area of lumen loss at the narrowest part of the carotid artery), vulnerable plaque, homocysteine, left/right carotid artery, smoke, coronary heart disease, atrial fibrillation, myocardial infarction, statin, antiplatelet and anticoagulant. Fasting blood samples were taken from all participants and biochemical measurements were analyzed. Serum biochemical parameters were determined with a biochemical auto-analyzer (Hitachi7600, Tokyo, Japan). The TyG index was determined using the formula: ln [TG (mg/dL) ×FBG (mg/dL)/2].
Assessment of carotid vulnerable plaque
The composition of plaques on CT is mainly classified based on attenuation density (Hounsfield units, HU): low attenuation plaques (< 60HU), mixed plaques (60-130HU), and calcified plaques (> 130HU). The main components of low attenuation plaque were lipid and intracavitary hemorrhage. Vulnerable plaques were defined as low attenuation plaques and mixed plaques23.
Statistical analysis
Values are presented as frequencies or percentages for categorical factors and were calculated using the chi-square test or Mann–Whitney U test. The mean ± standard deviation (SD) for continuous variables was analyzed using Student’s t-test. Correlations among variables, such as the TyG index and vulnerable plaque, were evaluated using Pearson’s correlation coefficients. In addition, a heatmap was created to visually represent each correlation coefficient.
The dose-response association between the TyG index and stroke outcomes in patients with carotid artery stenosis was illustrated through the use of restricted cubic splines (RCS) curve. Logistic regression models were employed to assess the relationship between the TyG index, vulnerable plaque, and the incidence of stroke events. Several subgroup analyses were conducted to explore whether the predictive utility of the TyG index and vulnerable plaque remained consistent across patients with diverse demographic characteristics or comorbidities. To elucidate the potential interactions between these subgroups and the risk of stroke, we also conducted interaction analyses concurrently.
The mediating role of vulnerable plaque between TyG index and stroke outcome was further analyzed. In all adjusted models, Model I was adjusted for age, smoke and hypertension. Model II was adjusted for age, BMI, HDL-C and homocysteine. Model III was adjusted for age, BMI, HDL-C, smoke, homocysteine and hypertension. The R software (version4.3.3) was applied for the analyses in the current study and a level of P <0.05 was considered as statistically significant.
Results
Baseline characteristics
A total of 505 carotid artery stenosis patients were included in this study. There are a total of 184 (36.4%) stroke patients. Nineteen people had had stroke previously. The majority of patients in this study were male. The mean age of the population was 64.86 ± 12.30 years. Patients with stroke are generally older, mostly smokers, and most of them are vulnerable plaques. The levels of FBG, TG, LDL-C, TyG index and homocysteine were higher, and BMI and HDL-C were lower. In addition, a heat map was drawn to visually display the correlation between different variables, showing a significant positive correlation between TyG index and vulnerable plaque (r = 0.62, P < 0.001, Fig. 2). The demographic and clinical characteristics of all patients were shown in Table 1.
Association between the TyG index, vulnerable plaques, and the incidence of stroke
The RCS curve shows that TyG index (non-linear, P=0.860) has a positive dose-response relationship with the incidence of stroke (Fig. 3). The univariate and multivariate Logistic regression analysis is shown in Table 2. The TyG index and vulnerable plaques were included in multivariate Logistic regression analysis. After adjusting for multiple confounding factors, TyG index (Model I: OR 1.2145, 95% CI: (1.1021 ~ 1.3384),P<0.001;Model II: OR 1.2290,95% CI: 1.0943 ~ 1.3804, P<0.001;Model III: OR 1.2283,95% CI: 1.0936 ~ 1.3797, P<0.001) and vulnerable plaques (Model I: OR 4.1860,95% CI: 2.4997 ~ 7.0101, P<0.001;Model II: OR 5.1487, 95% CI: 2.7947 ~ 9.4851, P<0.001; Model III: OR 5.1536, 95% CI: 2.7462 ~ 9.6713, P<0.001) were associated with an increased risk of stroke in patients with carotid artery stenosis (Table 3).
Subgroup analysis and interaction analysis
We also conducted various subgroup analyses to assess whether the TyG index and vulnerable plaque are consistent in different demographic characteristics. To elucidate the potential interactions between these subgroups and the risk of stroke, we also conducted interaction analyses concurrently. The results showed that the relationship between TyG index and stroke was not significant when the area<50. Besides, TyG index was an independent risk factor for stroke in other subgroups (Fig. 4). The results showed that vulnerable plaque was an independent risk factor for stroke in all subgroups (Fig. 5). Interaction analysis showed that the TyG index and vulnerable plaques did not interact with these eight factors in collectively affecting stroke. This means that TyG index and vulnerable plaques are an independent factor contributing to an increased risk of stroke.
Mediation analysis
As demonstrated in Table 4, the mediation analysis revealed that the vulnerable plaque exerted a significant partial mediating effect on the relationship between TyG index and the incidence of stroke across multiple adjusted models. In particular, the mediation proportions of an elevated vulnerable plaque were 18.3%, 15.8%, 13.9%, and 11.6% in the unadjusted, adjusted Model I, adjusted Model II, and adjusted Model III analyses, respectively.
Discussion
In this retrospective study of 505 patients with carotid stenosis, a significant association was found between increased baseline TyG index, vulnerable plaques and an increased incidence of stroke in patients with carotid artery stenosis. These associations remained statistically significant even after accounting for established stroke risk factors in different models. The results showed that the relationship between TyG index and stroke was not significant when the lost area<50. Besides, TyG index was an independent risk factor for stroke in other subgroups, and vulnerable plaque was an independent risk factor for stroke in all subgroups. Interaction analysis showed that there was no interaction between TyG index and vulnerable plaque in the common factors affecting stroke, which means that increased TyG index and vulnerable plaque are independent factors leading to the increase of stroke risk. In addition, the study revealed that vulnerable plaque partially mediates the association between TyG index and stroke outcomes in patients with carotid artery stenosis.
IR refers to the weakening of the physiological function of insulin in the body, which is a common pathological mechanism of many metabolic related diseases The causes of the disease mainly include genetic factors, environmental factors, increased hormone secretion and other diseases24,25. IR can lead to the development of metabolic disorders, including obesity, dyslipidemia, low-grade inflammation, endothelial dysfunction and hypertension, all of which are inducing factors of atherosclerosis26. TyG index is a direct and economical method to evaluate the level of IR27. TyG index is significantly correlated with cardiovascular death and stroke, indicating that IR plays a role in promoting the pathogenesis of cardiovascular and metabolic diseases15. A meta-analysis of 18 studies and 592,635 patients showed that the higher the TyG index, the higher the risk of ischemic stroke in the population. In addition, ischemic stroke patients with higher TyG index had a higher risk of stroke recurrence and increased risk of death compared with ischemic stroke patients with lower TyG index28. Our research is consistent with the existing research results. The results show that in different models, subgroup analysis and interaction analysis, there is an independent correlation between higher TyG index and stroke, which means that TyG is an independent factor leading to an increase in stroke risk. The results of TyG index are derived from the results of TG and FBG. Therefore, to reduce the incidence of stroke, TyG index must be reduced, and the levels of TG and FBG can be reduced through balanced dietary nutrition, scientific exercise, maintenance of ideal weight, psychological adjustment and rational use of medication29,30. In short, although TG and FBG are related to each other, through scientific and reasonable lifestyle adjustments and necessary drug interventions, it is entirely possible to take a two-pronged approach to effectively manage blood lipids and glucose and reduce the risk of stroke. The analysis of RCS curve shows that there is a positive dose-response relationship between TyG index and stroke, which indicates that the incidence of stroke increases with the increase of TyG index level.
Studies have shown that antithrombotic therapy plays a vital role in the prevention of stroke events. In patients with atherosclerosis, monoclonal or double antiplatelet therapy is usually the first choice, while anticoagulant therapy is recommended in the case of blood stasis or hypercoagulability31. The use of antiplatelet therapy or anticoagulant therapy can reduce the risk of ischemic stroke32.The use of statins is associated with a lower risk of stroke, especially ischemic stroke33. This study showed that there was no significant difference in the incidence of stroke between the use of antiplatelet therapy, statin therapy, and anticoagulation therapy. The reasons were as follows: First, this study was a cross-sectional study, and the causal relationship could not be judged. Secondly, not all patients with carotid artery stenosis in this study received formal drug management, only some or a small number of patients received drug treatment. Finally, even with drug treatment, the incidence of stroke will be different due to the stability of carotid plaque34. These three reasons may have led to the lack of statistically significant differences in the results of this study. However, according to the guidelines, the best medical treatment (BMT) is recommended for the management of carotid artery stenosis35. A meta-analysis of 39 studies (n = 359,783) showed that people with diabetes had a higher incidence of stroke, and most studies found that diabetes was associated with worse outcomes after stroke36,37 According to recent evidence from clinical studies and randomized controlled trials (RCTs), hypertension increases the risk of stroke, and the decline in stroke mortality observed over the past 50 years has been attributed in part to improved implementation of hypertension treatment and control strategies38,39. Hyperlipidemia is a risk factor for stroke, especially the increase of LDL-C, which can increase the risk of stroke, and the use of statin drugs has been shown in several studies to reduce the incidence of stroke40,41. This study did not find a significant relationship between diabetes and hyperlipidemia and stroke, and even the prevalence of hypertension in the non-stroke group was even higher than that in the stroke group. The reasons may be related to the defects of the cross-sectional study itself, the use of drugs, the stability of carotid plaque, the control level of hypertension, diabetes and hyperlipidemia. The incidence of stroke in this study was higher than that reported in previous research. We speculate that factors such as carotid stenosis rates, sex, age, and smoking may account for these differences. Previous studies have revealed that increased rates of carotid artery stenosis, older age, being male, and smoking can raise the incidence of stroke42,43,44,45.In our study, we concentrated on patients with moderate to severe carotid stenosis. Notably, there was a higher percentage of male patients, making up 79.60% of the cohort. Additionally, the participants were older on average. Furthermore, smoking is recognized as a significant risk factor for stroke, and in our study, the proportion of smokers was high, reaching 38.22%. These factors may have contributed to the increased incidence of stroke observed in this study.
Our study reaffirmed the clear association between the TyG index and the increased risk of stroke, and confirmed the previous research results. Vulnerable plaques are easily ruptured and unstable plaques, which are more likely to lead to ischemic events in clinic46.More and more studies have shown that the degree of carotid artery stenosis is not significant to the sensitivity of stroke, but the properties of carotid artery plaque is significantly correlated with stroke47,48,49. TyG index, as a sensitive index reflecting IR, is considered as an important risk factor for stroke50.IR will lead to the development and deterioration of atherosclerosis, which is the main cause of stroke51. When unstable carotid plaque forms, the risk of stroke is even greater52. Zhang et al. found that the incidence of carotid plaque gradually increased with the increase of TyG index53. Studies have shown that vulnerable plaque is a strong predictor of stroke outcome in patients with carotid artery stenosis and is closely associated with increased TyG index levels54. Therefore, unstable carotid plaque may be a potentially important mediator between TyG index and stroke risk. As far as we know, this study represents a groundbreaking analysis to explore the mediating role of carotid plaque properties in the association between TyG index and stroke events.
This study clarifies the mediating role of vulnerable plaque in the correlation between TyG index and stroke outcome in patients with carotid stenosis, thus combining previous evidence into an overall approach to clinical decision-making. Globally, the number of patients with carotid artery stenosis continues to rise, especially those with diabetes or carotid artery stenosis with vulnerable plaque characteristics, and it is more likely to have a stroke event34,55,56. Therefore, it is necessary to identify high-risk stroke patients in time. In clinical practice, evaluating TyG in patients with carotid stenosis can enhance the overall assessment of the disease and help develop more effective and personalized treatment and management strategies. In addition, understanding the correlation between TyG and the properties of carotid plaque, as well as stroke adverse events, enables doctors to better assess patients’ risk and identify and manage potential complications in a timely manner.
Although this study provides valuable information, it also has some limitations, which are worthy of careful consideration. First, this is a single-center retrospective study, not a prospective study, which limits our ability to establish a causal relationship between TyG index, vulnerable plaques and stroke events in patients with carotid stenosis. Second, this study assessed carotid plaque properties using carotid CTA rather than high-resolution MRI or pathological section, which may limit the ability to identify carotid plaque properties. In addition, while adjustments were made for known stroke risk factors, not all confounding variables were taken into account, leaving room for potential residual confounding effects. It is necessary to further study the complex relationship between these factors in the future.
Conclusions
TyG index and vulnerable plaque are associated with a higher risk of stroke in patients with carotid artery stenosis. The study also pointed out that vulnerable plaques partially mediated the relationship between TyG index and stroke outcomes.
Data availability
The datasets used and analyzed in the study are available from the corresponding author upon reasonable request.
References
Global and National age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet 392(10159), 1736–1788 (2018).
Wu, S. et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 18 (4), 394–405 (2019).
Gu, H. Q. et al. Clinical characteristics, management, and in-hospital outcomes in patients with stroke or transient ischemic attack in China. JAMA Netw. Open 4 (8), e2120745 (2021).
Wang, Y. et al. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke 44 (5), 1232–1237 (2013).
Pan, Y. et al. Residual risk and its risk factors for ischemic stroke with adherence to guideline-based secondary stroke prevention. J. Stroke 23 (1), 51–60 (2021).
Herrington, W. et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 118 (4), 535–546 (2016).
Roy, P., Orecchioni, M. & Ley, K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol. 22 (4), 251–265 (2022).
Li, Z. et al. China’s response to the rising stroke burden. BMJ, 364l879 (2019).
White, H. et al. Ischemic stroke subtype incidence among whites, blacks, and hispanics: the Northern Manhattan study. Circulation 111(10):1327–1331 (2005).
Zhou, X. et al. Study on insulin resistance and ischemic cerebrovascular disease: a bibliometric analysis via CiteSpace. Front. Public Health 11, 1021378 (2023).
Di Pino, A. & De Fronzo, R.A. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr. Rev. 40 (6), 1447–1467 (2019).
Simental-Mendía, L. E. , Rodríguez-Morán, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6 (4), 299–304 (2008).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28 (7), 412–419 (1985).
Tao, L. C. et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc. Diabetol. 21 (1), 68 (2022).
Lopez-Jaramillo, P. et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 4 (1), e23–e33 (2023).
Wang, A. et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc. Diabetol. 20 (1), 46 (2021).
Zhao, Y. et al. Elevated triglyceride-glucose index predicts risk of incident ischaemic stroke: the rural Chinese cohort study. Diabetes Metab. 47 (4), 101246 (2021).
Saba, L. et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 18 (6), 559–572 (2019).
Saba, L. et al. Carotid stenosis and cryptogenic stroke. J. Vasc Surg. 79 (5), 1119–1131 (2024).
Naim, C. et al. Vulnerable atherosclerotic carotid plaque evaluation by ultrasound, computed tomography angiography, and magnetic resonance imaging: an overview. Can. Assoc. Radiol. J. 65 (3), 275–286 (2014).
Jiang, Z. Z. et al. A high triglyceride-glucose index value is associated with an increased risk of carotid plaque burden in subjects with prediabetes and new-onset type 2 diabetes: A real-world study. Front. Cardiovasc. Med. 9, 832491 (2022).
Miao, M. et al. Triglyceride-glucose index and common carotid artery intima-media thickness in patients with ischemic stroke. Cardiovasc. Diabetol. 21 (1), 43 (2022).
Murgia, A. et al. CT imaging features of carotid artery plaque vulnerability. Ann. Transl. Med. 8 (19), 1261 (2020).
Lee, S. H., Park, S. Y. & Choi, C.S. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab. J. 46 (1), 15–37 (2022).
Yaribeygi, H. et al. Insulin resistance: review of the underlying molecular mechanisms. J. Cell. Physiol. 234 (6), 8152–8161 (2019).
Kosmas, C. E. et al. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 51 (3), 3000605231164548 (2023).
Sánchez-García, A. et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance. Syst. Rev. Int. J. Endocrinol. 2020, 4678526.
Yang, Y. et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc. Diabetol. 22 (1), 2 (2023).
The prevention of diabetes mellitus. JAMA 325(2), 190 (2021).
Chait, A. Hypertriglyceridemia. Endocrinol. Metab. Clin. N. Am. 51(3), 539–555 (2022).
Greco, A. et al. Antithrombotic therapy for primary and secondary rrevention of ischemic stroke: JACC state-of-the-art review. J. Am. Coll. Cardiol. 82 (15), 1538–1557 (2023).
Bhatia, K. et al. Contemporary antiplatelet and anticoagulant therapies for secondary stroke prevention: a narrative review of current literature and Guidelines. Curr. Neurol. Neurosci. Rep. 23 (5), 235–262 (2023).
Xu, T. et al. Statin adherence and the risk of stroke: a dose-response meta-analysis. CNS Drugs 31 (4), 263–271 (2017).
Saba, L. et al. Carotid plaque-RADS: a novel stroke risk classification system. JACC Cardiovasc. Imaging 17 (1), 62–75 (2024).
Bonati, L. H. et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur. Stroke J. 6 (2), I–xlvii (2021).
Alloubani, A. & Saleh, A. & Abdelhafiz, I. Hypertension and diabetes mellitus as a predictive risk factors for stroke. Diabetes Metab. Syndr. 12 (4), 577–584 (2018).
Lau, L. H. et al. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J. Diabetes Investig. 10 (3), 780–792 (2019).
Turin, T. C. et al. Hypertension and lifetime risk of stroke. J. Hypertens. 34 (1), 116–122 (2016).
Lackland, D. T. et al. Implications of recent clinical trials and hypertension guidelines on stroke and future cerebrovascular research. Stroke 49(3), 772–779 (2018).
Balling, M. et al. Elevated LDL triglycerides and atherosclerotic risk. J. Am. Coll. Cardiol. 81 (2), 136–152 (2023).
Alloubani, A. & Nimer, R. & Samara, R. Relationship between hyperlipidemia, cardiovascular disease and stroke: a systematic review. Curr. Cardiol. Rev. 17 (6), e051121189015 (2021).
Howard, D. P. J., Gaziano, L. & Rothwell, P.M. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 20 (3), 193–202 (2021).
Tsai, C. F. et al. Incidence, subtypes, sex differences and trends of stroke in Taiwan. PLoS One 17 (11), e0277296 (2022).
Boehme, A. K. & Esenwa, C. & Elkind, M.S. Stroke risk factors. Genet. Prev. Circ. Res. 120 (3), 472–495 (2017).
Hackshaw, A. et al. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 360, j5855 (2018).
Kramer, C. M. & Treiman, G.S. Vulnerable plaque in carotid arteries without significant stenosis: unmasking the hidden links to stroke. J. Am. Coll. Cardiol. 76 (19), 2223–2225 (2020).
Mossa-Basha, M. & Wasserman, B.A. Low-grade carotid stenosis: implications of MR Imaging. Neuroimaging Clin. N. Am. 26 (1), 129–145 (2016).
Tao, L. et al. Vulnerable plaque of the petrous internal carotid artery in embolic stroke of undetermined source. Eur. J. Neurol. 30 (3), 648–658 (2023).
Howard, D. P. et al. Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke 46 (1), 182–189 (2015).
Ding, X. et al. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc. Diabetol. 20 (1), 76 (2021).
Beverly, J. K. & Budoff, M. J. A. Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J. Diabetes 12 (2), 102–104 (2020).
Massara, M. et al. Unstable atherosclerotic plaque in the common carotid artery: diagnosis and treatment strategy. Semin. Vasc. Surg. 31 (2–4), 88–90 (2018).
Zhang, Y. et al. Association between the triglyceride-glucose index and carotid plaque incidence: a longitudinal study. Cardiovasc. Diabetol. 21 (1), 244 (2022).
Marnane, M. et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke 45 (3), 801–806 (2014).
Li, R. Y. et al. Association of pre-diabetes and type 2 diabetes mellitus with intracranial plaque characteristics in patients with acute ischemic stroke. Br. J. Radiol. 96 (1143), 20220802 (2023).
Campbell, B. C. V. et al. Ischaemic stroke. Nat. Rev. Dis. Primers 5 (1), 70 (2019).
Funding
This work was supported by the Suzhou “Science and Education Revitalize Health” Youth Science and Technology Project ( KJXW2021031).
Author information
Authors and Affiliations
Contributions
GH and CJ drafted the manuscript, and were major contributors in the collection, analysis and interpretation of data. DZ, ZY, YZ, JZ, YT, JH, and ZL were major contributors in the acquisition and interpretation of data and contributed to revision of the manuscript. GH designed the study and provided constructive suggestions for revisions of the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the ethics committee of The Affiliated Suzhou Hospital of Nanjing Medical University and strictly complied with the Declaration of Helsinki.
Consent for publication
All participants provided written/oral informed consent for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, J., Zhou, D., Yao, Z. et al. Insulin resistance, vulnerable plaque and stroke risk in patients with carotid artery stenosis. Sci Rep 14, 30453 (2024). https://doi.org/10.1038/s41598-024-81967-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81967-x