Abstract
We report on the potential application of crystalline thin metal oxide films (TiOx, SnOx) with varying stoichiometries in perovskite solar cell devices. The oxides were deposited via reactive e-beam evaporation, involving the sublimation of pure metals under different pressures of pure oxygen, followed by thermal annealing at 200 °C. Variable angle spectroscopic ellipsometry, X-ray diffraction (XRD), contact angle measurements, scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDS) were used to characterize the films. XRD findings confirmed the crystalline phases of SnOx thin films treated at 200 °C for the most oxygen-rich films (deposited at 2e-4 Torr), while TiOx layers exhibited an amorphous phase. FESEM results confirmed that uniform and dense films were generated across the entire substrate surface. Using the measured refractive indices in a computational model, it was demonstrated that optimizing the device design with these films could result in power conversion efficiencies surpassing 25%.
Similar content being viewed by others
Introduction
Given their outstanding electrical, structural, and optical qualities, thin films of metal oxides are necessary for microelectronic and optoelectronic devices1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. These films act as buffer layers to control the mobility of charge carriers. The structure and morphology of these films greatly affect the performance of energy conversion devices. Oxide materials that are either n-type or p-type and inorganic, which can be produced at low temperatures, offer benefits such as lower fabrication costs and improved device stability owing to their moisture resistance. Metal oxides like Titatnium oxide (TiOx) and Tin oxide (SnOx) have proven effective in meeting device requirements. Inorganic materials used for carrier transport need to have a clean electrical interface with the absorbing layer and high carrier mobility to minimize carrier recombination16,17,18,19,20,21,22,23,24,25.
Metal oxide layers have been employed as carrier transport materials in different perovskite device configurations. Achieving high-efficiency devices requires further optimization of these layers, particularly regarding processing temperature and optical properties alignment. Heterojunction-based solar cells use n-type or p-type semiconductors with low electron affinity, high carrier mobility, and a wide bandgap to efficiently transfer charge. Sputtering, evaporation, and atomic layer deposition (ALD) are commonly used methods for preparing these thin films. The resulting films often demonstrate p-type or n-type conductivity as a result of inherent defects, including metal or oxygen vacancies. TiOx and SnOx are recognized as metal-deficient semiconductors with inherent p-type behavior, showing varying optical properties due to stoichiometric deviations from different preparation methods. With optical bandgap energies above 3 eV, these materials are suitable for use as windows in solar energy conversion devices26,27,28,29,30,31,32,33,34,35.
In this research, the growth of oxide films via e-beam evaporation was accomplished by adjusting the oxygen flow rate, which subsequently affected the chamber pressure. E-beam evaporation was selected due to its precise control over the deposition rate, making it possible to cost-effectively produce films over large areas. This study investigates how surface morphology, optical properties, and microstructure of reactively produced thin metal oxide films are affected by oxygen pressure. Utilizing information from optical spectroscopy, such as absorbance, ellipsometry, and absorption coefficient measurements, the optical bandgaps of the films were ascertained. Furthermore, a relationship between surface morphology and various deposition pressures was discovered. Using numerical simulations with the SCAPS-1D simulator, the impact of oxide layers on solar cell device performance was evaluated36,37,38,39,40,41,42,43,44,45. This allowed for the computational screening of oxide materials prior to experimental testing in a solar cell.
Methodology
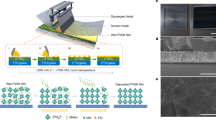
Soda lime glass (SLG) is the substrate material that was utilized. Acetone, isopropanol, and deionized water were used to clean the glass slides in an ultrasonic bath for five minutes each, and then nitrogen was used to dry them. The evaporation was carried out while reacting with oxygen flow using the Denton Vacuum Explorer™ e-beam evaporator at three distinct deposition pressures: 2 × 10− 4 Torr, 9 × 10− 5 Torr, and 2 × 10− 5 Torr. The base pressure of the deposition chamber is approximately 3 × 10− 6 Torr and it uses Ti and Sn as evaporation sources, both obtained from Kurt J Lesker with 99.995% purity. The films were deposited at a rate of 1 Å/s after being grown at room temperature. The films were post-annealed at 200 °C in air for 1 h after the evaporation process. The structural properties were analyzed at 2θ diffraction angles using a Rigaku™ X-ray diffractometer. A JEOL SEM 7610™ was used for morphological and EDS studies, and ellipsometry was used to examine the heat-treated films’ optical characteristics. In addition, ellipsometry was used to analyze the optical characteristics of the annealed films and contact angle measurement was used to understand the hydrophilicity or hydrophobicity of the films.
The application SCAPS-1D, a 1D-solar cell capacitance simulator, was utilized for numerical study. SCAPS was developed in the Department of Electronics and Information Systems at the University of Gent in Belgium. The basic governing equations for the carriers generated during the process, including transport, Poisson, and continuity equations, can be handled by this software. It functions as a useful instrument for examining any thin-film device structure. The assessments are predicated on the optical properties of the materials obtained from the experiments carried out in this study, such as bandgap and absorption coefficient. Electron affinity, electron mobility, hole mobility, density of states, dielectric permittivity, carrier density, and thermal velocity are additional metrics that were extracted from pertinent literature.
Results and discussions
Characteristics of the metal oxide thin films’ structure
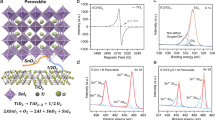
Annealing tin oxide (SnOx) can significantly impact its crystalline structure, as observed in X-ray diffraction (XRD) patterns. During the annealing process, which involves heating the material to elevated temperatures, atoms within the SnOx lattice can migrate and reorient, leading to enhanced crystallinity and potentially larger grain sizes. This improvement is often reflected in the XRD spectrum by sharper and more intense peaks at the characteristic 2θ positions. Additionally, annealing can reduce structural defects and residual stresses in the SnOx lattice, yielding a more defined and phase-pure tetragonal rutile structure. Such changes are advantageous for applications in sensors and photodetectors, as they can lead to improved electron mobility and material stability, thereby enhancing device performance and sensitivity. The annealed SnOx thin films show five noticeable peaks at (111), (101), (200), (211), (220), (221), (301), and (321) orientations in the XRD pattern seen in Fig. 1, suggesting that they are polycrystalline. Every noticed XRD peak matches the ICDD database’s standard XRD pattern. The clustering and development of grains throughout the heating process may have caused the alterations in the crystal structure. Compared to bigger nuclei, smaller nuclei can rotate more easily to minimize interfacial energy. As the temperature rises, the atoms in the crystal lattice realign, producing a structure that is more stable and has better crystalline quality. The Debye-Scherrer method was used to determine the average dimensions of the crystals in each configuration.
In nanometres, D and λ represent the mean crystallite size, X-ray wavelength, radians for the integral breadth or peak complete width at half maximum, degrees for the angle where the maximum intensity peak is located, and B and θ, respectively. The typical crystallite size is 19 nm, and there is a lattice strain of 0.25%. A greater annealing temperature may be required to induce the transition into the crystalline phase, as indicated by the amorphous phases of the treated TiOx samples.
An analysis of the surface morphology of annealed metal oxide thin films
The FESEM was used to examine the surface structure of the oxides. Large particles or grains, void- and discontinuity-free oxide coatings that increase cell efficiency without shunting are essential. There should be few crystalline faults in the film’s microstructure. To investigate surface morphology, 500 nm thin layer FESEM images were taken. The annealed thin films that were grown are displayed in Fig. 2 through FESEM images. It is possible to distinguish between thin films grown under oxygen-poor and oxygen-rich pressures based on their surface morphology characteristics. The production of consistent, dense films throughout the substrate is confirmed by the FESEM results. The optimized deposition parameters made it possible to achieve a surface free of pinholes and cracks, which is crucial for the intended devices’ electrical properties. In general, thin films deposited at lower temperatures are often subject to tensile stress, which can result in smaller grain sizes and a denser structural arrangement. On the other hand, annealing can lead to strain relaxation, influencing both growth dynamics and film thickness. Additionally, atomic surface diffusion rates increase with temperature, enabling atoms to spread more effectively across the substrate surface. This can lead to improved coalescence and a more even thickness, though excessively high temperatures may cause re-evaporation or material loss. The morphology of the films varies with annealing temperature, driven by the complex interactions of atomic mobility, film stress, surface diffusion, and deposition dynamics, each of which can influence growth either positively or negatively, resulting in the observed variations.
Table 1 shows the metal to oxygen atomic concentration as studied by EDS, which confirms that higher oxygen flow rate besides post annealing affect the growth orientation of the films by making them more oxygen rich and metal poor. Such films become more transparent due to oxidation, so it is possible to control the stoichiometry by regulating the level of oxygen present in the chamber during evaporation. These findings indicate the potential to improve the fabrication of photovoltaic devices by incorporating a suitable metal oxide layer to adjust the quality of the interface.
Annealed metal oxide thin-film optical properties.
The optical properties of the annealed samples were investigated using the ellipsometer spectrophotometer as shown in Figs. 3 and 4. It is established that the original films were either metal-rich or metal-poor based on their spectrum. In particular, at a deposition pressure of 2 × 10− 4 Torr, the films show decreased absorbance in the visible region. Conversely, TiOx films produced at 2e-4 Torr exhibit greater transparency up to 450 nm. There is a noticeable shift in the spectrum based on the deposition pressures. The combined impacts of disorder at grain boundaries, quantum size effect, and stoichiometric deviation might influence the transmission and absorbance of polycrystalline semiconductors45. The results are well aligned as reviewed46,47,48,49,50,51,52. To extract the optical constants (refractive index and extinction coefficient) of SnOx and TiOx from experimental data, various models are employed, each suited to different spectral characteristics of these materials53,54. The Tauc-Lorentz model is widely used for amorphous and polycrystalline films, as it effectively describes both the optical band gap (through Tauc’s approach) and the dielectric response above the band gap using Lorentz oscillators. This model is particularly valuable for SnOx and TiOx due to its accuracy in representing their absorption edge and dispersion. For regions where the material is transparent, the Cauchy model or Sellmeier model can be applied to describe the wavelength dependence of the refractive index in non-absorbing ranges, especially for TiOx. In cases where free carrier absorption is significant, often observed in doped or conductive oxides, the Drude model is used to account for free electron contributions to the extinction coefficient, particularly in the IR range. These models are fit to spectroscopic ellipsometry or reflectance/transmittance data through nonlinear regression, iteratively refining parameters to closely match the measured optical response across relevant wavelengths. This combination of models allows for a comprehensive representation of SnOx and TiOx optical behavior, from transparent to absorbing regions. As observed values of the extinction coefficient (k) and refractive index (n) for the films deposited at varying pressures at a wavelength of 632 nm. The graph shows that metal-rich films have higher k values, most likely as a result of the increased absorption brought on by the development of these films. Moreover, nearly all of the films (Table 2) show a high refractive index value (n > 2)40,41. Generally, the refractive index and extinction coefficient of thin films vary significantly with deposition pressure due to changes in the film’s microstructure, density, and optical path. At lower deposition pressures, the mean free path of sputtered atoms is longer, allowing them to reach the substrate with higher kinetic energy. This can lead to denser films with fewer voids, which generally have a higher refractive index. At higher pressures, atoms have a shorter mean free path and may collide more frequently, resulting in a film with increased porosity and lower density. A porous or less dense film will typically have a lower refractive index. Also, deposition pressure influences the growth mode and morphology of thin films. Lower pressures can produce more columnar or crystalline structures, which impact the refractive index by increasing the film’s compactness. Conversely, higher pressures often produce more granular or amorphous structures, which alter light propagation, potentially lowering the refractive index and changing the extinction coefficient. Higher deposition pressures can result in rougher surfaces and grain boundaries within the film. These features scatter light, increasing the extinction coefficient, which describes the material’s ability to absorb and scatter light. In denser films, with fewer scattering centers, the extinction coefficient may be lower, as light absorption rather than scattering becomes the dominant mode of energy dissipation. As studied, deposition pressure affects the kinetic energy and mobility of atoms during film growth, impacting density, microstructure, porosity, and even chemical composition. Each of these factors plays a role in determining the film’s optical properties, specifically the refractive index and extinction coefficient.
Measurements of the annealed metal oxide thin films’ contact angles.
The metal-oxide thin films’ hydrophobic characteristics play a key role, impacting how water droplets condense on the surface. Figure 5 shows different patterns of hydrophobic behavior in various metal oxides. Achieving hydrophobicity usually requires minimizing the impact of the substrate, as higher surface tension tends to push the interaction toward super hydrophilicity.
Numerical simulation results
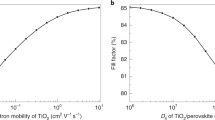
In general, the power conversion efficiency (PCE) of perovskite solar cells (PSCs) can be effectively predicted using numerical simulations, by implementing device physics models that accurately represent the material and device structure. In our work, to achieve reliable PCE estimations, we have used SCAPS-1D, which models the electronic and optical properties of the PSC layers based on parameters such as bandgap, electron and hole mobilities, defect densities, and recombination rates. Additionally, optical simulations account for light absorption, reflection, and scattering within the device layers, providing insights into photon management that impacts the PCE. We have used the realistic values reported in the literature to predict how various conditions—such as layer thicknesses, interface qualities, and defect passivation levels—affect the PCE. Sensitivity analysis or parametric sweeps within the simulation further help isolate the impact of individual factors, like trap densities or interface recombination, on the overall performance. A possible device that, in ideal conditions, makes use of solar cell absorber, ETM, and HTM layers is depicted in Fig. 6’s diagram. In these energy conversion devices, matched band energy alignment is crucial for every layer. The photo-carriers produced in the absorber layer will be transported to the ETM and HTM for electrons and holes, respectively. Charge carrier transfer is made possible by the lower conduction band edge of the N-type TiOx and SnOx layers that are produced experimentally compared to the absorber layer of the solar cell. NiOx and MoOx have the potential to function as the HTM layer due to their greater valence band level, which makes it easier to inject hole carriers.
SnOx is widely favored in perovskite cells for its high transparency, stability, and optimal band alignment, which together enhance charge extraction and reduce recombination losses—key factors that contribute to high PCEs in experimental settings. If the simulated model accurately reflects the material properties and interfaces within the cell, including optimized SnOx layers, then the predicted PCE could indeed be higher. Differences in the simulated and reported experimental PCEs may stem from underrepresented improvements in interface quality, defect passivation, or light management techniques, which are often refined in experimental setups. Therefore, to align more closely with state-of-the-art PCEs, the simulation parameters could be further adjusted to mirror recent advances in perovskite film quality, interface engineering, and device architecture that push efficiencies beyond the 26% threshold.
In addition to using observed optical parameters from the literature, such as the band gap and absorption coefficient, we also gathered additional variables for our research. These results will undoubtedly help optimize the electrical properties for the development of high-efficiency devices. Figure 6 illustrates how employing TiOx (3.75 eV) as an ETM layer with a 100 nm NiOx (3.5 eV) HTM layer can boost performance by more than 25%. This effect could be caused by improved hole carrier transport across aligned NiOx layers with a 3.5 eV bandgap and enhanced photon energy transmission via the ETM layer for absorber absorption.
Conclusions
Numerous studies have examined the structural properties of metal oxide thin films formed by reactive evaporation and annealing at different pressures, focusing on crystal orientation, grain size, and lattice parameters. The presence of vague peaks enhances the performance of anti-reflection coatings and optical filters for photovoltaic applications by improving photon reflection and increasing photon scattering. Oxidation effects on the surface and at deeper depths during deposition have led to stoichiometry deviations, as demonstrated by energy-dispersive X-ray spectroscopy (EDS). Scanning electron microscopy (SEM) images show that the films are uniformly dense and pinhole-free, which is crucial for reducing recombination losses in energy conversion devices. SEM images also reveal that the surface morphology of the films is significantly influenced by deposition pressure, with slower deposition rates resulting in smoother surfaces. The optical characteristics of annealed samples prepared under different process parameters are shown to correlate with the oxygen content in the films. Numerical analysis suggests that eliminating bulk and interface defects, along with carefully selecting electron transport material (ETM) and hole transport material (HTM) layers with optimal optical properties, could lead to the development of a high-performance device. Consequently, if good electrical properties are achieved, these optimized layers can be directly used as carrier transport layers and windows for energy devices, eliminating the need for additional fine-tuning.
Data availability
The data that support the findings of this study are available from the corresponding author, [MIH], upon reasonable request.
References
Green, M. A., Ho-Baillie, A. & Snaith, H. J. The emergence of perovskite solar cells. Nat. Photon. 8(7), 506 (2014).
Zhou, Q., Liu, X., Liu, Z., Zhu, Y., Lu, J., Chen, Z., Li, C., et al. Annual research review of perovskite solar cells in 2023. Materials Futures (2024).
Zuo, C. et al. Natural drying yields efficient perovskite solar cells. DeCarbon 2, 100020 (2023).
Yu, Z. et al. Micro–nano water film enabled high-performance interfacial solar evaporation. Nano-Micro Letters 15(1), 214 (2023).
Zhang, L. et al. Major strategies for improving the performance of perovskite solar cells. Energy 2(3), 172–199 (2023).
Ye, M. et al. Recent advances in interfacial engineering of perovskite solar cells. J. Phys. D Appl. Phys. 50(37), 373002 (2017).
Zhao, Y., Nardes, A. M. & Zhu, K. Effective hole extraction using MoOx-Al contact in perovskite CH3NH3PbI3 solar cells. Appl. Phys. Lett. 104(21), 213906 (2014).
Tseng, Z.-L. et al. Efficient inverted-type perovskite solar cells using UV-ozone treated MoOx and WOx as hole transporting layers. Solar Energy 139, 484–488 (2016).
You, J. et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nature Nanotechnol. 11(1), 75 (2016).
Chen, W. et al. Cesium doped NiOx as an efficient hole extraction layer for inverted planar perovskite solar cells. Adv. Energy Materials 7(19), 1700722 (2017).
Wang, J.T.-W. et al. Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 14(2), 724–730 (2013).
Pinpithak, P. et al. Low-temperature and ambient air processes of amorphous SnOx-based mixed halide perovskite planar solar cell. Chem. Lett. 46(3), 382–384 (2016).
Liu, C. et al. Ultra-thin MoOx as cathode buffer layer for the improvement of all-inorganic CsPbIBr 2 perovskite solar cells. Nano Energy 41, 75–83 (2017).
Cao, K. et al. Efficient screen printed perovskite solar cells based on mesoscopic TiO2/Al2O3/NiO/carbon architecture. Nano Energy 17, 171–179 (2015).
Hossain, M. I., Alharbi, F. H. & Tabet, N. Copper oxide as inorganic hole transport material for lead halide perovskite based solar cells. Solar Energy 120, 370–380 (2015).
Baloch, A. A. B, Hossain, Tabet, N. & Alharbi, F. H. Practical efficiency limit of methylammonium lead iodide perovskite (CH3NH3PbI3). Solar Cells 9, 426–434 (2018).
Hossain, M. I., Fahhad H. Alharbi, Fedwa El-Mellouhi & Nouar Tabet. Design optimization of solar cell with molybdenum sulfide as light absorber. J. Photon. Energy 8(2), 025501 (2018).
Subbiah, A. S. et al. Inorganic hole conducting layers for perovskite-based solar cells. J. Phys. Chem. Lett. 5, 1748–1753 (2014).
Groeneveld, B. et al. Improved efficiency of NiO x -based p-i-n perovskite solar cells by using PTEG-1 as electron transport layer. APL Mater. 5, 076103. https://doi.org/10.1063/1.4992783 (2017).
Tang, L. J. et al. A solution-processed transparent NiO hole-extraction layer for high-performance inverted perovskite solar cells. Chem. A Eur. J. 24(12), 2845–2849 (2018).
Tang, J. et al. High-performance inverted planar perovskite solar cells based on efficient hole-transporting layers from well-crystalline NiO nanocrystals. Solar Energy 161, 100–108 (2018).
Abzieher, Tobias, Somayeh Moghadamzadeh, Fabian Schackmar, Helge Eggers, Florian Sutterlüti, Amjad Farooq, Danny Kojda et al. "Electron‐Beam‐Evaporated Nickel Oxide Hole Transport Layers for Perovskite‐Based Photovoltaics." Advanced Energy Materials (2019): 1802995.
Guo, X. et al. A 16.5% efficient perovskite solar cells with inorganic NiO film as hole transport material. IEEE J. Photovoltaics 8(4), 1039–1043 (2018).
Baroch, P., Musil, J., Vlcek, J., Nam, K. H. & Han, J. G. Reactive magnetron sputtering of TiOx films. Surf. Coat. Technol. 193(1–3), 107–111 (2005).
Leja, E., Korecki, J., Krop, K. & Toll, K. Phase composition of SnOx thin films obtained by reactive dc sputtering. Thin Solid Films 59(2), 147–155 (1979).
Fernandes Cauduro, André L., Zacarias E. Fabrim, Mehrad Ahmadpour, Paulo FP Fichtner, Søren Hassing, Horst-Günter Rubahn, and Morten Madsen. "Tuning the optoelectronic properties of amorphous MoOx films by reactive sputtering." Applied Physics Letters 106, no. 20 (2015): 202101.
Lu, Y. M., Hwang, W.-S. & Yang, J. S. Effects of substrate temperature on the resistivity of non-stoichiometric sputtered NiOx films. Surface Coat. Technol. 155(2–3), 231–235 (2002).
Velevska, J. & Ristova, M. Electrochromic properties of NiOx prepared by low vacuum evaporation. Solar Energy Mater. Solar Cells 73(2), 131–139 (2002).
Bullock, J., Cuevas, A., Allen, T. & Battaglia, C. Molybdenum oxide MoOx: A versatile hole contact for silicon solar cells. Appl. Phys. Lett. 105(23), 232109 (2014).
Dai, C. M., Su, C. S. & Chuu, D. S. Growth of highly oriented tin oxide thin films by laser evaporation deposition. Appl. Phys. Lett. 57(18), 1879–1881 (1990).
Yao, J., Shao, J., He, H. & Fan, Z. Optical and electrical properties of TiOx thin films deposited by electron beam evaporation. Vacuum 81(9), 1023–1028 (2007).
Yang, X., Zheng, P., Bi, Q. & Weber, K. Silicon heterojunction solar cells with electron selective TiOx contact. Solar Energy Mater. Solar Cells 150, 32–38 (2016).
Hoffmann, L., et al. "Atmospheric pressure plasma enhanced spatial atomic layer deposition of SnOx as conductive gas diffusion barrier." Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 36, no. 1 (2018): 01A112.
Macco, B., et al. Low‐temperature atomic layer deposition of MoOx for silicon heterojunction solar cells. Physica Status Solidi (RRL) Rapid Res. Lett. 9(7), 393–396.
Singh, A. et al. Fabrication and current–voltage characteristics of NiOx/ZnO based MIIM tunnel diode. Appl. Surf. Sci. 334, 197–204 (2015).
Hammond, S. R. et al. Low-temperature, solution-processed molybdenum oxide hole-collection layer for organic photovoltaics. J. Mater. Chem. 22(7), 3249–3254 (2012).
Amin, N., Hossain, M. I., Chelvanathan, ASM Mukter Uzzaman, P. & Sopian, K. Prospects of Cu2ZnSnS4 (CZTS) solar cells from numerical analysis.In International Conference on Electrical & Computer Engineering (ICECE 2010) 730–733 (IEEE, 2010).
Hossain, M. I., Chelvanathan, P., Alam, M. M., Akhtaruzzaman, M., Sopian, K. & Amin, N. Potential buffer layers for Cu2ZnSnS4 (CZTS) solar cells from numerical analysis. In 2013 IEEE Conference on Clean Energy and Technology (CEAT) 450–454 (IEEE, 2013).
Hossain, M. I. et al. Hydrophilic antireflection and antidust silica coatings. ACS Omega 6(8), 5276–5286 (2021).
Hossain, M. I. & Alharbi, F. H. Recent advances in alternative materials photovoltaics. Mater. Technol. 28, 88–97 (2013).
Alfihed, S., Hossain, M., Alharbi, A., Alyamani, A. & Alharbi, F.H. PLD grown polycrystalline tungsten disulphide (WS2) films. J. Mater. (2013).
Payne, B. P., Biesinger, M. C. & McIntyre, N. S. J. Electron Spectrosc. Relat. Phenom. 184, 29–37 (2011).
Zhang, T. et al. Applied Physics Letters 113, 262102 (2018).
Ponchel, A. Alain DÏHuysser, Carole Lamonier and Louise Jalowiecki-Duhamel - Phys. Chem. Chem. Phys. 2, 303–312 (2000).
Lokhande, C. D., Ubale, A. U. & Patil, P. S. Thickness dependent properties of chemically deposited Bi2S3 thin films. Thin Solid Films 302(1–2), 1–4 (1997).
El-Rahman, A. M. A., Rabia, M. & Mohamed, S. H. Nitrogen doped TiO2 films for hydrogen generation and optoelectronic applications. J. Mater. Sci.: Mater. Electron. 34(14), 1149 (2023).
Mohamed, S. H., Khan, M. T., Almohammedi, A. & Awad, M. A. Synthesis, structural and photophysical properties of mixed Zn: SnO2 nanowires. Mater. Sci. Semiconductor Process. 123, 105573 (2021).
Abd El-Rahman, A. M. & Mohamed, S. H. Properties of SnO2 and SnO2-xNx grown on the boat walls using vapor transport method. Appl. Phys. A 126, 1–8 (2020).
Mohamed, S. H., El-Hagary, M. & Althoyaib, S. Photocatalytic and optical properties of nanocomposite TiO2-ZnO thin films. Eur. Phys. J.-Appl. Phys. 57(2), 20301 (2012).
Mohamed, S. H. SnO2 dendrites–nanowires for optoelectronic and gas sensing applications. J. Alloys Compds. 510(1), 119–124 (2012).
Mohamed, S. H. & Shaaban, E. R. Microstructural, optical and photocatalytic properties of CdS doped TiO2 thin films. Physica B: Condensed Matter 406(22), 4327–4331 (2011).
Mohamed, S. H., Kappertz, O., Leervad Pedersen, T. P., Drese, R. & Wuttig, M. Properties of TiOx coatings prepared by dc magnetron sputtering. Physica Status Solidi 198(1), 224–237 (2003).
Mohamed, S. H. Synthesis, structural and ellipsometric evaluation of oxygen-deficient and nearly stoichiometric zinc oxide and indium oxide nanowires/nanoparticles. Philos. Mag. 91(27), 3598–3612 (2011).
Mohamed, S. H. et al. Effect of heat treatment on structural, optical and mechanical properties of sputtered TiOxNy films. Thin Solid Films 468(1–2), 48–56 (2004).
Author information
Authors and Affiliations
Contributions
Mohammad Istiaque Hossain: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Roles/Writing - original draft.Puvaneswaran Chelvanathan: Writing - review & editing.Brahim Aissa: Supervision, Validation.Amith Khandakar: Writing - review & editing.Ahasanur Rahman: Writing - review & editing.Said Mansour: Project administration, Resources, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hossain, M.I., Chelvanathan, P., Aissa, B. et al. Enhanced perovskite solar cells performance with TiOx and SnOx thin films as electron transport layers. Sci Rep 15, 7709 (2025). https://doi.org/10.1038/s41598-024-83600-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83600-3