Abstract
Herein, a corrosion inhibitor called the Gast Reg drug (GRD) was used to prevent the copper from corroding. The aggressive solution used in this investigation was HCl acid solution. A mix of electrochemical and quantum investigations are used to assess GRD’s anti-corrosion properties. It has been discovered that the GRD is essential for stopping copper from corroding in a 2 M HCl solution. The study’s results indicated that GRD shown considerable corrosion prevention capabilities for copper in 2 M HCl solution. The inhibitory effectiveness of GRD was seen to rise with higher concentrations of GRD. It is noteworthy that the maximum levels of inhibitory effectiveness (82.1%) for HCl solution were obtained at 123.87 × 10− 5 M. The primary cause of GRD’s anti-corrosion properties is its propensity to adsorb on the surface of copper via its heteroatoms. The inhibitor’s adsorption behavior was described using the Langmuir model. Surface assessments with Energy Dispersive X-ray (EDX), Scanning Electron Microscope (SEM), and Atomic Force Microscope (AFM) demonstrated the development of a prominent adsorbed film on the copper surface. The correlation between molecule structure and its inhibitory effect has been investigated and analyzed using DFT and Monte Carlo simulation. The actual adsorption occurs through a variety of active centers and physical and chemical processes that are coordinated with the calculated quantum parameters. The outcomes gathered from electrochemical, surface, and theoretical studies are well correlated.
Similar content being viewed by others
Introduction
Copper and copper-based alloys are highly sought after in certain applications because of their exceptional resistance to corrosion, good physical qualities, workability, and advantageous thermal and electrical conductivities1. They can be employed in heat exchangers, water transport systems, water treatment units, saltwater condensers, tubes, valves, and fittings, as well as deployment in chemical, petrochemical industries, and electricity generation and desalination plants2,3,4,5. Despite the numerous utilities of copper, the challenge of mitigating its susceptibility to corrosion remains paramount. An established and cost-effective approach to address this challenge involves the application of corrosion inhibitors, with a particular emphasis on organic inhibitors6,7. Organic inhibitors typically function by adhering to the metal surface, thereby creating protective barriers that impede the corrosive action of the surrounding environment and attenuate metal degradation8,9. Contemporary research has extended the repertoire of corrosion inhibitors to include medicinal compounds employed in aqueous solutions10,11. Notably, pharmaceuticals, characterized by structural features such as aromatic rings and heteroatom active sites conducive to facile adsorption, emerge as promising candidates for corrosion inhibition in metal systems12. Extensive investigation into the corrosion inhibitory potential of various drugs has unfolded13,14,15,16, with their blocking effect due to attaching to the metal surface and stopping further breakdown.
This study aims to examine the efficacy of the Gast Reg drug (GRD) in mitigating the corrosion of copper electrodes immersed in 2 M HCl. Employing a diverse suite of electrochemical measurements, including open-circuit potential (OCP), potentiodynamic polarization (PP), and electrochemical impedance spectroscopy (EIS), along with surface studies using SEM/EDX and AFM, this gives a full picture of how the rust is stopped. By using equivalent circuit models, actual impedance data may be accurately matched with theoretical values. This enables a detailed comprehension of the electrochemical behavior occurring at the interface between a metal and a solution across varying conditions.
The inhibitor’s adsorption free energy on the copper surface is also calculated, revealing the chemical interaction between the inhibitor and the copper surface. DFT computations and Monte Carlo (MC) simulations are used to explore the inhibitor’s molecular structure and protective properties. This multifaceted approach aims to unravel the intricate interplay between the Gast Reg drug and copper surfaces, offering significant observations into the development of effective methods for preventing corrosion.
Materials and methods
The tested solutions
The prepared solutions for experimentation were meticulously crafted using analytical-grade chemical reagents and distilled water. Introducing a novel inhibitor, the Gast Reg drug (GRD), further enhanced the scope of the study. Manufactured by the Medical Union Pharmaceuticals in Abu-Sultan, Ismailia, Egypt, under license from Newport Pharmaceuticals Limited in Dublin, Ireland, Gast Reg is a pharmaceutical compound containing 24 mg of Trimebutine per 5 ml in a total volume of 125 ml. The required volumes to achieve the desired inhibitor concentrations were meticulously determined and subsequently prepared through dilution processes.
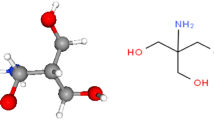
Trimebutine, identified chemically as 2-(Dimethylamino)-2-phenylbutyl 3,4,5-trimethoxybenzoate, possesses a molar mass of 387.5 g/mol, and its chemical formula is denoted as C22H29NO5. Figure 1 illustrates the structural representation of Trimebutine.
Preparation of the electrode
The working electrode, derived from a copper plate, was meticulously fashioned by securing it within glass tubing possessing a suitable internal diameter. This secure fixation was achieved through the application of chemically resistant epoxy resin, ensuring a robust bond. The resultant electrode configuration exhibited a front surface area of 0.12 cm², which was designated for direct contact with the corrosive solutions under investigation. A prerequisite to each experimental iteration involved a systematic preparatory procedure for the copper electrode surface. This involved successive abrasion using 1200/2000/2500 grit emery papers, tightly wrapped around a smooth polishing cloth. Subsequently, the electrode underwent thorough washing with tri-distilled water. Post-cleansing, the electrode was promptly dipped in the designated corrosive solutions.
Electrochemical cell
The electrochemical cell used in this study was a three-electrode configuration, consisting of an all-glass setup. The reference electrode employed was a saturated calomel electrode (SCE), while a platinum wire counter electrode was utilized for electrical connectivity. The electrochemical measurements were conducted within a 2 M hydrochloric acid solution, both in the absence and presence of diverse concentrations of the corrosion inhibitor (GRD). All experiments transpired at a controlled temperature of 25 °C, in stagnant 2 M HCl solutions. Each experimental iteration utilized a freshly prepared solution to ensure methodological rigor and consistency.
Electrochemical measurements
The copper electrode’s open circuit potential was measured individually against the saturated calomel electrode (SCE) reference electrode (Eo = 244 mV compared with the standard hydrogen electrode, SHE). After that, the Versa STAT 4 equipment and the Versa Studio electrochemistry software program were used to do potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) experiments, maintaining constant experimental conditions. For the potentiodynamic polarization experiments, a quasi-stationary state was achieved with a scan rate of 5 × 10− 3 V s− 1. The total impedance (Z) and phase shift plots were acquired at the Open Circuit Potential (OCP) within a frequency range from 105 to 10− 1 Hz, employing a sine wave of 10 mV amplitude.
Surface examination
Copper coupons were abrasively treated with different emery sheets up to a 2500 grade, and then immersed in 2 M HCl solutions, both with and without the presence of 123.87 × 10− 5 M of Gast Reg drug (GRD), for a duration of 24 h. After this exposure period, the samples were subjected to drying and, subsequently, were placed within a desiccator after being cleansed with distilled water. The surface examination was examined via scanning electron microscopy (SEM) analysis utilizing the BED-C 10.0VKV instrument manufactured by Jeol. This SEM apparatus was augmented with an energy-dispersive X-ray (EDX) unit, facilitating a comprehensive analysis of the surface composition.
DFT computations and MC simulations
Utilizing Accelrys Materials Studio 7.0, theoretical investigations were conducted utilizing the DMol3 module for Density Functional Theory (DFT) calculations and the adsorption locator module for Monte Carlo simulations. The geometric optimization of the inhibitor Gast Reg drug (GRD) was conducted in DFT calculations using the B3LYP method with a DNP basis set and COSMO solvation conditions17. The Forcite module was first used to optimize the structure of the inhibitor for Monte Carlo simulations. After Monte Carlo searches, the adsorption finder module found the inhibitor’s optimal adsorption configurations on the Cu (1 1 1) surface, allowing an evaluation of the inhibitor molecules’ protective efficiency18. The inhibitor’s adsorption, water molecules, and the copper (111) interface were all included in the simulation box, delineated with dimensions of 25.56 Å × 25.56 Å × 38.78 Å, utilizing the COMPASS force field19. It is pertinent to note that all specifics and inputs pertinent to the theoretical calculations have been comprehensively detailed in prior publications17,18, ensuring transparency and reproducibility of the computational methodologies employed in this study.
Results and discussion
OCP measurements
OCP curves were constructed by measuring the copper electrode potential relative to the SCE. As shown in Fig. 2, the Cu electrode’s OCP was measured after electrode dipped in a 2 M HCl solution with and without varying dosages of GRD to achieve a steady state. Every measurement was done at a temperature of 25 °C. The presence of GRD in 2 M HCl shifts Ess to negative potentials compared to the Ess of the copper electrode in inhibitor - free acid solution. The Ess shift may reflect that the heteroatoms (N & O) present on GRD compound are binding to active corrosion sites, slowing copper breakdown20,21,22.
PDP measurements
Potentiodynamic polarization studies of the Cu electrode were performed in 2 M HCl medium, both with and without different Gast Reg drug (GRD) doses. Figure 3 illustrates a representative presentation demonstrating the impact of GRD concentrations on the polarization curve of a copper electrode. Various corrosion parameters, including the corrosion potential (Ecorr), corrosion current density (icorr), and the anodic (βa) and cathodic (βc) Tafel slopes, were calculated from distinct polarization curves. These values are tabulated in Table 1 for solutions without and with GRD. A discernible and consistent trend emerges, as icorr exhibits a marked reduction in the presence of GRD. The value of corrosion potential (Ecorr) slightly affected in the presence of the inhibitor, which suggests a mixed adsorption process. Figure 3a further illustrates that while the presence of GRD doesn’t alter the general features of the polarization curves, it significantly diminishes the corrosion current density, indicative of a lowered corrosion rate23. This suggests that GRD molecules do not modify the underlying mechanism of the corrosion process but effectively reduce both cathodic and anodic current densities, affirming the role of GRD as a mixed inhibitor. Measured current densities in the presence (icorr(inh)) and absence (icorr) of GRD were used to quantify corrosion inhibition efficiency (η), according to the formula24: η = [(\(\:\frac{icorr-icorr\:\left(inh\right)}{icorr}\))× 100]. The estimated inhibition efficiencies, reported in Table 1, underscore the robust corrosion inhibition properties of GRD. The anodic dissolving process is hindered, and the cathodic reaction is retarded when GRD is added to the corrosive environment. A minimal concentration is sufficient to achieve a significant level of inhibition. One possible reason for this observation is that a small number of inhibitor molecules were able to cover a large area on the metal substrate by attaching to it horizontally. The figure demonstrates that, at this juncture, the inhibition efficiency value is mostly unaffected by the inhibitor concentration. After the surface becomes nearly saturated with adsorbed molecules, it is plausible to conclude that the inhibition efficacy remains unchanged with increasing inhibitor concentration25. Furthermore, noticeable alterations in cathodic Tafel slope values suggest the prevalence of the cathodic process over the anodic one26.
Electrochemical impedance spectroscopic measurements, EIS
Electrochemical Impedance Spectroscopy (EIS) stands as a formidable methodology for assessing corrosion rates, particularly in the context of coatings, passive films, and adsorbed layers. A notable advantage of EIS lies in its utilization of a minute alternating current signal, which minimally disrupts the electrode surface morphology, or the characteristics being measured. Furthermore, the approach has the possibility of simulating experimental impedance outputs utilizing pure electrical models. This process facilitates the deduction of numerical values that correspond to the pertinent physical and/or chemical characteristics of the electrochemical system and helps to validate or refute mechanistic models27,28,29,30,31,32. The copper electrode’s impedance data in 2 M HCl solutions with and without Gast Reg drug (GRD) concentrations is shown as Nyquist and Bode curves in Fig. 4 and summarized in Table 2. The Bode curves (Fig. 4a and b) prominently exhibit a two-phase maximum, with an increase in the phase angle and broadening of the phase maximum correlating with higher inhibitor concentrations, indicative of a concurrent reduction in the corrosion rate of copper. The Nyquist curve (Fig. 4c) manifests a characteristic profile featuring a depressed two capacitive loop at high and low frequencies, along with two semicircles. This behavior is attributed to a dual-time constant, encompassing relaxation effects related to adsorption phenomena. It signifies that the corrosion process is primarily under the influence of charge transfer control20,24,27. The high-frequency semicircle is usually linked with double-layer capacitance relaxation, with its diameter signifying charge-transfer resistance30,31. The second loop at low-to-intermediate frequency considers the formation of an adsorption inhibitor layer, and the resistance increases with increasing the inhibitory concentrations20. In the analysis of impedance data, the provided software associated with the impedance system was employed, utilizing a dispersion formula. As shown in the inset of Fig. 4d, utilizing a fundamental equivalent circuit model, the experimental impedance measurements were matched to the theoretical data. This comprehensive electrochemical analysis provides valuable insights into the corrosion processes and the efficacy of the inhibitor, GRD, in altering the charge transfer dynamics at the copper electrode interface.
SEM/EDX and AFM studies
Figure 5a presents a SEM picture of the copper surface following 24 h of immersion in 2 M HCl, whereas Fig. 5b displays a SEM image of a different copper specimen surface after 24 h of immersion in 2 M HCl combined with 123.87 × 10− 5 M of GRD. The micrographs indicate significant surface damage due to corrosion without the inhibitor (blank), but the presence of the inhibitor results in much reduced surface damage. This is due to the development of an effective protective layer on the copper surface. The EDX spectrum of the copper sample immersed in 2 M HCl indicated failure in the absence of inhibitor molecules due to significant external corrosion, as seen in Fig. 6a. The introduction of 123.87 × 10− 5 M of GRD inhibitor significantly enhanced the copper sample’s surface through the formation of a protective film of surfactant molecules, as evidenced by the reduction of the copper peak in Fig. 6b, indicating that the protective film was strongly adherent to the copper surface, resulting in a high level of inhibition efficiency32,33.
The copper surface microstructure was examined using atomic force microscopy (AFM), and further investigation into the GRD’s ability to inhibit corrosion was also done. Figure 7 shows the copper surface following corrosion testing with and without a GRD concentration of 123.87 × 10− 5 M. On the basis of Fig. 7a, the copper surface in 2 M HCl has an average roughness and root mean square of 64.25 nm and 79.53 nm, which are larger than those of a copper sample in GRD (Fig. 7b), which are 26.06 nm and 34.01 nm, respectively. The findings of this investigation show that GRD molecules adsorb on copper’s surface, forming a protective layer that successfully protects the metal from undesirable ions34,35.
Adsorption Isotherm
Gast Reg drug (GRD) molecule adsorption onto metallic surfaces is the key corrosion inhibition mechanism. Two common adsorption processes, namely physisorption and chemisorption can be expected. The occurrence of physical adsorption requires the existence of metal surfaces that possess electrical charges, as well as charged species that are present within the solution’s bulk33. However, in chemisorption, the inhibitor molecule transfers some of its charges to the metal surface. Adsorption can be classified as physisorption, chemisorption, or a combination of both, depending on the existing forces. To clarify the adsorption mode of GRD molecules on the copper surface in acidic solution, the degree of surface coverage (θ) at various concentrations of GRD in 2 M HCl solution was determined from corresponding electrochemical polarization measurements using the formula: θ = (\(\:\frac{icorr-icorr\:\left(inh\right)}{icorr}\)). These θ values were fitted to different isotherms, such as Langmuir, Temkin, and Frumkin isotherms34,35. As represented in Fig. 8, the Langmuir adsorption isotherm, expressed as KC = \(\:\frac{Ɵ}{(1-\:Ɵ)}\), emerged as the most suitable fitting, where C is the concentration of GRD, θ is the surface coverage and K is the equilibrium constant of the GRD adsorption process constant related to the free energy of adsorption ΔGads as36: K = \(\:\frac{1}{Csolvent}\text{exp}\left(-\frac{\varDelta\:G}{Rt}\right)\), where T is the absolute temperature, R is the universal gas constant, and csolvent, which stands for the water concentration, is equal to 55.5 mol dm−3. This isotherm assumes equivalent adsorption sites and independent binding events at these sites, forming a linear relationship when C/θ is plotted against C according to KC= \(\:\frac{\theta\:}{1-\theta\:}\), which can be rearranged to get the following formularization23,37: \(\:\frac{C}{\theta\:}\) = \(\:\frac{1}{K}\)+ C. The experimentally derived equilibrium constant (K) is 1111.11, and the standard free energy of adsorption (ΔG°ads) can be calculated using the equation7,38: ΔG°ads = -RT ln(55.5 Kads), The calculated ΔG°ads value is -33.04 kJ mol−1, demonstrating the spontaneity of the adsorption process. It further indicates that the major mode of adsorption is mixed type as represented in Table 339,40.
Theoretical approaches
DFT studies
We examined how Gast Reg drug (GRD) molecules’ active parts interact with copper metal using Density Functional Theory (DFT). GRD’s optimized structures and HOMO and LUMO orbitals are shown in Fig. 9. The appropriate quantum chemical parameters are provided in Table 4. According to the Frontier Molecular Orbital (FMO) hypothesis, the HOMO and LUMO energies of an inhibitor molecule dictate whether it can donate or absorb electrons when it comes into contact with the metal surface41. Higher EHOMO and lower ELUMO values signify a highly effective corrosion inhibitor. Table 4; Fig. 9 show that the phenyl ring and amino group have the highest HOMO level, suggesting that the π-bonds and nitrogen atoms are ideal for electrophilic assaults on the copper surface. LUMO exhibits significant electronic density distributed over most of the molecules. These results back up the idea that inhibitor molecules can effectively adsorb on the surface of the copper, which is in line with the results of the studied measurements. The energy gap, denoted as ΔE, plays a pivotal role as an important indication, where lower values of ΔE are linked to increased competence in corrosion prevention shown by the inhibitor molecule42. Additionally, low electronegativity (X) values indicate increased potential reactivity of the GRD molecules in providing electrons to the copper surface43. Furthermore, the stability and reactivity of the molecule, measured through hardness (η) and softness (σ), highlight that the soft molecule, characterized by high reactivity, offers effective corrosion inhibition through smooth electron delivery to the metal surface through adsorption44. The dipole moment (µ) is a major indication determining corrosion inhibition45, where a higher dipole moment increases deformation energy and metal surface molecule adsorption. The calculated dipole moment (µ = 3.9) supports an increase in reactivity in comparison to a medium without a solvent, suggesting improved corrosion inhibition efficacy46.
The values of the Mulliken charge distribution and the polar direction (moment) are displayed in Fig. 10 and documented in Table 5. The high electronic intensity regions, particularly on some carbon atoms and heteroatoms (N, O), denote active cores vulnerable to electrophilic attacks47. According to previous research, some heteroatoms with highly negatively charged regions form a thin deposit on the metal surface via donor-acceptor-style reactions48,49.
The Dmol3 module was used to perform Molecular Electrostatic Potential Mapping (MEP) for the purpose of identifying the active sites of inhibitor compounds. MEP mapping, a three-dimensional visual descriptor, shows the net electrostatic influence on a molecule by showing charge distribution50. As shown in Fig. 11, red colors indicate maximum electron density regions (nucleophilic reaction), with the highest negative areas primarily over carbonyl groups51. The presence of a lower electron density above the phenyl ring and amino group indicates the existence of potential electrophilic reaction sites. In contrast, the molecular electrostatic potential (MEP) demonstrates the highest magnitude of positive charge distribution in inhibitor compounds specifically localized around hydrogen atoms. The inhibitor molecules possess areas of heightened electron density, shown by the red parts. These locations are considered favorable for establishing contacts with the copper surface, resulting in the formation of a strongly adsorbed shielding layer.
MC simulations
The inhibitor molecules’ attachment to the copper metal surface and its interactions were thoroughly understood using Monte Carlo (MC) computer simulations. Figure 12 shows the adsorption locator module’s best inhibitor molecule adsorption configurations on copper. These designs’ virtually planar patterns suggest improved adsorption and maximum surface coverage52. The MC simulation adsorption energies parameters are shown in Table 6. Notably, GRD exhibits a higher negative adsorption energy value (-3371.0 kcal mol− 1), indicative of robust adsorption on the copper surface. This suggests the development of a strongly adsorbed layer that effectively inhibits the copper corrosion, which is consistent with the results obtained in experimental studies53. Adsorption energy estimates for GRD are shown in Table 6 for both the unrelaxed state (-3244.3 kcal mol− 1) and the relaxed state (-126.7 kcal mol− 1) before and after geometry adjustment, respectively. The higher inhibition proficiency for GRD is supported by these values. The dEads/dNi values, representing the metal-adsorbates configuration energy when the adsorbed inhibitors or water molecules are omitted, are crucial in understanding the strength of adsorption54. For GRD, the dEads/dNi value is (-122.5 kcal mol− 1), as presented in Table 6, which demonstrates exceptional adsorptive properties. In addition, the surrounding water has a low dEads/dNi ratio (-16.3 kcal mol− 1), indicating that inhibitor molecules are being adsorbed more strongly than water molecules. Hence, GRD exhibits strong adhesion to the surface of the copper, developing in the formation of a durable protective layer. This layer effectively safeguards the copper surface against corrosion when exposed to corrosive environments. Both empirical and computational analyses support this result.
The inhibition mechanism
The organic compounds that make up our inhibitor bind to metal surfaces, preventing further metal breakdown by cathodic or anodic processes, or both. Organic inhibitors have the ability to form unbreakable complexes or chelates with the metal ions present on their surface55. The assessment of the corrosion inhibition effectiveness of GRD (major constituents) in the presence of copper corrosion in a 2 M hydrochloric acid solution can be comprehended by considering various factors. These factors include the quantity of adsorption sites, the density of their charge, the size of the molecules, the way they interact with the copper surface, and their capacity to form a complex with the metallic substrate. The π-electrons and free electrons associated with the oxygen and nitrogen atoms establish chemical interactions with the copper metal surface. Based on the potential-pH diagram56, it has been shown that copper oxide coatings with protective properties, namely Cu2O and CuO, tend to readily dissolve under conditions of low pH. The following steps of actions may be used to explain how copper dissolves in an acidic environment:
Cu − e−→ Cu(I)ads (fast).
Cu(I)ads − e−→ Cu(II) (slow).
Cu(I)ads denote a species that has been adsorbed onto the copper surface and doesn’t exhibit diffusion into the bulk solution. The process of reducing oxygen at the cathode may be represented by the following equation:
O2 + 4 H+ + 4e−→ 2H2O.
Two mechanisms have been hypothesized in the literature to explain the high inhibitory efficiency seen for the inhibitors (INH)57. One possible explanation for this phenomenon is the creation of a layer of INH that is adsorbed onto the surface of the metal.
Cu − e−→ Cu(I)ads (fast).
Cu(I)ads − e−→ Cu(II) (slow).
Cu(S) + INH → Cu∶INH(ads).
where Cu: INH(ads) refers to INH adsorbed on the copper surface. The other mechanism proposes the formation of a protective coating composed of Cu(I) IN on the surface, which effectively hinders the anodic dissolution process., i.e.,
Cu+(aq) + INH(aq) → Cu(I)IN(S) + H+(aq)
Less acidic media prefer the Cu(I)IN complex at higher anodic potentials, whereas more acidic media favor the formation of the adsorbed species at higher cathodic potentials.
Conclusions
This work used theoretical and empirical methods to investigate Gast Reg drug (GRD)’s suppression of copper corrosion during acid pickling. GRD demonstrated effective inhibition against copper corrosion, with its inhibitory effects increasing with higher concentrations. The maximum protection capacity reached 85.5%. Polarization investigations suggested that GRD operated as a mixed-type inhibitor. GRD adsorbed onto the metal surface, successfully preventing copper corrosion in HCl solution, according to SEM, EDX, and AFM investigations. The Langmuir isotherm model was employed to describe the adsorption behavior on the metal surface. Density Functional Theory (DFT) calculations illustrated that GRD interacted with the metal interface through donor–acceptor attractions. Additionally, Monte Carlo (MC) simulations suggested that GRD spontaneously adsorbed via its electron-rich sites.
Data availability
All data generated or analysed during this study are included in this published article.
References
Jones, D. A. Principles and prevention. Corrosion 2, 168 (1996).
Shih, H. & Tzou, R. Effect of benzotriazole on the stress corrosion cracking and the electrochemical polarization of 70/30 brass in fluoride solutions. J. Electrochem. Soc. 138 (4), 958 (1991).
Abbas, M. Effects of temperature on dezincification and electrochemical behaviour of 70–30 brass in sulphuric acid. Br. Corros. J. 26 (4), 273–278 (1991).
Al-Matrouk, F. et al. Industrial Corrosion and Corrosion Control Technology, 567–579 (Kuwait Institute for Scientific Research, 1996).
Quartarone, G. et al. Using indole to inhibit copper corrosion in aerated 0.5 M sulfuric acid. Corrosion 54 (8), 606–618 (1998).
Sobhi, M. & Eid, S. Chemical, electrochemical and morphology studies on methyl hydroxyethyl cellulose as green inhibitor for corrosion of copper in hydrochloric acid solutions. Prot. Met. Phys. Chem. Surf. 54, 893–898 (2018).
Eid, S. et al. Surface, electrochemical, and theoretical investigation on utilizing olive leaf extract as green inhibitor for copper corrosion in alkaline environment. Arab. J. Sci. Eng. 49 (1), 147–164 (2024).
Althobaiti, I. O. et al. Evaluation of the impact of two thiadiazole derivatives on the dissolution behavior of mild steel in acidic environments. Molecules 28 (9), 3872 (2023).
Attia, M. et al. Experimental and theoretical study on some azo chromotropic acid dyes compounds as inhibitor for carbon steel corrosion in sulfuric acid. J. Iran. Chem. Soc. 19 (2), 655–664 (2022).
Eid, S. Expired Desloratidine drug as inhibitor for corrosion of carbon steel pipeline in hydrochloric acid solution. Int. J. Electrochem. Sci. 16 (1), 150852 (2021).
Fouda, A. et al. Tenormin drug as save corrosion inhibitor for 304 stainless steel in hydrochloric acid solutions. Der Pharma Chem. 7 (4), 22–33 (2015).
Khudhair, N. A., Kadhim, M. M. & Khadom, A. A. Effect of trimethoprim drug dose on corrosion behavior of stainless steel in simulated human body environment: experimental and theoretical investigations. J. Bio Tribo-Corros. 7 (3), 124 (2021).
Ganapathi Sundaram, R., Vengatesh, G. & Sundaravadivelu, M. Adsorption behavior and anticorrosion capability of antibiotic drug nitroxoline on copper in nitric acid medium. J. Bio Tribo-Corros. 3, 1–13 (2017).
Maduelosi, N. J. & Iroha, N. B. Insight into the adsorption and inhibitive effect of spironolactone drug on C38 carbon steel corrosion in hydrochloric acid environment. J. Bio Tribo-Corros. 7 (1), 6 (2021).
Eddy, N. O., Ebenso, E. E. & Ibok, U. J. Adsorption, synergistic inhibitive effect and quantum chemical studies of ampicillin (AMP) and halides for the corrosion of mild steel in H 2 SO 4. J. Appl. Electrochem. 40, 445–456 (2010).
Geethamani, P. et al. Corrosion inhibition and adsorption properties of mild steel in 1 M hydrochloric acid medium by expired ambroxol drug. J. Bio Tribo-Corros. 5, 1–18 (2019).
El-Katori, E. E. & Abousalem, A. S. Impact of some pyrrolidinium ionic liquids on copper dissolution behavior in acidic environment: experimental, morphological and theoretical insights. RSC Adv. 9 (36), 20760–20777 (2019).
El-Katori, E. E., Ahmed, M. & Nady, H. Imidazole derivatives based on glycourils as efficient anti-corrosion inhibitors for copper in HNO3 solution: synthesis, electrochemical, surface, and theoretical approaches. Colloids Surf. A Physicochem. Eng. Aspects 649, 129391 (2022).
Farahati, R. et al. Evaluation of corrosion inhibition of 4-(pyridin-3-yl) thiazol-2-amine for copper in HCl by experimental and theoretical studies. J. Mol. Struct. 1205, 127658 (2020).
Badawy, W. A., Ismail, K. M. & Fathi, A. M. Corrosion control of Cu–Ni alloys in neutral chloride solutions by amino acids. Electrochim. Acta 51 (20), 4182–4189 (2006).
Radovanović, M. B. et al. Electrochemical and DFT studies of brass corrosion inhibition in 3% NaCl in the presence of environmentally friendly compounds. Sci. Rep. 9 (1), 16081 (2019).
Ismail, K. M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 52 (28), 7811–7819 (2007).
Syam, S. et al. An examination of the effectiveness of the expired drug isoprinosine in preventing aluminum corrosion in alkaline solutions using both computational and experimental techniques. RSC Adv. 14 (16), 11244–11257 (2024).
Abd El-Hafez, G. M. & Badawy, W. A. The use of cysteine, N-acetyl cysteine and methionine as environmentally friendly corrosion inhibitors for Cu–10Al–5Ni alloy in neutral chloride solutions. Electrochim. Acta 108, 860–866 (2013).
Salah Eid, K. A., Soliman, A. Y., El–Etre, E., Gad & Nady, H. Combination of experimental and computational insight into the anti-corrosion performance of 1-(4-tert-butylphenyl)-4-(4-(benzhydryloxy)piperidin-1-yl)butan-1-one onto C-steel in acidic environments. J. Bio Tribo-Corrosion 9(61), 1–14 (2023).
Khadom, A. A. & Yaro, A. S. Mass transfer effect on corrosion inhibition process of copper–nickel alloy in hydrochloric acid by Benzotriazole. J. Saudi Chem. Soc. 18 (3), 214–219 (2014).
Macdonald, J. WB Johnson in: JR Macdonald. Impedance Spectroscopy, 150–170 (Wiley, 1987).
Mansfeld, F. & Shih, H. Detection of pitting with electrochemical impedance spectroscopy. J. Electrochem. Soc. 135, 1171–1172 (1988).
Badawy, W., El-Rabiei, M. & Nady, H. Synergistic effects of alloying elements in Cu-ternary alloys in chloride solutions. Electrochim. Acta 120, 39–45 (2014).
Ma, H. et al. Inhibition of copper corrosion by several Schiff bases in aerated halide solutions. J. Appl. Electrochem. 32, 65–72 (2002).
Sherif, E. & Park, S. M. Inhibition of copper corrosion in acidic pickling solutions by N-phenyl-1, 4-phenylenediamine. Electrochim. Acta 51 (22), 4665–4673 (2006).
Chaubey, N. et al. A comparative study of leaves extracts for corrosion inhibition effect on aluminium alloy in alkaline medium. Ain Shams Eng. J. 8 (4), 673–682 (2017).
Quartarone, G. et al. Investigation of the inhibition effect of indole-3-carboxylic acid on the copper corrosion in 0.5 M H2SO4. Corros. Sci. 50 (12), 3467–3474 (2008).
Khaled, K., Fadl-Allah, S. A. & Hammouti, B. Some benzotriazole derivatives as corrosion inhibitors for copper in acidic medium: experimental and quantum chemical molecular dynamics approach. Mater. Chem. Phys. 117 (1), 148–155 (2009).
Quartarone, G. et al. Inhibitive action of gramine towards corrosion of mild steel in deaerated 1.0 M hydrochloric acid solutions. Corros. Sci. 64, 82–89 (2012).
Ross, P. N. Adsorption of molecules at metal electrodes (1992).
Abdallah, M. et al. Animal glue as green inhibitor for corrosion of aluminum and aluminum-silicon alloys in sodium hydroxide solutions. J. Mol. Liq. 220, 755–761 (2016).
Abdallah, M. et al. Natural occurring substances as corrosion inhibitors for tin insodium bicarbonate solutions. J. Korean Chem. Soc. 53 (5), 485–490 (2009).
Syam, S. et al. Combination of practical and theoretical measurements of albumin egg as an eco-friendly inhibitor for copper corrosion in alkaline solutions. RSC Adv. 13 (48), 33929–33942 (2023).
Solmaz, R., Altunbaş, E. & Kardaş, G. Adsorption and corrosion inhibition effect of 2-((5-mercapto-1, 3, 4-thiadiazol-2-ylimino) methyl) phenol Schiff base on mild steel. Mater. Chem. Phys. 125(3), 796–801 (2011).
Boulhaoua, M. et al. Synthesis, structural analysis and corrosion inhibition application of a new indazole derivative on mild steel surface in acidic media complemented with DFT and MD studies. Colloids Surf. A Physicochem. Eng. Aspects 617, 126373 (2021).
Abd El-Lateef, H. M., Shalabi, K. & Tantawy, A. H. Corrosion inhibition and adsorption features of novel bioactive cationic surfactants bearing benzenesulphonamide on C1018-steel under sweet conditions: combined modeling and experimental approaches. J. Mol. Liq. 320, 114564 (2020).
Palaniappan, N., Cole, I. & Kuznetsov, A. Experimental and computational studies of graphene oxide covalently functionalized by octylamine: Electrochemical stability, hydrogen evolution, and corrosion inhibition of the AZ13 mg alloy in 3.5% NaCl. RSC Adv. 10 (19), 11426–11434 (2020).
Obot, I., Macdonald, D. & Gasem, Z. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: an overview. Corros. Sci. 99, 1–30 (2015).
Lukovits, I., Kalman, E. & Zucchi, F. Corrosion inhibitors—correlation between electronic structure and efficiency. Corrosion 57(1), 3–8 (2001).
Upadhyay, A. et al. Verification of corrosion inhibition of mild steel by some 4-Aminoantipyrine-based Schiff bases–impact of adsorbate substituent and cross-conjugation. J. Mol. Liq. 333, 115960 (2021).
Abd El-Lateef, H. M., Shalabi, K. & Tantawy, A. H. Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: experimental and computational investigations. New J. Chem. 44 (41), 17791–17814 (2020).
Oyebamiji, A. & Adeleke, B. Quantum chemical studies on inhibition activities of 2, 3-dihydroxypropyl-sulfanyl derivative on carbon steel in acidic media. Int. J. Corros. Scale Inhib. 7 (4), 498–508 (2018).
Madkour, L. H., Kaya, S. & Obot, I. B. Computational, Monte Carlo simulation and experimental studies of some arylazotriazoles (AATR) and their copper complexes in corrosion inhibition process. J. Mol. Liq. 260, 351–374 (2018).
Gece, G. & Bilgiç, S. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros. Sci. 51 (8), 1876–1878 (2009).
Shalabi, K. et al. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: experimental and theoretical studies. J. Mol. Liq. 293, 111480 (2019).
El Aadad, H. et al. Improvement of the corrosion resistance of mild steel in sulfuric acid by new organic-inorganic hybrids of Benzimidazole-Pyrophosphate: facile synthesis, characterization, experimental and theoretical calculations (DFT and MC). Surf. Interfaces. 24, 101084 (2021).
Dehghani, A. et al. A detailed study on the synergistic corrosion inhibition impact of the quercetin molecules and trivalent europium salt on mild steel; electrochemical/surface studies, DFT modeling, and MC/MD computer simulation. J. Mol. Liq. 316, 113914 (2020).
Haque, J. et al. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: a combined experimental and theoretical approach. J. Ind. Eng. Chem. 49, 176–188 (2017).
Mistry, B. et al. Experimental and quantum chemical studies on corrosion inhibition performance of quinoline derivatives for MS in 1 N HCl. Bull. Mater. Sci. 35, 459–469 (2012).
Mzioud, K. et al. Inhibition of copper corrosion by the essential oil of Allium sativum in 0.5 MH 2 SO 4 solutions. SN Appl. Sci. 2, 1–13 (2020).
Khaled, K. & Amin, M. A. Dry and wet lab studies for some benzotriazole derivatives as possible corrosion inhibitors for copper in 1.0 M HNO3. Corros. Sci. 51 (9), 2098–2106 (2009).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S. M. Syam, H. Nady, Salah Eid and Emad E. El-Katori did the electrochemical experiments, calculations, and drafting of the electrochemical study part. S. M. Syam, H. Nady, Salah Eid and Emad E. El-Katori provided the concept and wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
All procedures were performed in accordance with the Guidelines for Care and Use of Laboratory of Benha University and approved by the Ethics Committee of faculty of science (BUFS - REC- 2024 − 169 chm).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Syam, S.M., Nady, H., Eid, S. et al. Experimental and theoretical assessment to investigate the impact of Gast Reg drug on the copper corrosion control in an acidic environment. Sci Rep 15, 5216 (2025). https://doi.org/10.1038/s41598-024-84041-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84041-8