Abstract
Ferroptosis is of great significance in carcinogenesis as it interconnects with a multiplicity of biological processes. Meanwhile, its function and regulatory role in lung cancer remains ambiguous. In this study, we discovered by WB and IHC that ALYREF has a higher expression in lung adenocarcinoma (LUAD) tissues compared with normal ones. By the direct observation of mitochondria with electron microscopy, we also identified that the knockdown of ALYREF limits the proliferation of LUAD in vitro as well as in vivo and prompts LUAD cells to go through ferroptosis. Mechanistically, our RNA-immunoprecipitation experiment combined with qRT-PCR analysis confirmed that ALYREF could interact with SLC7A11. ALYREF mainly binds to the 3’UTR region of SLC7A11 mRNA, increasing the mRNA stability of SLC7A11 and reducing intracellular Reactive Oxygen Species (ROS) and Methylene diphenyl diamine (MDA) thereby inhibiting ferroptosis and eventually promoting the proliferation and metastasis of LUAD cells. Importantly, our study reveals for the first time that ALYREF can regulate LUAD by inhibiting ferroptosis. Based on the evidences mentioned above, we have good reason to believe that the role of ALYREF as a new oncological factor could be proved and, acting along the ALYREF-SLC7A11-ferroptosis axis, utilized as a promising therapeutic target for LUAD.
Similar content being viewed by others
Introduction
Lung cancer, as the second most commonly diagnosed cancer worldwide, is the main reason for cancer-related deaths1. Accounting for the largest proportion of 85% among classified lung cancers, advanced non–small cell lung cancer (NSCLC) carries a extremely low 5-year survival rate2. Due to their high efficacy, targeted therapies are still considered as one of the most important, irreplaceable treatment methods for lung cancer patients3. Thus, exploring more potential molecular targets and achieving a better understanding of the mechanism of lung cancer development is critical in the pursuit of therapies yielding longer survival.
Ferroptosis, a form of cell death distinct from apoptosis, pyroptosis, parthanatos, and necroptosis4, is known to play a significant role in cancer, neurodegeneration, tissue injury, inflammation, and infection5. Nonetheless, its specific mechanism in lung cancer remains unexplored. With the requirements of available ROS and suitable lipid substrates6, ferroptosis has consistently enjoyed the attention of researchers since the intriguing mechanism of this novel form of cell death was unveiled in 20127. Although the popularity of ferroptosis-related studies remains unremitting, the understanding of ferroptosis is currently incomplete. Assuming the possibility of discovering potential treatment targets, among which SLC7A11 has proven to be a key molecule, ferroptosis can repress the tumorigenesis and development of multiple cancers8. SLC7A11 is thought to execute the main duty of exporting intracellular glutamate in exchange for extracellular cystine at a 1:1 ratio9, so that the redox dynamical balance could be maintained at the normal range, which is a necessity for the survival of cancer cells10. However, the mechanism behind SLC7A11-meditated ferroptosis in the context of lung cancer has not been comprehensively described.
RNA-binding proteins (RBPs) are associated with a range of human diseases involving cancer11, with crucial roles in post-transcriptional gene expression regulation, such as by modulating microRNA (miRNA) processing, and the alternative splicing, alternative polyadenylation, subcellular localization, stability, and translation of RNAs12. It has been reported that the knockdown of RNA-binding protein RBMS1 (as a translation-promoting factor for SLC7A11) induces ferroptosis in lung cancer cells, leading to the inhibition of lung cancer cell growth13. Zhang, Z. et al. also figured out that the overexpression of RBP ZFP36 conferred resistance to HSC ferroptosis, while boosted ubiquitination degradation resulted in the reduction of ZFP expression when ferroptosis occurred under the induction of sorafenib, erastin and RSL314. The research conducted by Wang, Q. et al. concluded that DAZAP1 promotes HCC progression by physically interacting with SLC7A11 and influencing the SLC7A11/GPX4 pathway to regulate ferroptosis15. The above studies all suggest that RBPs possibly function in cancerogenesis partly by relying on ferroptosis, principally intermediated by SLC7A11. Thus, it is reasonable to assert that the crosstalk between RBPs and ferroptosis in cancers is an urgent topic that needs to be further explored.
ALYREF, a demanding RNA binding protein, has been found to be significantly upregulated in various tumors and a predictor of poor progonosis among these cancer types16. In this article, for the first time, we report that the knockdown of RBP ALYREF activates LUAD cells in a number of cancer phenotypes, regarding their proliferation, migration, invasion, etc., partly resulting from its effect on SLC7A111-mediated ferroptosis, which suggests that ALYREF-SLC7A11-mediated ferroptosis might be a potential treatment target to consider for LUAD patients.
Results
ALYREF presents higher expression in LUAD tissues and is associated with poorer outcomes
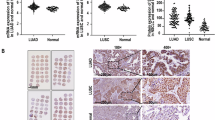
TCGA database analysis showed ALYREF was upregulated in various cancers involving LUAD (Fig. 1A). The box picture further displays the expression difference (Fig. 1B). Next, we observed that ALYREF is with higher expression in LUAD tissues compared to the normal lung tissues by WB (Fig. S1A). Subsequently, we investigated the relationship between ALYREF expression and patient clinicopathological features in TCGA database, which showed that higher expression of ALYREF was significantly correlated with higher clinical stage (p < 0.001) and larger tumor size (p < 0.001) but not with other clinicopathological characteristics, such as sex, age or metastasis (Fig.S1B). Importantly, Kaplan–Meier analysis based on the level of ALYREF showed that higher expression of ALYREF predicted poorer FPS (log-rank p < 0.05), PPS (log-rank p < 0.05) and OS (log-rank p < 0.05) outcomes (Fig. 1C- E). The analysis of tissue microarray of 90 patients further verified the consistent conclusions (Fig. 1F-G). These results emphasize the significance of ALYREF in LUAD.
ALYREF is highly expressed in LUAD and predicts poorer clinical outcomes. A: The image of expression difference of ALYREF in pan-cancer was obtained by TCGA database analysis. B: The expression difference plot of ALYREF in LUAD in TCGA was shown. C: The Kaplan-Meier Curve for OS (Overall Survival) were plotted based on the TCGA. D and E: The FPS (Progression-Free Survival) and PPS (Progressive survival) of ALYREF in LUAD from KM-plotter (http://kmplot.com/) were displayed respectively. F: The expression of ALYREF in 90 pairs of tissue microarray was analyzed. G: The representative images of IHC between cancer and normal tissues were displayed. Scale bar:100 μm and 10 μm. H: The Kaplan-Meier Curve for OS were plotted based on tissue microarray.
Silencing of ALYREF represses the proliferation and metastasis of LUAD cells in vitro
The expression of ALYREF between four LUAD cell lines and human normal lung epithelial cells BEAS-2B was detected by WB and qPCR to select the appropriate cell lines for further experiments (Fig. 2A and S1C). Eventually, the H1975 and PC9 lines were chosen according to the expression levels. Next, we silenced the ALYREF in the selected cell lines, so as to observe whether and how this affected the LUAD phenotypes. The silencing rate was verified by qPCR and WB; the former revealed that ALYREF was prominently knocked down by more than 90%, which also presented an obvious downward trend based on the latter (Fig. 2B and S1D). As shown in the graph, the knockdown of ALYREF significantly inhibited the viability of both H1975 and PC9 cells while overexpression of ALYREF promotes that, measured by CCK-8 assay at OD450 (Fig. 2C and S1E). The clone formation ability of cells was statistically significantly weakened after the knockdown of ALYREF and strengthened after the overexpression (Fig. 2D and S1F-G). The silencing of ALYREF inhibits the proliferation while overexpression of ALYREF promotes that by EDU assay (Fig. 2E-F and S1H). The migration and invasion ability of LUAD cells was obviously undermined following the silencing of ALYREF, as measured by transwell assays (Fig. 2G and H), and these results were statistically significant (Fig. 2I and J). Overexpressed ALYREF prompted the metastasis compared with negative control group (Fig.S1I-J). Besides, the wound healing experiment indicated a consistent trend with the transwell assay—the knockdown of ALYREF exerted a crippling effect on the migration ability of LUAD cells, further evidenced by statistical analysis (Fig. 2K and L).
Knockdown of ALYREF inhibits the proliferation and metastasis of lung cancer cells in vitro. A: The expression of ALYREF among BEAS-2B, PC9, H1299, H1975, and A549 was detected by WB (original blots are presented in Supplementary Figure S5). B: The silencing rate of ALYREF in H1975 and PC9 cell lines was verified. C and D: The proliferation rate difference between diverse groups was detected by CCK8 (cell counting kit-8) and clone formation (N = 3). E and F: The representative pictures of EDU(5-ethynyl-2’-deoxyuridine). Scale bar: 100 μm. G: The ability of migration was tested by transwell assay and the statistical results are shown in Figure J (N = 3). Scale bar:20 μm. H: Representative pictures revealing the invasion ability of cells from the different treated groups are shown. Scale bar:20 μm. I: The statistic results of H. K and L: Representative images of wound healing analysis for assessing the migration of transfected cells at 0, 24, and 48 h and corresponding statistic results . (N = 3) Scale bar: 200 μm.
Knockdown of ALYREF inhibits the proliferation of LUAD in vivo
We next aimed to find out whether the above experiments would bring similar results to those with mice; therefore, a subcutaneous implantation experiment was conducted. A xenograft model was constructed by using stably transfected H1975 and PC9 cells. The qPCR and WB results suggested that the model construction was successful—ALYREF was knocked down by more than 70% as far as LV-sh1 was concerned, which was chosen for the following steps (Fig. 3A). In the end, we found that the tumor volume decreased significantly compared to the control group and the growth of subcutaneous tumor was significantly inhibited after the silencing of ALYREF (Fig. 3B and C). The tumors removed from the control group had lower weight (Fig. 3D). The IHC images showed the decrease in ki-67 expression level in the knockdown group of ALYREF and also substantiated the successful knockdown of ALYREF (Fig. 3E and F). Collectively, ALYREF promoted the growth of LUAD in vivo.
Silencing of ALYREF represses tumor growth in vivo. A: The silencing rate of stably transfected cell lines was detected by qPCR and WB (original blots are presented in Supplementary Figure S5). LV-sh1 was selected to conduct further research. B: The images of formed tumors and removed ones were displayed. C: The growth curve was obtained based on the recorded data by GraphPad Prism8.0. D. The weight of removed tumor was determined to obtain the graph. E and F: IHC photos of ALYREF and ki-67 stained with the tumor tissues of nude mice were shown respectively. Scale bar: 200 μm and 20 μm.
Silencing of ALYREF promotes the occurrence of ferroptosis
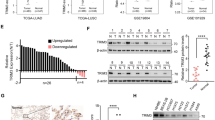
RNA sequencing was performed to analyze the downstream gene regulated by ALYREF in LUAD. The results indicated that SLC7A11 was downregulated in the ALYREF-knockdown LUAD cells compared to the control ones (Fig. 4A). SLC7A11, ALOX5, KLHDC3, GDF15, LONP1, CDKN1A, SMPD1 and IL6 were identified and imaged by analyzing the overlap between differentially expressed genes (DEGs) and ferroptosis (Fig. 4B and C). The plot from RNA-Seq and LUAD tissues illustrated that SLC7A11 and ALYREF were positively related (Fig. 4D and E). At the same time, SLC7A11 is possibly the only prognostic marker in LUAD among 8 genes (Fig. 4F and S4). We also detected the expression of ALYREF and SLC7A11 in paired LUAD tissues (Fig. S1A). WB and qRT-PCR were further conducted to detect the expression of SLC7A11, and the results revealed that the expression of SLC7A11 decreased significantly in the si-ALYREF groups compared with the control ones (Fig. 4G and H). We also conducted the IHC staining of SLC7A11 in removed subcutaneous tumor and found that the expression of SLC7A11 decreased after the knockdown of ALYREF(Fig. 4I). Therefore, we were prompted to find out whether SLC7A11 was the crucial downstream of ALYREF and the knockdown of ALYREF induced the occurrence of ferroptosis. For more sufficient and direct evidence, the mitochondrial morphology was observed under an electron microscope, and the results proved our speculation. It was apparent that the volume of mitochondria from the treated groups decreased obviously compared with the control groups and mitochondrial crests were reduced at the same time, validating that the knockdown of ALYREF did indeed induce the occurrence of ferroptosis in both H1975 and PC9 cell lines (Fig. 5A and B). Furthermore, we confirmed by flow cytometry and fluorescence microscope that the level of cellular ROS and C11 ROS soared considerably with the knockdown of ALYREF while decreased with the overexpression (Fig. 5C-D and Fig.S2C). The representative fluorescence pictures of ROS were displayed in Fig.S2A. Notably, the levels of ROS and C11 ROS can be reversed after adding the inhibitor of ferroptosis ferr-1(Fig.S2B). Likewise, the intercellular MDA showed striking elevation compared to the control groups in both cell lines (Fig. 5E) and the content of GSH underwent the opposite trend (Fig. 5F). The level of cellular Fe2+ increased with the knockdown of ALYREF and decreased partly after the addition of Fe2+ chelating agent DFOM (Fig. 5G). Whereas, the cell line presented the opposite trend with the overexpression of ALYREF-the decrease of MDA and and Fe2+ as well as increase of GSH (Fig.S2D). The confocal pictures also showed the corresponding changes of fluorescence intensity (Fig. 5H and S2E). We also conducted the expression of FTH1 and TRF after the silencing of ALYREF (Fig. S3K). In short, the above evidences pointed out that LUAD cells underwent ferroptosis after the knockdown of ALYREF.
SLC7A11 is the downstream target of ALYREF and is positively associated with ALYREF. A: Volcano plot representing the down/upregulated gene after the knockdown of ALYREF. B and C: 8 genes were obtained and imaged by analyzing the overlap between differentially expressed genes (DEGs) and ferroptosis. D and E: Pictures were plotted based on RNA-Seq and 17 pairs of LUAD tissues from patients displaying the correlation between ALYREF and SLC7A11. F: The Kaplan-Meier Curve for OS was displayed. G and H: Western blotting and qRT-PCR were performed to evaluate the expression of SLC7A11 after ALYREF was silenced in H1975 and PC9 (original blots are presented in Supplementary Figure S5). I: The representative pictures of SLC7A11 stained in removed subcutaneous tumor of mice. Scale bar:200 μm and 20 μm.
Decreased expression of ALYREF prompts LUAD cells to undergo ferroptosis. A: Representative images of mitochondrial morphology taken by electron microscopy at the scales of 5 μm and 500 μm in H1975. B: The mitochondrial morphology of PC9 cell line was imaged at the scales of 2 μm and 500 nm. The red arrows mark normal mitochondria, the green ones point at the mitochondrial morphology of ferroptosis. C: The level of C11 ROS of cells was measured by flow cytometric detection after cells were transfected for 72 h. The statistical results are shown in the volume charts. D: Intracellular ROS level was detected by flow cytometry and the quantified results were demonstrated. E: The level of MDA was measured (N = 3). F and G: The level of GSH (GSH: Glutathione) and Fe2+ among different treated groups was assessed. H: The confocal representative images of Fe2+ probe.
ALYREF functions during the ferroptosis process through impacting the RNA stability of SLC7A11
The ENCORI database (https://rnasysu.com/encori/) revealed that SLC7A11 mRNA is the target of ALYREF. Given this fact, RNA-immunoprecipitation assay was completed and the results suggested that the SLC7A11 could successfully bind with ALYREF (Fig. 6A). Thus, we speculated that SLC7A11 is possibly the key downstream gene of ALYREF by which it induces ferroptosis. To explore how SLC7A11 functions in this process, cells were co-transfected with 3’UTR-mutated or WT plasmids of SLC7A11 with si-RNA or corresponding si-NC (overexpressed plasmid of ALYREF or empty vector plasmid of pcDNA3.1) of ALYREF for 48 h, then the Dual-Luciferase® Reporter Assay System was applied to detect the fluorescence intensity. The results unveiled that the downregulation of ALYREF repressed the luciferase activities of H1975 and PC9 cells featured by the wild-type SLC7A11 3’UTR reporter gene, while this effect was absent when the 3’UTR region of SLC7A11 was mutated (Fig. 6B). The overexpressed group also verified this logic (Fig. 6C). Furthermore, actinomycin was applied to explore if ALYREF maintained the RNA stability of SLC7A11. The results indicated that SLC7A11 RNA stability obviously decreased after the knockdown of ALYREF compared to the negative control group and overexpressing group saw the opposite trend (Fig. 6D). Importantly, altered characteristics of ferroptosis could be rescued with the overexpression of SLC7A11 in ALYREF-silenced cancer cell lines (Fig. S3H-J). In summary, the silencing of ALYREF weakened the stability of SLC7A11 mRNA to promote the occurrence of ferroptosis.
ALYREF interacts with SLC7A11 and the altered phenotypes could be partly rescued by the overexpression of SLC7A11. A: RNA-immunoprecipitation assay was conducted to explore the interactions between ALYREF and SLC7A11. B and C: Double luciferase reporter assay was conducted on the H1975 and PC9 cells co-transfected with SLC7A11 3’UTR WT or SLC7A11 3’UTR MUT reporter and si-ALYREF/si-NC or oe-ALYREF/pcDNA3.1. D: The RNA ability was compared using 2 µmol/mL actinomycin. E: CCK-8 curves measuring the proliferation ability of cells. F: Representative images of colonies formed in H1975 and PC9. G and H: The migration and invasion ability of cells was investigated by transwell assay. Scale bar:20 μm. I: The number of formed colonies was statistically analyzed (N = 3). J and K: The corresponding statistics of graph G and H. NB: Transfected with si-NC, named NC, or with si-ALYREF, called si, or with si-ALYREF + SLC7A11, referred to as si + slc group.
Altered phenotypes of cells resulting from being transfected by si-ALYREF could be partly rescued by overexpressing SLC7A11 or adding ferrostatin-1
The viability of H1975 and PC9 cells at different time points were detected by CCK-8 assay. We discovered that the knockdown of ALYREF decreased the OD value at 450 nm, and this effect was partially reversed after co-transfection with SLC7A11 overexpression plasmid for 48 h or adding ferrostatin-1 (Fig. 6E and S3A). The colony formation ability of the H1975 and PC9 cells was determined by colony formation assay, which showed that decreased colony formation ability after ALYREF silencing could be partially recovered by co-transfection with the overexpression plasmid of SLC7A11 or by adding ferrostatin-1 (Fig. 6F and S3B). Notably, the result was statistically significant, revealed by the volume chart (Fig. 6I and S3C). Furthermore, the rescue experiments (migration and invasion assays) were completed using transwell assay and the data confirmed accordant trends—the reduced migration and invasion abilities after silencing ALYREF could be reversed partly by adding ferroptosis inhibitor ferr-1 or the overexpression of SLC7A11 (Fig. 6G-H and S3D-E). The corresponding volume chart also showed a statistically significant result (Fig. 6J-K and S3F-G).
Discussion
RBPs are critically important in assorted human diseases, including cancer, the leading cause of human disease-related deaths worldwide17, while the explicit mechanism remains largely elusive. The relationship between RBPs and ferroptosis has been reported in cancers, with the former mediating the ferroptosis process that could induce cancer cell death, which suggests that the RBPs-ferroptosis axis might be a new therapeutic target for cancers. Given that related studies are not yet sufficient, more research is required to clarify the link between RBPs and ferroptosis for the discovery of potential molecular targets. Our study is the first to reveal that ALYRE, a RNA-binding protein, regulates the phenotypes of LUAD cells partly by SLC7A11-mediated ferroptosis.
ALYREF has been shown to play multiple roles in biological processes, acting as a transcriptional coactivator, being responsible for mRNA export and transcription, and maintaining the nuclear RNA stability18. Initially identified as a transcriptional coactivator lacking an intrinsic transcriptional activation domain19, ALYREF could bind two transcriptional regulatory proteins, LEF-1 and AML, to stimulate transcription in the context of TCRc enhancer20. It is also worthwhile to mention that ALYREF, as a human counterpart of essential yeast protein Yra1, has a role in the mRNA export of eukaryotic cells21. MRNA export can regulate a series of crucial biological processes involving DNA repair, gene expression, maintenance of pluripotency, haematopoiesis, proliferation and cell survival, resulting from bridging transcription with translation22. Recently, ALYREF has drawn researchers’ attention in the field of tumorigenesis for its abilities to recognize and bind to modified RNA methylation sites (m5c-related RBPs), leading to the dysregulation of mRNA23 and eventually resulting in the occurrence of diseases, with particular reference to cancers. Taken together, the ALYREF gene is undoubtedly a promising target as it influences the survival of cells mainly transcriptionally and post-transcriptionally; however, the precise mechanisms of ALYREF are waiting to be unmasked.
Given that the function of ALYREF has remained largely enigmatic, further and extensive research is urgently needed to clarify the complicated roles of this gene in carcinogenesis. In this paper, we discovered that the knockdown of ALYREF promotes the malignant phenotypes of LUAD in vitro, consistent with the results reported by Yang et al.24. Our cell-derived xenograft experiment also verified that silencing ALYREF significantly repressed the growth of tumor in vivo. Based on previous studies, some RBPs13,15can regulate the proliferation of cancers by ferroptosis; our RNA-seq also revealed that ALYREF impacts ferroptosis-related genes, particularly SLC7A11-mediated ferroptosis pathways. Research has assumed the responsibility of ALYRE in mediating nuclear mRNA export25, maintaining their stability and involvement in the process of pre-mRNA splicing26. Nevertheless, studies highlighting this function of ALYREF in LUAD have been scarce. Wang, Q. et al. discovered that the RNA binding protein DAZAP1 regulates ferroptosis by interacting with the 3’UTR region of SLC7A11 mRNA in HCC progression15. This might imply that this region of SLC7A11 mRNA might be an important therapeutic target for cancer, as it builds a bridge between RBPs and ferroptosis. These reasons prompted us to direct our research interest on the interactions between ALYREF and SLC7A11 in the context of ferroptosis.
When referring to the development and treatment of lung cancer, the importance of ferroptosis is beyond doubt27. It plays a pivotal role in chemotherapy, radiotherapy and immunotherapy28and bears a potential to reverse or even overcome the drug resistance dilemma in lung cancer17. It has been reported that 44% of ferroptosis-related genes were differentially expressed in lung cancer compared with adjacent normal tissues, and the constructed risk model revealed that five ferroptosis-related hub genes, ALOX5, DPP4, FANCD2, GCLC, and SLC7A11, are associated with the OS of lung cancer patients29. It was uncovered that the loss of RBP RBMS1 made cells susceptible to ferroptosis because it could bridge the 3′- and 5′-UTR ends of SLC7A11 by binding to the eIF3d complex to inhibit the expression of SLC7A11 13. Moreover, Wang, X. et al. figured out that in lung cancer, SOX2 prevents cancer cells from undergoing ferroptosis by upregulating the expression of SLC7A11 at the transcriptional level. More specifically, the binding between SOX2 and SLC7A11 promoter makes cancer cells more vulnerable to ferroptosis, indicating the importance of the SOX2–SLC7A11 axis in lung cancer30. Another study has demonstrated that YTHDF1 interacts with SLC7A11 to stabilize its mRNA, disturbing its translation and resulting in the development of lung cancer31, which further evidences that the crosstalk between RNA modifications and ferroptosis is probably a decisive breakthrough in cancer treatment. Herein, we found a strong connection between ferroptosis and the development of lung cancer. Going forward, the importance of more fundamental researches may be overshadowed by the need to focus on delineating the precise role of ferroptosis in the development and progression of lung cancer. However, in addition to its role in maintaining cellular redox homeostasis and regulating ferroptosis, SLC7A11 is also involved in amino acid metabolism, which can significantly influence cellular growth and proliferation. Further studies should consider excluding the effects of cystine and glutamate metabolism on cell growth in the context of the ALYREF-SLC7A11 axis, as these metabolic pathways may confound the interpretation of the results related to ferroptosis.
To conclude, the knockdown of RNA-binding protein ALYREF downregulates the expression of SLC7A11 by binding to the 3’UTR region of SLC7A11 mRNA to weaken its stability, leading to the ferroptosis of lung cancer cells and eventually regulating the development of lung cancer. Undeniably, however, our research has the limitation of insufficient quantity of clinical samples, which needs to be addressed in future research.
Conclusion
In summary, our work has revealed for the first time that ALYREF, as an RNA-binding protein, can regulate LUAD cell phenotypes partly via 3’UTR SLC7A11-mediated ferroptosis, highlighting the ALYREF-SLC7A11-ferroptosis axis as a potential target in LUAD therapy.
Materials and methods
Clinical specimens and ethics statement seventeen LUAD tissues, including the corresponding adjacent non-tumor tissues were collected from First Affiliated Hospital of Zhejiang University. One set of tissue microarray chip containing 180 LUAD tissue dots was obtained from Shanghai Qutdo Biotech Company, which was utilized for analysis of overall survival (Table S1). The research was approved by the ethics committee of the first affiliated hospital of Zhejiang University (Approval Number: IIT20240263B-R2). This study conforms to the Helsinki Declaration of the World Medical Association and all patients provided written informed consent.
Cell lines H1975, PC9, A549, H1299 and BEAS-2B cells were purchased from the Meisen Chinese Tissue Culture Collections, Hangzhou, China. All cells were cultured in RPMI 1640 Medium(L121-500, BD BIO, Shenzhen, China) containing 10% FBS (FSD500, ExCell Bio, Shanghai, China) at 37 °C and 5% CO2.
qRT-PCR The total RNA of tissue samples and cells was extracted using NucleoZOL reagent (740404.200, MACHEREY-NAGEL, Germany), and cDNA was synthesized using the HiScript III All-in-one RT SuperMix Perfect for qPCR (R33301, Vazyme Biotech, Nanjing, China). Quantitative real-time PCR was performed on an Applied Biosystems 7500HT Fast Real-Time PCR System (Applied Biosystems, USA) using ChamQ Universal SYBR qPCR Master Mix (Q711-02, Vazyme Biotech, Nanjing, China). The procedure was set to 40 cycles, with initial activation for 3 min at 95℃, 10 s at 95℃ and 30 s at 60℃, followed by melting curve analysis. The primers for qRT-PCR were as follows: -actin, forward 5-AGCGAGCATCCCCCAAAGTT-3 and reverse 5-GGGCACGAAGGCTCATCATT-3; ALYREF, forward 5-GCAGGCCAAAACAACTTCCC-3 and reverse 5-AGTTCCTGAATATCGGCGTCT-3; SLC7A11, forward 5-TCTCCAGGTTATTCTATGTTGC-3 and reverse 5-GCATTATCATTGTCAAAGGGTG-3; PKM2, forward 5- ATGTCGAAGCCCCATAGTGAA-3 and reverse5- TGGGTGGTGAATCAATGTCCA-3.
Western blotting Cells or tissues were treated with RIPA lysis buffer (P0013C, Beyotime, Shanghai, China) containing protease inhibitors (20137ES05, YEASEN, Shanghai) and phosphatase inhibitor (20109ES05, YEASEN, Shanghai). The concentration of proteins was measured by a bicinchoninic acid kit (FD2001, FUDE Biological Technology, Hangzhou, China). The extracted proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane (ISEQ00010, Merkmillipore, China). The membrane was blocked with 5% non-fat milk (Beyotime, China) and incubated with primary antibodies anti-ALYREF (1:2000, ab202894, Abcam, USA), anti-actin (1:20000, AC026, ABclonal, China), anti-SLC7A11 (1:1000, ab307601, Abcam, USA), anti- Transferrin (1:1000, A19130, Abclonal, China) and anti-FTH1(1:1000, A19544, Abclonal, China). The membrane was then incubated with anti-mouse secondary antibodies (1:5000, RS0001, Immunoway, USA) or anti-rabbit secondary antibodies (1:5000, RS0002, Immunoway, USA).
Immunohistochemistry In brief, tissue samples were collected, embedded in paraffin, cut into 5 μm slides and then deparaffinized in gradient alcohol and gradient xylene successively. This was followed by exhausting endogenous peroxidase activity in the solution by adding 3% H2O2 for 10 min. For the next step, these slides were incubated in mixed solution containing 4% BSA (FD0030-10, FUDE BIOLOGICAL TECHNOLOGY, Hangzhou, China) and 0.5% Triton X-100 (T8200, Solarbio, Beijing, China) for 30 min after the antigen retrieval was performed with 1×EDTA solution (ZLI-9079, ZSGB-BIO, Beijing, China). After cooling to RT without any intervention measures, these slides were then incubated with the primary antibody at 4℃ overnight and the next day incubated with the biotinylated secondary antibody (PV-8000, USAZSG-BIO, Beijing, China) for 30 min at 37℃. The signal was developed with DAB (PV-800). The subsequent processes involved hematoxylin staining, dehydration and sealing with the neutral resin. The following antibodies were used: anti ALYREF (Abcam), anti-ki67 (Abcam) and anti-SLC7A11 (Abcam). The staining intensity scores were evaluated according to the following standard: 0, negative staining; 1, mild staining; 2, moderate staining; 3, intense staining. The positive rate of colored cells was scored as follows: 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%) or 4 (> 75%). The two numbers were multiplied to obtain a final score value ranging from 0 to 12. Scores of 0–7 were identified as low expression and 8–12 as high expression in every group. Protein expression was evaluated by two pathologists blinded regarding the clinical data.
Cell counting kit-8 (CCK-8) assay Cells were cultivated on 96-well plates, with 5000 cells for H1975 or 4000 cells for PC9 per well. The optical density (OD) values at 450 nm were detected by CCK-8 assay (HY-K0301, MCE, Shanghai, China) after 0, 24, 48, 72, and 96 h as described in the manual. At each time point, CCK-8 was diluted to 1/10 initial concentration with serum-free 1640 medium and incubated for 1 h at 37℃. The absorbance at 450 nm was determined using a Varioskan microplate reader (Thermo Scientific). The cell proliferation rate was standardized to 1 according to the number of 0 h among different groups.
EDU(5-ethynyl-2’-deoxyuridine) assay EDU reagent was added to treat cells and incubated at 37 ° C for 4 h. The cells were fixed with 4% paraformaldehyde at room temperature for 15 min, then 0.3% triton was used to penetrate the cells. The reaction solution was prepared according to the system of the reagent kit (C0078L, Beyotime) and the cells were observed under a fluorescence microscope.
Colony formation assay Transfected cells after 24 h were seeded into 6-well plates (5000 cells/well for H1975 and 4000 cells/well for PC9) and kept in 1640 containing 10% FBS for 7–14 days to obtain at least 50 cells for each colony. The colonies were fixed with 4% paraformaldehyde for 15 min before being stained with crystal violet.
Wound-healing assay Briefly, transfected cells in the exponential phase of growth were harvested and seeded in a 6-well plate. When the cell confluence reached 90% or more as seen under the microscope, a sterile 10 µL pipette tip was used to scratch a vertical wound along the line earlier drawn with the marker pen on the bottom of the dish. Next, the cells were gently rinsed twice with PBS to remove floating cells. Images of the scratches were taken using an inverted microscope at ×10 magnification at 0, 24 and 48 h of incubation.
Migration and invasion assays 8.0 μm pore inserts (CLS3422, Corning) were pre-coated with Matrigel (356234, Corning) for 1 h at 37℃before the cell suspension was added to the chamber, so as to conduct the invasion assay smoothly. More specifically, cells were digested and resuspended in FBS-free 1640 culture medium. The lower chamber was filled with 750 µL complete culture medium (1640 with 10% FBS), while a total of 100 µL (containing 1 × 105 cells/100 µL) cell suspension was seeded into the upper chamber. This step lasted for 48 h and then 4% paraformaldehyde was used to fix the cells for 15 min before they were stained with 1% crystal violet for another 20 min. The stained chambers were cut and fixed with neutral resin before performing microscopic imaging (AX10). Each experiment was performed in triplicate, and the cells of different groups were analyzed by ImageJ and GraphPad. For the migration assays, a total of 5 × 104 LUAD cells were seeded into 8.0 μm pore inserts without Matrigel. The rest of the steps were identical with those in the invasion assays.
Animal experiments All Animal experiments were approved by the Animal Experimental Research Ethics Committee of Zhejiang University and conducted according to its guidelines (Approval Number: 2024 − 1173). Female BALB/c nude mice (4–5 weeks, 14–18 g) were purchased from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd (Hangzhou, China). They were bred in specific pathogen-free conditions under a 12 h light/dark cycle. We randomly divided the mice into two groups: the “NC” group and the “sh” group. 2 × 106 tumor cells (0.10mL) were resuspended in PBS and injected subcutaneously into the right flanks of mice. Tumor size was measured every 3 days using calipers, and the volume was calculated using the formula (length × width2)/2. Two weeks later, mice were humanely euthanized, and tumor size, tumor weight as well as body weight were measured.
Electron Microscopy Cells transfected for 96 h were collected into a 1.5 mL centrifuge tube and fixed in 2.5% glutaraldehyde for at least 4 h at 4℃. Subsequently, 0.1 mL PBS and 1% osmium tetroxide was used to rinse and fix the samples, respectively. Next, the samples were stained with 2% uranyl acetate lasting for half an hour, then being washed 3× by 1 mL distilled water, lasting for 10 min each. Gradient ethanol (50%, 70%, 90%, 100%) and 100% acetone were prepared to treat these cells before they went through the embedding process in resin, and they were baked overnight at 60℃. The carbon-coated grids were observed under an electron microscope (Talos L120C, Thermo Scientific) and images were taken for further analysis.
ROS detection The level of intracellular ROS was determined by using a ROS kit (CA1410-100T, Solarbio, Beijing, China). Serum-free culture medium at the ratio of 1000:1 with DCFH-DA was used to prepare the working solution. The collected cells were suspended in this solution and transferred to 37 °C cell culture incubator for 25 min for H1975, while 45 min was needed for PC9. After the staining process, rinsing was performed using serum-free cell culture medium or PBS, which was necessary to remove DCFH-DA thoroughly that had not entered the cell. Finally, flow cytometry was applied to quantify the fluorescence intensity.
C11 ROS assay The level of C11 ROS was detected using a C11 BODIPY 581/591 reagent (GC40165, GLPBIO, USA). For the concrete steps, cells transfected for 72–96 h were collected and rinsed by PBS twice. Then, they were incubated at room temperature for about 30 min for H1975 and 1 h for PC9 with the 5 µmol/mL C11 BODIPY 581/591 reagent, which was diluted by PBS in advance. The incubated cells were rinsed by PBS for another 2 times, followed by testing by flow cytometry. The entire process was protected from light.
MDA assay MDA testing kit (S0131S, Beyotime, China) was applied in our study. The detailed procedures were as follows: cells were lysed using Western and IP cell lysates (P0013, Beyotime), and centrifuged at 12,000×g for 10 min at 4℃ before the supernatant was taken for subsequent assays. The protein concentration in the prepared sample was determined with the BCA protein concentration assay kit (FD2001, FUDE biological technology) prepared for the subsequent calculation of the MDA content per unit protein weight in a cell. After adding PBS as a blank control to a 1.5 mL centrifuge tube, 0.1 mL of the standards included in the kit were diluted to different concentrations to obtain a standard curve, along with 0.1 mL of sample for determination. To these tubes, 0.2 mL of MDA detection solution was added for 15 min at 37℃ after being vortexed completely. Samples were then put in the water bath to cool to room temperature and centrifuged at 1,000×g for 10 min at room temperature. Next, 200 µL of supernatant was sucked to put into a 96-well plate and the absorbance was subsequently measured with a multimode microplate reader (0906052G, Thermo Vasiscan) at 532 nm.
GSH measurement The intracellular level of GSH was determined using a GSH and GSSG Assay Kit (S0053, Beyotime) according to the manufacturer’s instructions. Briefly, cells were lysed in protein M solution equal to triple the volume of cells (i.e., if the cell pellet was 10 µL, 30 µL of the protein M solution was added) after being washed once in PBS and fully vortexed. The volume of the cell pellet could be estimated based on the weight of the cell pellet. The centrifuge tubes were weighed before and after the collection of cells, so that the weight of the cell pellet could be determined. According to this assay kit, the volume of 10 mg cell pellet can be roughly seen as 10 µl. The samples were then subjected to rapid freeze-thaw periods 3× using liquid nitrogen and a 37℃ water bath. Subsequently, they were left at 4℃ or in an ice bath for another 5 min before being centrifuged at 10,000×g for 10 min at 4℃. The supernatant was used for the determination of total glutathione. Eventually, the microplate reader was used to measure the absorbance of total GSH and GSSG levels and their standards at 412 nm wavelength. Finally, according to the formula “GSH = Total Glutathione-GSSG×2”, the concentration of GSH could be calculated.
Fe2 + assay The level of cellular Fe2 + was assayed with Iron ion detection probe - FerroOrange (F374, Dojindo). 10,000 cells were inoculated in a 96 well transparent blackboard overnight. 10 umoL/ml DFOM(HY-B0988, MCE) was added to the cells for 24 h. 1 µ mol/l FerroOrange working solution was added in the cells at 37 ℃ for 30 min before FBS-free MEM medium were used to rinse the cells. Finally, multifunctional microplate reader was used to detect the fluorescence intensity of each sample (Ex: 543 nm/Em: 580 nm).
RIP (RNA immunoprecipitation) An RNA immunoprecipitation kit (Geneseed, Guangzhou, China) was used in this article to complete this assay. 200 µL of protein A + G beads were taken and washed by Buffer A by vortexing twice, lasting 10 s each time. 1 mL Buffer A (1×) as well as 20 µL Buffer D were added and the reaction was rotated at 4℃ at a speed of 10 rpm for 30 min. When the reaction completed, the solution with beads was divided into 2 parts, with 500 µL each, labeled as IP group and IgG group, respectively. These 2 parts were washed with BufferA another 2 times and placed to the magnetic frame to be used. Next, 1 mL BufferA (1×) was added to each of the two tubes of pretreated beads, and 5 µg of IP antibody was added to the IP group and 5 µg IgG antibody was added to the IgG group at 4℃, with 10 rpm/min rotation to facilitate the reaction overnight. The next day, cells were collected and transferred to a centrifuge tube for centrifugation at 500×g for 5 min. After being rinsed by pre-chilled PBS, the cell deposition was lysed with a mixture of 1 mL Buffer A (1×) working solution, including 10 µL protease inhibitor and 10 µL RNase on ice, for 10 min while vortexing twice for 5 s each during waiting. After being centrifuged at 10,000×g 4℃ for 10 min, the supernatants were transferred to another tube for further determination. In the following stage, 350 µL of Buffer A (1×) and 400 µL cell lysis sample were added to magnetic beads conjugated with IP antibody or IgG antibody, respectively, at 4℃, and rotated at 10 rpm/min overnight for the reaction to complete. On the last day, WB and qPCR were applied to measure and analyze the separated proteins and captured RNA, respectively.
Dual-Luciferase assay The Dual-Luciferase® Reporter Assay System (E1910, Promega, USA) was applied in this study. Specifically, cells transfected with siRNA and plasmids for 48 h were lysed by 1× passive lysis buffer after being rinsed by PBS 2 times at 4℃ for approximately 20 min. Lysates were placed at room temperature after the samples were thoroughly vortexed. 40 µL luciferase assay reagent was mixed with 10 µL sample very quickly and completely before measuring the luminescence for 10 s. Then, 40 µL Stop&Glo® reagent was added straight away to the mixture and stirred at a fast speed before measuring the Renilla luminescence.
RNA stability Transfected cells were digested for 72 h and seeded in the 6-well plate again. 2 µmol/mL actinomycin D (HY-17559, MCE) was added to cells at the same time before cells were collected at 0 h, 2 h, 4 h. The RNA stability of diverse groups and time points was analyzed by qRT-PCR.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software, San Diego, USA). A two-tailed unpaired Student t-test was adopted to analyze the differences between two experimental groups, while ANOVA by non-parametric alternatives was applied for the multiple ones. All data from 3 independent experiments were presented as mean ± SD. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Data availability
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249 (2021).
Hirsch, F. R. et al. Lung cancer: current therapies and new targeted treatments. Lancet 389, 299–311 (2017).
Kris, M. G. et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA 311, 1998 (2014).
Zhao, L. et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 42, 88–116 (2022).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell. Res. 31, 107–125 (2021).
Kazan, K., Kalaipandian, S. & Ferroptosis Yet Another Way to Die. Trends Plant. Sci. 24, 479–481 (2019).
Dixon, S. J. et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 149, 1060–1072 (2012).
Xing, N. et al. Ferroptosis in lung cancer: a novel pathway regulating cell death and a promising target for drug therapy. Cell. Death Discov. 9, 110 (2023).
Bannai, S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J. Biol. Chem. 261, 2256–2263 (1986).
Combs, J. A. & DeNicola, G. M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 11, 678 (2019).
Lukong, K. E., Chang, K., Khandjian, E. W. & Richard, S. RNA-binding proteins in human genetic disease. Trends Genet. 24, 416–425 (2008).
Kang, D., Lee, Y. & Lee, J. S. RNA-Binding Proteins in Cancer: Functional and Therapeutic Perspectives. Cancers 12, 2699 (2020).
Zhang, W. et al. RBMS1 regulates lung cancer ferroptosis through translational control of SLC7A11. J. Clin. Invest. 131, e152067 (2021).
Zhang, Z. et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 16, 1482–1505 (2020).
Wang, Q. et al. RNA binding protein DAZAP1 promotes HCC progression and regulates ferroptosis by interacting with SLC7A11 mRNA. Exp. Cell. Res. 399, 112453 (2021).
Zhao, Y., Xing, C. & Peng, H. ALYREF (Aly/REF export factor): A potential biomarker for predicting cancer occurrence and therapeutic efficacy. Life Sci. 338, 122372 (2024).
Zhang, T. et al. Targeting histone deacetylase enhances the therapeutic effect of Erastin-induced ferroptosis in EGFR-activating mutant lung adenocarcinoma. Transl Lung Cancer Res. 10, 1857–1872 (2021).
Stubbs, S. H. & Conrad, N. K. Depletion of REF/Aly alters gene expression and reduces RNA polymerase II occupancy. Nucleic Acids Res. 43, 504–519 (2015).
Virbasius, C. M. A., Wagner, S. & Green, M. R. A Human Nuclear-Localized Chaperone that Regulates Dimerization, DNA Binding, and Transcriptional Activity of bZIP Proteins. Mol. Cell. 4, 219–228 (1999).
Laurakay Bruhn, A. & Munnerlyn and Rudolf Grosschedl 1. ALY, a context-dependent coactivator of LEF-1 and AML-1 is required for TCR enhancer function. GENES Dev. 11, 640–653 (1997).
Sträßer, K. et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417, 304–308 (2002).
Wickramasinghe, V. O. & Laskey, R. A. Control of mammalian gene expression by selective mRNA export. Nat. Rev. Mol. Cell. Biol. 16, 431–442 (2015).
Nulali, J. et al. ALYREF-mediated RNA 5-Methylcytosine modification Promotes Hepatocellular Carcinoma Progression Via Stabilizing EGFR mRNA and pSTAT3 activation. Int. J. Biol. Sci. 20, 331–346 (2024).
Yang, Q. et al. LINC02159 promotes non-small cell lung cancer progression via ALYREF/YAP1 signaling. Mol. Cancer. 22, 122 (2023).
Chang, C. T. et al. Chtop is a component of the dynamic TREX mRNA export complex. EMBO J. 32, 473–486 (2013).
Wang, N. et al. m5C-dependent cross-regulation between nuclear reader ALYREF and writer NSUN2 promotes urothelial bladder cancer malignancy through facilitating RABL6/TK1 mRNAs splicing and stabilization. Cell. Death Dis. 14, 139 (2023).
Wu, S. et al. The role of ferroptosis in lung cancer. Biomark. Res. 9, 82 (2021).
Wang, W. et al. CD8 + T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Liu, T., Luo, H., Zhang, J., Hu, X. & Zhang, J. Molecular identification of an immunity- and Ferroptosis-related gene signature in non-small cell lung Cancer. BMC Cancer. 21, 783 (2021).
Wang, X. et al. Stem Cell Factor SOX2 Confers Ferroptosis Resistance in Lung Cancer via Upregulation of SLC7A11. Cancer Res. 81, 5217–5229 (2021).
Xu, Y. et al. METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m6A modification. Cancer Cell. Int. 22, 11 (2022).
Funding
This research was supported by National Natural Science Foundation of China (82070100), Zhejiang Provincial Natural Science Foundation of China(LQ21H030006)and National Natural Science Foundation of China (82103073).
Author information
Authors and Affiliations
Contributions
W.H. conceived and designed the research. J.C. provided administrative support and revision suggestion. Y.C. performed most of the experiments and data analysis with the assistance from T.Z. and H.C. Y.C. wrote the manuscript and Ting Zhao revised it. W.H., J.C. and H.C. collected and provided clinical samples and data. All authors have read and approved this version of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Zhao, T., Chen, H. et al. RNA binding protein ALYREF regulates ferroptosis to facilitate LUAD growth and metastasis via promoting SLC7A11 mRNA stability. Sci Rep 15, 1351 (2025). https://doi.org/10.1038/s41598-024-83276-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83276-9